Abstract
Immune checkpoint inhibitors such as pembrolizumab, ipilimumab, and nivolumab, now FDA-approved for use in treating several types of cancer, have been associated with immune-related adverse effects. Specifically, the antibodies targeting the programmed-cell death-1 immune checkpoint, pembrolizumab and nivolumab, have been rarely reported to induce the development of type 1 diabetes mellitus. Here we describe a case of a patient who developed antibody-positive type 1 diabetes mellitus following treatment with pembrolizumab in combination with systemic chemotherapy for metastatic adenocarcinoma of the lung. We will also provide a brief literature review of other rarely reported cases of type 1 diabetes presenting after treatment with pembrolizumab and nivolumab, as well as discussion regarding potential mechanisms of this adverse effect and its importance as these drugs continue to become even more widespread.
Keywords: Pembrolizumab, Nivolumab, Ipilimumab, Diabetes mellitus, Immunomodulatory, Immune-related adverse effect
Introduction
The immune checkpoint inhibitors ipilimumab, nivolumab, pembrolizumab, and atezolizumab, have been FDA-approved for the treatment of multiple malignancies in recent years. These immunomodulating agents target CTLA-4, PD-1, or PD-L1 and have been associated with several immune-related adverse effects, including autoimmune pneumonitis, colitis, hepatitis, nephritis, and several endocrinopathies [1–5]. CTLA-4 is predominantly expressed on T cells, PD-1 is expressed on T cells, B cells, monocytes, natural killer cells, dendritic cells, and many lymphocytes, and PD-L1 is expressed on resting T cells, B cells, dendritic cells, macrophages, vascular endothelial cells, and pancreatic islet cells [6, 7]. Pembrolizumab is an IgG4 monoclonal antibody targeting the PD-1 T cell receptor and is FDA-approved for treatment of metastatic melanoma, non-small cell lung cancer, and renal cell carcinoma. Pembrolizumab has rarely been associated with the development of type 1 diabetes mellitus (T1DM) [8–10]. As immunotherapy continues to be more widely used for cancer treatment, it is important to identify possible rare but significant immune-related side effects, such as T1DM, associated with these targeted therapies.
We present the case of a 76-year-old male with metastatic adenocarcinoma of the lung, who developed antibody-positive T1DM following treatment with pembrolizumab in combination with systemic chemotherapy. We will also provide a brief literature review of reported cases of type 1 diabetes presenting after treatment with pembrolizumab or nivolumab.
Case presentation
The patient is a 76-year-old male with an approximately 10 pack-year smoking history (quit smoking 35 years prior to presentation) and no history of diabetes mellitus, who presented in June 2015 with shortness of breath. He underwent computed tomography (CT) of his chest which demonstrated a large right-sided pleural effusion, as well as several right-sided lung nodules involving both the upper and lower lobes. Diagnostic thoracentesis demonstrated cytology positive for metastatic carcinoma. Immunohistochemistry stains were positive for cytokeratin (CK) CAM 5.2, vimentin and variable CD10 positivity. CT scan of the abdomen and pelvis demonstrated several enlarged retroperitoneal lymph nodes, as well as a hyperdense lesion in the upper pole of the right kidney and an enhancing lesion in the lateral aspect of the spleen. Given his symptomatic pleural effusion, 1 month following presentation the patient underwent robotic video-assisted thoracic surgery with right upper lobe wedge resection and biopsy, as well as talc pleurodesis. Immunohistochemistry was positive for CK7 and negative for thyroid transcription factor 1 (TTF1), paired box gene (PAX), and CK20, consistent with lung adenocarcinoma. The tumor showed wild type for anaplastic lymphoma kinase (ALK), ROS1, Kirsten rat sarcoma (KRAS), and epidermal growth factor receptor (EGFR).
Two months after presentation, the patient was started on systemic treatment as part of a clinical trial in 21 day cycles. Treatment consisted of pembrolizumab, carboplatin, and nab-paclitaxel on day one, followed by nab-paclitaxel on days eight and fifteen. The patient presented in September 2015 for cycle two, day eight and was found to have a blood glucose level of 616 mg/dL. He was subsequently admitted to the hospital (Fig. 1). He was asymptomatic without polyuria or polydipsia. He did not have an anion gap. Prior to starting treatment, his random blood glucose levels ranged from 120 to 140 mg/dL. He was not being treated for pre-diabetes. Hemoglobin A1c measured 1 month prior to starting treatment was 6.3 %. It was 5.8 % 1 month after starting treatment.
