Abstract
Introduction
Lung cancer is the most common cancer worldwide. Every year, as many people die of lung cancer as of breast, colon and rectum cancers combined. Because most patients are being diagnosed in advanced, not resectable stages and therefore have a poor prognosis, there is an urgent need for alternative therapies. Since it has been demonstrated that a high number of tumor- and stromal-infiltrating cytotoxic T cells (CTLs) is associated with an increased disease-specific survival in lung cancer patients, it can be assumed that immunotherapy, e.g. peptide vaccines that are able to induce a CTL response against the tumor, might be a promising approach.
Methods
We analyzed surgically resected lung cancer tissues with respect to HLA class I- and II-presented peptides and gene expression profiles, aiming at the identification of (novel) tumor antigens. In addition, we tested the ability of HLA ligands derived from such antigens to generate a CTL response in healthy donors.
Results
Among 170 HLA ligands characterized, we were able to identify several potential targets for specific CTL recognition and to generate CD8+ T cells which were specific for peptides derived from cyclin D1 or protein-kinase, DNA-activated, catalytic polypeptide and lysed tumor cells loaded with peptide.
Conclusions
This is the first molecular analysis of HLA class I and II ligands ex vivo from human lung cancer tissues which reveals known and novel tumor antigens able to elicit a CTL response.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1454-2) contains supplementary material, which is available to authorized users.
Keywords: Lung cancer, Immunotherapy, Epitope, HLA, Antigen processing
Introduction
Lung cancer is the most common cancer worldwide with more than 1.6 million new cases each year. More people die of lung cancer than of colon, breast and prostate cancer combined. The combined 5-year survival rate for all tumor stages is around 15 % for men and 18 % for women [1] (World Cancer Report 2008).
There are two types of lung cancer: 80 % of all lung cancers are nonsmall-cell lung cancers (NSCLC), 20 % are small-cell lung cancers (SCLC). Prognosis of the latter is extremely poor, and surgical intervention is performed very rarely. Since most lung cancers do not cause symptoms until they have spread, the majority of cases (60 %) are being diagnosed in locally advanced or metastasized stages with poor prognosis in spite of multimodality treatment regimes. Therefore, lung cancer still ranks among the most aggressive forms of cancer.
Previous studies demonstrated that HLA class I expression is associated with a favorable prognosis and less lymph node metastases in NSCLC [2, 3]. It has been shown that CTLs preferentially migrate into the tumor epithelium, which may be evidence for an antitumor response mediated by CTLs in NSCLC [4]. Moreover, a high number of tumor- and stromal-infiltrating CTLs was associated with an increased disease-specific survival [5]. These results suggest a crucial role of T-cell-mediated immune response for the course of disease and raise hope that immunotherapy, e.g. peptide vaccines that induce a CTL response against the tumor, might be a promising approach.
The aim of this study was to identify HLA class I- and II-presented peptides which are processed from proteins typically expressed in lung cancer tissue, and therefore are characteristic for the tumor in vivo, and to analyze their potential as immunotherapeutic agents.
A frequently applied classification of tumor-associated antigens (TAA) relies on gene expression analysis that allows for the detection of proteins highly overexpressed in tumor tissue accompanied by relatively low or even no expression in normal tissues, so that we can assume tumor association. Using mass spectrometry (LC/MS)-based peptide sequencing, HLA-presented peptides are identified directly after extraction from tumor tissue, so that these peptides are presented on tumor or tumor stroma cells, thus eventually being able to mediate an immune response against the tumor. The strength of our approach lies in combining these two powerful and well-established analytical tools, so we are able to identify candidate peptides for immunotherapy by mass spectrometry, whose source proteins are then evaluated for their gene expression levels in tumor tissue and different normal tissues with the aim to identify antigens that are either selectively expressed or highly overexpressed in tumors.
Here, we describe—among many other HLA ligands identified from solid lung cancer tissues—two candidate peptides for immunotherapy able to generate a peptide-specific CTL response.
Materials and methods
Patients and tumor samples
Tissue samples from lung cancer patients were provided by the Department for Thoracic, Cardiac and Vascular Surgery, University of Tübingen, Germany, after written informed consent had been obtained from each patient. Specimens were frozen in liquid nitrogen immediately after surgery. HLA typing was carried out by the Institute for Clinical and Experimental Transfusion medicine; pathological staging was performed by the Department of Pathology, University Hospital of Tübingen. This study has been approved by the local Institutional Ethical Review Board.
Elution of HLA-presented peptides
HLA-presented peptides were isolated from frozen tumor samples by immunoprecipitation of HLA molecules using the HLA-A-, HLA-B- and HLA-C-specific antibody W6/32 (American Type Culture Collection (ATCC), HB-95) and the HLA-DR-specific antibody L243 (ATCC, HB-55, both produced in-house from hybridoma cultures following standard protocols) as described previously [6, 7]. HLA yield was determined by Edman degradation using a pulsed-liquid protein sequencer Procise 494A (Applied Biosystems).
Peptide analysis and sequence determination
Microcapillary LC–MS/MS (liquid chromatography–tandem mass spectrometry) peptide analysis was carried out using a reversed-phase HPLC system (Eksigent) coupled directly to a hybrid quadrupole orthogonal acceleration time of flight mass spectrometer (Q-TOF Ultima, Micromass), equipped with a micro-ESI (electrospray) source. Sequence analysis was carried out manually as described elsewhere in detail [8]. Database searches (National Center for Biotechnology Information, Expressed Sequence Tag) were carried out using the multiple alignment system for protein sequences based on three-way dynamic programming (MASCOT, http://matrixscience.com). It has to be noted that mutated peptides cannot be characterized by this strategy, since only sequences available in public databases can be used for fragment spectra interpretation. Assignment of peptide sequences to specific HLA allotypes was based upon known peptide motifs in agreement with the HLA typing of the samples using the epitope prediction program SYFPEITHI. All natural HLA ligands will be included in the next update of the HLA ligand database SYFPEITHI (http://www.syfpeithi.de).
HLA class II immunohistology
Tumors were fixed in 4 % phosphate buffered formaldehyde, embedded in paraffin, stained with H&E and examined by light microscopy. Diagnosis of the NSCLC was carried out according to routine histopathologic and immunohistologic investigations. For immunohistologic detection of HLA class II molecules, 5-μm paraffin-embedded tissue sections were pretreated with 10 mM citrate buffer (pH 6) followed by incubation either with a mouse anti-HLA-DR α chain monoclonal antibody (Dako, Hamburg, clone TAL.1B5, 1:50) or mouse IgG1 (2 μg/ml, BD Biosciences PharMingen, San Diego, CA) and visualized using the Ventana iView 3,3 diaminobenzidine detection kit (Nexes System, Ventana Medical Systems, Illkirch, France). Tissue sections were counterstained with hematoxylin and finally embedded in Entellan.
Gene expression analysis by high-density oligonucleotide microarrays
RNA isolation from lung cancer tissue specimens was carried out by the Microarray Facility, University of Tübingen. Gene expression analysis using Affymetrix Human Genome (HG) U133 Plus 2.0 oligonucleotide microarrays was performed as previously described [9].
Total RNA from healthy human tissues (“bulk” RNA isolations = mixture of all cell types contained in the respective tissue) was obtained commercially (Ambion, Huntingdon, UK; Clontech, Heidelberg, Germany; Stratagene, Amsterdam, Netherlands; BioChain, Hayward, CA, USA). The RNA from several individuals (between 2 and 123 individuals) was mixed such that RNA from each individual was equally weighted. RNA quality for all RNA samples was determined using the RNA 6000 Pico LabChip kit on a 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany).
