Abstract
Multiple myeloma (MM) is characterized by a malignant proliferation of plasma cells in the bone marrow with associated organ damage. Although the prognosis of MM has improved recently, the disease remains incurable for the large majority of patients. The eradication of residual disease in the bone marrow is a main target on the road toward cure. Immune cells play a role in the control of cancer and can be tools to attack residual MM cells. However, the myeloma-associated immune deficiency is a major hurdle to immunotherapy. We evaluated ex vivo the effects of low doses of the immunomodulatory drugs (IMiDs) lenalidomide and pomalidomide on several immune cell types from MM patients after autologous stem cell transplantation and with low tumor burden. We observed that these drugs increased CD4+ and CD8+ T-cell proliferation and cytokine production, enhanced the lytic capacity of cytotoxic T lymphocytes and reduced the suppressive effects of regulatory T cells on CD8+ T-cell responses. In addition, we found that functional dendritic cells (DCs) can be generated from mononuclear cells from MM patients. The presence of IMiDs improved the quality of antigen-specific T cells induced or expanded by these DCs as evidenced by a higher degree of T-cell polyfunctionality. Our results provide a rationale for the design of early phase clinical studies to assess the efficacy of DC-based immunotherapy in combination with posttransplant maintenance treatment with IMiDs in MM.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1571-6) contains supplementary material, which is available to authorized users.
Keywords: Multiple myeloma, Immunomodulatory drugs, Lenalidomide, Pomalidomide, Immunotherapy, Dendritic cells
Introduction
The introduction of novel agents such as proteasome inhibitors and immunomodulatory drugs (IMiDs) in combination with high-dose therapy and autologous stem cell transplantation (ASCT) has markedly improved the prognosis of multiple myeloma (MM) in younger (<65 years) patients [1, 2]. Very good partial remissions (VGPR) or complete remissions (CR) can be obtained in 60–70 % of patients, and the median duration of these responses exceeds 24–30 months. Maintenance treatment with IMiDs has been shown to prolong progression-free and overall survival after ASCT. However, the emergence of resistant subclones will eventually lead to disease progression [3] in the vast majority of cases [4]. Active immunotherapy through induction of T lymphocytes targeting malignant cells may be an adjuvant strategy to eradicate residual disease after ASCT. A number of studies explored immunotherapeutic strategies in MM patients that underwent ASCT, for instance, vaccination with idiotype-pulsed dendritic cells (DCs) [5, 6], with DC/tumor fusions [7] or with DCs electroporated with mRNA encoding myeloma-associated antigens [8], administration of IL-2 and GM-CSF [9], combination therapy with adoptive T-cell transfer and tumor antigen vaccination [10] or adoptive cellular therapy with NKG2D+ CD3+ CD8+ T cells [11]. These immunotherapies resulted in augmented cellular or humoral immune responses, thus showing the potency of this approach. DCs are specialized antigen-presenting cells capable of inducing strong and long-term T-cell responses. Therefore, DC vaccination against targets on malignant plasma cells could be an option for immunotherapy in MM. However, MM is characterized by a profound immunosuppression including inhibition of DC function by enhancing the expression of the immune-suppressive enzyme indoleamine 2,3-dioxygenase (IDO) [12] and suppressing the antigen-presenting function through the production of hepatocyte growth factor [13]. In addition, increased frequencies of immune-suppressive cell types have been noted in MM patients, such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) [14–19]. Hence, disease-related immunosuppression is an important hurdle that needs to be overcome in order for immunotherapeutic strategies for MM to be successful. The current availability in the clinic of IMiDs may offer opportunities to reach this goal. In addition to variable degrees of anti-angiogenic [20] and direct anti-tumoral [21] effects, the so-called second generation IMiDs, lenalidomide and pomalidomide exert a number of powerful immune stimulatory effects [22]. They stimulate T-cell proliferation and enhance natural killer (NK) cell toxicity. The mechanism of these actions includes modulation of Rho GTPases in T cells [23], which is critical for immune synapse formation. In addition, lenalidomide promotes cereblon-dependent destruction of Ikaros proteins, which are known to inhibit IL-2 production by T cells [24, 25]. We hypothesize that the possibility to achieve minimal residual disease state after ASCT in combination with IMiD-based maintenance treatment may facilitate immunotherapeutic interventions in MM patients. Here, we report the results of a preclinical study performed on samples obtained from MM patients in CR/VGPR after ASCT without IMiD maintenance treatment. We evaluated the ex vivo effects of IMiDs on patient-derived T cells, Tregs and MDSCs. We also assessed the feasibility to generate functional DCs from these patients, and we investigated the effects of IMiDs on DC-induced T-cell responses. Our results provide a rationale for proceeding with DC-based anti-MM vaccination strategies.
Materials and methods
Study subjects
MM patients were recruited from the Clinical Hematology Department of the Universitair Ziekenhuis Brussel (UZ Brussel, Brussels, Belgium). Approval for this study was obtained from the institutional review board, and informed consent was provided according to the Declaration of Helsinki. A total of nine MM patients autografted after high-dose (200 mg/m2) melphalan were included; there were three male and six female patients, and the median age was 60 years (range 29–62). Induction treatment before ASCT consisted to 4 courses of an IMiD-containing regimen in eight patients and VAD (vincristine + adriamycin + dexamethasone) in one patient. Patients were in VGPR (4/9) or CR (5/9) at a median of 21 months (range 5–54 after ASCT). To obtain sufficient amounts of immune cells as well as mononuclear cell to produce DCs, patients underwent a single leukapheresis using the Cobe Spectra device. A total blood volume of 10 l was processed, and a median of 6.17 billion peripheral blood mononuclear cells (PBMCs) (range 2.98–10.6 billion cells) was obtained for ex vivo studies and DC production. Melanoma patients were recruited at the UZ Brussel within the context of DC immunotherapy trials performed at our institution [26, 27].
IMiDs
Lenalidomide (CC-5013, IMiD3) and pomalidomide (CC-4047, IMiD1) were obtained from Selleck Chemicals LLC, dissolved in DMSO (Sigma) at a concentration of 80 mM and stored in aliquots at −20 °C.
T-cell isolation and manipulation
PBMCs were obtained through leukapheresis. Monocytes were isolated through plastic adherence for DC generation (see further). Non-adherent cells (NACs) were frozen as a T-cell source in heat-inactivated autologous plasma supplemented with 10 % DMSO. Before their use in T-cell stimulations, NACs were thawed and rested overnight in Iscove’s Modified Dulbecco’s medium (IMDM, Cambrex) supplemented with 10 % heat-inactivated human AB serum (PAA laboratories), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, 0.24 mM l-asparagine and 0.55 mM l-arginine (all from Cambrex), further referred to as lymphocyte medium, in the presence of 25 U/ml IL-2 (Chiron) and 10 U/ml DNase I (Sigma-Aldrich).