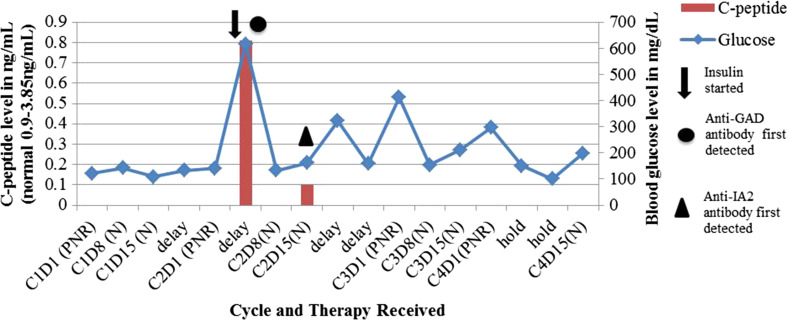
Fig. 1.
Blood glucose and c-peptide levels were measured throughout treatment with pembrolizumab, nab-paclitaxel, and carboplatin for adenocarcinoma of the lung. The data points represent 7-day intervals. Treatment was delayed several times for cytopenias, in addition to hyperglycemia after C2D1. C4D8 was held completely for cytopenias. C cycle, D day, P pembrolizumab, N nab-paclitaxel, R carboplatin, Anti-GAD anti-glutamic acid decarboxylase, Anti-IA2 anti-islet antigen 2
On admission, further workup demonstrated random c-peptide levels that were 0.81 ng/mL (normal range 0.9–3.85 ng/mL) and then less than 0.10 ng/mL after initiation of insulin therapy. Thyroid and cholesterol studies were within normal limits. He demonstrated elevated anti-glutamic acid decarboxylase antibodies (anti-GAD, 23.8units/mL, normal < 1.1 units/mL) and islet antigen 2 antibodies (anti-IA2, 0.32 nmol/L, normal < 0.02 nmol/L). Insulin antibodies and islet cell immunoglobulin G (IgG) antibodies were negative. HLA-typing was not performed. The patient was started on both long-acting insulin glargine and prandial short-acting insulin with improved glycemic control. Although not standard practice, given that many immune-related adverse events are controlled with the use of systemic steroids, the patient was started on an empiric trial of prednisone at 10 mg per day. This was started 21 days after his presentation with hyperglycemia. The prednisone was stopped after 25 days when no improvement was seen in the patient’s glycemic control. Of note, there was no significant worsening of the patient’s hyperglycemia on prednisone. The patient’s blood glucose was controlled on long-acting insulin glargine and prandial short-acting insulin.
The patient received a total of four cycles of the chemotherapy regimen, including pembrolizumab, with an overall decrease in size and near resolution of his right lung nodules, as shown in Fig. 2. The patient tolerated therapy well without any other immune-related side effects. There were no dose-limiting toxicities in cycle 1. Cycle 2, day 8 was delayed 1 week due to hyperglycemia; however, due to rapid correction and control with insulin, it was felt safe to proceed with continued pembrolizumab treatment. His treatment was also delayed 1–2 weeks at three other points due to cytopenias (neutropenia and thrombocytopenia), which were likely hematologic toxicities of carboplatin and/or nab-paclitaxel. The patient currently remains on maintenance pembrolizumab every 3 weeks. He remains on insulin therapy with stable insulin requirements and fasting blood glucose levels.
