Abstract
Transforming growth factor beta (TGF-β) promotes tumor growth, invasion and metastasis in established tumors. In this study, we analyzed the effect of overexpressing an anti-TGF-β peptide fused to apolipoprotein A-I (ApoA-I) as a scaffold molecule. We generated and characterized stable MC38 colon carcinoma clones expressing ApoA-I fused to the anti-TGF-β peptide P144 and ApoA-I as control cells. We evaluated in vitro the gene expression profile, cell cycle and anchorage-independent growth. The in vivo tumorigenic potential and immunogenicity were analyzed inoculating the MC38 clones into C57BL/6 mice, recombination-activating gene 1 knockout mice or mice deficient in NK cells either subcutaneously or intrasplenically to generate hepatic metastases. While overexpression of ApoA-I had no effect on the parameters analyzed, ApoA-I fused to P144 markedly diminished the tumorigenic capacity and metastatic potential of MC38 in vitro and in vivo, thus generating a highly immunogenic cell line. MC38 cells transfected with ApoA-I fused to P144 triggered memory T cell responses able to eliminate the parental cell line upon re-challenge. In summary, expression of ApoA-I fused to P144 is a novel strategy to modulate TGF-β in tumor cells. These results highlight the potential of TGF-β as a target in the development of new antitumor treatments.
Keywords: Transforming growth factor beta, Apolipoprotein A-I, Colon cancer, Liver, Antitumor immune response
Introduction
Transforming growth factor beta (TGF-β) is a pleiotropic cytokine, which exerts a variety of functions in maintaining tissue homeostasis including cell proliferation, migration, differentiation and apoptosis [1]. However, abnormalities in its pathway result in carcinogenesis. These changes lead to abnormal tissue homeostasis, where TGF-β acts as tumor growth promoter, is a lead molecule in the tumor microenvironment and is critical for the initiation of the metastatic process [1, 2]. Interestingly, TGF-β is used as a clinical biomarker and its expression correlates with poor prognosis in patients within a large number of cancer-related malignancies [3–7]. This would suggest TGF-β to be a classic target for immunotherapeutic approaches. However, despite several anti-TGF-β strategies being implemented in clinical trials [2], TGF-β blockade in patients with cancer has not shown a clear clinical benefit. In fact, given that TGF-β affects the activity and differentiation of numerous cell types, there is a need for strategies designed to deliver TGF-β inhibitors to either the tumor microenvironment or the site of metastasis. To this aim, we selected apolipoprotein A-I (ApoA-I) to deliver the anti-TGF-β peptide P144 [8]. ApoA-I is the main protein component of high-density lipoproteins (HDL). HDL are generated in the liver and remove cholesterol from peripheral tissues for delivery to hepatocytes [9]. The main ApoA-I receptor, a scavenger receptor class B type I (SR-B1), is expressed at high levels in several organs such as the liver, adrenal glands, ovaries, testes and endothelial cells. Interestingly, SR-B1 expression is also upregulated in tumor cells [10–12]. Therefore, HDL and other ApoA-I decorated particles are ideal candidates as vehicles for tumor- and liver-targeted drug delivery [13–16]. P144 is a 14mer peptide that blocks TGF-β [17]. Peptide P144 inhibitory activity has been characterized in vitro and in vivo in different models of liver fibrosis and scleroderma [18, 19] and has entered a phase II clinical trial for the treatment of scleroderma. Moreover, the peptide is able to enhance immunotherapeutic strategies [20–22].
We have previously shown that liver expression of the fusion protein of ApoA-I and P144 (ApoLinkerP144) by an adeno-associated vector is able to reduce liver metastasis and improve survival in a mouse model of spontaneous melanoma by modulating the TGF-β signaling pathway [8]. To gain further mechanistic insight into the antitumor activity of the novel molecule, herein we characterize the biological effects of the overexpression of ApoLinkerP144 on MC38 colon cancer cells.
Materials and methods
Cell lines
The mouse colon carcinoma cell line MC38 was maintained in RPMI 1640 with GlutaMAX and supplemented with 10 % heat-inactivated fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin and 5 × 10−5 mol/l 2-mercaptoethanol (RPMI complete medium). MC38 Apo and MC38 ApoLinkerP144 transfected cell lines were obtained by transfecting MC38 cells with the plasmids pApo [23] or pApoLinkerP144 [8] using Lipofectamine 2000 (Invitrogen, Carlsbed, CA) following the manufacturer’s instructions. Twenty-four hours later, transfected clones were selected with 800 µg/ml G418 (Invitrogen). For in vitro cell proliferation assessment, 6 × 103 cells/well (six replicates per sample) were seeded in 96-well plates. Seventy-two hours later, cell proliferation was quantified by luminometry using ViaLight Plus Kit (Lonza, Rockland, ME, USA).