DC generation, maturation and antigen loading
PBMCs were seeded in Cell Factories® using X-vivo 15 medium (BioWhittaker) supplemented with 1 % heat-inactivated autologous plasma, allowing plastic adherence of monocytes. After removal of the NACs, monocytes were cultured in RPMI 1640 medium supplemented with 1 % heat-inactivated autologous plasma in the presence of GM-CSF and IL-4 for 6 days. GM-CSF and IL-4 were added on days 2 and 4. On day 6, DCs were harvested and either frozen in an immature state or electroporated with a combination of constitutively active Toll-like receptor 4 (caTLR4), CD40 ligand (CD40L) and CD70 (‘TriMix1’) and MAGE-C1 encoding mRNAs in order to evaluate the quality of DCs from MM patients. DCs used for T-cell stimulations were either used immature (‘iDCs’) or matured, either by adding a mixture of inflammatory cytokines (IL-6, IL-1β, TNF-α and PGE2) for 24 h (cytokine cocktail matured DCs, ‘CM-DCs’) or through electroporation with TriMix1 or TriMix2 (caTLR4, CD40L and 4-1BB ligand (4-1BBL/CD137L) encoding mRNA). For antigen-specific T-cell stimulations, DCs were co-electroporated with TriMix and influenza nuclear protein (Flu-NP)- or Melan-A-encoding mRNA. DC electroporations were performed using the Gene Pulser Xcell Electroporation System (Bio-Rad).
T-cell stimulations
T-cell stimulations were performed in lymphocyte medium at a density of 2 million T cells/ml. For some experiments, CD8+ T cells were sorted using CD8 coated microbeads (Miltenyi Biotec). One hour before they were stimulated, and the T cells were pre-treated with the IMiDs (0.5 μM) or an equal volume of lymphocyte medium (‘no IMiDs’ cultures).
Polyclonal T-cell stimulations
T cells were stimulated with either anti-CD3 and anti-CD28-coated microbeads (Invitrogen) or anti-CD3 beads in combination with autologous DCs (DC/T-cell ratio 1/10). Anti-CD3-coated microbeads were prepared using tosyl-activated dynabeads (Invitrogen) and anti-CD3 OKT-3 (in-house preparation). Every 48 h, IMiDs or an equal volume of lymphocyte medium (‘no IMiDs’ cultures) was added.
Flu-NP-specific T-cell stimulations
Purified CD8+ T cells were stimulated with CM-, TriMix1- or TriMix2-DCs (co-) electroporated with Flu-NP-DC-LAMP encoding mRNA (DC/T-cell ratio 1/10). After 48 h, lenalidomide (0.5 μM), pomalidomide (0.5 μM) or an equal volume of lymphocyte medium was added.
Melan-A-specific T-cell stimulations
Melan-A specific T-cell stimulations were performed with purified CD8+ T cells and autologous DCs from healthy HLA-A2+ donors. CD8+ T cells were stimulated with DCs co-electroporated with TriMix1- and Melan-A-DC-LAMP-encoding mRNA (DC/T-cell ratio 1/10). Every 48 h, lenalidomide (0.5 μM), pomalidomide (0.5 μM) or an equal volume of lymphocyte medium were added. CD8+ T cells were restimulated weekly with the same stimulator DCs as were used in the primary stimulation. After three rounds of stimulation, CD8+ T cells were harvested and their antigen specificity and function were determined. Melan-A-specific T-cell stimulations were screened upon overnight co-culture with T2 cells pulsed with Melan-A-A2 or Gag-A2 peptide.
Treg isolation
CD4+ T cells were isolated using CD4-coated microbeads (Miltenyi Biotec) and stained with anti-CD4 PerCP-Cy5.5, anti-CD127 FITC (both from eBioscience) anti-CD25 PE (Miltenyi Biotec) and anti-CD127 FITC (eBioscience). CD4+ CD25high CD127low Tregs were sorted using a FACS Aria III (BD Biosciences).
MDSC isolation
CD14+ cells were isolated using CD14-coated microbeads (Miltenyi Biotec) and stained with biotinylated anti-CD11b (BD Pharmingen), anti-CD14 APC-Cy7 (Biolegend) and anti-HLA-DR PE (BD Biosciences). CD11b+ CD14+ HLA-DRlow/− and CD11b+ CD14+ HLA-DRhigh cells were sorted using a FACS Aria III.
Treg and MDSC suppression assays
CD8+ T cells were labeled with CellTrace Violet (as described in ‘Proliferation assay’) and incubated with IMiDs at a concentration of 0.5 μM for 1 h. Afterward, the CD8+ T cells were stimulated with anti-CD3 and anti-CD28-coated microbeads and co-cultured with purified autologous Tregs or MDSCs, at different ratios. Co-cultures were performed in round-bottom 96-well plates at a density of 100,000 CD8+ T cells/well in a total volume of 100 μl lymphocyte medium/well. IL-2 was added to the Treg suppression assays at a concentration of 25 U/ml. IMiDs were added at a concentration of 0.5 μM every 48 h. After 6 days of co-culture, the supernatants and cells were harvested and used to measure cytokine production and CD8+ T-cell proliferation, respectively.
Flow cytometry
DC phenotyping was performed using a FACSCanto flow cytometer (BD Biosciences). Other flow cytometry analyses were performed on an LSR Fortessa flow cytometer (BD Biosciences). Automatic compensation was performed using CompBeads (BD Biosciences).
Tetramer staining
Cells were stained with a FITC-labeled anti-CD8 (BD Biosciences) and with 10 nM PE-labeled HLA-A2 tetramers (prepared in-house) containing Melan-A (ELAGIGILTV) peptides.
CD107a/4-1BB staining
Sixteen hours before the cells were stained, a PE-Cy5 labeled CD107a antibody (eBioscience) was added to the co-cultures together with monensin (GolgiStop, BD Biosciences). The next day, T cells were harvested and stained using the following antibodies: 4-1BB PE (BD Pharmingen), CD3 Horizon V450 (eBioscience) and CD8 FITC. Aspecific T-cell responses, measured upon culture in the absence of T-cell stimulus, were considered as background.
Intracellular cytokine staining
Sixteen hours before the cells were stained, brefeldin (GolgiPlug, BD Biosciences) was added to the T-cell cultures. For some experiments, a Brilliant Violet 421 labeled CD107a antibody (BioLegend, respectively) was added together with monensin (GolgiStop, BD Biosciences). The next day, T cells were harvested and stained using the following antibodies: CD4 PerCP-Cy5.5 (eBioscience) and CD8 APC-eFluor 780 (eBioscience). Fixation and permeabilization of the cells was performed using the Cytofix/Cytoperm kit (BD Biosciences), according to the manufacturer’s instructions. Intracellular stainings were performed using the following antibodies: IFN-γ PE (eBioscience), TNF-α FITC (eBioscience), IL-2 APC (eBioscience), MIP-1β PE-Cy7 (BD Pharmingen) and/or perforin FITC (Abcam). Aspecific T-cell responses, measured upon culture in the absence of T-cell stimulus, were considered as background.