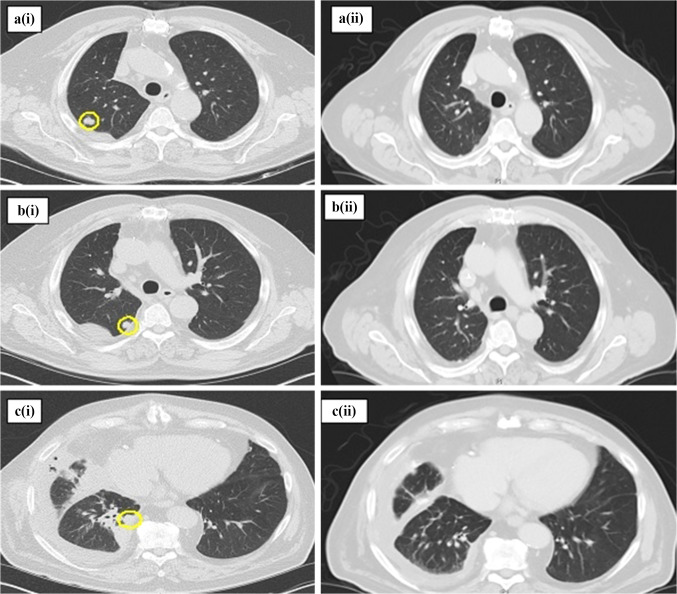
Fig. 2.
Pre- (i) and post-treatment (ii) chest computed tomography (CT) scans demonstrating an overall decrease in size of the patient’s right upper and right lower lobe nodules after 4 cycles of treatment with pembrolizumab, nab-paclitaxel, and carboplatin. Interval between pre- and post-treatment scans was approximately 5 months. a Pre-treatment CT scan with a lobular, partially spiculated peripheral right upper lobe nodule, measuring 13 mm in the long axis. Resolved on post-treatment scan. b Pre-treatment CT scan with a lobular, partially spiculated right upper lobe nodule, measuring 10 mm in the long axis. Resolved on post-treatment scan. c Pre-treatment CT scan with a well-circumscribed right lower lobe peripheral nodule, measuring 15 mm in the long axis. Resolved on post-treatment scan
Discussion
Although our patient had no prior history of autoimmune disease or diabetes mellitus, his presentation suggests the development of autoimmune T1DM in association with pembrolizumab treatment for adenocarcinoma of the lung. Currently, there have been rare reports of T1DM occurring in association with pembrolizumab treatment (Table 1). In one report, the patient developed fulminant T1DM and DKA with negative autoantibodies and undetectable c-peptide levels 2 weeks after receiving a second injection of pembrolizumab for metastatic melanoma [9]. In another report, a patient with a history of autoimmune thyroid disease and psoriasis presented with ketonuria and was diagnosed with antibody-negative T1DM, with low c-peptide levels, less than 1 month after receiving treatment with pembrolizumab for melanoma [8]. A third report described a patient developing DKA and anti-GAD-positive autoimmune T1DM after receiving three injections of pembrolizumab for metastatic melanoma. The third patient had also been previously treated with ipilimumab [10]. Additionally, in another report of a patient previously treated with ipilimumab, the patient developed T1DM 3 weeks after initiation of pembrolizumab therapy for melanoma [11]. Recently, a case report described a patient who developed anti-GAD-positive, insulin-dependent diabetes after 17 cycles of treatment with pembrolizumab for metastatic melanoma. The patient had previously received interferon alpha 2B, vemurafenib, high-dose IL-2, and ipilimumab therapy. His fasting blood glucose levels and insulin requirements decreased after discontinuation of pembrolizumab [12]. Ours is the first case demonstrating the association of T1DM with pembrolizumab treatment and concurrent standard chemotherapy for patients with lung adenocarcinoma.
Table 1.