Mice
Five-week-old female C57BL/6 mice (Harlan Laboratories, Barcelona, Spain) or recombination-activating gene 1 (Rag-1) knockout mice deficient in T and B cells (C57BL/6 J-Rag1tm1Mom) were kept in the Center for Applied Medical Research animal facilities and cared for according to the institutional guidelines for animal care. For the subcutaneous tumor model, 5 × 105 cells per mice were injected subcutaneously in the left flank. Mice which rejected MC38 ApoLinkerP144 tumors were rechallenged subcutaneously in the right flank with the MC38 parental cell line 35 days after tumor rejection. Tumor occurrence and tumor size, presented as the average of two perpendicular diameters (millimeters), were measured at regular intervals. For the experimental liver metastasis model, liver metastases were generated by injecting cells into the spleens of syngeneic C57BL/6. Briefly, the spleen was exposed by an incision in the left upper abdomen of mice. MC38 cells or the different stable transfectants (5 × 105) in 50 µl of phosphate-buffered saline were injected into the spleens of C57BL/6 mice. Mice were then euthanized after 2 weeks, and livers were removed, weighed, and examined for metastases, using Matlab image analysis to quantify the tumor area. NK cells from C57BL/6 mice were depleted by administration of 100 µg anti-NK1.1 antibody at days −2, 0 and 3.
RT-PCR synthesis of total cDNA
Total RNA from cells was isolated using TRI reagent (Sigma, Dorset, UK). Sample concentration and purity were determined in a spectrophotometer. RNA was treated with DNase I and reverse-transcribed to cDNA with MMLV RT in the presence of RNase OUT (all reagents from Invitrogen) according to the manufacturer’s instructions. Samples were stored at −20 °C.
Quantitative PCR analysis
Mouse apolipoprotein A-I (ApoA-I), toll-like receptor 4 (Tlr4), matrix metallopeptidase 2 (Mmp2), matrix metallopeptidase 9 (Mmp9), forkhead box O3 (Foxo3a), cyclooxygenase 2 (Cox2) and histone H3.3 (H3f3a) expression were determined by quantitative real-time PCR using SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) and specific primers for each gene. (ApoA-I-Fw: 5′-cccagtcccaatgggaca-3′ and ApoA-I-Rv: 5′-caggagattcaggttcagctgtt-3′; Tlr4-Fw: 5′-agctcctgaccttggtcttg-3′ and Tlr4-Rv: 5′-cgcaggggaactcaatgagg-3′; Mmp2-Fw: 5′-gaagaagaaaatggaccccg-3′ and Mmp2-Rv: 5′-gcagcgatgaagatgatagg-3′; Mmp9-Fw: 5′-ccaaaccttcaaaggcctca-3′ and Mmp9-Rv: 5′-cggttgaagcaaagaaggag-3′; Foxo3a-Fw: 5′-gtttctcaagcactgccaag-3′ and Foxo3a-Rv: 5′-tgtggaagaactctgggaag-3′; Cox2-Fw: 5′-caaaagctgggaagccttctc-3′ and Cox2-Rv: 5′-cctcgcttatgatctgtcttg-3′). As histone H3f3a transcript levels remain unchanged across different experimental conditions, the expression of this housekeeping gene was used to standardize gene expression (H3f3a-s444pan: 5′-aaagccgctcgcaagagtgcg-3′ and H3f3a-a665pan: 5′-acttgcctcctgcaaagcac-3′).
The amount of each transcript was expressed by the formula where C t is the point at which gene fluorescence rises significantly above background fluorescence. Real-time PCR reactions were performed using BioRad reagents in accordance with the manufacturer’s recommended protocol.
Microarray analysis
RNA from MC38 Apo cells and MC38 ApoLinkerP144 cells was extracted using TRIzol reagent (Invitrogen). Samples were then processed following Affymetrix recommendations, and cRNA was hybridized to the Affymetrix Mouse Gene 1.0 ST array (Affymetrix, Santa Clara, CA, USA). Both background correction and normalization were performed using the Robust Multi-array Average normalization algorithm [24]. Then, a filtering process was performed to eliminate probe sets with low expression levels. Applying the criterion of an expression value greater than 5 in at least one of the experimental conditions, 30,763 probe sets were selected for the fold change (FC) calculation. Genes of interest were identified based on a logFC cutoff (|logFC| > 1), and functional enrichment analysis of Gene Ontology categories [25] was carried out using a standard hypergeometric test. R and Bioconductor were used for preprocessing and statistical analysis [26]. The biological knowledge extraction was complemented through the use of Ingenuity Pathway Analysis (Ingenuity Systems, www.ingenuity.com), a database which includes manually curated and fully traceable data derived from literature sources.
Analytical determinations
Splenic T cells producing interferon gamma were enumerated by ELISPOT according to manufacturer’s instructions (BD Biosciences, San Jose, CA). Spots were counted using an automated ELISPOT reader (CTL, Aachen, Germany).
Flow cytometry
MC38 size was analyzed using forward scatter by flow cytometry in a FACSCalibur flow cytometer (BD). Cell cycle was assessed using the Click-it EdU Cell Proliferation Assay (Invitrogen) according to manufacturer’s instructions, and the assay was performed in a FACS Canto II flow cytometer (BD).
Soft agar colony formation assay
Anchorage-independent growth was assayed by seeding 7000 cells per 35-mm plate into complete RPMI medium with 800 µg/ml G418 containing 0.25 % noble agar (Difco Laboratories, Detroit, MI, USA). Then, cells were covered with a layer of complete RPMI medium with 800 µg/ml G418 containing 0.5 % noble agar. After 20 days, colonies were counted under a microscope (100× magnification).