Proliferation assay
In order to measure T-cell proliferation, the CellTrace Violet cell proliferation kit (Invitrogen) was used. T cells were resuspended at 1 million cells/ml in PBS and were incubated with 0.5 μM CellTrace Violet for 20 min at 37 °C. Afterward, the cells were washed thoroughly with lymphocyte medium and stimulated as described. On day 6 or 7, the T cells were harvested and stained with CD3 APC (Biolegend), CD4 PerCP-Cy5.5, CD8 FITC (Biolegend) and/or CD8 APC-eFluor 780 (eBioscience). Aspecific T-cell proliferation, measured upon culture in het absence of T-cell stimulus, was considered as background.
DC phenotyping
DCs were stained with anti-CD14 PE, anti-CD40 PE, anti-CD80 PE, anti-CD83 PE (all from BioLegend), anti-CD70 FITC (BD Pharmingen) and anti-CCR7 APC (eBioscience). DC phenotyping was performed 24 and 48 h after electroporation with TriMix1 and MAGE-C1 encoding mRNA.
ELISA
Enzyme-linked immunosorbent assays (ELISA) were performed to quantify IL-10 and IL-12p70 production by DCs and TNF-α, IL-2 and IFN-γ production by T cells. Following ELISA kits were used: IL-10: R&D Systems, IL-12p70, TNF-α and IL-2: eBioscience and IFN-γ: Endogen. ELISA were performed according to the manufacturer’s instructions. Aspecific cytokine production by T cells, measured after culture in the absence of T-cell stimulation, was considered as background.
Data analysis
Flow cytometry data were analyzed using FACS DIVA (BD Biosciences) and FlowJo (Tree Star inc.) software. Unless the background is shown, data were corrected for the background by subtraction of the responses measured in the negative control conditions. Statistical analysis was performed using GraphPad Prism version 5.0 software (GraphPad Software). Statistical analysis was performed by repeated measures ANOVA. Post hoc Bonferroni tests were performed to compare individual groups.
Results
IMiDs exert a costimulatory effect on T cells from MM patients
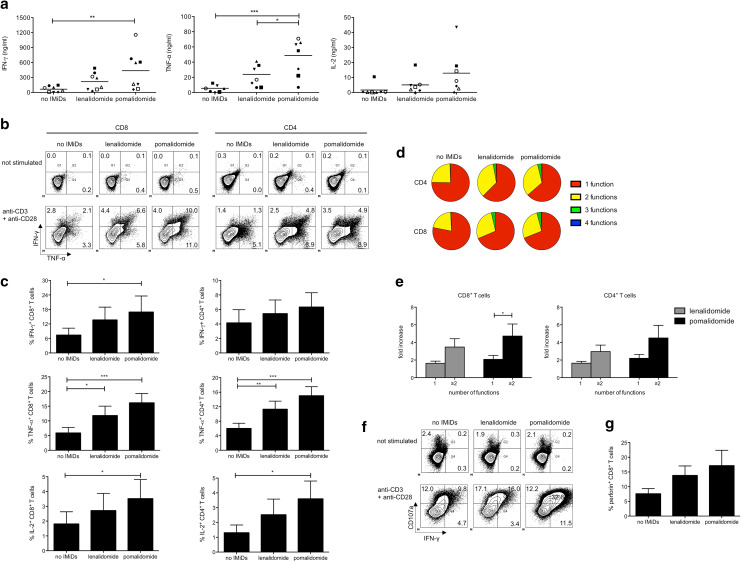
In the presence of IMiDs, the CD8+ T-cell cytokine production upon polyclonal stimulation was significantly higher and this effect was more pronounced for pomalidomide compared to lenalidomide (Fig. 1a). T-cell cytokine production was further investigated by performing intracellular flow cytometry after 72 h of stimulation. The presence of IMiDs in the T-cell cultures resulted in significantly higher percentages of cytokine-producing T cells (Fig. 1b, c). The effects of pomalidomide on the percentage of cytokine-producing T cells were generally more profound compared to lenalidomide. To assess polyfunctionality of T cells, as reflected by the concomitant production of more than one cytokine per cell, we also evaluated the simultaneous production of IFN-γ, TNF-α, IL-2 and MIP-1β by both CD8+ and CD4+ T cells from MM patients. In particular, the proportion of polyfunctional T cells (exerting more than 1 of the following T-cell functions: IFN-γ, TNF-α, IL-2 and MIP-1β production) upon stimulation was increased in the presence of IMiDs (Fig. 1b, d, e). The lytic capacity of CD8+ T cells was also boosted by stimulation in the presence of IMiDs, as evidenced by a higher CD107a mobilization and an increased perforin expression (Fig. 1f, g).
Fig. 1.