Patient characteristics in immune-modulator associated type 1 diabetes mellitus
| Agent | Disease | Antibodies detected | Time to development | C-peptide (ng/mL) | DKA | Prior treatment | Relevant history | High-risk HLA genotype | References |
|---|---|---|---|---|---|---|---|---|---|
| Nivolumab | Melanoma | None | 5 months | <0.1 | + | Ipilimumab | Autoimmune thyroiditis | A02:01, DRB1*04 | [8] |
| Nivolumab | NSCLC | +anti-GAD | <1 month | <0.1 | + | None | None | A02:01, DRB1*04 | [8] |
| Nivolumab | RCC | +anti-GAD, anti-ICA512, anti-IA2 | 4 months | 1.3 | – | Proleukin, bevacizumab, interferon | None | A02:01, DRB1*04 | [8] |
| Nivolumab | SLC | +anti-GAD | 1 week | <0.1 | + | Carboplatin, etoposide, paclitaxel | Type 2 DM | A02:01 | [8] |
| Nivolumab | Melanoma | None | 7 months | 0.08 | + | Dacarbazine | None | NR | [13] |
| Nivolumab | Melanoma | None | 4 months | UD | + | None | None | None | [14] |
| Nivolumab | Melanoma | None | 12 months | 1 | – | Dacarbazine, nimustine, cisplatin, tamoxifen | None | DRB1*04:05, DQB1*04:01 | [15] |
| Nivolumab | Melanoma | None | 6 weeks | <0.1 | – | None | None | NR | [11] |
| Nivolumab | Melanoma | +anti-GAD | 3 weeks | low | + | Dacarbazine, Ipilimumab | Type 2 diabetes mellitus | NR | [11] |
| Nivolumab | Melanoma | NR | 6 weeks | NR | NR | Dacarbazine, Ipilimumab | NR | NR | [11] |
| Pembrolizumab | Melanoma | +anti-GAD | 3 weeks | Low | – | Ipilimumab | None | NR | [11] |
| Pembrolizumab | Melanoma | None | 2 weeks after 2nd injection | UD | + | Ipilimumab | Autoimmune thyroiditis | None | [9] |
| Pembrolizumab | Melanoma | +anti-GAD | After 3rd infusion | NR | + | Ipilimumab | None | DRB1*04, DQB1*03:02 | [10] |
| Pembrolizumab | Melanoma | None | <1 month | 0.5 | – | None | Autoimmune thyroiditis, psoriasis | Not reported | [8] |
| Pembrolizumab | Melanoma | +anti-GAD | 51 weeks | 2.4 | – | IFN alpha 2B, vemurafenib, high-dose IL-2, ipilimumab | Treatment-related hypothyroidism | Not reported | [12] |
| Pembrolizumab | NSCLC | +anti-GAD, anti-IA2 | 1 week after 2nd injection | <0.1 | – | Concurrent carboplatin, nab-paclitaxel | None | Not tested | Current report |
Normal c-peptide level range: 0.9–3.85 ng/mL
NSCLC non-small cell lung cancer, RCC renal cell carcinoma, SCLC small cell lung cancer, anti-GAD anti-glutamic acid decarboxylase, anti-ICA512 islet cell autoantigen 512, anti-IA2 anti-islet antigen 2, DKA diabetic ketoacidosis, UD undetectable, NR not reported
Nivolumab, another immunologic agent targeting the PD-1 cell receptor, has also been reported to be associated with the development of T1DM. With nivolumab, type 1 diabetes has presented anywhere from 1 week to 7 months after treatment initiation, with various clinical presentations, including diabetic ketoacidosis (DKA) (Table 1) [8, 11, 13–15]. Additionally, type 1 diabetes has been associated with other immune therapies, with multiple reports of this association with interferon alpha 2B and IL-2. In one study of patients receiving combination anti-melanoma vaccine and low-dose IL-2 therapy, 1 patient out of 91 evaluable patients developed new onset insulin-dependent diabetes mellitus which resolved after discontinuation of IL-2. Anti-islet cell autoantibodies were not measured [16]. Another patient who received single-agent low-dose IL-2 therapy for advanced colorectal cancer developed insulin-dependent diabetes mellitus during treatment. His blood glucose levels improved upon cessation of IL-2 and recurred when IL-2 therapy was restarted. He had positive anti-islet cell autoantibodies [17]. There have been many reports of IFN-associated type 1 diabetes mellitus in patients treated for hepatitis C, most involving the development of positive autoantibodies [18–23]. Several studies have shown that IFN-alpha is involved in the initiation of T1DM in non-obese diabetic (NOD) mice by demonstrating that blocking IFN-alpha receptors can delay the onset and decrease the incidence of T1DM in NOD mice [24, 25]. To our knowledge, the platinum and taxane classes of chemotherapy have not been reported to be associated with the development of T1DM, and therefore it is unlikely that concurrent treatment with these agents contributed to the development of T1DM in our patient.