Gene expression analysis of all tumor and normal tissue RNA samples was performed by Affymetrix Human Genome (HG) U133A (e.g., bladder, bone marrow, brain, colon, heart, liver, lung, placenta) or HG-U133 Plus 2.0 (all NSCLC samples, e.g., artery, breast, esophagus, gall bladder, ovary, pancreas, skin, vein) oligonucleotide microarrays (Affymetrix, Santa Clara, CA, USA). The same normal kidney sample was hybridized to both array types to achieve direct comparability of all samples. All steps were carried out according to the Affymetrix manual (http://www.affymetrix.com/support/technical/manual/expression_manual.affx). Images were scanned with the Agilent 2500A GeneArray Scanner (U133A) or the Affymetrix Gene-Chip Scanner 3000 (U133 Plus 2.0), and data were analyzed with the GCOS software (Affymetrix), using default settings for all parameters. Pairwise comparisons were calculated using the respective normal kidney array as baseline. For normalization, 100 housekeeping genes provided by Affymetrix were used (http://www.affymetrix.com/support/technical/mask_files.affx). Relative expression values were calculated from the signal log ratios given by the software, and the normal lung sample was arbitrarily set to 1.0.
All expression data were deposited according to the guidelines of the microarray gene expression data society (MGED) following the minimum information about a microarray experiment (MIAME) standards. Gene expression data can be found under GSE27556.
In vitro stimulation of human CD8+ T cells using dendritic cells
T-cell culture medium consisted of Iscove’s modified Dulbecco’s medium, 50 U penicillin ml−1, 50 μg streptomycin ml−1 and 25 μg gentamicin ml−1 (all from Lonza), 50 μM β-mercaptoethanol and 10 % heat-inactivated human AB serum (CC pro).
Peripheral blood mononuclear cells (PBMCs) were isolated from leukapheresis products by standard ficoll gradient separation and cryopreserved in fetal calf serum with 10 % DMSO until use. Dendritic cells were isolated from PBMCs by plastic adherence, as described elsewhere in detail [7, 10].
Autologous CD8+ T cells were positively selected by MACS separation and in vitro cultured for 1 week with 2 ng/ml IL-2 (BD Biosciences) and 5 μg/ml IL-7 (BD Biosciences). After 6 days of culture, T cells were harvested, pooled and counted.
Mature DCs were pulsed for 2–3 h with 25 μg/ml of peptide in T-cell culture medium. In vitro stimulations were initiated in 24-well plates with 30 × 105 CD8+ T cells plus 5 × 105 DCs per well in 2 ml of T-cell culture medium. After 7 days of incubation, T cells were restimulated with autologous DCs. IL-2 (BD Biosciences) was added every other day, and IL-7 (BD Biosciences) was added every 6 days. Cryopreserved, peptide-loaded, irradiated autologous PBMCs were used for later restimulation cycles. After 3 restimulations, T cells were analyzed by intracellular cytokine staining.
Intracellular IFN-γ staining
Autologous PBMCs were pulsed with 10 μg peptide ml−1 and incubated in the presence of Brefeldin A (Sigma Aldrich) for at least 6 h. Cells were analyzed using Cytofix/Cytoperm (BD Biosciences), CD3-FITC, CD8-PerCP, CD4-APC, TNFα-PacificBlue and IFN-γ-PE (BD Biosciences). For negative controls, cells were incubated with HIV peptide (GSEELRSLY, A*01, because leukapheresis donors were A*01 positive), respectively. Stimulation with phorbol-12-myristate-13-acetate (PMA)/ionomycin was used as a positive control. Cells were analyzed on an eight-color FACS Canto II.
51Cr release assay
The standard 51Cr-release assay was performed as described [11]. Target cells were pulsed with 10 μg/ml peptide for 1 h and labeled with [51Cr]-sodium chromate in X-Vivo 15 for 1 h at 37 °C. Target cells (104) were transferred to a well of a round-bottomed 96-well plate. Varying numbers of CTL were added to give a final volume of 200 μl and incubated for 4–19 h at 37 °C. At the end of the assay, supernatants (50 μl/well) were harvested and counted in a beta-plate counter. The percentage of specific lysis was calculated as follows: 100 × (experimental release—spontaneous release/maximal release—spontaneous release). Spontaneous and maximal release were determined in the presence of either medium or 1 % Triton X-100, respectively.
Results
Patient-individual analysis of peptides presented on lung cancer tissue
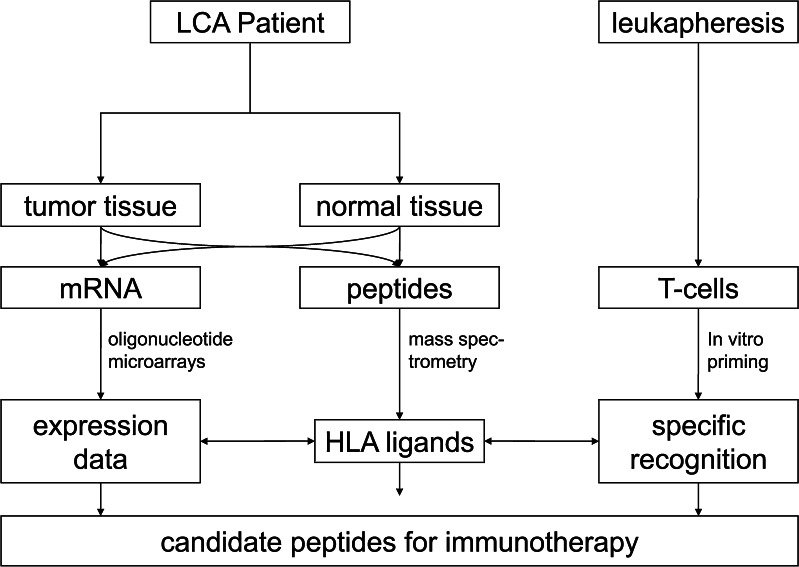
The general outline of the study is depicted in Fig. 1. While HLA ligand identification reveals the primary structure of HLA ligands, complementary information from gene expression profiling and immunogenicity testing identified candidate peptides that may serve as targets for immunotherapeutic approaches. Two analyzed NSCLC tumor samples included one primary squamous cell carcinoma and one primary adenocarcinoma. In addition, one metastasis of a colon carcinoma in the lung was analyzed. Tumors were moderately to poorly differentiated and the patients’ disease ranged from pT2 to pT4. Additional lung cancer samples were used for gene expression analysis only (Table 1).
Fig. 1.
Patient-individual analysis of HLA-presented peptides and gene expression patterns. HLA ligands were extracted from primary tumor tissue of each lung cancer patient. Characterization of HLA peptide presentation was done by performing on-line tandem mass spectrometry. In addition, gene expression profiling was performed using oligonucleotide microarrays. HLA ligands classified as tumor-associated were used to elicit CTL responses in vitro
Table 1.
List of lung cancer specimens studied
| Tissue | Histology | Stage (TNM), grading | HLA typing |
|---|---|---|---|
| LCa008 | Squamous cell carcinoma | pT2pN1Mx, G2 | A*01, A*02, B*14, B*35, DRB1*01, DRB1*15, DRB5 |
| LCa010 | Metastasis of a colon carcinoma | pT3N0M1, G2 | A*03, A*29, B*35, B*44, DRB1*01, DRB1*07, DRB4 |
| LCa011 | Adenocarcinoma | pT1NxM1, G2 | A*02, B*35, B*38, DRB1*11, DRB1*12, DRB3 |
| LCa018 | Adenocarcinoma | TNM unknown, G3 | A*24, B*18, B*35, DRB1*11, DRB1*14, DRB3 |
| LCa020 | Adenocarcinoma | pT4pN0pMx, G3 | A*03, A*24, |
| B*35, B*4901, DRB1*01, DRB1*13, DRB3 | |||
| LCa021 | Adenocarcinoma | pT1NxM1, G2 | A*02, B*35, B*38, DRB1*11, DRB1*12, DRB3 |
The lung cancer tissues weighed around 2–3 g, and the HLA yield determined by Edman degradation ranged between 160 and 197 pmol/g for HLA class I, and from 22 to 160 pmol/g for MHC class II, which in our experience is well within a normal range for tumor tissues [12]. After HLA immunoprecipitation and peptide extraction, we were able to identify more than 170 HLA ligands from three cancer tissue preparations (supplemental Table 1). To our knowledge, this is the first report on the characterization of naturally presented HLA ligands in lung cancer tissue. These peptides derive from more than 140 source proteins and were presented by various HLA allotypes. There was only one peptide overlapping with HLA-A*02 ligands from lung cancer cell lines reported recently [13], indicating considerable differences between primary tissues and permanent cell lines.