Statistical analysis
Prism software (GraphPad Software, Inc.) was employed for statistical analysis. We used the log-rank test to determine the significance of differences in survival curves. Mean differences between two groups were compared with a t test, and for three group comparisons we used a one-way ANOVA followed by Tukey´s posttest. Finally, tumor growth was fitted to an exponential growth equation and compared with the extra sum-of-squares F test. P values <0.05 were considered to be statistically significant.
Results
Apolipoprotein A-I and ApoLinkerP144 transfected cells differ in their metastatic gene expression signature
To analyze the effect of ApoLinkerP144 in MC38 colon cancer cells, we generated stable clones transfecting the plasmids encoding for ApoA-I (MC38 Apo) or for ApoA-I linked to the anti-TGF-β peptide P144 by a long and flexible linker (GAPAPAETKAEPMT) (MC38 ApoLinkerP144). While MC38 ApoA-I clones overexpress ApoA-I 200-fold more than WT cells, ApoLinkerP144 transfection of MC38 cells produced fewer clones than ApoA-I and lower levels of transgene expression were detected (Fig. 1a). These data provide evidence that MC38 cells stably expressing high levels of ApoLinkerP144 were toxic. We therefore analyzed the effect of clones expressing low levels of ApoLinkerP144.
Fig. 1.
ApoLinkerP144 expression by MC38 colon carcinoma cell lines modifies mRNA levels. Quantitative RT-PCR of MC38 parental cell line (MC38) or MC38 stably transfected with Apolipoprotein A-I (MC38 Apo) or MC38 transfected with ApoLinkerP144 (MC38 ApoLinkerP144). Gene expression of different genes is represented. a Apolipoprotein A-I (ApoA-I); b toll-like receptor 4 (Tlr4); c matrix metallopeptidase 2 (Mmp2); d matrix metallopeptidase 9 (Mmp9); e forkhead box O3 (Foxo3a) and f cyclooxygenase 2 (Cox2). Mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001
To gain initial insight into the cellular processes induced by the overexpression of the TGF-β inhibitor, we performed a microarray analysis to compare the gene expression profile between cells expressing either ApoA-I or ApoLinkerP144. We identified several genes with differential expression. These genes are involved in pathways that allowed us to predict a reduced tumorigenic capacity of the MC38 ApoLinkerP144 cells. Among these pathways, we found “colorectal cancer metastasis signaling,” “autoimmune disease,” “colitis,” “decrease in tumor size,” “decrease in tumor growth,” “tumorigenesis,” “blood vessel development,” “formation of vessels,” “invasion of tumor cells,” “metastasis of tumor cell lines,” “metastasis of cells” and “decrease of metastasis.” Table 1 shows a selection of upregulated or downregulated genes that were involved in several of these categories. Furthermore, we validated Tlr4, Mmp2, Mmp9, Foxo3a and Cox2. As shown in Fig. 1b–f, quantitative RT-PCR confirmed significant differences in gene expression between tumor cells expressing ApoA-I or ApoLinkerP144. Finally, the MC38 Apo cell line did not modify the expression of these genes as compared to the parental MC38 cells.
Table 1.
Microarray analysis to compare the gene expression profile between cells expressing either ApoAI or ApoLinkerP144
| Abbreviation | Gene name | No categories | Fold change |
|---|---|---|---|
| Col18a1 | Collagen, type XVIII, alpha 1 | 6 | −1.511 |
| Hgf | Hepatocyte growth factor | 9 | −1.681 |
| Mmp9 | Matrix metallopeptidase 9 | 8 | −2.734 |
| Itga1 | Integrin, alpha 1 | 6 | −1.067 |