Several studies with mouse models have provided insight into the potential mechanism for the development of autoimmune TIDM related to blockade of the PD-1 pathway. Ansari et al. [26] found that PD-1 and programmed death ligand-1 (PD-L1) inhibition precipitated diabetes, with an increased frequency of GAD-reactive splenocytes, in non-obese prediabetic female mice. Another study found that blockade of the PD-1 pathway in s mice models appears to undermine their otherwise significant genetic protection from type 1 diabetes. The PD-1 blockade in the mouse model rapidly precipitated type 1 diabetes in nearly all of the mouse strains tested [27]. Guleria et al. [28] demonstrated that PD-L1 blockade in NOD mice leads to the expansion of autoreactive CD4+ and CD8+ T cells in vivo which likely play a role in the development of T1DM. Based on these preclinical studies, it appears that the PD-1 pathway is an inhibitory pathway in the development of T1DM. Therefore, disinhibition of this pathway with PD-1 and PD-L1 inhibitors allows for the expansion of T cells which are autoreactive and may lead to the development of T1DM.
It has been established that T1DM is precipitated by the destruction of pancreatic beta cells by autoreactive T cells. Regulatory T cells (Treg) inhibit the activation and effector functions of these autoreactive T cells in healthy control subjects. Studies have shown conflicting results regarding the number of Treg cells found in healthy controls versus diabetic patients. In a recent study, peripheral blood mononuclear cells (PBMC) from both long-standing diabetic patients and healthy control subjects were stimulated with CD3/CD28 to induce proliferation of Treg cells. After stimulation, diabetic patients were found to have decreased proliferation of Treg cells, as well as lower expression of PD-1 on the surface of these cells [29]. This study supports the previous findings by Fujisawa et al. [30] which found that there was a significant decrease in the frequency of CD4+ T cells which express PD-1 in patients with T1DM compared to healthy control subjects. Therefore, a proposed mechanism for the development of T1DM is that lower PD-1 expressing CD4+ cells may lead to activation of autoreactive T cells that can infiltrate pancreatic islet cells and ultimately lead to T1DM. At this point, it is unclear whether decreased proliferation or function of Treg cells due to PD-1 inhibition leads to activation of autoreactive T cells, or if PD-1 inhibition allows these autoreactive T cells to be directly activated through removal of the inhibitory pathway.
At this time, it is thought that risk factors for the development of T1DM include both high-risk HLA genotypes as well as environmental factors [31]. It has been established that the primary risk factor, especially in Caucasian patients, appears to occur in individuals with either class II HLA-DR3-DQ2 (DR3) or HLA-DR4-DQ8 (DR4) haplotypes, or even higher risk with both, in addition to an environmental trigger [32]. Additionally, there have also been high-risk HLA class I genotypes that have been associated. Therefore, it is reasonable to suspect that having a high-risk haplotype may place patients at an increased risk of developing T1DM in the setting of treatment with immune modulators. Although not measured routinely prior to starting treatment with immunomodulators, high-risk HLA genotypes have been present in several of the case reports of patients who developed T1DM after treatment with nivolumab or pembrolizumab (Table 1). Testing HLA genotypes prior to the administration of immunotherapy may help to identify patients at risk for developing autoimmune side effects, therefore allowing for increased monitoring of these patients to identify these adverse effects sooner. As suggested previously, recognizing those at risk for developing these adverse effects may not affect the decision to treat, as the potential for increased survival with these drugs is likely greater than the risk of adverse effects, it may help to identify these adverse effects earlier and prevent significant morbidity [33]. As immunomodulating agents become more widely used and standardized parts of cancer treatment regimens, the importance of understanding any potential unique adverse effects becomes even greater. Although our patient continues on insulin therapy, it is uncertain whether he will have lifelong T1DM. Based on prior reports of patients with preexisting autoimmune disease later developing immune-related adverse effects after treatment with immunomodulators, we suspect that having a prior autoimmune disease or adverse effect may be a risk factor for the development of future immune-related adverse effects. Potential risk factors for the development of T1DM after administration of pembrolizumab may become better elucidated as more reports of this effect are seen.
