Abstract
The accumulation of regulatory T cells (Tregs) at high density in various human carcinomas is generally associated with a poor prognosis, as expected from their capacity to inhibit antitumor immunity. Surprisingly, in patients bearing colorectal carcinoma (CRC), high regulatory T-cell infiltration is associated with a favorable prognosis, as shown by the analysis of seven clinical studies. To explain this paradox, we emphasize a putative role of the dense microbiological flora present in the large intestine with a trend toward translocation through the tumor. This microbiological hazard requires a T-cell-mediated inflammatory anti-microbial response that involves Th17 cells and can thereby promote cancer growth. This Th17-cell-dependent proinflammatory and tumor-enhancing response can be attenuated by Tregs, thus constituting a possible explanation for their favorable role in CRC prognosis. The link between a high density of FOXP3-positive cells in CRC immune infiltrates and favorable prognosis should lead us to consider tumor infiltrating Tregs as allies to be respected, rather than enemies to be destroyed during trials of CRC treatment.
Keywords: FOXP3, Regulatory T cells, Colorectal cancer, Prognosis, Immune response
Introduction
Regulatory T cells (Tregs) were initially identified in both mice [1] and humans [2] as CD4+ T cells constitutively expressing the α receptor to IL-2 (CD25) and inhibiting the immune response of effector T cells, notably their production of interferon-γ. These cells are known to be a key contributor to the maintenance of immune tolerance, preventing the emergence of organ-specific auto-immune disease. However, in cancer-bearing animals or patients, Tregs expand, migrate to tumor sites and suppress antitumor immune response mediated by NK cells, CD4+ and CD8+ T cells, and myeloid cells, through different molecular mechanisms [3]. The discovery that the transcription factor forkhead box P3 (FOXP3) was a more specific marker for distinguishing Tregs from other populations of T lymphocytes [4] strengthened their identity. Thus, nuclear immunolabeling with anti-FOXP3 antibodies has been widely used to characterize Treg cells on tissue sections, notably in human tumors.
In experimental tumor models as well as in cancer-bearing patients, accumulation of Tregs generally progresses during tumor growth. Treg accumulation was first described among peripheral blood leukocytes in cancer-bearing patients [5], in the spleen of tumor-bearing rodents and also in the tumor itself [6]. These Tregs have the capacity to block the T-cell-mediated immune response against human tumors [7] including colorectal cancer (CRC) [8]. Therefore, Tregs are usually considered as a major cell population involved in immune tolerance that protects cancer cells from antitumor immunity.
We will limit this review to carcinomas, i.e., cancers consisting of transformed epithelial cells, a group including many of the most common and also most severe cancers in humans. Consequently, we will not consider the prognostic effect of Treg invasion in hematopoietic malignancies in which lymphoid or myeloid cancer cells can exert particular interactions with the surrounding immune system.
In most varieties of human carcinomas, a high density of FOXP3+ tumor-infiltrating cells is associated with unfavorable clinical prognosis
Due to their suppressive effect on antitumor immune response, it is not surprising that a high density of tumor-infiltrating Treg, identified after FOXP3 immunolabeling, was found to be associated with unfavorable outcome. This relation has been reported in a wide range of localized or metastatic human carcinomas, including breast [9], ovarian [6], lung [10], hepatocellular [11], renal cell [12], pancreatic [13], gastric [14], ovarian [15], and cervical [16] carcinomas (Table 1). These findings attracted considerable interest, as they supported the appealing hypothesis that the presence of Tregs in the tumor bed could be a major escape mechanism of human cancers to the immune response, and therefore could represent a new prognostic biomarker, and a potential target for immunotherapy.
Table 1.