| Foxo3 | Forkhead box O3 | 3 | −1.058 |
| Vegfc | Vascular endothelial growth factor C | 3 | −1.519 |
| Cox2 | Cyclooxygenase 2 | 6 | −1.145 |
| Mmp2 | Matrix metallopeptidase 2 | 4 | −2.057 |
| Nfkbia | Nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor, alpha | 4 | −1.215 |
| Fas | TNF receptor superfamily, member 6 | 4 | −2.651 |
| C3 | Complement component 3 | 3 | −2.398 |
| Nos2 | Nitric oxide synthase 2, inducible | 4 | −3.024 |
| Sema4d | Semaphorin 4D | 6 | 1.258 |
| Tgfbr2 | Transforming growth factor, beta receptor II | 7 | 1.293 |
| Thbs1 | Thrombospondin 1 | 7 | 2.269 |
| Wisp1 | Wnt1 inducible signaling pathway protein 1 | 5 | 1.207 |
| Tlr4 | Toll-like receptor 4 | 4 | 1.413 |
A selection of upregulated and downregulated genes involved in several functional categories and the fold change are shown
Stable expression of ApoLinkerP144 modifies tumor cell biology in the MC38 colon carcinoma cell line
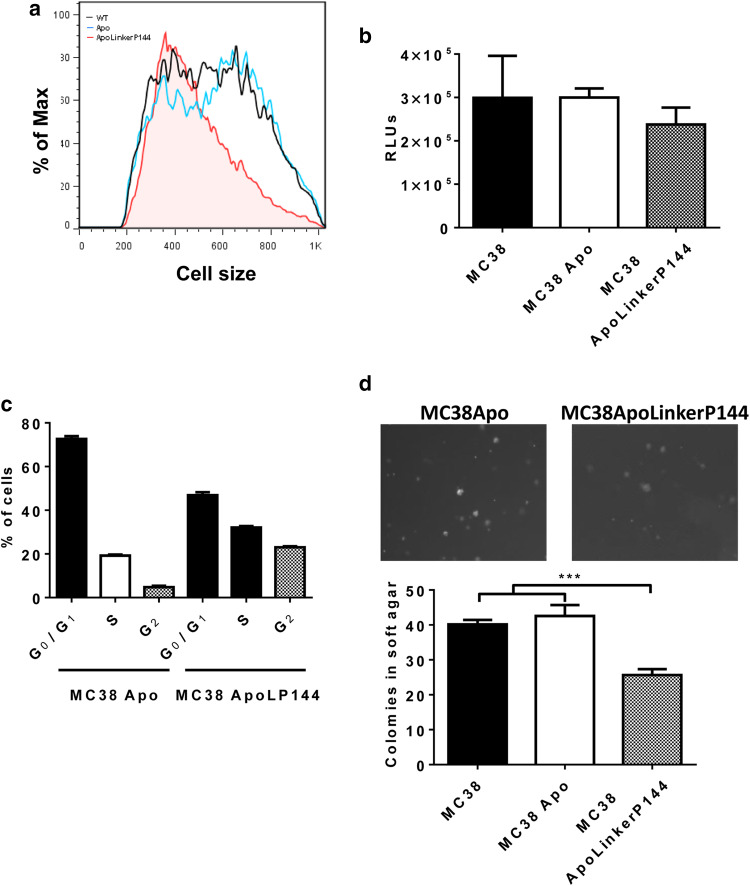
Next, we studied whether the differences in mRNA levels translated into phenotypic changes in vitro. ApoLinkerP144 expression altered the morphology of the cell lines, while cell lines expressing ApoA-I remained similar to the parental cell line. MC38 ApoLinkerP144 cells appeared smaller than the MC38 Apo cells. To demonstrate this reduced cell size, we represented the forward scatter obtained by flow cytometry, a parameter directly related to cell size (Fig. 2a).
Fig. 2.
ApoLinkerP144 expression by MC38 colon carcinoma cell lines modifies in vitro cell biology. a Changes in cell size after ApoLinkerP144 transfection. Size was determined by flow cytometry. Black lines correspond to MC38, blue to MC38 Apo and tinted red to MC38 ApoLinkerP144. b Cell proliferation of MC38 transfected cell lines. A total of 6000 cells per well were seeded, and proliferation was measured after 72 h; Mean ± SEM. c Cell cycle was analyzed by flow cytometry; Mean ± SEM. d Colonies in soft agar were counted under a ×100 magnification. Mean ± SEM ***P < 0.001
Cell proliferation was not significantly impaired (Fig. 2b), but a G2 phase cell cycle arrest was detected in MC38 ApoLinkerP144 cells (Fig. 2c). Finally, we analyzed the invasive phenotype of the clones by studying their capacity to grow in soft agar. MC38 Apo cells showed the same ability to form colonies in soft agar as the parental cells, but this capacity was impaired in MC38 ApoLinkerP144 cells (Fig. 2d), corroborating the hypothesis generated with the gene expression analysis. Therefore, stable expression of ApoLinkerP144 reduced the metastatic capacity of MC38 cells in vitro.
Expression of ApoLinkerP144 reduced in vivo tumor formation
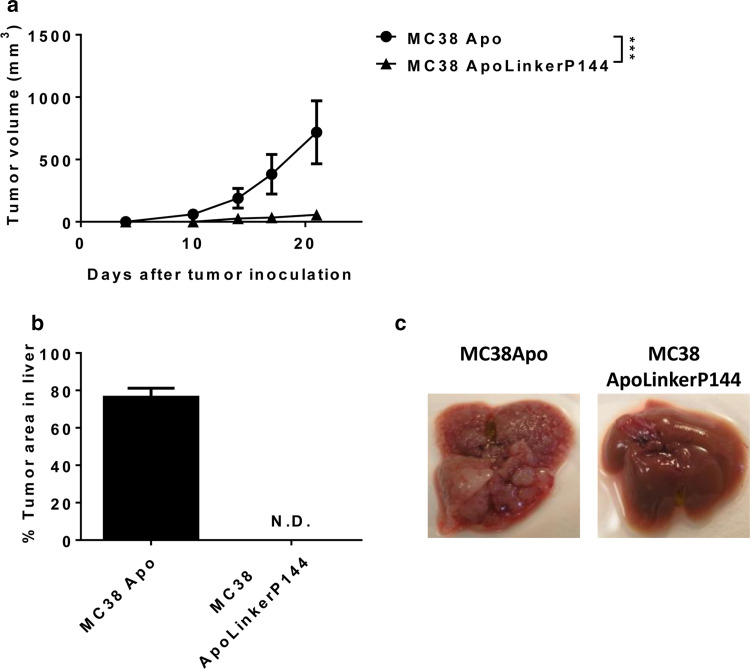
To determine the effect of ApoLinkerP144 on tumor growth in vivo, we injected the MC38 clones subcutaneously into C57BL/6 mice. Interestingly, while MC38 Apo grafted in all mice, MC38 ApoLinkerP144 did not grow in 7 out of 11 littermates (Fig. 3a). We next tested whether ApoLinkerP144 expression would affect the ability of MC38 cells to metastasize to the liver by intrasplenic injection of the MC38 clones. Interestingly, the effect of ApoLinkerP144 was even more dramatic in this liver metastasis model. There were no metastases when MC38 ApoLinkerP144 cells were injected (Fig. 3b, c). These data support that endogenous TGF-β blockade in stable-transfected tumor cell lines proportionally decreased their tumorigenicity and metastatic potential in vivo.