The package inserts and prescribing information for nivolumab and pembrolizumab both list T1DM as a rare immune-related side effect. Interestingly, although the CTLA-4 inhibitor ipilimumab has been associated with a variety of other immune-related side effects, there has been a lack of reported association with T1DM [4, 5]. The association may be rare and might have occurred and not yet been described. However, we hypothesize that as with previous mouse and human studies, PD-1 expression plays a role specifically in the development of T1DM, but the role (if any) of CTLA-4 is unclear. These findings are supported by the fact that although CTLA-4 and PD-1 receptors both negatively regulate T cell activation, they appear to do so by separate and distinct mechanisms [34]. Additionally, it has been demonstrated that CTLA-4 may play a role in the development of T1DM only in neonate NOD mice, but not later in their lifespan [26].
As noted previously, our patient was found to have a lower HbA1c level once starting treatment compared to his baseline, although his blood glucose levels were significantly higher. It has been shown that in rare settings of rapidly evolving type 1 diabetes mellitus, the HbA1c levels may not have time to catch up with the acute elevations in blood glucose levels, which may have been a contributing factor in our patient’s case [35]. Although to our knowledge the accuracy of HbA1c levels in the setting of chemotherapy-induced anemia has not been studied, it is reasonable to suspect that HbA1c levels may also be less reliable in this setting.
Ideal management of these complications has yet to be determined. There are currently no widely accepted guidelines regarding treatment of pembrolizumab-induced type 1 diabetes mellitus other than insulin therapy. Given the autoimmune nature of the adverse effect and the formation of autoantibodies, we hypothesized that steroids may be a useful treatment. Although not beneficial in our patient, we suspect that a higher dose of systemic steroids given sooner after the diagnosis of T1DM was made may have improved his glycemic control.
Conclusion
Anti-PD-1 immunomodulating agents, specifically pembrolizumab, now FDA-approved for use in melanoma, non-small cell lung cancer, and renal cell carcinoma, have been reported to have immune adverse effects. Recently, rare case reports have demonstrated an association between pembrolizumab and T1DM. However, our case is the first which demonstrates a patient who developed antibody-positive T1DM after combination treatment with chemotherapy and pembrolizumab for lung adenocarcinoma. Further research is needed to help delineate the frequency of this adverse effect, as well as evaluate potential risk factors and ideal management strategies.
Abbreviations
- ALK
Anaplastic lymphoma kinase
- CK
Cytokeratin
- CT
Computed tomography
- DKA
Diabetic ketoacidosis
- EGFR
Epidermal growth factor receptor
- GAD
Glutamic acid decarboxylase
- HbA1c
Hemoglobin A1c
- IA
Islet antigen
- ICA
Islet cell autoantigen
- KRAS
Kirsten rat sarcoma
- NOD
Non-obese diabetic
- NR
Not reported
- NSCLC
Non-small cell lung cancer
- PAX
Paired box gene
- RCC
Renal cell carcinoma
- SCLC
Small cell lung cancer
- T1DM
Type 1 diabetes mellitus
- Treg
Regulatory T cell
- TTF
Thyroid transcription factor
- UD
Undetectable
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Young Kwang Chae and Lauren Chiec have contributed equally to this work.