Effect of high versus low tumor-infiltrating FOXP3+ cell accumulation on prognosis of patients with various carcinomas (to the exclusion of colorectal and head and neck carcinomas)
| References | N | Stage | Univariate HR and P value | Multivariate HR and P value |
|---|---|---|---|---|
|
Breast carcinoma Bates et al. [9] |
299 | Invasive and non-invasive |
HR = 1.58 P = 0.04 (RFS) |
|
|
Non-small cell lung carcinoma Petersen et al. [10] |
64 | I |
FoxP3/CD3 ratio HR = 3.0–6.3 P = 0.017–0.15 (DSS) |
FoxP3/CD3 ratio HR = 3.3–8.2 P = 0.05–0.007 (DSS) |
|
Hepatocellular carcinoma Gao et al. [11] |
302 | Non-metastatic |
HR = 1.50 P = 0.006 (OS) |
|
|
Renal-cell carcinoma Li et al. [12] |
125 | I–IV |
Foxp3+ peritumoral HR = 6.88 P < 0.001 Foxp3+ intratumoral HR = 1.384 P = 0.405 (OS) |
Foxp3+ peritumoral HR = 4.26 P = 0.017 (OS) |
|
Pancreatic carcinoma Hiraoka et al. [13] |
198 | I–IV |
HR = 2.56 P < 0.0001 (OS) |
HR = 2.45 P < 0.0001 (OS) |
|
Gastric carcinoma Perrone et al. [14] |
110 |
Radical resection for stage II–III |
P = 0.008 (RFS) P = 0.002 (OS) |
HR = 2.00 P = 0.024 (RFS) HR = 2.34 P = 0.006 (OS) |
|
Ovarian carcinoma Wolf et al. [15] |
99 | I–III |
HR = 2.44 P = 0.004 (OS) HR = 2.56 P = 0.004 (PFS) |
|
|
Cervical carcinoma Jordanova et al. [16] |
115 |
Radical hysterectomy for stage I–II |
HR = 2.76 P = 0.048 (OS) |
HR = 2.25 P = 0.187 (OS) |
N number of patients analyzed, HR hazard ratio comparing high versus low density of tumor-infiltrating FOXP3+ cells for survival, OS overall survival, DFS disease-free survival, DSS disease-specific survival, PFS progression-free survival
However, conflicting data have accumulated suggesting that high tumor infiltration by FOXP3+ Tregs is not always associated with a poor prognosis, but, on the contrary, can be associated with an improved prognosis in some cancer types, notably in CRC. We will comment on these surprising results, and put forward some biological hypotheses to explain them.
The paradox of colorectal cancer
In this context, three recent studies about Foxp3+ T cell infiltrates in large patient populations came to surprising conclusions, contrary to those expected from the aforementioned studies in other tumor types. Salama et al. [17] studied the density of tumor-infiltrating lymphocytes bearing FOXP3 (for Tregs), CD45RO (for memory T cells), or CD8 in 967 patients with stage II or III CRC. Slides were treated with a high-resolution scanner to evaluate the density of stained cells. The density of cells bearing each marker was assessed quantitatively using digitized, high-resolution images and was found to be significantly increased in tumors compared with adjacent normal mucosa. In accordance with previous reports [18], univariate analysis confirmed the favorable prognostic importance of CD8+ and CD45RO+ T-cell density, but more interestingly also showed that high tumor infiltration with FOXP3+ T cells was also associated with better survival. Moreover, by multivariate analysis, only high FOXP3+ T-cell tumor infiltration was independently associated with improved survival (P = 0.001). This counter-intuitive observation was recently confirmed by Frey et al. [19] in a study including 1,420 non-metastatic CRC patients. In view of the established relationship between favorable CRC prognosis and microsatellite instability (MSI) reflecting inactivation or loss of function of the mismatch repair (MMR) genes [20], this population was stratified in two groups according to their MMR status. A significantly better 5-year survival rate was observed in the MMR-deficient group, compared with the MMR-proficient tumor group (71% vs. 54%). When compared by univariate survival analysis, the 5-year survival rate was significantly higher among patients with high vs low tumor infiltration by FOXP3+ cells in both MMR-proficient (61 and 45%, respectively; P < 0.004) and MMR-deficient (83 and 67%, respectively; P < 0.029) tumor group, excluding the possibility that the significant relation between high FOXP3+ cell density and favorable prognosis could be explained by MMR status. By multivariate analysis, the favorable prognostic value of high versus low tumor infiltration by FOXP3+ cells remained significant in MMR-proficient patients (Table 2), but not in MMR-deficient CRC (P = 0.13) possibly because of lack of power. In a third study, Nosho et al. [21] analyzed the relation between the density of various tumor-infiltrating T cells (CD3+, CD8+, CD45RO+, and FOXP3+) and the prognosis in 768 CRC from two prospective cohort studies. Densities of T-cells infiltrating CRC were determined on tissue microarrays. By univariate analysis, high densities of CD8+, CD45RO+, and FOXP3+ cells were associated with longer cancer-specific and overall survival (P ≤ 0.0003 for high FOXP3+ T infiltrates). By multivariate analysis, FOXP3+ cell density was not significantly associated with survival (P = 0.095). Only CD45RO+ cell density was associated with overall survival (P = 0.015). However, the interpretation of these results raised some questions, because FOXP3+ T cells mostly expressed CD45RO+ in humans [22]. Consequently, a significant interaction between CD45RO+ and FOXP3+ T cells among tumor-infiltrating cells should be considered, and FOXP3+ and CD45RO+ T-cell infiltration could not be independently analyzed in a multivariate model.
Table 2.