The largest group of peptides derived from classical self-proteins, i.e. constitutively expressed enzymes and receptors, structural proteins, serum proteins and various others without obvious tumor association. A second group of peptides derived from well-known and already described tumor antigens such as cyclin D1 (CCND1), proliferating cell nuclear antigen (PCNA), napsin A aspartic peptidase (NAPSA), epoxide hydrolase 1, microsomal (xenobiotic) (EPHX1), sequestosome 1 (SQSTM1) or member of RAS oncogene family (RAN) [14–19], some of which have already been used in clinical vaccination studies for other tumor entities. The third category consists of peptides whose source proteins have not yet been described to play an important role in lung carcinogenesis or have not been demonstrated to induce an immune response against lung cancer cells. These represent novel candidate TAAs for NSCLC, for example signal transducer and activator of transcription 1 (STAT1) or protein-kinase, DNA-activated, catalytic polypeptide (PRKDC) (Table 2).
Table 2.
Candidate peptides for immunotherapy of lung cancer
| Peptide | HLA | Gene symbol | Gene | Reference |
|---|---|---|---|---|
| DRVLRAML | B*14 | CCND1 | Cyclin D1 | This paper, [19, 29, 30] |
| DRLLALNSL | B*14 | PRKDC | Protein kinase, DNA-activated, catalytic polypeptide | This paper, [49–51] |
| IADMGHLKY | A*01 | PCNA | Proliferating cell nuclear antigen | [18, 28] |
| VLWDRTFSL | A*02 | STAT1 | Signal transducer and activator of transcription 1 | [25–27] |
| ARKLIGDPNLEF | DRB1*15 | RAN | RAN, member RAS oncogene family | [14, 22, 23] |
| FSDEGGWLTRL | DRB1*15 | SQSTM1 | Sequestosome 1 | [17] |
| GPTEEIRALHAAIGGIP | DRB1*01 | NAPSA | Napsin A aspartic peptidase | [15, 24] |
| WTTGTIISSQRF | DRB1*01 | EPHX1 | Epoxide hydrolase 1, microsomal (xenobiotic) | [16] |
All peptides have been eluted from lung cancer tissue; HLA association was assigned by their anchor residues according to the database SYFPEITHI (www.syfpeithi.de). Source proteins have been reported to play an important role in carcinogenesis. Moreover, some showed overexpression in lung cancer tissue compared to healthy tissues
Regarding allotypic peptides that are reliably found on cell lines and tissues expressing a given HLA allotype, we identified YLLPAIVHI (HLA-A*02) and KIADRFLLY (HLA-A*03), corresponding to the HLA typing of the tissue samples. From several antigens, different peptides were identified, e.g. from clathrin, heavy chain (CLTC), complement component 1, q subcomponent, B chain (C1QB), collagen, type VI, alpha 3 (COL6A3) or vimentin (VIM), or length variants were presented such as peptides derived from lymphocyte cytosolic protein 1 (LCP1) or CD14. Moreover, we were able to identify peptides from SQSTM1, cytochrome c oxidase subunit VIIIA (COX8A) and CDGSH iron sulfur domain 2 (CISD2), which are presented on HLA class I as well as on HLA class II. We only identified three peptides (MPVGPDAILRY derived from BCL2-associated athanogene 6 (BAT3), GVAPFTIAR derived from COL6A3 and NVIRDAVTY derived from histone cluster 1, H4a (HIST1H4A)) that were abundantly presented on different tissue samples, which underlines the importance of a patient-individual concept of immunotherapy.
Identification of HLA class II-extracted peptides from lung cancer tissues
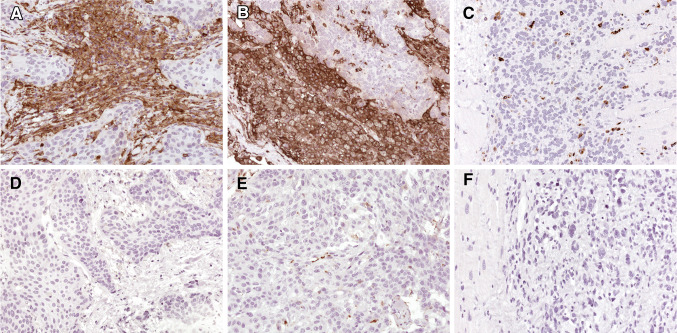
Since CD8+ and CD4+ T cells should be activated to elicit a long-lasting antitumor immune response, we isolated HLA-DR ligands from two of three lung cancer samples. Similar to the results of HLA class I ligand analysis, we mainly found peptides without obvious tumor association, among them most probably HLA-DR ligands from infiltrating immune cells, for example, derived from CD14 (supplemental Table 1). On the other hand, we were able to identify peptides from known and novel tumor antigens such as RAN, EPHX1, SQSTM1 or NAPSA (Table 1). So our data suggest that the eluted peptides derive from HLA class II-positive tumor cells and infiltrating leukocytes, although a clear distinction between the sources of HLA class II ligands was not possible. Under normal, noninflammatory conditions, class II molecules should only be expressed by cells of the hematopoietic system and by thymic epithelium. In inflammation, HLA class II expression can be induced in most cell types and tissues by IFN-γ [20]. In a recent study, we observed HLA class II expression in renal cell carcinoma [21] and therefore performed immunohistochemical staining of the lung cancer tissues. As exemplarily shown in two cases, we found an intense staining of HLA-DR molecules primarily on tumor cells (Fig. 2).
Fig. 2.
Immunohistochemistry of lung cancer tissues. Expression of HLA class II molecules in NSCLC of two patients was analyzed using HLA-DR-specific antibodies. Both LCa008 (a) and LCa010 (b) demonstrated strong HLA-DR expressing on tumor cells. A rhabdomyosarcoma of the heart (c) was included as an example of a HLA-DR-negative tumor control, with only infiltrating macrophages being positive for MHC class II. Incubation with mouse IgG instead of specific antibodies consistently revealed negative staining results (d–f). Magnification was 200 fold
All HLA class II ligands identified should have been presented by HLA-DR molecules since the DR-specific antibody L243 was used for immunoprecipitation. Among 30 HLA-DR ligands, four were derived from TAA. Peptide ARKLIGDPNLEF is contained in the sequence of the GTPase RAN, which is associated with local invasion and metastasis, e.g. in human clear cell renal cell carcinoma [14] and a poor prognosis in serous epithelial ovarian cancer [22]. Moreover, T-cell epitopes derived from RAN have been identified which were able to induce tumor-reactive CTLs in cancer patients [23].
HLA-DR ligand GPTEEIRALHAAIGGIP derives from NAPSA (Napsin A), an aspartic proteinase which can be found in kidneys and lungs, where it is expressed in type II pneumocytes and involved in the maturation of surfactant protein B. It is overexpressed in renal cell carcinomas and sometimes in papillary thyroid carcinomas. Moreover, it has been identified as a sensitive marker for pulmonary adenocarcinoma where its expression is associated with a high degree of differentiation [15, 24].
From EPHX1, the HLA-DR ligand WTTGTIISSQRF was identified. EPHX1 is a critical biotransformation enzyme which plays a dual role in the detoxification and activation of tobacco procarcinogens. It is a phase II metabolic enzyme catalyzing the hydrolysis of epoxides, thus making them water soluble and less reactive. However, from some hydrocarbon species present in tobacco smoke, more reactive and mutagenic compounds are generated. Because of this dual role, EPHX1 can be considered as a risk factor for lung cancer as well as a protective factor, depending on the respective carcinogen. Moreover, a reduced EPHX1 activity could be protective against lung cancer [16].