IMiDs exert a costimulatory effect on T cells from MM patients. a Purified CD8+ T cells from MM patients were stimulated with anti-CD3 and anti-CD28-coated microbeads. After 6 days, culture supernatants were harvested and cytokine concentration was determined by ELISA. Each symbol represents one experiment, and the mean of all experiments is indicated by the line. b–e T cells from MM patients were stimulated with anti-CD3 and anti-CD28-coated microbeads. After 3 days of T-cell stimulation, intracellular flow cytometry stainings were performed to evaluate the IFN-γ, TNF-α, IL-2 and MIP-1β production by the CD4+ and CD8+ T-cell compartment. b Flow cytometry plots showing the percentages of IFN-γ+, TNF-α+ and IFN-γ+ TNF-α+ CD8+ and CD4+ T cells of one representative out of 4 experiments. c The bar graphs cytokine production by CD8+ and CD4+ T cells. Data are from 5 independent experiments, represented as mean ± standard error of the mean (SEM). d The pie charts indicate the proportion of polyfunctional T cells (one representative out of five experiments is shown). e The bar graphs show the fold increase of CD8+ T cells and CD4+ T cells exerting either 1 or ≥ 2 of the following functions: IFN-γ, TNF-α, IL-2 and MIP-1β production compared to the ‘no IMiDs’ condition. Data are from 5 independent experiments, represented as mean ± standard error of the mean (SEM). f–g Purified CD8+ T cells from MM patients were stimulated with anti-CD3 and anti-CD28-coated microbeads. After 3 days of T-cell stimulation, intracellular flow cytometry stainings were performed to evaluate the CD107a, IFN-γ and perforin expression by the CD8+ T cells. f Flow cytometry plots showing the percentages of IFN-γ+, CD107a+ and IFN-γ+ CD107a+ CD8+ T cells of one representative out of 3 experiments. g The bar graphs show the percentages of perforin+ CD8+ T cells. Data are from 3 independent experiments, represented as mean ± standard error of the mean (SEM). All statistically significant differences are indicated: ***p < 0.001; **p < 0.01; *p < 0.05
IMiDs reduce the suppressive effects of Tregs on Teffs
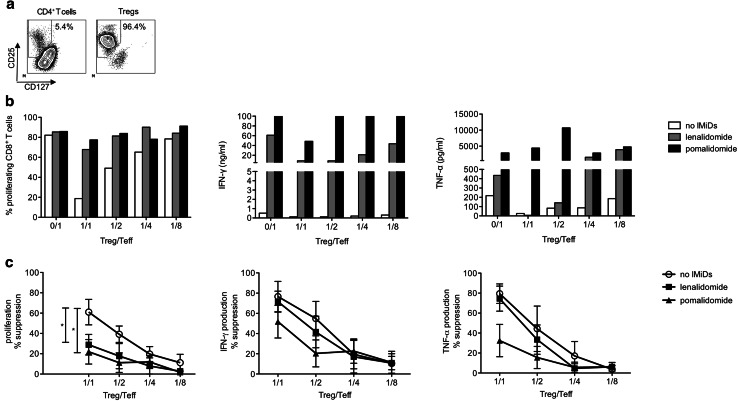
Significant controversy exists in the literature about the number and functionality of Tregs in MM patients. In eight out of nine MM patients, we detected a significant percentage of CD4+ CD25high CD127low Foxp3+ cells in the leukapheresis product (Supplementary figure 1a). We therefore examined whether the suppressive effects of these Tregs on effector T-cell (Teff) responses would be affected by IMiDs. Tregs were isolated by FACS, resulting in a high purity of CD4+ CD25high CD127low cells (>95 % pure) (Fig. 2a). Purified Tregs and CD8+ Teffs were co-cultured at different ratios. Tregs suppressed both CD8+ T-cell proliferation and cytokine production, but their suppressive effects on cytokine production were much more pronounced (Fig. 2b, c). The addition of either lenalidomide or pomalidomide resulted in a significantly (p < 0.05) reduced suppression of CD8+ T-cell proliferation by the Tregs. The suppressive effects of Tregs on T-cell cytokine production were also less pronounced when pomalidomide was added to the co-cultures, although this difference did not reach statistical significance (Fig. 2c).
Fig. 2.
IMiDs reduce the suppressive effects of Tregs on Teffs. a Tregs were purified by FACS based on a CD4+ CD127low CD25high phenotype. The expression of CD25 and CD127 on either purified CD4+ T cells or purified Tregs is shown on the flow cytometry plots. b Purified CD8+ T cells from MM patients (‘Teffs’) were labeled with CellTrace Violet, stimulated with anti-CD3 and anti-CD28-coated microbeads and co-cultured with FACS purified autologous Tregs at different ratios. After 6 days of co-culture, the CD8+ T-cell proliferation was determined by flow cytometry and the cytokine concentration in the culture supernatants was determined by ELISA. In panels A–B, one representative out of four experiments is shown. c The percentage suppression of CD8+ T-cell proliferation and cytokine production was calculated as 100 % − (T-cell response in condition with Tregs/T-cell response in condition without Tregs) * 100 %. Each symbol represents the mean of four experiments, and the error bar represents the SEM. All statistically significant differences are indicated: *p < 0.05
Effects of IMiDs on the suppressive effects of MDSCs on Teffs
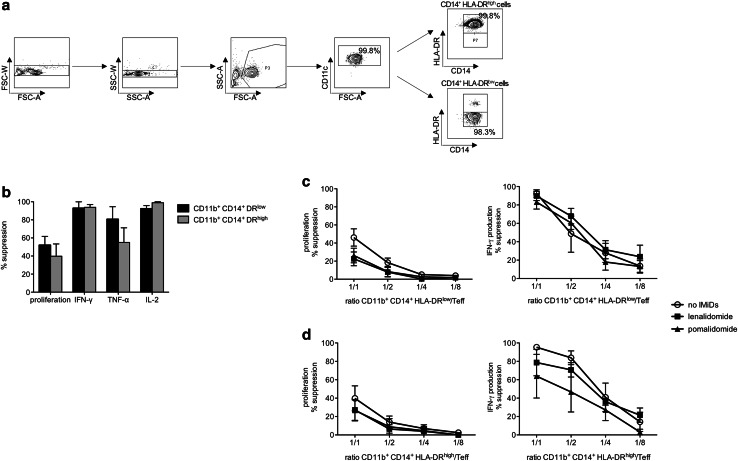
MM patients show increased frequencies of MDSCs [15, 18, 19]. A large number of MDSC phenotypes have been described in many different human diseases. Recently, CD14+ HLA-DRlow/− MDSCs were shown to be present in several cancer types. We identified a population of on average 3.5 % CD14+ HLA-DRlow/− cells within the PBMCs from MM patients (Supplementary figure 1b). To test the immune-suppressive capacity of these cells, we sorted CD11b+ CD14+ HLA-DRlow/− cells by FACS. CD11b+ CD14+ HLA-DRhigh cells were used as a control (Fig. 3a). We found that not only CD11b+ CD14+ HLA-DRlow/− cells, but also CD11b+ CD14+ HLA-DRhigh cells from MM patients were T-cell suppressive, reducing T-cell proliferation and cytokine production (Supplementary figure 2, Fig. 3b–d). As was observed for the Tregs, MDSCs suppressed T-cell cytokine secretion to a larger extent compared to T-cell proliferation (Fig. 3b–d). In all the experiments performed, the addition of IMiDs during the MDSC/T-cell co-culture resulted in a reduced suppression of the CD8+ T-cell proliferation (Fig. 3c, d), but these effects were not as pronounced as the effects of the IMiDs on the Treg-mediated CD8+ T-cell suppression and did not reach statistical significance. No clear effects of IMiDs on the suppressive effects of monocytic MDSCs on CD8+ T-cell cytokine production could be observed.
Fig. 3.