Effect of high versus low tumor-infiltrating FOXP3+ cell accumulation on colorectal cancer prognosis
| References | N | Stage | 5-year survival | Univariate HR and P value | Multivariate HR and P value |
|---|---|---|---|---|---|
| Salama et al. [17] | 967 | II and III |
HR = 0.78 P < 0.0001 |
HR = 0.54 P < 0.001 |
|
|
Sinicrope et al. [25] Intraepithelial Treg Stromal Treg |
160 | II and III |
68 versus 75% (OS) P = 0.239 72 versus 67% (OS) P = 0.751 |
HR = 0.81 P = 0.44 HR = 1.15 P = 0.65 |
|
|
Frey et al. [19] MMR-proficient, group 1 MMR-proficient, group 2 MMR-deficient |
1,420 613 584 223 |
I–III |
62 versus 46% (DFS) P = 0.004 60 versus 44% (DFS) P < 0.001 83 versus 67% (DFS) P = 0.029 |
HR = 0.73 P = 0.019 HR = 0.70 P = 0.007 HR = 0.63 P = 0.13 |
|
| Suzuki et al. [26] | 94 | I–III | P = 0.342 (OS) |
HR = 1.46 P = 0.34 |
|
| Correale et al. [24] | 57 | IV | P = 0.0025 (OS) |
HR = 0.28 P = 0.02 |
|
| Lee et al. [23] | 87 | II |
100 versus 79% (OS) P = 0.04 |
HR = 0.225 P = 0.24 |
|
| Nosho et al. [21] | 768 | I–IV |
80 versus 64% (DSS) P < 0.0001 |
HR = 0.48 P < 0.0001 |
HR = 0.89 P = 0.76 |
N number of patients analyzed, MMR mismatch repair, HR hazard ratio comparing high versus low density of tumor-infiltrating FOXP3+ cells for survival, OS 5-year overall survival, DFS 5-year disease-free survival, DSS 5-year disease-specific survival
This surprising relationship between high FOXP3+ T-cell infiltration and favorable prognosis in CRC patients led us to investigate all published papers referring to FOXP3+ T-cell infiltration and prognosis in patient cohorts of at least 50 CRC (Table 1). We found no study concluding that high FOXP3+ Treg infiltration is associated with adverse prognosis. Three additional studies supported a statistically significant relation between favorable prognosis in CRC patients and a high density of tumor-infiltrating FOXP3+ T cells. In one study, among 87 patients who had stage II CRC, the 5-year overall survival rate for patients with high FOXP3+ T-cell infiltrates was 100% compared with 79% for patients with low-FOXP3+ T-cell tumor density. The authors concluded that the determination of FOXP3+ tumor-infiltrating T cells could help to select a subpopulation of stage II CRC patients that may require adjuvant treatment [23]. Interestingly, also in a metastatic setting, another study performed in 57 CRC patients treated with chemotherapy, found that higher FOXP3+ T-cell tumor infiltration scores were significantly associated with better prognosis, with an overall survival of 43 months versus 29 months (P = 0.0005) in patients with low FOXP3+ tumor infiltration [24]. Of note, in the last two studies we examined [25, 26], no significant relation was observed between the absolute number of FOXP3+ T cells infiltrating the tumors and prognosis in colorectal cancer patients. One reason for this may be the low number of patients included in these two studies, indicating that they may have lacked sufficient power to reach statistical significance. These two studies together included only 10% of all patients studied in the literature.
Finally, taken together (Table 2) and contrary to the findings observed in most other human carcinomas, these results strongly suggest that a high frequency of tumor-infiltrating FOXP3+ regulatory T cells is associated with favorable prognosis in patients with CRC.
Are colorectal cancer-infiltrating FOXP3+ cells functional immunosuppressive Tregs?
In all the papers quoted above, cells infiltrating CRC were considered as Tregs because they expressed FOXP3. However, the reliability of FOXP3 as a specific marker of human Treg cells has been recently questioned [27]. Tregs were initially defined as a functionally distinct subpopulation of CD4+ T cells expressing CD25 at high levels and endowed with their capacity of preventing autoimmunity, but also controlling tumor immunity. Anti-FOXP3 antibodies not only label CD4+CD25+, but also CD8+CD25+ T cells, which have been reported to be present in CRC [28]. However, CD8+CD25+FOXP3+ T cells represent only a minor fraction of tumor-infiltrating FOXP3+ cells found in colorectal cancer. Indeed, when colorectal cancer sections were double-labeled with antibodies against FOXP3 and CD8 or CD4, the majority of FOXP3+ cells expressed CD4, not CD8 [25, 26]. Moreover, CRC-infiltrating CD8+CD25+FOXP3+ T cells still exert suppressive functions on the antitumor immune response [28].