The peptide FSDEGGWLTRL derives from SQSTM1, a protein that directly contributes to tumorigenesis, for example in multiple myeloma [17]. It was overexpressed in one of our patients and showed relevant expression only in normal skeletal muscle and placenta. This suggests that SQSTM1-derived peptides can be used for vaccination in particular cases in a patient-individual concept of immunotherapy.
Gene expression analysis
Antigens were considered as overexpressed when the mRNA expression of the source protein was increased at least twofold in the respective tumor tissue compared with normal lung tissue and also markedly increased compared with other healthy tissues. In seven lung cancer samples analyzed for gene expression, about 20 proteins showed a more than 400-fold overexpression compared with normal lung tissue (Table 3) in a quite individual manner. Interestingly, one of the most prominent tumor antigens, melanoma antigen family A, 1 (MAGEA1), was abundantly expressed by two lung cancer tissues (LCa008 and LCa018) while it was not among the highly overexpressed antigens in all other samples, which again reflects the individual molecular differences in human tumors. Since a number of functional T-cell epitopes from MAGEA1 have been reported, immunotherapy for LCa008 and LCa018 might include such epitopes according to the respective HLA typing. In addition to MAGEA1, other well-known TAAs demonstrated dramatic overexpression in the range of several hundredfold increased values (Table 2).
Table 3.
Ranking of the highest gene expression values in seven lung cancer tissues
| Gene symbol | LCA003 | LCA008 | LCA010 | LCA011 | LCA018 | LCA020 | LCA021 |
|---|---|---|---|---|---|---|---|
| MAGEA1 | 4,705 | 4,390 | |||||
| WDHD1 | 315 | 891 | 676 | 128 | |||
| TOP2A | 388 | 478 | 446 | ||||
| MELK | 512 | 294 | |||||
| IGHD | 119 | 630 | |||||
| SYCP2 | 362 | ||||||
| ATP2A3 | 147 | 128 | |||||
| PLK4 | 119 | 338 |
Given are the overexpression ratios as compared to normal lung tissue
Evaluation of tumor-associated HLA ligands by complementary analysis of gene expression
Regarding their expression profiles, the source proteins of the identified HLA ligands could be divided into three groups. Due to the house-keeping character of most source proteins (see above), overexpression in tumors was neither expected nor observed. In contrast to that, a subset of proteins was found to be repeatedly upregulated in lung cancer tissues and showed a very low or even no expression in normal lung or other healthy tissues. Some of these proteins are already known as TAAs in NSCLC, such as CCND1 or PCNA. Others, like PRKDC or STAT1, have not been described as TAAs in NSCLC yet, but play an important role in the carcinogenesis of other tumor entities, as it is the case for STAT1.
Being derived from STAT1 (signal transducer and activator of transcription 1), we could identify the peptide VLWDRTFSL, which carries an HLA-A*02 motif. It belongs to the STAT protein family, which plays a key role in interferon signaling, embryonic development, innate and adaptive immune function and the regulation of cell differentiation, growth and apoptosis. Although STAT1 has been shown to be overexpressed in several human cancers, it also has been associated with tumor growth suppression, contributing to an interferon-dependent tumor surveillance system [25]. On the other hand, STAT1 was found to be overexpressed in radioresistant melanomas, where its knockdown significantly impaired the migratory and invasive capacity of metastatic melanoma cells [26]. It also seems to play a crucial role for radioresistance in renal cell carcinomas [27]. In our experiments, STAT1 was overexpressed in nearly all tumor tissue samples (up to 9-fold), but also showed relevant expression in the normal tissues, so one could expect some adverse effects by targeting this antigen.
Peptide IADMGHLKY (HLA-A*01), derived from PCNA, is an enzyme involved in DNA replication and DNA repair. It was found to be overexpressed in breast cancer [28] and lung cancer patients. Moreover, patients whose tumors contained a high proportion of PCNA-positive cells had significantly shorter median survival times [18]. In our experiments, PCNA was overexpressed up to 7.5-fold in nearly all tumor samples but showed significant expression in normal bone marrow (4-fold), testis (3-fold), thymus (3.5-fold) and ovary (3.5-fold).
Two HLA class I ligands encompassing the HLA-B*14 peptide motif attracted our interest most, one derived from CCND1 and the other from PRKDC.
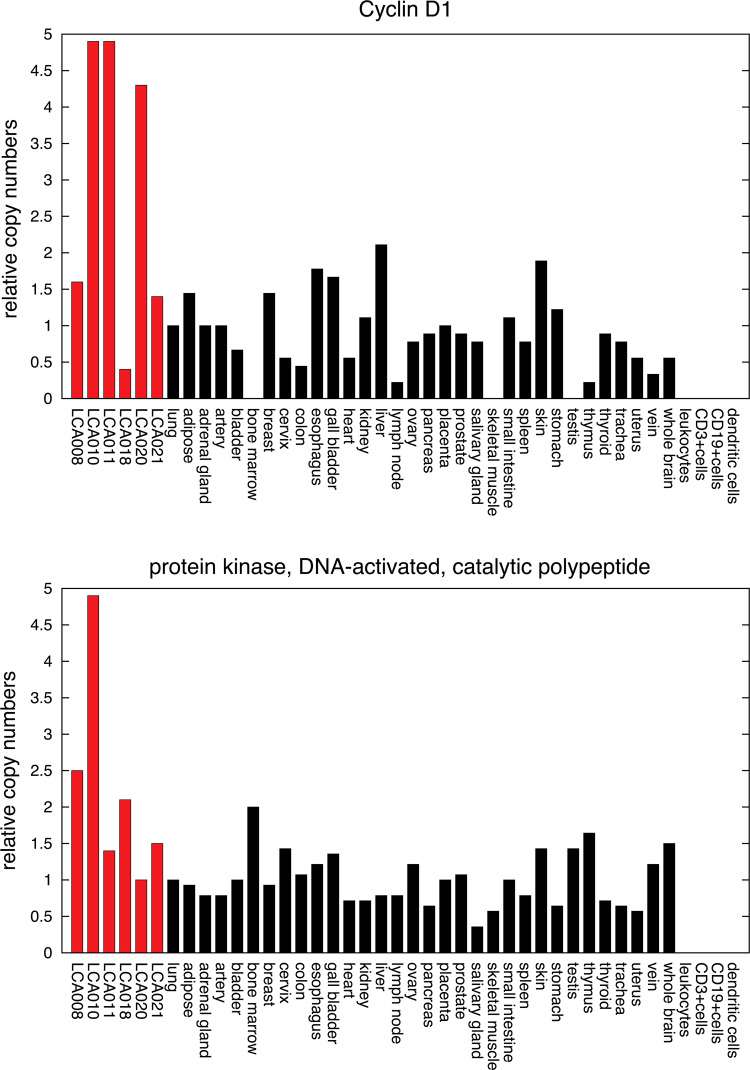
Mutations, amplification and overexpression of CCND1 have been observed frequently in a variety of tumors. For example, it is overexpressed in most breast cancers, and its ablation can protect against breast cancer development [19]. Moreover, CCND1 has been identified as a key driver of malignant transformation in lung cancer, and its overexpression is an indicator of poor prognosis in NSCLC [29, 30]. In our investigations, CCND1 was upregulated in most lung cancer specimens with no relevant expression in healthy tissues (Fig. 3). Several peptides derived from CCND1 have been identified as T-cell epitopes and have been included in clinical vaccination trials. Therefore, we determined the immunogenicity of peptide DRVLRAML in the context of HLA-B*14 by stimulating T cells from healthy donors in vitro.