Fig. 3.
ApoLinkerP144 reduces the tumorigenic capacity of MC38 cells. a 5 × 105 MC38 Apo or MC38 ApoLinkerP144 transfected cell lines were injected subcutaneously into C57BL/6 mice. Tumor growth was measured at regular intervals. Mean ± SEM ***P < 0.001. b 5 × 105 MC38 Apo or MC38 ApoLinkerP144 transfected cell lines were intrasplenically injected. Mice were killed at day 15 after tumor cell inoculation, and tumor area in the liver was measured. c Representative images of the liver in each group are shown. Mean ± SEM. ND non detectable
Expression of ApoLinkerP144 allows activation of an antitumor immune response
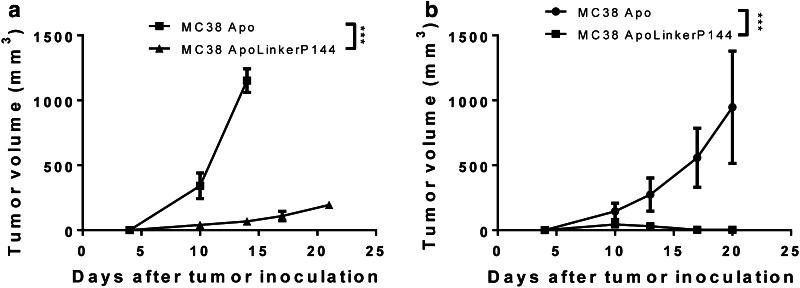
One of the main roles of TGF-β as a tumor promoter is suppression of antitumor immunity. Since MC38 ApoLinkerP144 tumor growth was dramatically impaired in wild-type mice, we implanted these cell lines in Rag-1-deficient mice—which lack mature B and T cells—to analyze whether ApoLinkerP144 renders the transfected cells more immunogenic. In these mice, MC38 ApoLinkerP144 tumors grew in all injected mice, although a significant delay in tumor growth was still observed (Fig. 4a). To analyze whether NK cells played any role, we depleted NK cells from C57BL/6 mice challenged with the MC38 clones using a depleting anti-NK1.1 antibody. In these mice, we detected the development of small tumors in all animals, but these were later eradicated (Fig. 4b). Therefore, NK cells were able to reduce tumor growth in the initial phase, but a T cell-mediated immune response was likely to be mainly responsible for the reduced tumor growth and metastatic potential of the transfected tumor cells.
Fig. 4.
ApoLinkerP144-expressing tumor cells triggers an antitumor immune response. a Rag-1 immune-deficient mice received subcutaneously 5 × 105 MC38 Apo or MC38 ApoLinkerP144 transfected cell lines. Tumor growth was measured at regular intervals. Mean ± SEM ***P < 0.001. b NK cells from C57BL/6 mice were depleted by administration of 100 µg anti-NK1.1 antibody at days −2, 0 and 3. On day 0, 5 × 105 MC38 Apo or MC38 ApoLinkerP144 transfected cell lines were inoculated subcutaneously. Tumor growth was measured at regular intervals. Mean ± SEM ***P < 0.001
Induction of a memory T cell response in mice challenged with MC38 ApoLinkerP144
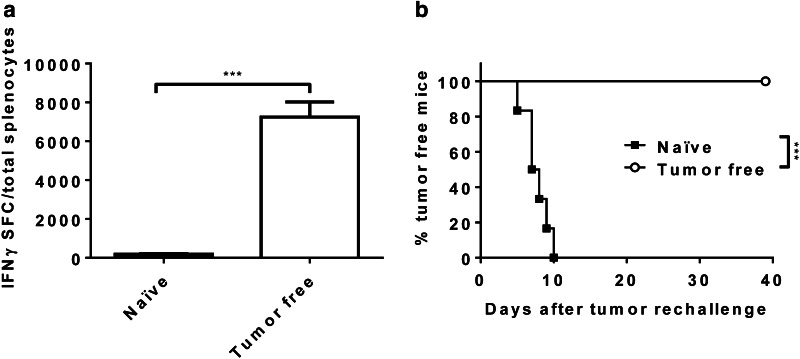
To confirm that a T cell response was developed in mice that rejected the MC38 tumors, we analyzed the number of T cells that secrete interferon gamma after stimulation of splenocytes with tumor cells. A positive response was detected in tumor-free mice (Fig. 5a). Moreover, these mice had developed a memory response since they were able to reject the parental MC38 cell line on re-challenge (Fig. 5b).
Fig. 5.