FOXP3 expression in humans is not confined to functionally regulatory T cells, but can also be transiently expressed in conventionally activated CD4+CD25+effector T cells [29]. These induced FOXP3+ T cells are not functionally suppressive. It is important to note that very few studies in the literature have examined the exact functional properties of FOXP3+ T cells isolated from human CRC. However, in one of the few available studies, CD4+FOXP3+ T cells sorted from colorectal cancer have the capacity to suppress T-cell proliferation and IFN-γ production [30]. Interestingly, in a study conducted in a mouse model of intestinal polyposis, CD4+CD25+ regulatory T lymphocytes were able to induce regression of intestinal tumors [31], raising the possibility of broader roles for Tregs in colorectal carcinoma. Human Tregs may also express cytotoxic molecules such as granzyme and perforin and could in this way induce death of cancer cells [32]. However, the capability of FOXP3+ cells isolated from human CRC to destroy cancer cells has not yet been investigated and these cells expressed negligible amounts of cytotoxic molecule granzyme B [30].
Taken together, in apparent contradiction with their favorable prognostic role in CRC, these results indicate that a large majority of FOXP3+ T cells infiltrating human CRC are bona fide functionally suppressive Tregs that could impair rather than enhance immune response.
Colorectal cancer grows in a septic microenvironment. Treg cells that infiltrate colorectal cancers may preferentially control T-cell immune response driven by microbial rather than by tumor antigens
In healthy conditions, the human intestine contains more than 500 different types of microorganisms and the colon contains more than 1013 bacteria [33]. The physical structure of the intestinal mucosal barrier comprises a monolayer of epithelial cells linked together by tight junctions. This barrier limits access of the commensal microflora to the mucosa, with the help of lymphoid and myeloid cells accumulated in the lamina propria or infiltrated between epithelial cells. Many bacterial species were found to be enriched in CRC samples [34]. Bacterial translocation across the mucosal surface is frequent in CRC, possibly due to increased permeability of tight junctions and necrosis or ulceration of the tumor surface [35]. Many gastrointestinal bacteria are known to trigger the production of proinflammatory cytokines [36, 37], with subsequent proangiogenic [38] and tumor-enhancing effects through activation of transcription factors like NF-κB or STAT3 [39, 40]. Unlike other carcinomas, in which many infiltrating T cells have specificity for tumor-specific antigens, T-cells infiltrating CRC could be more specific for the commensal microflora (Fig. 1).
Fig. 1.
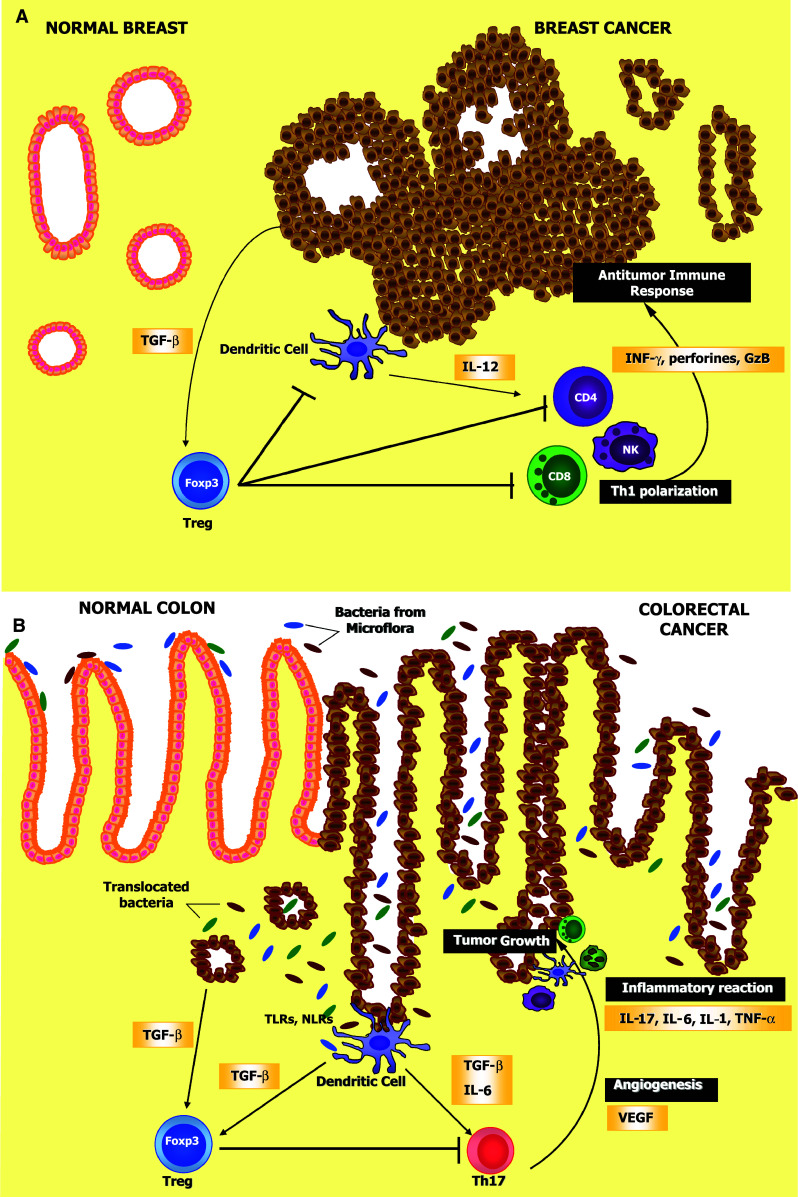
Opposite prognostic effect of Foxp3+ Treg cells according to the tumor type: In tumors growing in a non-infected microenvironment like breast carcinoma (a), tumor cells promote Treg accumulation notably through TGF-β secretion, which blunts Th1-dependent antitumor immune response, which normally involves secretion of IL-12 by dendritic cells, and secretion of INF-γ, perforines and GzB by T cells and NK cells. Conversely, in tumors growing in a septic environment, like CRC (b), bacterial translocation across the mucosal surface due to increased permeability of tight junctions and/or ulceration of the tumor triggers the production of proinflammatory cytokines by dendritic cells. This inflammatory anti-microbial response notably involves Th17 cells, which can stimulate angiogenesis through VEGF production and induce inflammatory reaction triggered by cytokines like IL-17, IL-1, IL-6, and TNF-α, thus contributing to tumor promotion. This proinflammatory and tumor-enhancing response could be attenuated by Tregs, thus constituting a possible explanation for their favorable role in CRC prognosis TGF-β transforming growth factor-β, NK natural killer cells, Th1 T helper type 1 cells, INF-γ interferon γ, GzB granzyme B, VEGF vascular endothelial growth factor, TLR toll-like receptor, NLR nod-like receptor, TNF-α tumor necrosis factor α