Fig. 3.
mRNA expression of CCND1 (Probeset ID 208712_at) and PRKDC (Probeset ID 208694_at) in lung cancer tissues and various healthy human tissues. Relative expression values were normalized to lung (expression = 1). CCND1 showed overexpression in most lung cancer specimens with no relevant expression in healthy tissues, and PRKDC was overexpressed in three tumor tissue samples and showed low expression in healthy tissues
A second peptide assigned to HLA-B*14 and used for T-cell stimulation was derived from PRKDC, which was overexpressed in three lung cancer tissue samples and had a relatively low expression regarding the normal tissue samples (Fig. 3). By searching the gene expression omnibus (GEO) database, we found several data that showed PRKDC to be highly expressed in all disease stages of lung cancer. Moreover, it was significantly overexpressed in pulmonary adeno- and squamous cell-carcinoma samples, which were compared to their adjacent normal tissues, and in most cases of malignant pleural mesotheliomas [31–35]. PRKDC is essential for DNA repair, thus playing a role in the development of chemo- and radioresistance of cancer cells, and is frequently observed to be overexpressed in various cancer entities (e.g., radioresistant cervical cancers) [36]. A frequent overexpression of this molecule has also been reported in NSCLC, where it was associated with a significantly shorter median survival and an increased risk of death. Most important, to our knowledge, no T-cell epitopes derived from PRKDC have been described yet. In summary, PRKDC seems to play a crucial role in lung tumorigenesis, justifying further investigations of the peptide DRLLALNSL in the context of HLA-B*14 for suitability in immunotherapy.
Induction of CD8+ T cells specific for HLA ligands from CCND1 and PRKDC
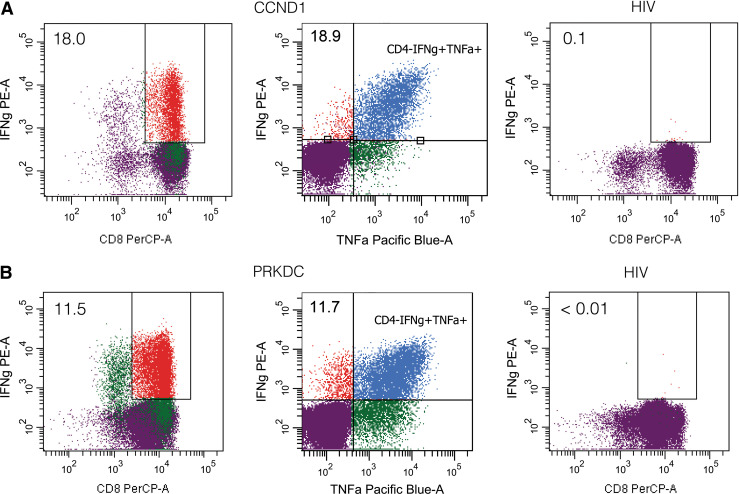
Both peptides have been eluted from a HLA-B*14 positive lung tumor and encompass the B*14 peptide motif (R in position 2, L at the C-terminus). To investigate whether these peptides can be recognized by functional cytotoxic T cells (CTLs), we stimulated CD8+ T cells of HLA-B14+ healthy blood donors in vitro with peptide-loaded DCs and subsequently with autologous peptide-loaded PBMCs. After four stimulations, intracellular cytokine levels were assessed by flow cytometry. T cells were stained with CD3-FITC, CD4-APC and CD8-PerCP antibodies and intracellularly with IFN-γ-PE and TNFα-PacificBlue to determine the percentage of cytokine-producing cells in specific T-cell subpopulations. Stimulations with an irrelevant peptide or without any peptide served as negative controls in every experiment. We considered an IFN-γ response as positive if the percentage of IFN-γ producing CD8+ T cells was >2-fold higher compared to the negative controls.
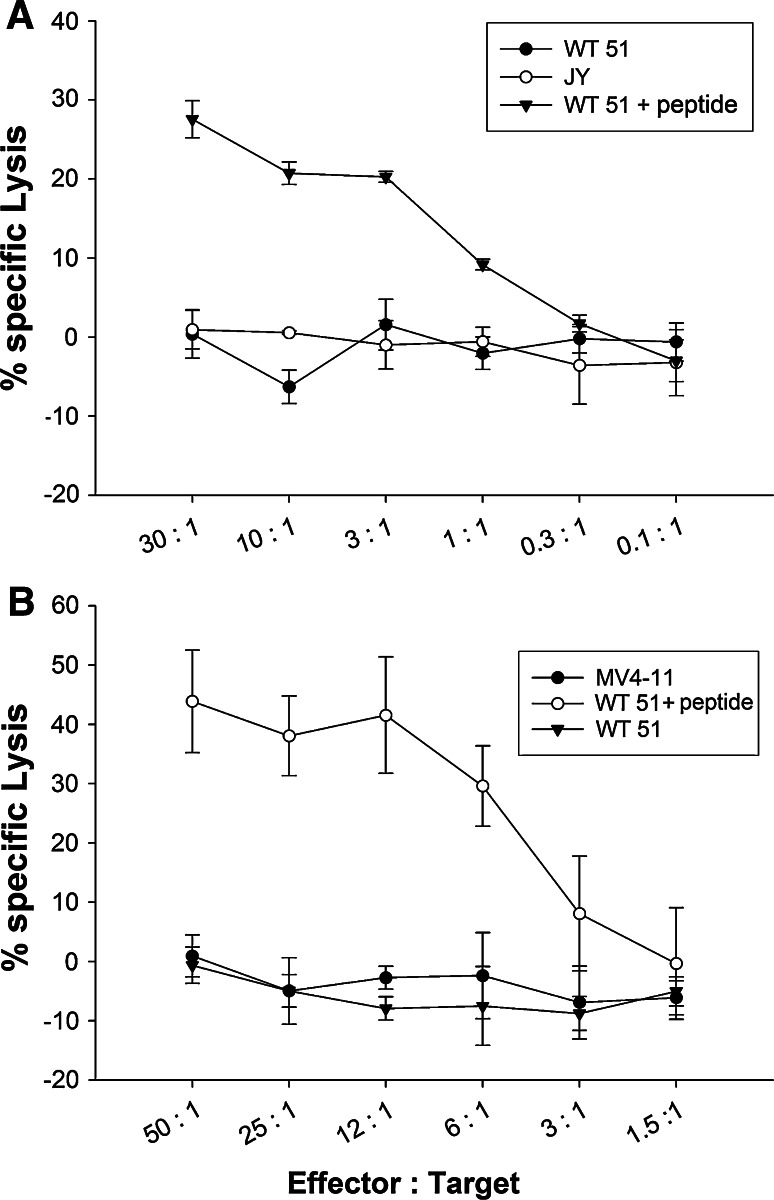
Both peptides were able to induce IFN-γ-producing cells in 4 different healthy blood donors. The frequency of DRVLRAML (CCND1)-specific CD8+ T cells producing IFN-γ ranged between 0.1 and 18 %. For the DRLLALNSL(PRKDC)-specific cells, frequencies of IFN-γ producing cells between 0.2 and 16.3 % were obtained. Most IFN-γ producing CD8+ cells were simultaneously secreting TNFα. Representative flow cytometry dot blots are shown in Fig. 4. Furthermore, we analyzed the cytolytic potential of the peptide-specific and cytokine secreting CTL lines in a chromium release assay (Fig. 5). The DRVLRAML (CCND1)-specific CD8+ T cells were able to lyse the B*14 positive tumor cell line WT 51 loaded with DRVLRAML peptide, but did not lyse unloaded WT 51 cells (Fig. 5a) nor the B*14 negative cell line JY. The DRLLALNSL (PRKDC)-specific CD8+ T cells were also able to lyse the B*14 positive.
Fig. 4.
Intracellular cytokine staining of in vitro stimulated CD8+ T cells from HLA-B14+ healthy blood donors. The peptides DRVLRAML (CCND1) and DRLLALNSL (PRKDC) were used for at least 6 h stimulation prior to fluorescent staining. a, b IFN-γ and TNF-α production, assessed after three in vitro restimulations with peptide-loaded DCs or PBMCs. Reactivities against DRVLRAML (CCND1, a) or DRLLALNSL (PRKDC, b) and a HIV peptide (negative control) are shown. Dot plots are gated on the CD4− fraction, and percentages of CD8+ IFN-γ+ T cells are shown
Fig. 5.