Effects of IMiDs on the suppressive effects of MDSCs on Teffs. a MDSCs were purified by FACS based on a CD11b+ CD14+ HLA-DRlow/− phenotype. The expression of CD14 and HLA-DR on either purified CD11b+ CD14+ HLA-DRhigh or purified CD11b+ CD14+ HLA-DRlow/− cells is shown on the flow cytometry plots. One representative out of five experiments is shown. b Overview graph showing the suppression of CD8+ T-cell proliferation and cytokine production by either autologous CD11b+ CD14+ HLA-DRlow/− or autologous CD11b+ CD14+ HLA-DRhigh cells upon a myeloid cell/T cell co-culture performed at a 1/1 ratio. The bars indicate the mean of four independent experiments, and the error bars indicate the SEM. c, d Purified CD8+ T cells from MM patients (‘Teffs’) were stimulated with anti-CD3 and anti-CD28-coated microbeads and co-cultured with FACS purified CD11b+ CD14+ HLA-DRlow/− or CD11b+ CD14+ HLA-DRhigh cells at different ratio’s. After 6 days of co-culture, the CD8+ T-cell proliferation was determined by flow cytometry and the cytokine concentration in the culture supernatants was determined by ELISA. The percentage suppression of CD8+ T-cell proliferation and cytokine production was calculated as 100 % − (T-cell response in condition with myeloid cells/T-cell response in condition without myeloid cells) * 100 %. Each symbol represents the mean of 4 (HLA-DRhigh cells) or 5 (HLA-DRlow/− cells) experiments, and the error bar represents the SEM
Quality of in vitro generated DCs from MM patients
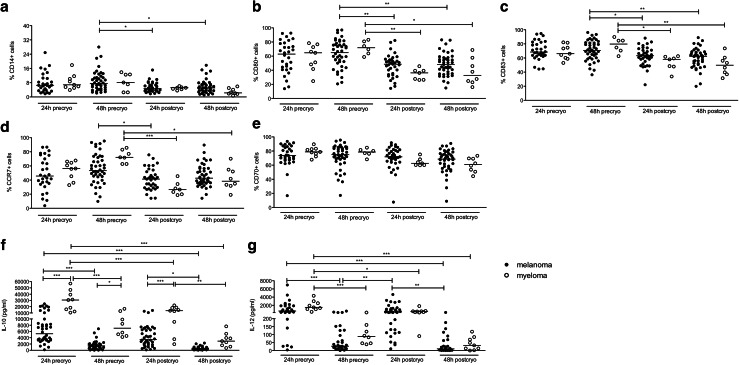
To assess the quality of DCs generated from MM patients, we decided to evaluate the phenotype and cytokine production of DCs generated according to the protocol used in our laboratory for the preparation of DC-based vaccines for melanoma patients. These DCs are matured by electroporation with a mixture of mRNAs encoding a constitutively active TLR4, CD40L and CD70 (TriMix1) and were shown to have a superior T-cell stimulatory capacity compared to other DC types [28–30]. We found that the purity, the maturation (based on the expression of CD80, CD83 and CCR7) and the electroporation efficiency (based on the expression of CD70, encoded by TriMix1 mRNA) of DCs from MM and melanoma patients were largely comparable (Fig. 4a–e). Interestingly, the IL-10 production by DCs from MM patients was significantly higher compared to the IL-10 production by DCs from melanoma patients (Fig. 4f), whereas the IL-12 production by the DCs was similar for both patient groups (Fig. 4g).
Fig. 4.
Quality of in vitro generated DCs from MM patients. Monocytes were isolated from PBMCs through plastic adherence and DCs were generated. On day 6, the immature DCs were harvested and electroporated with mRNA encoding TriMix1 and MAGE-C1. Twenty-four and 48 h after the electroporation, the DC phenotype was analyzed and supernatants were collected to measure cytokine secretion. In addition, part of the DCs was stored in nitrogen for quality control after a freeze/thaw cycle. The graphs show the phenotype of the DCs as determined by flow cytometry (panels a–e) and the DC cytokine production as determined by ELISA (panels f, g) from melanoma compared to MM patients 24 and 48 h after electroporation, either before (‘precryo’) or after (‘postcryo’) cryopreservation. Each symbol represents one experiment, and the median of all experiments is indicated by the line. All statistically significant differences are indicated: ***p < 0.001; **p < 0.01; *p < 0.05
Costimulatory effect of IMiDs is enhanced when combined with TriMix DCs
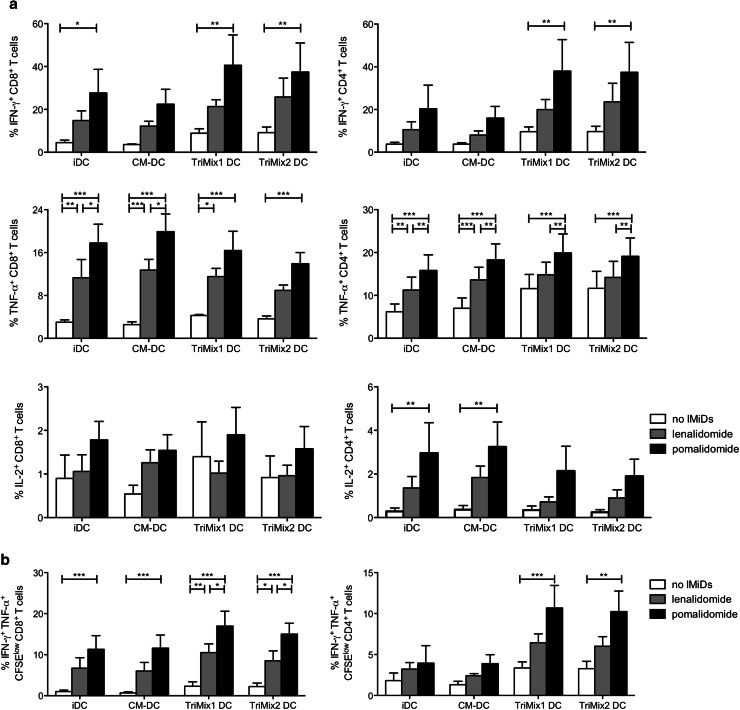
Since we showed that IMiDs enhance T-cell responses and that it is feasible to generate high-quality DCs from MM patients, we wanted to evaluate which type of DC-based vaccination would be best suited to combine with IMiDs in order to obtain potent T-cell responses. Therefore, we stimulated T cells with anti-CD3-coated microbeads together with either iDCs, CM-DCs, TriMix1 DCs (matured by electroporation with mRNA encoding a constitutively active TLR4, CD40L and CD70) [28] or TriMix2 DCs (matured by electroporation with mRNA encoding a constitutively active TLR4, CD40L and 4-1BBL/CD137L) [30]. We observed that the addition of IMiDs to T-cell stimulations always resulted in more functional CD8+ and CD4+ T cells, irrespective of the DC type used during the T-cell stimulation (Supplementary figure 3, Fig. 5a, b). When we performed an intracellular cytokine staining after 72 h of T-cell stimulation, the type of DC used for the stimulation did not clearly affect the T-cell responses (Fig. 5a). However, when we evaluated both proliferation and cytokine production after 6 days of T-cell stimulation, CD4+ and CD8+ T cells with the highest degree of functionality, producing more cytokines on a per cell basis, were obtained when IMiDs were combined with one of the TriMix variants (Supplementary figure 3, Fig. 5b).
Fig. 5.