MC38 ApoLinkerP144 induces memory immune responses in vivo. C57BL/6 mice were subcutaneously injected with 5 × 105 MC38 ApoLinkerP144 cells/mice. 35 days later, most of the mice remained tumor-free. a An ELISPOT assay was performed with splenocytes of MC38 ApoLinkerP144 tumor-free mice. Results show interferon gamma spots among total splenocytes. Mean ± SEM ***P < 0.001. b 35-day MC38 ApoLinkerP144 tumor-free mice were rechallenged subcutaneously in the right flank with 5 × 105 MC38 parental cells. Survival results are displayed as a Kaplan–Meier plot of tumor occurrence. Treatment groups were compared using the log-rank test. ***P < 0.001
Discussion
In this study, we characterized the effect of overexpressing the anti-TGF-β peptide P144 on MC38 colon cancer cells. We have previously shown that the plasmid encoding the peptide alone had no effect, probably due to its hydrophobic nature that impairs the processing and exporting mechanism. To overcome this problem, we took advantage of ApoA-I as a molecular scaffold for expressing this hydrophobic anti-TGF-β peptide [8]. Since ApoLinkerP144 can be processed and exported after transfection, we were able to obtain stable cell lines overexpressing either the therapeutic peptide or ApoA-I as a control. The cell lines exhibited marked abnormalities, reflecting the toxicity of the expression of an anti-TGF-β molecule. In fact, only clones with low transgene expression could be established. Several anticancer drugs such as paclitaxel and herbal derivatives have been shown to induce tumor cell cytotoxicity by inducing G2 cell cycle arrest [27]. These results are in line with our cell cycle analysis and the low expression of ApoLinkerP144 in the tumor cell clones. Gene expression profiling highlighted that many processes involved in tumor growth and invasion were impaired. We selected a panel of the differentially expressed genes. Among the genes upregulated in the MC38 ApoLinkerP144 cells, we validated the overexpression of Tlr4. This is one of the receptors that sense danger-associated molecular patterns and may render cells more immunogenic [28]. Among the downregulated genes, two metallopeptidases, 2 and 9, where differentially expressed in the clone expressing ApoLinkerP144. Metallopeptidases are required for tumor stroma remodeling and therefore are involved in the invasion and metastatic potential of tumor cells [29]. Two other genes were validated. Foxo3a is a transcription factor that promotes the expression of metallopeptidases and the induction of apoptosis [30, 31]. Cox2 is the rate-limiting enzyme in the synthesis of prostaglandins that participates in the development of an immune suppressive microenvironment [32].
The phenotype predicted by gene expression profiling was confirmed both in vitro and in vivo. In vitro, MC38 ApoLinkerP144 exhibited an altered morphology, showed abnormalities in the cell cycle and a reduced capacity to form colonies in soft agar. Consequently, this cell line exhibited reduced tumorigenicity and metastatic potential in vivo. A total of 63 % of mice did not develop tumors after subcutaneous implantation of tumor cells, and no liver metastasis were detected 15 days after intrasplenic injection. The main mechanism involved in the immune system since tumor-free mice possessed memory T cells able to reject a second tumor challenge. Moreover, tumors grew in all mice deficient in mature B and T cells.
These results are in line with previous reports that blocked TGF-β. Indeed, immunization with tumor cells deficient in TGF-β has been proposed as a strategy to develop therapeutic antitumor vaccines [33, 34]. Furthermore, we previously demonstrated using viral-based vectors in two unrelated tumor models that overexpression of ApoLinkerP144 by hepatocytes resulted in decreased tumor progression [8]. Although long-term suppression of TGF-β may be detrimental in normal tissues due to the pleiotropic effects of this cytokine [2], it is important to highlight that our strategy limits the activity of the TGF-β inhibitor to the SR-B1-overexpressing tissues, mainly tumor and liver [35]. Therefore, this strategy may minimize the adverse effects associated with long-term inhibition of TGF-β.
In conclusion, these results highlight the relevance of TGF-β in tumor cell biology. Targeting TGF-β may have profound effects both at the tumor cell level and in promoting effector antitumor immune responses.
Acknowledgments
We are grateful to Dr. Paul Miller for English editing. This work was supported by the grant PI13/00207 from Instituto de Salud Carlos III, financed by the FEDER program of the European Union. Pedro Berraondo was supported by a Miguel Servet contract from Instituto de Salud Carlos III.
Conflict of interest
The authors declare that there are no conflicts of interest.