Cytolytic potential of DRVLRAML (CCND1)- and DRLLALNSL (PRKDC)-specific CTLs. CD8+ T cells from HLA-B*14 positive, healthy blood donors were stimulated four times in vitro with peptide-loaded DCs or autologous peptide-loaded PBMCs, respectively. Cytotoxic activity of induced CTL was determined in a standard 51Cr-release assay. a WT51 (HLA-B*14 positive) cells, pulsed for 1 h with 10 μg/ml DRVLRAML peptide, were lysed by CTLs. Unloaded WT51 cells were not lysed. JY (HLA-B*14 negative) cells were used as a negative control. b WT51 (HLA-B*14+/PRKDC+) cells, pulsed for 1 h with 10 μg/ml DRLLALNSL peptide, were lysed by CTLs. Unloaded WT51 cells were not lysed. MV4-11 (HLA-B*14+/PRKDC−) cells were used as a negative control
EBV-transformed B cell line WT51 (which is weakly expressing PRKDC protein, supplemental Fig. 1) loaded with DRLLALNSL peptide, but did not lyse unloaded WT 51 cells (Fig. 5b). The cell line MV4-11, which does not express PRKDC (supplemental figure 1), remained unlysed.
Discussion
Patient-individual analysis of peptides presented on lung cancer tissue
In 1996, Chen et al. [37] reported a loss of MHC class I in human lung cancer, which is generally assumed as an escape mechanism from a cytotoxic T-cell response directed against cancer cells, and thus could be indicative of an ongoing immune response against the tumor. Moreover, functional CD8 + T cells were found to infiltrate into the tumor epithelium of NSCLCs, thus indicating that an antitumor immune response can be present in NSCLC offering possibilities for specific immunotherapy. Thus, the aim of our work was to identify HLA-presented peptides that might represent the molecular basis of T-cell-mediated immune responses against lung cancer [4].
We identified a large number of HLA class I and II ligands presented to T cells in the tumor tissue. Most of these peptides were derived from abundantly expressed housekeeping proteins, but some derived from known TAA or could be proposed as novel tumor-associated peptides. Through this direct analysis of peptides presented on solid tumor tissues, we are able to obtain information on the peptides that are actually processed by and presented on a significant proportion of cancer cells, also indicating sufficient expression of MHC class I and II in our tumor samples. This report represents a pilot study limited by the small number of analyses. Further studies will have to show whether our results can be generalized.
The role of CCND1 and PRKDC in lung cancer
Cyclin D1 (CCND1) belongs to the highly conserved family of cyclins, which are crucial for cell cycle progression and regulation. In addition, CCND1 acts as a modifier of gene transcription [38]. Given its role in promoting cellular proliferation and modulating transcription, it is not surprising that CCND1 is deregulated in cancer. Interestingly, CCND1 alterations are tumor-type specific and occur through chromosomal translocation, gene amplification, mutations or excessive accumulation of the protein [39–42].
In NSCLC, overexpression of CCND1 was found to be progressively increased from metaplasia over carcinoma in situ to invasive carcinoma of the lung. Moreover, it was associated with a poor prognosis in NSCLCs and lymph node metastases [30, 43]. These results suggest that overexpression of CCND1 might play an important role in the multistep process of lung tumorigenesis. HLA-A*02-restricted T-cell epitopes from CCND1 have been identified, e.g. in mantle cell lymphoma [44], and are already in use in vaccination studies.
In advanced stages of NSCLC and SCLC, chemo- and radiotherapy become more and more important. However, during these treatments, many tumors develop resistance mechanisms, often through enhanced DNA repair.
Double-strand breaks, which can arise, e.g., by exposure to irradiation or radiomimetic drugs like bleomycin, are generally regarded as the most lethal of all DNA lesions, which if unrepaired severely threaten not only the integrity of the genome but also survival of the organism [45]. In mammalians, double-strand breaks are generally repaired via nonhomologous end joining, in which DNA-dependent protein kinase (PRKDC), a serine/threonine kinase, plays a key role [46]. PRKDC has a dual role: it senses DNA damage and repairs it, but if repair fails, it induces apoptosis and therefore avoids passing damaged DNA to progeny cells [47]. Hence, PRKDC-deficient cells have a radiosensitive phenotype and are defective in the repair of chromosomal double-strand breaks [48]. Several studies have reported that components of the DNA-PK complex are upregulated in cancer cells [36, 49].
In NSCLC, overexpression of PRKDC was significantly associated with a shorter median survival and an increased risk of death [50]. In lung cancer cell lines, a high content/activity of PRKDC was found in the most radioresistant cells [51].
Evaluation of tumor-associated HLA ligands by complementary analysis of gene expression
HLA ligands optimally suited for immunotherapy should be derived from TAA of high expression in tumor tissue and very low or absent expression in any other tissue. The two antigens that we decided to investigate further, CCND1 and PRKDC, only partly fulfill these criteria: low expression in normal tissues was not always accompanied by overexpression in lung cancer tissue. This observation reflects the high degree of individuality found in human tumor tissues and the need for individual solutions [52].
Cyclin D1 (CCND1) is a widely known tumor antigen and has been implicated to play a key role in lung tumorigenesis. PRKDC is a critical enzyme in DNA double-strand repair, so that by targeting this antigen, chemo- and radiosensitivity of lung cancer could be increased. As immunotherapy is supposed to work best in the adjuvant setting, PRKDC is an ideal antigen to increase the effectiveness of chemo- and/or radiotherapy. Both antigens were highly overexpressed in data stored in the GEO database. In our analyses, CCND1 and PRKDC were both overexpressed in three out of six lung cancer samples, which underlines the importance of a patient-individual immunotherapy. It has to be mentioned that a correlation between HLA ligand levels and mRNA levels is not always given [8].
Induction of CD8+ T cells specific for HLA ligands from CCND1 and PRKDC
The identification of CTL epitopes from tumor-associated proteins still is a critical step on the way towards a specific immunotherapy of cancer. Since immunogenicity is a prerequisite for employing natural HLA ligands in immunotherapy, we investigated whether the identified peptides from CCND1 and PRKDC could serve as targets for a T-cell-mediated immune response. Since we had no access to blood from lung cancer patients, we generated human CTLs from healthy blood donors, which were able to recognize the two peptides in an HLA*B14-restricted manner.
Due to the lack of HLA-B*14-positive lung cancer cell lines, cytotoxicity assays were carried out using the B-lymphoblastoid cell line WT51 as a target, which was lysed by the generated CTLs after peptide loading. The lack of recognition of unloaded cells may be due to low expression of CCND1 or PRKDC, leading to a presentation too weak to trigger a CTL response. Another explanation might be a low avidity of the T-cell receptors to the MHC-peptide complexes, which reflects a common observation in tumor immunology. In addition, low affinity of the peptide to the restricting HLA class I molecule or the downregulation of MHC class I surface antigens on the tumor cells [53, 54] might explain the failure of recognition. In many tumor types and cancer cell lines, a downregulation of APM (antigen processing machinery) components has been observed [55], e.g. downregulation of protease subunits responsible for the generation of the majority of antigenic peptides, or the transporter associated with antigen processing 1 (TAP1), which transports the peptides from the cytosol into the ER [37, 56]. Moreover, aberrant expression of peptidases in the cytosol or in the ER necessary for the correct trimming of the epitope might be the reason for the lack of recognition of unloaded tumor cells [57].