Costimulatory effect of IMiDs is enhanced when combined with TriMix DCs. T cells from MM patients were labeled with CellTrace Violet, stimulated with anti-CD3-coated microbeads. To some of the conditions, autologous iDCs, CM-DCs, TriMix1 DCs or TriMix2 DCs were added. After 6 days of T-cell stimulation, proliferation was measured and an intracellular flow cytometry staining was performed to evaluate the IFN-γ, TNF-α and IL-2 production by the CD4+ and CD8+ T-cell compartment. a The bar graphs indicate the mean percentage of cytokine-producing T cells, and the error bars indicate the SEM (n = 4). b The percentages of IFN-γ+ TNF-α+ cells within the CellTrace Violetlow (proliferating) CD8+ or CD4+ T-cell population are shown on the overview graphs. The bar graphs indicate the mean percentage of cytokine-producing T cells, and the error bars indicate the SEM (n = 4). TriMix1 = caTLR4 + CD40L + CD70, TriMix2 = caTLR4 + CD40L + 4-1BBL. Statistically significant differences resulting from the addition of IMiDs are indicated: ***p < 0.001; **p < 0.01; *p < 0.05
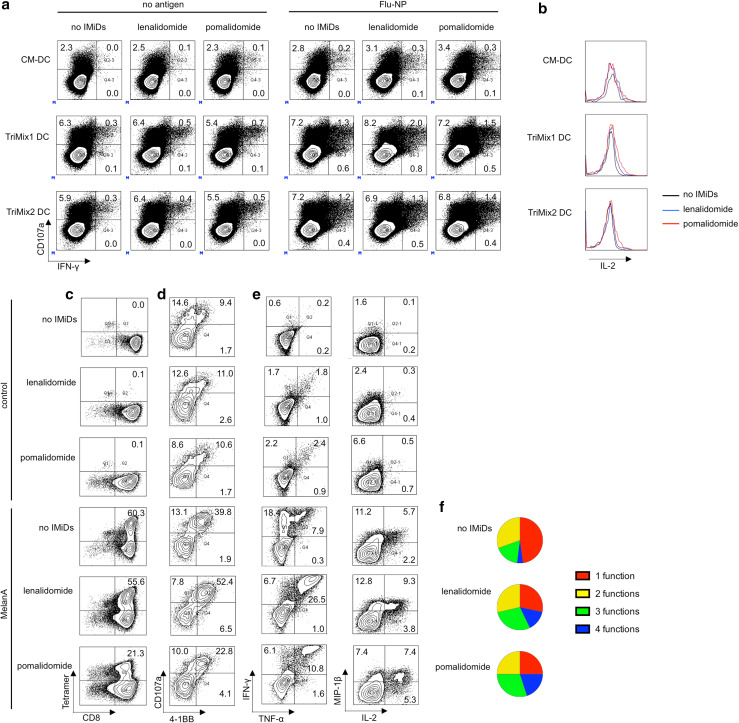
Finally, we evaluated the effect of IMiDs on the stimulation of CD8+ T cells by autologous DCs. We observed that the lytic capacity, as measured by CD107a membrane expression, as well as the cytokine production by Flu-NP-specific CD8+ T cells from MM patients was enhanced upon stimulation with TriMix1 or TriMix2 DCs as compared to CM-DCs (Fig. 6a) and that the degree of polyfunctionality was improved when IMiDs were added to the co-cultures, as more IL-2 was produced by CD107a+ IFN-γ+ CD8+ T cells upon addition of lenalidomide or pomalidomide (Fig. 6b). Next, we assessed the effects of IMiDs on the induction of Melan-A-specific T-cell responses by TriMix1 DCs. We observed that IMiDs did not increase the proliferation of Melan-A-specific CD8+ T cells, as the percentage of Melan-A tetramer+ CD8+ T cells was the same for the ‘no IMiDs’ condition and upon addition of lenalidomide during the T-cell stimulation. The number of tetramer+ cells was even reduced upon stimulation in the presence of pomalidomide (Fig. 6c). Lenalidomide and pomalidomide had differential effects on the frequency of antigen-specific (4-1BB+) CTLs (CD107a+ CD8+ T cells) and cytokine-producing (IFN-γ+, TNF-α+, IL-2+ and/or MIP-1β+) CD8+ T cells, which were increased in the lenalidomide, but decreased in the pomalidomide conditions (Fig. 6d, e). However, we observed that the addition of IMiDs during the DC-mediated T-cell stimulation consistently resulted in a higher degree of polyfunctionality of the induced Melan-A-specific CD8+ T cells.
Fig. 6.
IMiDs enhance the quality of antigen-specific T cells stimulated or induced by TriMix DCs. a, b Purified CD8+ T cells from MM patients were stimulated with DCs electroporated with Flu-NP-encoding mRNA or electroporated without antigen-encoding mRNA (negative control). After 3 days, intracellular flow cytometry stainings were performed to evaluate the CD107a, IFN-γ and IL-2 expression by the CD8+ T cells. a Flow cytometry plots showing the percentages of IFN-γ+, CD107a+ and IFN-γ+ CD107a+ CD8+ T cells. b Expression of IL-2 in CD107a+ IFN-γ+ CD8+ T cells. Results in panels a, b are representative of two experiments. c–f Purified CD8+ T cells from healthy donors were stimulated with TriMix1 DCs co-electroporated with Melan-A encoding mRNA. After three rounds of stimulation, T cells were co-cultured with Melan-A or Gag (control) peptide pulsed T2 cells and the functionality of the Melan-A-specific T cells was examined. c Flow cytometry plots showing the percentage of Melan-A-specific CD8+ T cells. d Flow cytometry showing the percentage of CD107a+ 4-1BB+ Melan-A-specific CD8+ T cells. e Flow cytometry pots showing the percentage of cytokine-producing Melan-A-specific T cells. f Pie charts indicating the proportion of polyfunctional Melan-A-specific CD8+ T cells. Results in panel c–f are representative of at least two experiments
Discussion
The introduction of novel agents in combination with ASCT with or without maintenance treatment has led to prolonged progression-free survival in younger MM patients. However, most patients still relapse and alternative approaches to eradicate minimal residual disease should be explored. Immunotherapy targeting MM-associated antigens may be an option. Several potential MM-associated antigens have been identified as potential targets for immunotherapy [31]. DC-based antitumor vaccination has shown promise in several malignancies including prostate cancer and melanoma and may be the preferred way to induce strong and durable antitumor immunity in MM. However, the typical disease- and treatment-related immunosuppression might be difficult to overcome. We hypothesize that the minimal disease state and the re-expanding T-cell repertoire after high-dose chemotherapy in combination with additional immunostimulation and the use of a validated DC vaccination technique offers the best conditions for successful immunotherapy. Since IMiDs are increasingly used as maintenance treatment after ASCT, they could play an important role to enhance DC vaccination strategies.