Abbreviations
- ApoA-I
Apolipoprotein A-I
- ApoLinkerP144
Fusion protein of apolipoprotein A-I and P144
- Cox2
Cyclooxygenase 2
- FC
Fold change
- Foxo3a
Forkhead box O3
- HDL
High-density lipoproteins
- H3f3a
Histone H3.3
- MC38 Apo
MC38 stable clones overexpressing apolipoprotein A-I
- MC38 ApoLinkerP144
MC38 stable clones overexpressing the fusion protein of apolipoprotein A-I and P144
- Mmp2
Matrix metallopeptidase 2
- Mmp9
Matrix metallopeptidase 9
- Rag-1
Recombination-activating gene 1
- SR-B1
Scavenger receptor class B type I
- Tlr4
Toll-like receptor 4
- TGF-β
Transforming growth factor beta
References
- 1.Massague J. TGFbeta in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacman D, Merkel S, Croner R, Papadopoulos T, Brueckl W, Dimmler A. TGF-beta receptor 2 downregulation in tumour-associated stroma worsens prognosis and high-grade tumours show more tumour-associated macrophages and lower TGF-beta1 expression in colon carcinoma: a retrospective study. BMC Cancer. 2007;7:156. doi: 10.1186/1471-2407-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruna A, Darken RS, Rojo F, Ocana A, Penuelas S, Arias A, Paris R, Tortosa A, Mora J, Baselga J, Seoane J. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11:147–160. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Ivanovic V, Todorovic-Rakovic N, Demajo M, Neskovic-Konstantinovic Z, Subota V, Ivanisevic-Milovanovic O, Nikolic-Vukosavljevic D. Elevated plasma levels of transforming growth factor-beta 1 (TGF-beta 1) in patients with advanced breast cancer: association with disease progression. Eur J Cancer. 2003;39:454–461. doi: 10.1016/S0959-8049(02)00502-6. [DOI] [PubMed] [Google Scholar]
- 6.Krasagakis K, Tholke D, Farthmann B, Eberle J, Mansmann U, Orfanos CE. Elevated plasma levels of transforming growth factor (TGF)-beta1 and TGF-beta2 in patients with disseminated malignant melanoma. Br J Cancer. 1998;77:1492–1494. doi: 10.1038/bjc.1998.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robson H, Anderson E, James RD, Schofield PF. Transforming growth factor beta 1 expression in human colorectal tumours: an independent prognostic marker in a subgroup of poor prognosis patients. Br J Cancer. 1996;74:753–758. doi: 10.1038/bjc.1996.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medina-Echeverz J, Fioravanti J, Diaz-Valdes N, Frank K, Aranda F, Gomar C, Ardaiz N, Dotor J, Umansky V, Prieto J, Berraondo P. Harnessing high density lipoproteins to block transforming growth factor beta and to inhibit the growth of liver tumor metastases. PLoS ONE. 2014;9:e96799. doi: 10.1371/journal.pone.0096799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 10.Cao WM, Murao K, Imachi H, Yu X, Abe H, Yamauchi A, Niimi M, Miyauchi A, Wong NC, Ishida T. A mutant high-density lipoprotein receptor inhibits proliferation of human breast cancer cells. Cancer Res. 2004;64:1515–1521. doi: 10.1158/0008-5472.CAN-03-0675. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Voutilainen R, Heikkila P, Kahri AI. Ribonucleic acid expression of the CLA-1 gene, a human homolog to mouse high density lipoprotein receptor SR-BI, in human adrenal tumors and cultured adrenal cells. J Clin Endocrinol Metab. 1997;82:2522–2527. doi: 10.1210/jcem.82.8.4123. [DOI] [PubMed] [Google Scholar]
- 12.Jiang J, Nilsson-Ehle P, Xu N. Influence of liver cancer on lipid and lipoprotein metabolism. Lipids Health Dis. 2006;5:4. doi: 10.1186/1476-511X-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fioravanti J, Medina-Echeverz J, Berraondo P. Scavenger receptor class B, type I: a promising immunotherapy target. Immunotherapy. 2011;3:395–406. doi: 10.2217/imt.10.104. [DOI] [PubMed] [Google Scholar]
- 14.Lacko AG, Nair M, Prokai L, McConathy WJ. Prospects and challenges of the development of lipoprotein-based formulations for anti-cancer drugs. Expert Opin Drug Deliv. 2007;4:665–675. doi: 10.1517/17425247.4.6.665. [DOI] [PubMed] [Google Scholar]
- 15.Lou B, Liao XL, Wu MP, Cheng PF, Yin CY, Fei Z. High-density lipoprotein as a potential carrier for delivery of a lipophilic antitumoral drug into hepatoma cells. World J Gastroenterol. 2005;11:954–959. doi: 10.3748/wjg.v11.i7.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McConathy WJ, Nair MP, Paranjape S, Mooberry L, Lacko AG. Evaluation of synthetic/reconstituted high-density lipoproteins as delivery vehicles for paclitaxel. Anticancer Drugs. 2008;19:183–188. doi: 10.1097/CAD.0b013e3282f1da86. [DOI] [PubMed] [Google Scholar]