To our knowledge, we identified for the first time naturally presented HLA class I and II ligands in patients with lung cancer. Moreover, we were able to generate CCND1 and PRKDC peptide-specific CTLs from healthy blood donors by stimulating T cells with DCs pulsed with the corresponding peptide. Thus, our study may pave the way for additional preclinical and clinical studies to evaluate the safety, feasibility and efficacy of peptide-based immunotherapy in the treatment of lung cancer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr. Martina Sauter for performing the immunohistochemical stainings. This work was supported by the Deutsche Forschungsgemeinschaft (DFG, SFB 685 and GK 794) and the European Union (Cancer Immunotherapy, LSHC-CT-2006, 518234).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Boyle P, Levin B (2008) World Cancer Report 2008. http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/wcr_2008_1.pdf. Accessed 03 March 2013
- 2.Passlick B, Pantel K, Kubuschok B, Angstwurm M, Neher A, Thetter O, Schweiberer L, Izbicki JR. Expression of MHC molecules and ICAM-1 on non-small cell lung carcinomas: association with early lymphatic spread of tumour cells. Eur J Cancer. 1996;32A:141–145. doi: 10.1016/0959-8049(95)00551-X. [DOI] [PubMed] [Google Scholar]
- 3.Kikuchi E, Yamazaki K, Torigoe T, Cho Y, Miyamoto M, Oizumi S, Hommura F, Dosaka-Akita H, Nishimura M. HLA class I antigen expression is associated with a favorable prognosis in early stage non-small cell lung cancer. Cancer Sci. 2007;98:1424–1430. doi: 10.1111/j.1349-7006.2007.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdegaal EM, Hoogstraten C, Sandel MH, Kuppen PJ, Brink AA, Claas FH, Gorsira MC, Graadt van Roggen JF, Osanto S. Functional CD8 + T cells infiltrate into nonsmall cell lung carcinoma. Cancer Immunol Immunother. 2007;56:587–600. doi: 10.1007/s00262-006-0214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 6.Falk K, Rötzschke O, Stevanovic S, Jung G, Rammensee HG. Allel-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991;351:290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 7.Weinzierl AO, Maurer D, Altenberend F, Schneiderhan-Marra N, Klingel K, Schoor O, et al. A cryptic vascular endothelial growth factor T-cell epitope: identification and characterization by mass spectrometry and T-cell assays. Cancer Res. 2008;68:2447–2454. doi: 10.1158/0008-5472.CAN-07-2540. [DOI] [PubMed] [Google Scholar]
- 8.Weinzierl AO, Lemmel C, Schoor O, Müller M, Krüger T, Wernet D, et al. Distorted relation between mRNA copy number and corresponding major histocompatibility complex ligand density on the cell surface. Mol Cell Proteomics. 2007;6:102–113. doi: 10.1074/mcp.M600310-MCP200. [DOI] [PubMed] [Google Scholar]
- 9.Krüger T, Schoor O, Lemmel C, Kraemer B, Reichle C, Dengjel J, et al. Lessons to be learned from primary renal cell carcinomas: novel tumor antigens and HLA ligands for immunotherapy. Cancer Immunol Immunother. 2005;54:826–836. doi: 10.1007/s00262-004-0650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 11.Brossart P, Stuhler G, Flad T, Stevanovic S, Rammensee HG, Kanz L, Brugger W. Her-2/neu derived peptides are tumor-associated antigens expressed by human renal cell and colon carcinoma lines and are recognized by in vitro induced specific cytotoxic T lymphocytes. Cancer Res. 1998;58:732–736. [PubMed] [Google Scholar]
- 12.Stickel JS, Stickel N, Hennenlotter J, Klingel K, Stenzl A, Rammensee HG, Stevanovic S. Quantification of HLA class I molecules on renal cell carcinoma using Edman degradation. BMC Urol. 2011;11:1–11. doi: 10.1186/1471-2490-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shetty V, Sinnathamby G, Nickens Z, Shah P, Hafner J, Mariello L, et al. MHC class I-presented lung cancer-associated tumor antigens identified by immunoproteomics analysis are targets for cancer-specific T cell response. J Proteomics. 2011;74:728–743. doi: 10.1016/j.jprot.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Abe H, Kamai T, Shirataki H, Oyama T, Arai K, Yoshida K. High expression of Ran GTPase is associated with local invasion and metastasis of human clear cell renal cell carcinoma. Int J Cancer. 2008;122:2391–2397. doi: 10.1002/ijc.23400. [DOI] [PubMed] [Google Scholar]
- 15.Ueno T, Linder S, Elmberger G. Aspartic proteinase napsin is a useful marker for diagnosis of primary lung adenocarcinoma. Br J Cancer. 2003;88:1229–1233. doi: 10.1038/sj.bjc.6600879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gsur A, Zidek T, Schnattinger K, Feik E, Haidinger G, Hollaus P, et al. Association of microsomal epoxide hydrolase polymorphisms and lung cancer risk. Br J Cancer. 2003;89:702–706. doi: 10.1038/sj.bjc.6601142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiruma Y, Honjo T, Jelinek DF, Windle JJ, Shin J, Roodman GD, Kurihara N. Increased signaling through p62 in the marrow microenvironment increases myeloma cell growth and osteoclast formation. Blood. 2009;113:4894–4902. doi: 10.1182/blood-2008-08-173948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volm M, Koomägi R. Relevance of proliferative and pro-apoptotic factors in non-small-cell lung cancer for patient survival. Br J Cancer. 2000;82:1747–1754. doi: 10.1054/bjoc.1999.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- 20.LeibundGut-Landmann S, Waldburger JM, Krawczyk M, Otten LA, Suter T, Fontana A, Acha-Orbea H, Reith W. Mini-review: specificity and expression of CIITA, the master regulator of MHC class II genes. Eur J Immunol. 2004;34:1513–1525. doi: 10.1002/eji.200424964. [DOI] [PubMed] [Google Scholar]
- 21.Dengjel J, Nastke MD, Gouttefangeas C, Gitsioudis G, Schoor O, Altenberend F, et al. Unexpected abundance of HLA class II presented peptides in primary renal cell carcinomas. Clin Cancer Res. 2006;12:4163–4170. doi: 10.1158/1078-0432.CCR-05-2470. [DOI] [PubMed] [Google Scholar]
- 22.Ouellet V, Guyot MC, Le Page C, Filali-Mouhim A, Lussier C, Tonin PN, Provencher DM, Mes-Masson AM. Tissue array analysis of expression microarray candidates identifies markers associated with tumor grade and outcome in serous epithelial ovarian cancer. Int J Cancer. 2006;119:599–607. doi: 10.1002/ijc.21902. [DOI] [PubMed] [Google Scholar]
- 23.Azuma K, Sasada T, Takedatsu H, Shomura H, Koga M, Maeda Y, et al. Ran, a small GTPase gene, encodes cytotoxic T lymphocyte (CTL) epitopes capable of inducing HLA-A33-restricted and tumor-reactive CTLs in cancer patients. Clin Cancer Res. 2004;10:6695–6702. doi: 10.1158/1078-0432.CCR-04-0818. [DOI] [PubMed] [Google Scholar]
- 24.Bishop JA, Sharma R, Illei PB. Napsin A and thyroid transcription factor-1 expression in carcinomas of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesothelioma. Hum Pathol. 2010;41:20–25. doi: 10.1016/j.humpath.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- 26.Khodarev NN, Beckett M, Labay E, Darga T, Roizman B, Weichselbaum RR. STAT1 is overexpressed in tumors selected for radioresistance and confers protection from radiation in transduced sensitive cells. Proc Natl Acad Sci USA. 2004;101:1714–1719. doi: 10.1073/pnas.0308102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui Z, Tretiakova M, Zhang Z, Li Y, Wang X, Zhu JX, et al. Radiosensitization by inhibiting STAT1 in renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2009;73:288–295. doi: 10.1016/j.ijrobp.2008.08.043. [DOI] [PubMed] [Google Scholar]