Our study includes a first in depth analysis of the ex vivo effects of IMiDs on several immune functions in MM patients as performed on enriched leukocytes isolated from peripheral blood samples obtained in CR/VGPR at various time points after ASCT. First, we showed that lenalidomide and pomalidomide enhance CD4+ and CD8+ T-cell responses upon polyclonal stimulation, including enhancement of cytokine production, lytic capacity and increased polyfunctionality as previously shown in HIV patients [32]. Recent studies showed that polyfunctional T cells might play a role in tumor eradication [33–35]. However, it remains to be demonstrated whether polyfunctional T-cell responses will correlate with more efficacious anti-tumoral activity in vivo.
Tregs and MDSCs are well characterized as suppressor cell types hampering antitumoral immune responses. It has been shown that relatively high doses of lenalidomide and pomalidomide inhibit the proliferation and suppressive capacity of Tregs isolated from healthy donors [36]. In our assay, we observed that IMiDs significantly reduce the suppressive effects of Tregs on T-cell proliferation. We found that MDSCs had a suppressive effect on T-cell function, which was more pronounced for T-cell cytokine production compared to T-cell proliferation. However, using the CD11b+ CD14+ HLA-DRhigh cells as a control in our assay, we found that this type of monocytes also had a suppressive capacity in the majority of the patients. Not much is known about the phenotype and the functionality of MDSCs in MM patients, and only one recent study evaluated the effects of lenalidomide on MDSCs in MM patients. In that study, the suppressive function of a granulocytic population of MDSCs, characterized by a CD11b+ CD14− HLA-DRlow/− CD33+ CD15+ phenotype, was shown. The authors showed that lenalidomide dramatically reduced the expression of IL-6, IL-10, IFN-γ and GM-CSF in LPS-stimulated granulocytic MDSCs, but that lenalidomide could not overcome T-cell suppression mediated by this MDSC type [18]. We investigated the T-cell suppressive effects of monocytic MDSCs (CD11b+ CD14+ HLA-DRlow/−) from MM patients. Unfortunately, most studies that investigated the functionality of monocytic MDSCs did not evaluate the suppressive capacity of CD14+ HLA-DRhigh cells [37–41]. Schilling et al. did not observe a suppressive function for CD14+ HLA-DRhigh cells [42], while Duffy et al. found a comparable T-cell suppressive capacity for CD14+ HLA-DRlow/− and CD14+ HLA-DRhigh cells when the MDSCs were added at similar MDSC/T-cell ratios as used in our experiments [43]. Serafini et al. [44] observed a suppressive effect of CD14+ cells on T-cell expansion in MM patients.
We previously developed a method to mature DCs and load them with an antigen of interest in one mRNA electroporation step [28]. For this purpose, DCs are co-electroporated with TriMix- and antigen-encoding mRNA. TriMix is an mRNA cocktail encoding for a constitutively active Toll-like receptor 4, CD40L and CD70. TriMix DCs were shown to have a superior capacity to induce naïve T-cell responses compared to DCs matured by co-incubation with a mixture of pro-inflammatory cytokines (CM-DCs), the ‘standard’ DC maturation stimulus [45]. TriMix DCs are successfully used at our institution as a melanoma immunotherapy [28, 29], and this type of DCs might also be suited as an anti-MM vaccine. Our study shows that it is feasible to generate TriMix DCs from MM patients with purity, electroporation efficiency and phenotype comparable to TriMix DCs generated from melanoma patients. TriMix DCs from myeloma patients produced more IL-10 compared to TriMix DCs from melanoma patients. This higher IL-10 production may hamper the induction of effective T-cell responses. Further studies are needed to evaluate whether blocking IL-10 production in DCs from MM patients may result in an improved T-cell stimulatory capacity. The quality of the DCs significantly deteriorated after cryopreservation. Some of the functional deficiencies that occur after cryopreservation may recover after infusion, but this is impossible to verify. Only measurement of induced immune responses may offer an answer to this question. One could argue for the use of freshly prepared vaccines, but that is logistically very difficult to realize. In addition, a reduced expression of some of the markers was also shown in DCs from melanoma patients. We previously showed that vaccination of these patients with thawed DCs resulted in clinical and immunological responses [46].
In a further step, we analyzed the effect of IMiDs on several DC subtypes including iDCs, CM-DCs, TriMix1 and TriMix2 DCs. TriMix1 (caTLR4, CD40L and CD70) electroporated DCs were shown to have a superior capacity to induce naïve T-cell responses [45]. We previously showed that a similar mRNA cocktail that contains 4-1BBL instead of CD70, TriMix2 (caTLR4, CD40L and 4-1BBL), is more potent for the stimulation of preexisting T-cell responses [30]. In this study, we clearly show that, irrespective of the DC type used during the T-cell stimulation, IMiDs are able to significantly boost the proliferation, cytokine secretion, lytic capacity and polyfunctionality of polyclonally stimulated T cells. The highest percentages of polyfunctional T cells were obtained upon stimulation with TriMix DCs in the presence of IMiDs. Importantly, CM-DCs, which are often used in cancer vaccine preparations, hardly resulted in improved T-cell responses compared to iDCs. We furthermore showed that TriMix DCs are able to boost influenza-specific T-cell responses, which are impaired in myeloma patients [47]. Furthermore, we showed that lenalidomide addition leads to an improved quality of Melan-A-specific T cells induced by TriMix1 DCs. Although pomalidomide addition resulted in a reduced number of Melan-A-specific T cells, it did also result in a higher degree of polyfunctionality of these tumor antigen-specific T cells. Future clinical studies will have to point out which is the most important goal in cancer immunotherapy: generating higher numbers of tumor antigen-specific T cells or improving their functionality: quantity or quality?
Taken together, our preclinical study on samples from MM patients in CR/VGPR after ASCT shows that IMiDs can reverse the immunosuppressive state in MM patients and can add to the potency of DC-based vaccine products. We are confident that the effects observed may be an underestimation with respect to the situation in a clinical trial. Patients could be vaccinated at an earlier time point after ASCT, at times of immune reconstitution after transplant. Also, in vivo effects of IMiDs may be more profound because more continuous exposure and targeting more components of the immune environment in the bone marrow of the patients, where the tumor mostly resides. A recent paper describing the effects of lenalidomide and therapeutic vaccination in mice-bearing lymphomas showed that the dose of lenalidomide is critical for its adjuvant effect: continuous administration of high-dose lenalidomide failed to potentiate the vaccine-induced tumor protection, which may be a result of T-cell exhaustion [48]. The maximum plasma lenalidomide concentration following a standard daily dose of 25 mg is 2.2 μM [49], thus the concentration of 0.5 μM tested in our experiments physiologically relevant. Thus far, only one clinical trial investigated DC vaccination in MM patients who receive also a treatment with IMiDs. In this study, 6 patients with asymptomatic MM were treated with α-galactosylceramide-loaded DCs and lenalidomide, with the aim to enhance NKT-cell responses. This therapy was well tolerated and the clinical responses, which were observed in some patients, correlated with antitumor T-cell immunity [50].