References
- 1.Yamazaki N, Kiyohara Y, Uhara H, Fukushima S, Uchi H, Shibagaki N, Tsutsumida A, Yoshikawa S, Okuyama R, Ito Y, Tokudome T. Phase II study of ipilimumab monotherapy in Japanese patients with advanced melanoma. Cancer Chemother Pharmacol. 2015;76(5):997–1004. doi: 10.1007/s00280-015-2873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Min L, Ibrahim N. Ipilimumab-induced autoimmune adrenalitis. Lancet Diabetes Endocrinol. 2013;1(3):e15. doi: 10.1016/S2213-8587(13)70031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol. 2011;164(2):303–307. doi: 10.1530/EJE-10-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keytruda (pembrolizumab) (2014) [package insert] Merck & Co., Inc., Whitehouse Station, NJ
- 5.Opdivo (nivolumab) (2014) [package insert] Bristol-Myers Squibb Company, Princeton, NJ
- 6.Sansom DM. CD28, CTLA-4 and their ligands: who does what and to whom? Immunology. 2000;101(2):169–177. doi: 10.1046/j.1365-2567.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J, Hu Y, Hu M, Li B. Development of PD-1/PD-L1 pathway in tumor immune microenvironment and treatment for non-small cell lung cancer. Sci Rep. 2015;5:13110. doi: 10.1038/srep13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes J, Vudattu N, Sznol M, Gettinger S, Kluger H, Lupsa B, Herold KC. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38(4):e55–e57. doi: 10.2337/dc14-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaudy C, Clevy C, Monestier S, Dubois N, Preau Y, Mallet S, Richard MA, Grob JJ, Valero R, Beliard S. Anti-PD1 pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care. 2015;38(11):e182–e183. doi: 10.2337/dc15-1331. [DOI] [PubMed] [Google Scholar]
- 10.Martin-Liberal J, Furness AJ, Joshi K, Peggs KS, Quezada SA, Larkin J. Anti-programmed cell death-1 therapy and insulin-dependent diabetes: a case report. Cancer Immunol Immunother. 2015;64(6):765–767. doi: 10.1007/s00262-015-1689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, Schmidgen MI, Gutzmer R, Utikal JS, Goppner D, Hassel JC, Meier F, Tietze JK, Thomas I, Weishaupt C, Leverkus M, Wahl R, Dietrich U, Garbe C, Kirchberger MC, Eigentler T, Berking C, Gesierich A, Krackhardt AM, Schadendorf D, Schuler G, Dummer R, Heinzerling LM. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–209. doi: 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Hansen E, Sahasrabudhe D, Sievert L. A case report of insulin-dependent diabetes as immune-related toxicity of pembrolizumab: presentation, management and outcome. Cancer Immunol Immunother. 2016;65(6):765–767. doi: 10.1007/s00262-016-1835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teramoto Y, Nakamura Y, Asami Y, Imamura T, Takahira S, Nemoto M, Sakai G, Shimada A, Noda M, Yamamoto A. Case of type 1 diabetes associated with less-dose nivolumab therapy in a melanoma patient. J Dermatol. 2016 doi: 10.1111/1346-8138.13486. [DOI] [PubMed] [Google Scholar]
- 14.Miyoshi Y, Ogawa O, Oyama Y. Nivolumab, an anti-programmed cell death-1 antibody, induces fulminant type 1 diabetes. Tohoku J Exp Med. 2016;239(2):155–158. doi: 10.1620/tjem.239.155. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto M, Okamoto M, Gotoh K, Masaki T, Ozeki Y, Ando H, Anai M, Sato A, Yoshida Y, Ueda S, Kakuma T, Shibata H. Fulminant type 1 diabetes mellitus with anti-programmed cell death-1 therapy. J Diabetes Investig. 2016 doi: 10.1111/jdi.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chianese-Bullock KA, Woodson EM, Tao H, Boerner SA, Smolkin M, Grosh WW, Neese PY, Merrill P, Petroni GR, Slingluff CL., Jr Autoimmune toxicities associated with the administration of antitumor vaccines and low-dose interleukin-2. J Immunother. 2005;28(4):412–419. doi: 10.1097/01.cji.0000171314.00924.2b. [DOI] [PubMed] [Google Scholar]
- 17.Soni N, Meropol NJ, Porter M, Caligiuri MA. Diabetes mellitus induced by low-dose interleukin-2. Cancer Immunol Immunother. 1996;43(1):59–62. doi: 10.1007/s002620050304. [DOI] [PubMed] [Google Scholar]
- 18.Shiba T, Morino Y, Tagawa K, Fujino H, Unuma T. Onset of diabetes with high titer anti-GAD antibody after IFN therapy for chronic hepatitis. Diabetes Res Clin Pract. 1995;30(3):237–241. doi: 10.1016/0168-8227(95)01188-9. [DOI] [PubMed] [Google Scholar]