Observations performed by Erdman and co-workers in experimental mouse models of colorectal cancer established important data about the interactions between gastrointestinal bacteria, colorectal cancer, and Treg. They demonstrated that T-cell deficient mice were highly susceptible to inflammatory bowel disease and subsequent colorectal carcinoma dependent on microbial infection. Adoptive transfer of regulatory T cells was able to prevent bacteria-driven inflammation and carcinogenesis, but only when Treg were previously exposed to enteric bacteria and could secrete IL-10 [41–44].
Thus, by suppressing inflammation and immune responses resulting from bacterial invasion, FOXP3+ Tregs could be in fact antitumorigenic in colorectal cancer, which could also explain the relationship between their abundance inside the tumor and favorable prognosis [34].
Interactions between Tregs and other varieties of T cells infiltrating colorectal cancers
Tumor-infiltrating T cells other than Tregs are also involved in the prognosis of colorectal cancer. In situ immunostaining analyses on colorectal cancers from 415 patients demonstrated that tumors from patients without recurrence were infiltrated by higher immune cell densities of total CD3+ T cells, CD8+ T cells and memory CD45RO+ T cells, than those from patients whose tumors had recurred [18]. A strong in situ immune reaction correlated with a favorable prognosis regardless of the local extent of the tumor and of invasion of regional lymph nodes. In their paper evaluating the significance of FOXP3+, CD8+, and CD45RO+ T cells infiltrating colorectal cancers, Salama et al. [17] observed that that only high FOXP3+ T cell infiltrates, but not high CD8+ or CD45RO+ T cells infiltrates were an independent prognostic factor by multivariate analysis. The authors conclude that high FOXP3+ T-cell infiltrates had stronger prognostic significance compared with CD8+ and CD45RO+ lymphocytes in CRC patients.
These results thus suggest that high-density infiltration of colorectal cancers by CD8+, CD45RO+, and FOXP3+ T cells has a favorable prognostic significance in CRC.
Interactions between Tregs and Th17 cells infiltrating colorectal cancers
Another layer of complexity was revealed by the recent discovery of another distinct lineage of CD4+ T helper cell differentiation, namely Th17 cells [45]. Th17 are characterized by the expression of the transcription factor RORC that is dependent on STAT-3. Like FOXP3+Treg, Th17 differentiation requires TGF-β1, but, in contrast to Treg, Th17 also need IL-6. Th17 cells constitute a distinct lineage of CD4+ T helper cells and produce the proinflammatory cytokine IL-17.
In healthy conditions, most Th17 cells are induced in the gut-associated lymphoid tissue (GALT) in response to intestinal microbiological flora [46]. The development of Th17 cells infiltrating normal intestinal mucosa depends on components of the commensal microflora [47]. These cells are considerably more abundant in colorectal cancers than in other types of malignancies, such as breast cancer or melanoma, possibly as the consequence of bacterial colonization resulting in dendritic cell activation by toll-like receptor ligands and other bacterial signals [48]. The precise role of tumor-infiltrating Th17 cells is still controversial. Th17 cells mediate protection against extracellular pathogens, by recruiting macrophages and neutrophils and through the production of cytokines and anti-bacterial proteins, thereby preventing pathogen dissemination [47]. Th17 cells have also pro-inflammatory effects linked to their release of cytokines, including IL-17 [49]. IL-17 exerts proangiogenic effects on surrounding endothelial cells and fibroblasts by inducing production of VEGF and other angiogenic mediators [38]. Through their proinflammatory activity and the release of various angiogenic mediators, Th17 cells have mostly been reported to promote cancer growth [49]. IL-17-dependent new blood vessel formation determines tumor cell survival and is a condition of tumor progression.