- 28.Terry G, Ho L, Londesborough P, Duggan C, Hanby A, Cuzick J. The expression of FHIT, PCNA and EGFR in benign and malignant breast lesions. Br J Cancer. 2007;96:110–117. doi: 10.1038/sj.bjc.6603512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gautschi O, Ratschiller D, Gugger M, Betticher DC, Heighway J. Cyclin D1 in non-small cell lung cancer: a key driver of malignant transformation. Lung Cancer. 2007;55:1–14. doi: 10.1016/j.lungcan.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Keum JS, Kong G, Yang SC, Shin DH, Park SS, Lee JH, Lee JD. Cyclin D1 overexpression is an indicator of poor prognosis in resectable non-small cell lung cancer. Br J Cancer. 1999;81:127–132. doi: 10.1038/sj.bjc.6690661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wachi S, Yoneda K, Wu R. Interactome-transcriptome analysis reveals the high centrality of genes differentially expressed in lung cancer tissues. Bioinformatics. 2005;21:4205–4208. doi: 10.1093/bioinformatics/bti688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon GJ, Rockwell GN, Jensen RV, Rheinwald JG, Glickman JN, Aronson JP, et al. Identification of novel candidate oncogenes and tumor suppressors in malignant pleural mesothelioma using large-scale transcriptional profiling. Am J Pathol. 2005;166:1827–1840. doi: 10.1016/S0002-9440(10)62492-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raponi M, Zhang Y, Yu J, Chen G, Lee G, Taylor JM, et al. Gene expression signatures for predicting prognosis of squamous cell and adenocarcinomas of the lung. Cancer Res. 2006;66:7466–7472. doi: 10.1158/0008-5472.CAN-06-1191. [DOI] [PubMed] [Google Scholar]
- 35.Stearman RS, Dwyer-Nield L, Zerbe L, Blaine SA, Chan Z, Bunn PA, et al. Analysis of orthologous gene expression between human pulmonary adenocarcinoma and a carcinogen-induced murine model. Am J Pathol. 2005;167:1763–1775. doi: 10.1016/S0002-9440(10)61257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beskow C, Skikuniene J, Holgersson A, Nilsson B, Lewensohn R, Kanter L, Viktorsson K. Radioresistant cervical cancer shows upregulation of the NHEJ proteins DNA-PKcs, Ku70 and Ku86. Br J Cancer. 2009;101:816–821. doi: 10.1038/sj.bjc.6605201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen HL, Gabrilovich D, Tampé R, Girgis KR, Nadaf S, Carbone DP. A functionally defective allele of TAP1 results in loss of MHC class I antigen presentation in a human lung cancer. Nat Genet. 1996;13:210–213. doi: 10.1038/ng0696-210. [DOI] [PubMed] [Google Scholar]
- 38.Ewen ME, Lamb J. The activities of cyclin D1 that drive tumorigenesis. Trends Mol Med. 2004;10:158–162. doi: 10.1016/j.molmed.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Knudsen KE, Diehl JA, Haiman CA, Knudsen ES. Cyclin D1: polymorphism, aberrant splicing and cancer risk. Oncogene. 2006;25:1620–1628. doi: 10.1038/sj.onc.1209371. [DOI] [PubMed] [Google Scholar]
- 40.Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, Barnes D, Peters G. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 1994;54:1812–1817. [PubMed] [Google Scholar]
- 41.Lamb J, Ramaswamy S, Ford HL, Contreras B, Martinez RV, Kittrell FS, et al. A mechanism of cyclin D1 action encoded in the patterns of gene expression in human cancer. Cell. 2003;114:323–334. doi: 10.1016/S0092-8674(03)00570-1. [DOI] [PubMed] [Google Scholar]
- 42.Bosch F, Jares P, Campo E, Lopez-Guillermo A, Piris MA, Villamor N, et al. PRAD-1/cyclin D1 gene overexpression in chronic lymphoproliferative disorders: a highly specific marker of mantle cell lymphoma. Blood. 1994;84:2726–2732. [PubMed] [Google Scholar]
- 43.Lonardo F, Rusch V, Langenfeld J, Dmitrovsky E, Klimstra DS. Overexpression of cyclins D1 and E is frequent in bronchial preneoplasia and precedes squamous cell carcinoma development. Cancer Res. 1999;59:2470–2476. [PubMed] [Google Scholar]
- 44.Wang M, Sun L, Qian J, Han X, Zhang L, Lin P, Cai Z, Yi Q. Cyclin D1 as a universally expressed mantle cell lymphoma-associated tumor antigen for immunotherapy. Leukemia. 2009;23:1320–1328. doi: 10.1038/leu.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vilenchik MM, Knudson AG. Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc Natl Acad Sci USA. 2003;100:12871–12876. doi: 10.1073/pnas.2135498100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith GC, Jackson SP. The DNA-dependent protein kinase. Genes Dev. 1999;13:916–934. doi: 10.1101/gad.13.8.916. [DOI] [PubMed] [Google Scholar]
- 47.Bernstein C, Bernstein H, Payne CM, Garewal H. DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat Res. 2002;511:145–178. doi: 10.1016/S1383-5742(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 48.Lieber MR, Ma Y, Pannicke U, Schwarz K. Mechanism and regulation of human non-homologous DNA end-joining. Nat Rev Mol Cell Biol. 2003;4:712–720. doi: 10.1038/nrm1202. [DOI] [PubMed] [Google Scholar]
- 49.Zhao HJ, Hosoi Y, Miyachi H, Ishii K, Yoshida M, Nemoto K, et al. DNA-dependent protein kinase activity correlates with Ku70 expression and radiation sensitivity in esophageal cancer cell lines. Clin Cancer Res. 2000;6:1073–1078. [PubMed] [Google Scholar]
- 50.Xing J, Wu X, Vaporciyan AA, Spitz MR, Gu J. Prognostic significance of ataxia-telangiectasia mutated, DNA-dependent protein kinase catalytic subunit, and Ku heterodimeric regulatory complex 86-kD subunit expression in patients with nonsmall cell lung cancer. Cancer. 2008;112:2756–2764. doi: 10.1002/cncr.23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sirzén F, Nilsson A, Zhivotovsky B, Lewensohn R. DNA-dependent protein kinase content and activity in lung carcinoma cell lines: correlation with intrinsic radiosensitivity. Eur J Cancer. 1999;35:111–116. doi: 10.1016/S0959-8049(98)00289-5. [DOI] [PubMed] [Google Scholar]
- 52.Rammensee HG, Weinschenk T, Gouttefangeas C, Stevanovic S. Towards patient-specific tumor antigen selection for vaccination. Immunol Rev. 2002;188:164–176. doi: 10.1034/j.1600-065X.2002.18815.x. [DOI] [PubMed] [Google Scholar]
- 53.Aptisauri N, Cabrera T, Mendez R, Garcia-Lora A, Ruiz-Cabello F, Garrido F. Role of altered expression of HLA class I molecules in cancer progression. Adv Exp Med Biol. 2007;601:123–131. doi: 10.1007/978-0-387-72005-0_13. [DOI] [PubMed] [Google Scholar]
- 54.Méndez R, Rodríguez T, Del Campo A, Monge E, Maleno I, Aptsiauri N, et al. Characterization of HLA class I altered phenotypes in a panel of human melanoma cell lines. Cancer Immunol Immunother. 2008;57:719–729. doi: 10.1007/s00262-007-0411-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol Today. 2000;21:455–464. doi: 10.1016/S0167-5699(00)01692-3. [DOI] [PubMed] [Google Scholar]
- 56.Seliger B, Höhne A, Knuth A, Bernhard H, Ehring B, Tampé R, Huber C. Reduced membrane major histocompatibility complex class I density and stability in a subset of human renal cell carcinomas with low TAP and LMP expression. Clin Cancer Res. 1996;8:1427–1433. [PubMed] [Google Scholar]
- 57.Fruci D, Ferracuti S, Limongi MZ, Cunsolo V, Giorda E, Fraioli R, et al. Expression of endoplasmic reticulum aminopeptidases in EBV-B cell lines from healthy donors and in leukemia/lymphoma, carcinoma, and melanoma cell lines. J Immunol. 2006;176:4869–4879. doi: 10.4049/jimmunol.176.8.4869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.