- 19.Kawazoe T, Araki M, Lin Y, Ogawa M, Okamoto T, Yamamura T, Wakakura M, Murata M. New-onset type 1 diabetes mellitus and anti-aquaporin-4 antibody positive optic neuritis associated with type 1 interferon therapy for chronic hepatitis C. Intern Med. 2012;51(18):2625–2629. doi: 10.2169/internalmedicine.51.7771. [DOI] [PubMed] [Google Scholar]
- 20.Yamazaki M, Sato A, Takeda T, Komatsu M. Distinct clinical courses in type 1 diabetes mellitus induced by peg-interferon-alpha treatment for chronic hepatitis C. Intern Med. 2010;49(5):403–407. doi: 10.2169/internalmedicine.49.2656. [DOI] [PubMed] [Google Scholar]
- 21.Lv YY, Shi BY, Guo H. Abrupt onset of type 1 diabetes mellitus during recombinant interferon-alpha 2b therapy in a patient with chronic hepatitis B. World J Gastroenterol. 2008;14(29):4713–4715. doi: 10.3748/wjg.14.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Recasens M, Aguilera E, Ampurdanes S, Sanchez Tapias JM, Simo O, Casamitjana R, Conget I. Abrupt onset of diabetes during interferon-alpha therapy in patients with chronic hepatitis C. Diabet Med. 2001;18(9):764–767. doi: 10.1046/j.1464-5491.2001.00562.x. [DOI] [PubMed] [Google Scholar]
- 23.Fabris P, Betterle C, Greggio NA, Zanchetta R, Bosi E, Biasin MR, de Lalla F. Insulin-dependent diabetes mellitus during alpha-interferon therapy for chronic viral hepatitis. J Hepatol. 1998;28(3):514–517. doi: 10.1016/S0168-8278(98)80328-0. [DOI] [PubMed] [Google Scholar]
- 24.Li Q, McDevitt HO. The role of interferon alpha in initiation of type I diabetes in the NOD mouse. Clin Immunol. 2011;140(1):3–7. doi: 10.1016/j.clim.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Xu B, Michie SA, Rubins KH, Schreriber RD, McDevitt HO. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci USA. 2008;105(34):12439–12444. doi: 10.1073/pnas.0806439105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai H, Khoury SJ, Auchincloss H, Jr, Sayegh MH. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198(1):63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kochupurakkal NM, Kruger AJ, Tripathi S, Zhu B, Adams LT, Rainbow DB, Rossini A, Greiner DL, Sayegh MH, Wicker LS, Guleria I. Blockade of the programmed death-1 (PD1) pathway undermines potent genetic protection from type 1 diabetes. PLoS One. 2014;9(2):e89561. doi: 10.1371/journal.pone.0089561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guleria I, Bupp MG, Dada S, Fife B, Tang Q, Ansari MJ, Trikudanathan S, Vadivel N, Fiorina P, Yagita H, Azuma M, Atkinson M, Bluestone JA, Sayegh MH. Mechanisms of PDL1-mediated regulation of autoimmune diabetes. Clin Immunol. 2007;125(1):16–25. doi: 10.1016/j.clim.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Perri V, Russo B, Crino A, Schiaffini R, Giorda E, Cappa M, Rosado MM, Fierabracci A. Expression of PD-1 molecule on regulatory T lymphocytes in patients with insulin-dependent diabetes mellitus. Int J Mol Sci. 2015;16(9):22584–22605. doi: 10.3390/ijms160922584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujisawa R, Haseda F, Tsutsumi C, Hiromine Y, Noso S, Kawabata Y, Mitsui S, Terasaki J, Ikegami H, Imagawa A, Hanafusa T. Low programmed cell death-1 (PD-1) expression in peripheral CD4(+) T cells in Japanese patients with autoimmune type 1 diabetes. Clin Exp Immunol. 2015;180(3):452–457. doi: 10.1111/cei.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pociot F, Lernmark A. Genetic risk factors for type 1 diabetes. Lancet. 2016;387(10035):2331–2339. doi: 10.1016/S0140-6736(16)30582-7. [DOI] [PubMed] [Google Scholar]
- 32.Noble JA, Valdes AM. Genetics of the HLA region in the prediction of type 1 diabetes. Curr Diabetes Rep. 2011;11(6):533–542. doi: 10.1007/s11892-011-0223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spain L, Larkin J, Martin-Liberal J. Determining predictive factors for immune checkpoint inhibitor toxicity: Response to Letter to the Editors “A case report of insulin-dependent diabetes as immune-related toxicity of pembrolizumab: presentation, management and outcome”. Cancer Immunol Immunother. 2016;65(6):769–770. doi: 10.1007/s00262-016-1845-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.International Expert, C. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]