Under physiological conditions, Treg cells, notably through their IL-10 production, maintain tissue homeostasis despite a continuous interaction with the multiple infectious agents from the gut microflora. However, under conditions of uncontrollable inflammation, IL-6 with TGF-β may inhibit Treg functions and polarize T-cell response toward a pathogenic Th17 inflammatory response. Blocking inflammation with anti-TNF-α antibodies or by supplementation with gut bacteria-primed Treg cells is able to induce tumor regression [50, 51].
Until now, the prognostic value of human colorectal cancer infiltration by Th17 cells had not been determined. However, in a very recent study, the group of Galon et al. found a poorer prognosis in patients with CRC highly infiltrated by IL17+ T cells (P = 0.0009), contrasting with a favorable prognosis when the tumor was highly infiltrated by Th1 and CD8+ cytotoxic cells. Th17 cell infiltration was quantified by determining the expression of a gene cluster including IL-17, but the poor prognosis linked to high infiltration by Th17 cells was also confirmed on immunohistochemistry analysis [52]. Interestingly, the same work confirmed the favorable prognosis linked to high infiltration with T cells expressing FOXP3 [52].
Thus, the relation between Tregs and proinflammatory immune cells like Th17 could be considered in explanation of the favorable effect of CRC-infiltrating FOXP3+ Tregs on cancer prognosis. Human Tregs are known to inhibit activation and function not only of Th1 and Th2 effector CD4+ T cells but also Th17 cells. By suppressing Th17 ability to proliferate and produce IL-17, and consequently their proinflammatory and tumor-promoting capacities, Treg may blunt their protumorigenic effects [53] (Fig. 1).
Beneficial role of Treg in carcinomas other than colorectal cancer
A similar relation between high tumor infiltration by FOXP3+ cells and favorable prognosis has also been observed in head and neck carcinomas. In 84 untreated patients with head and neck squamous cell carcinoma, high levels of tumor-infiltrating FOXP3+ CD4+ T cells were significantly associated with better locoregional control of the tumor (P = 0.026) but did not significantly influence overall survival (P = 0.07) [54]. Immunohistochemical staining was also performed in 106 biopsy specimens from newly diagnosed undifferentiated nasopharyngeal carcinoma, of which 47% positively stained for Ebstein Barr Virus (EBV) antigens. In this study, high T-cell density was correlated with improved survival [55]. The density of tumor-infiltrating FOXP3+ T cells was negatively associated with tumor stage (P < 0.05) and was associated with better overall and progression-free survival (P < 0.01). Squamous cell carcinoma and undifferentiated nasopharyngeal carcinoma represent two different types of head and neck cancer, associated with smoking and alcohol abuse and EBV infection, respectively. However, both occur in oral cavities permanently exposed to microbiological agents, even in the healthy state, and pathogen contamination seems to play a role in head and neck carcinogenesis and tumor progression [56]. Like CRC, squamous cell head and neck carcinomas are infiltrated by high number of Th17 cells [57]. It is tempting to postulate a link between this septic environment, the high prevalence of Th17 cells and the favorable impact of high density of FOXP3+ regulatory T cells in CRC and head and neck carcinomas.
Conclusions: tumor-infiltrating FOXP3+ T cells: to be eliminated or preserved?
In this review, we advance the hypothesis that the association of a favorable prognosis with a high density of FOXP3+ T cells infiltrating colorectal carcinomas, and probably also head and neck carcinomas; could be linked to their capacity to suppress tumor-promoting inflammatory immune response generated by infectious stimuli from bacteria translocated through the mucosal barrier, and notably promoted by Th17 cells. In the fields of fundamental and clinical oncology, FOXP3+ T cells are generally considered as one of the major cell populations in the tumor microenvironment capable of suppressing the antitumor immune response, thus blocking the success of immunotherapy. From this point of view, Tregs should be destroyed and their elimination, for instance by treatment with low doses of cyclophosphamide, restored efficacy of immunotherapy in experimental tumor models [7], but also in human, in small clinical studies in combination with vaccines [58, 59]. Other clinical trials targeted CD25 for the depletion of Treg cells in human carcinomas, using either a humanized monoclonal anti-CD25 antibodies (Dalizumab) [60] or a fusion protein of human IL-2 and diphteria toxin (denileukin diftitox) [61, 62]. These assays, as others performed in human cancers other than carcinomas such as melanoma and glioblastoma, demonstrated a selective depletion of circulating Treg cells and a relatively good tolerance of Treg-depleting treatment in these final-stage patients usually considered as escaping to any form of cancer therapy. No major objective clinical effect was reported but the low number of the patients in each trial and the very advanced stage of their disease have to be taken into consideration.