- 17.Dotor J, Lopez-Vazquez AB, Lasarte JJ, Sarobe P, Garcia-Granero M, Riezu-Boj JI, Martinez A, Feijoo E, Lopez-Sagaseta J, Hermida J, Prieto J, Borras-Cuesta F. Identification of peptide inhibitors of transforming growth factor beta 1 using a phage-displayed peptide library. Cytokine. 2007;39:106–115. doi: 10.1016/j.cyto.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Ezquerro IJ, Lasarte JJ, Dotor J, Castilla-Cortazar I, Bustos M, Penuelas I, Blanco G, Rodriguez C, Lechuga Mdel C, Greenwel P, Rojkind M, Prieto J, Borras-Cuesta F. A synthetic peptide from transforming growth factor beta type III receptor inhibits liver fibrogenesis in rats with carbon tetrachloride liver injury. Cytokine. 2003;22:12–20. doi: 10.1016/S1043-4666(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 19.Santiago B, Gutierrez-Canas I, Dotor J, Palao G, Lasarte JJ, Ruiz J, Prieto J, Borras-Cuesta F, Pablos JL. Topical application of a peptide inhibitor of transforming growth factor-beta1 ameliorates bleomycin-induced skin fibrosis. J Invest Dermatol. 2005;125:450–455. doi: 10.1111/j.0022-202X.2005.23859.x. [DOI] [PubMed] [Google Scholar]
- 20.Gil-Guerrero L, Dotor J, Huibregtse IL, Casares N, Lopez-Vazquez AB, Rudilla F, et al. In vitro and in vivo down-regulation of regulatory T cell activity with a peptide inhibitor of TGF-beta1. J Immunol. 2008;181:126–135. doi: 10.4049/jimmunol.181.1.126. [DOI] [PubMed] [Google Scholar]
- 21.Llopiz D, Dotor J, Casares N, Bezunartea J, Diaz-Valdes N, Ruiz M, Aranda F, Berraondo P, Prieto J, Lasarte JJ, Borras-Cuesta F, Sarobe P. Peptide inhibitors of transforming growth factor-beta enhance the efficacy of antitumor immunotherapy. Int J Cancer. 2009;125:2614–2623. doi: 10.1002/ijc.24656. [DOI] [PubMed] [Google Scholar]
- 22.Llopiz D, Dotor J, Zabaleta A, Lasarte JJ, Prieto J, Borras-Cuesta F, Sarobe P. Combined immunization with adjuvant molecules poly(I:C) and anti-CD40 plus a tumor antigen has potent prophylactic and therapeutic antitumor effects. Cancer Immunol Immunother. 2008;57:19–29. doi: 10.1007/s00262-007-0346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fioravanti J, Gonzalez I, Medina-Echeverz J, Larrea E, Ardaiz N, Gonzalez-Aseguinolaza G, Prieto J, Berraondo P. Anchoring interferon alpha to apolipoprotein A-I reduces hematological toxicity while enhancing immunostimulatory properties. Hepatology. 2011;53:1864–1873. doi: 10.1002/hep.24306. [DOI] [PubMed] [Google Scholar]
- 24.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blake JA, Dolan M, Drabkin H, Hill DP, Li N, Sitnikov D, et al. Gene Ontology annotations and resources. Nucleic Acids Res. 2013;41:D530–D535. doi: 10.1093/nar/gks1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, et al. Orchestrating high-throughput genomic analysis with bioconductor. Nat Methods. 2015;12:115–121. doi: 10.1038/nmeth.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiPaola RS. To arrest or not to G(2)-M Cell-cycle arrest : commentary re: A. K. Tyagi et al., Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth inhibition, G(2)-M arrest, and apoptosis. Clin. cancer res., 8: 3512–3519, 2002. Clin Cancer Res. 2002;8:3311–3314. [PubMed] [Google Scholar]
- 28.Yamazaki T, Hannani D, Poirier-Colame V, Ladoire S, Locher C, Sistigu A, Prada N, Adjemian S, Catani JP, Freudenberg M, Galanos C, Andre F, Kroemer G, Zitvogel L. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ. 2014;21:69–78. doi: 10.1038/cdd.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem. 1999;274:13066–13076. doi: 10.1074/jbc.274.19.13066. [DOI] [PubMed] [Google Scholar]
- 30.Storz P, Doppler H, Copland JA, Simpson KJ, Toker A. FOXO3a promotes tumor cell invasion through the induction of matrix metalloproteinases. Mol Cell Biol. 2009;29:4906–4917. doi: 10.1128/MCB.00077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HY, Youn SW, Kim JY, Park KW, Hwang CI, Park WY, Oh BH, Park YB, Walsh K, Seo JS, Kim HS. FOXO3a turns the tumor necrosis factor receptor signaling towards apoptosis through reciprocal regulation of c-Jun N-terminal kinase and NF-kappaB. Arterioscler Thromb Vasc Biol. 2008;28:112–120. doi: 10.1161/ATVBAHA.107.153304. [DOI] [PubMed] [Google Scholar]
- 32.Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 33.Nemunaitis J, Barve M, Orr D, Kuhn J, Magee M, Lamont J, et al. Summary of bi-shRNA/GM-CSF augmented autologous tumor cell immunotherapy (FANG) in advanced cancer of the liver. Oncology. 2014;87:21–29. doi: 10.1159/000360993. [DOI] [PubMed] [Google Scholar]
- 34.Liu P, Jaffar J, Zhou Y, Yang Y, Hellstrom I, Hellstrom KE. Inhibition of TGFbeta1 makes nonimmunogenic tumor cells effective for therapeutic vaccination. J Immunother. 2009;32:232–239. doi: 10.1097/CJI.0b013e318197ac86. [DOI] [PubMed] [Google Scholar]
- 35.Shahzad MM, Mangala LS, Han HD, Lu C, Bottsford-Miller J, Nishimura M, et al. Targeted delivery of small interfering RNA using reconstituted high-density lipoprotein nanoparticles. Neoplasia. 2011;13:309–319. doi: 10.1593/neo.101372. [DOI] [PMC free article] [PubMed] [Google Scholar]