The unexpected observation that tumor-infiltrating FOXP3+ T cells could be associated with favorable prognosis in some varieties of human carcinomas, including CRC and head and neck carcinomas, should lead us to question this strategy in these types of tumors. Presently we have no sufficient information to determine the clinical effect of an immunotherapy targeting regulatory T cells in patients with CRC or head and neck carcinomas. This should lead to a careful attitude in these types of tumors, at least as long as the mechanisms linking tumor-infiltrating FOXP3+ T reg density to a favorable outcome remain enigmatic.
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 2.Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25− subpopulations. J Immunol. 2000;165:3105–3110. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 4.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 5.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 6.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 7.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 8.Clarke SL, Betts GJ, Plant A, Wright KL, El-Shanawany TM, Harrop R, Torkington J, Rees BI, Williams GT, Gallimore AM, Godkin AJ. CD4+CD25+FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS One. 2006;1:e129. doi: 10.1371/journal.pone.0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RP, Campa MJ, Sperlazza J, Conlon D, Joshi MB, Harpole DH, Jr, Patz EF., Jr Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2668–2672. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 11.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 12.Li JF, Chu YW, Wang GM, Zhu TY, Rong RM, Hou J, Xu M. The prognostic value of peritumoral regulatory T cells and its correlation with intratumoral cyclooxygenase-2 expression in clear cell renal cell carcinoma. BJU Int. 2009;103:399–405. doi: 10.1111/j.1464-410X.2008.08151.x. [DOI] [PubMed] [Google Scholar]
- 13.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 14.Perrone G, Ruffini PA, Catalano V, Spino C, Santini D, Muretto P, Spoto C, Zingaretti C, Sisti V, Alessandroni P, Giordani P, Cicetti A, D’Emidio S, Morini S, Ruzzo A, Magnani M, Tonini G, Rabitti C, Graziano F. Intratumoural FOXP3-positive regulatory T cells are associated with adverse prognosis in radically resected gastric cancer. Eur J Cancer. 2008;44:1875–1882. doi: 10.1016/j.ejca.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 16.Jordanova ES, Gorter A, Ayachi O, Prins F, Durrant LG, Kenter GG, van der Burg SH, Fleuren GJ. Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: which variable determines survival of cervical cancer patients? Clin Cancer Res. 2008;14:2028–2035. doi: 10.1158/1078-0432.CCR-07-4554. [DOI] [PubMed] [Google Scholar]
- 17.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 18.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 19.Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, Oertli D, Kettelhack C, Terracciano L, Tornillo L. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126:2635–2643. doi: 10.1002/ijc.24989. [DOI] [PubMed] [Google Scholar]
- 20.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 21.Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, Giovannucci E, Dranoff G, Fuchs CS, Ogino S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–366. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee WS, Park S, Lee WY, Yun SH, Chun HK. Clinical impact of tumor-infiltrating lymphocytes for survival in stage II colon cancer. Cancer. 2010;116:5188–5199. doi: 10.1002/cncr.25293. [DOI] [PubMed] [Google Scholar]
- 24.Correale P, Rotundo MS, Del Vecchio MT, Remondo C, Migali C, Ginanneschi C, Tsang KY, Licchetta A, Mannucci S, Loiacono L, Tassone P, Francini G, Tagliaferri P. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother. 2010;33:435–441. doi: 10.1097/CJI.0b013e3181d32f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137:1270–1279. doi: 10.1053/j.gastro.2009.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki H, Chikazawa N, Tasaka T, Wada J, Yamasaki A, Kitaura Y, Sozaki M, Tanaka M, Onishi H, Morisaki T, Katano M. Intratumoral CD8(+) T/FOXP3 (+) cell ratio is a predictive marker for survival in patients with colorectal cancer. Cancer Immunol Immunother. 2010;59:653–661. doi: 10.1007/s00262-009-0781-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roncarolo MG, Gregori S. Is FOXP3 a bona fide marker for human regulatory T cells? Eur J Immunol. 2008;38:925–927. doi: 10.1002/eji.200838168. [DOI] [PubMed] [Google Scholar]
- 28.Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant JC, Menegaux F, Rosenzwajg M, Lemoine F, Klatzmann D, Taieb J. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009;58:520–529. doi: 10.1136/gut.2008.158824. [DOI] [PubMed] [Google Scholar]
- 29.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kryczek I, Liu R, Wang G, Wu K, Shu X, Szeliga W, Vatan L, Finlayson E, Huang E, Simeone D, Redman B, Welling TH, Chang A, Zou W. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res. 2009;69:3995–4000. doi: 10.1158/0008-5472.CAN-08-3804. [DOI] [PubMed] [Google Scholar]
- 31.Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, Fox JG, Schauer DB. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- 32.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 34.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 35.Soler AP, Miller RD, Laughlin KV, Carp NZ, Klurfeld DM, Mullin JM. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis. 1999;20:1425–1431. doi: 10.1093/carcin/20.8.1425. [DOI] [PubMed] [Google Scholar]
- 36.Ivanov II, de Frutos RL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 39.Wang S, Liu Z, Wang L, Zhang X. NF-kappaB signaling pathway, inflammation and colorectal cancer. Cell Mol Immunol. 2009;6:327–334. doi: 10.1038/cmi.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. CD4+CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poutahidis T, Haigis KM, Rao VP, Nambiar PR, Taylor CL, Ge Z, Watanabe K, Davidson A, Horwitz BH, Fox JG, Erdman SE. Rapid reversal of interleukin-6-dependent epithelial invasion in a mouse model of microbially induced colon carcinoma. Carcinogenesis. 2007;28:2614–2623. doi: 10.1093/carcin/bgm180. [DOI] [PubMed] [Google Scholar]
- 43.Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ, Sher A. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erdman SE, Poutahidis T. Cancer inflammation and regulatory T cells. Int J Cancer. 2010;127:768–779. doi: 10.1002/ijc.25430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blaschitz C, Raffatellu M. Th17 cytokines and the gut mucosal barrier. J Clin Immunol. 2010;30:196–203. doi: 10.1007/s10875-010-9368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- 49.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183:4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 50.Rao VP, Poutahidis T, Ge Z, Nambiar PR, Horwitz BH, Fox JG, Erdman SE. Proinflammatory CD4+ CD45RB(hi) lymphocytes promote mammary and intestinal carcinogenesis in Apc(Min/+) mice. Cancer Res. 2006;66:57–61. doi: 10.1158/0008-5472.CAN-05-3445. [DOI] [PubMed] [Google Scholar]
- 51.Erdman SE, Rao VP, Olipitz W, Taylor CL, Jackson EA, Levkovich T, Lee CW, Horwitz BH, Fox JG, Ge Z, Poutahidis T. Unifying roles for regulatory T cells and inflammation in cancer. Int J Cancer. 2010;126:1651–1665. doi: 10.1002/ijc.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pages F, Galon J. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 53.Crome SQ, Clive B, Wang AY, Kang CY, Chow V, Yu J, Lai A, Ghahary A, Broady R, Levings MK. Inflammatory effects of ex vivo human Th17 cells are suppressed by regulatory T cells. J Immunol. 2010;185:3199–3208. doi: 10.4049/jimmunol.1000557. [DOI] [PubMed] [Google Scholar]
- 54.Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, Mosseri V, Laccourreye O, Bruneval P, Fridman WH, Brasnu DF, Tartour E. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 55.Zhang YL, Li J, Mo HY, Qiu F, Zheng LM, Qian CN, Zeng YX. Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Mol Cancer. 2010;9:4. doi: 10.1186/1476-4598-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hooper SJ, Wilson MJ, Crean SJ. Exploring the link between microorganisms and oral cancer: a systematic review of the literature. Head Neck. 2009;31:1228–1239. doi: 10.1002/hed.21140. [DOI] [PubMed] [Google Scholar]
- 57.Kesselring R, Thiel A, Pries R, Trenkle T, Wollenberg B. Human Th17 cells can be induced through head and neck cancer and have a functional impact on HNSCC development. Br J Cancer. 2010;103:1245–1254. doi: 10.1038/sj.bjc.6605891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacLean GD, Miles DW, Rubens RD, Reddish MA, Longenecker BM. Enhancing the effect of THERATOPE STn-KLH cancer vaccine in patients with metastatic breast cancer by pretreatment with low-dose intravenous cyclophosphamide. J Immunother Emphasis Tumor Immunol. 1996;19:309–316. doi: 10.1097/00002371-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Berd D, Maguire HC, Jr, Mastrangelo MJ. Induction of cell-mediated immunity to autologous melanoma cells and regression of metastases after treatment with a melanoma cell vaccine preceded by cyclophosphamide. Cancer Res. 1986;46:2572–2577. [PubMed] [Google Scholar]
- 60.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann NY Acad Sci. 2009;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- 61.Gerena-Lewis M, Crawford J, Bonomi P, Maddox AM, Hainsworth J, McCune DE, Shukla R, Zeigler H, Hurtubise P, Chowdhury TR, Fletcher B, Dyehouse K, Ghalie R, Jazieh AR. A Phase II trial of Denileukin Diftitox in patients with previously treated advanced non-small cell lung cancer. Am J Clin Oncol. 2009;32:269–273. doi: 10.1097/COC.0b013e318187dd40. [DOI] [PubMed] [Google Scholar]
- 62.Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, Lyerly HK, Clay TM. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112:610–618. doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]