Some of our experiments show a high variation between the results of different patients. The sampling timing of the patients was quite variable. The common features for all patients were previous treatment including high-dose chemotherapy, low disease burden, the absence of concurrent infection and no other associated immunomodulating therapy. The basic immune status may differ between patients in this cohort. However, we demonstrated immune dysfunction in all cases and we show that this can be—at least ex vivo—reversed by the use of lenalidomide or pomalidomide. We conclude that our preclinical data offer a solid scientific rationale to proceed with phase 1 and phase 2 clinical trials to assess safety and efficacy of combined IMiD treatment and DC vaccination in MM patients achieving low tumor burden after ASCT.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank Bilal Khan, Chiraz Mahmoud, Steven Heynderickx, Angelo Willems, Elsy Vaeremans, Petra Roman and Xavier Debaere for excellent technical assistance and Sarah Maenhout for her advice regarding the MDSC suppression assays. This study was supported by the Stichting Tegen Kanker, the International Myeloma Foundation (Brian D Novis junior research award), the King Baudouin Foundation (Fund Catharina Weekers) and the Wetenschappelijk Fonds Willy Gepts (Universitair Ziekenhuis Brussel). Parts of this work were realized with a research grant Emmanuel van de Schueren of the Vlaamse Liga Tegen Kanker.
Conflict of interest
TriMix DCs are the topic of a current patent application (WO2009/034172). Kris Thielemans is mentioned as inventor of this application. None of the authors involved in this study receives any form of support or remuneration related to this platform.
Abbreviations
- 4-1BBL
4-1BB ligand
- ASCT
Autologous stem cell transplantation
- caTLR4
Constitutively active Toll-like receptor 4
- CD40L
CD40 ligand
- CM-DC
Cytokine cocktail matured dendritic cell
- CR
Complete response
- DC
Dendritic cell
- Flu-NP
Influenza nuclear protein
- iDC
Immature dendritic cell
- IDO
Indoleamine 2,3-dioxygenase
- IMiD
Immunomodulatory drug
- MDSC
Myeloid-derived suppressor cell
- MM
Multiple myeloma
- NAC
Non-adherent cell
- NK
Natural killer
- PBMC
Peripheral blood mononuclear cell
- Teff
Effector T cell
- Treg
Regulatory T cell
- VGPR
Very good partial remission
References
- 1.Schots R. Recent advances in myeloma treatment. Transfus Apheresis Sci. 2011;44:223–229. doi: 10.1016/j.transci.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Munshi NC, Anderson KC. New strategies in the treatment of multiple myeloma. Clin Cancer Res. 2013;19:3337–3344. doi: 10.1158/1078-0432.CCR-12-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munshi NC, Anderson KC, Bergsagel PL, et al. Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood. 2011;117:4696–4700. doi: 10.1182/blood-2010-10-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111:2521–2526. doi: 10.1182/blood-2007-08-104984. [DOI] [PubMed] [Google Scholar]
- 5.Reichardt VL, Okada CY, Liso A, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma—a feasibility study. Blood. 1999;93:2411–2419. [PubMed] [Google Scholar]
- 6.Liso A, Stockerl-Goldstein KE, Auffermann-Gretzinger S, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood progenitor cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2000;6:621–627. doi: 10.1016/S1083-8791(00)70027-9. [DOI] [PubMed] [Google Scholar]
- 7.Rosenblatt J, Avivi I, Vasir B, et al. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin Cancer Res. 2013;19:3640–3648. doi: 10.1158/1078-0432.CCR-13-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobo W, Strobbe L, Maas F, et al. Immunogenicity of dendritic cells pulsed with MAGE3, Survivin and B-cell maturation antigen mRNA for vaccination of multiple myeloma patients. Cancer Immunol Immunother. 2013;62:1381–1392. doi: 10.1007/s00262-013-1438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meehan KR, Wu J, Bengtson E, et al. Early recovery of aggressive cytotoxic cells and improved immune resurgence with post-transplant immunotherapy for multiple myeloma. Bone Marrow Transplant. 2007;39:695–703. doi: 10.1038/sj.bmt.1705665. [DOI] [PubMed] [Google Scholar]
- 10.Rapoport AP, Aqui NA, Stadtmauer EA, et al. Combination immunotherapy using adoptive T-cell transfer and tumor antigen vaccination on the basis of hTERT and survivin after ASCT for myeloma. Blood. 2011;117:788–797. doi: 10.1182/blood-2010-08-299396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meehan KR, Talebian L, Tosteson TD, et al. Adoptive cellular therapy using cells enriched for NKG2D+ CD3+ CD8+ T cells after autologous transplantation for myeloma. Biol Blood Marrow Transplant. 2013;19:129–137. doi: 10.1016/j.bbmt.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair JR, Carlson LM, Koorella C, et al. CD28 expressed on malignant plasma cells induces a prosurvival and immunosuppressive microenvironment. J Immunol. 2011;187:1243–1253. doi: 10.4049/jimmunol.1100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okunishi K, Dohi M, Nakagome K, et al. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J Immunol. 2005;175:4745–4753. doi: 10.4049/jimmunol.175.7.4745. [DOI] [PubMed] [Google Scholar]
- 14.Beyer M, Kochanek M, Giese T, et al. In vivo peripheral expansion of naive CD4+ CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood. 2006;107:3940–3949. doi: 10.1182/blood-2005-09-3671. [DOI] [PubMed] [Google Scholar]
- 15.Brimnes MK, Vangsted AJ, Knudsen LM, et al. Increased level of both CD4+ FOXP3+ regulatory T cells and CD14+ HLA-DR−/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand J Immunol. 2010;72:540–547. doi: 10.1111/j.1365-3083.2010.02463.x. [DOI] [PubMed] [Google Scholar]
- 16.Feyler S, von Lilienfeld-Toal M, Jarmin S, et al. CD4(+)CD25(+)FoxP3(+) regulatory T cells are increased whilst CD3(+)CD4(−)CD8(−)alphabetaTCR(+) double negative T cells are decreased in the peripheral blood of patients with multiple myeloma which correlates with disease burden. Br J Haematol. 2009;144:686–695. doi: 10.1111/j.1365-2141.2008.07530.x. [DOI] [PubMed] [Google Scholar]
- 17.Van Valckenborgh E, Schouppe E, Movahedi K, et al. Multiple myeloma induces the immunosuppressive capacity of distinct myeloid-derived suppressor cell subpopulations in the bone marrow. Leukemia. 2012;26:2424–2428. doi: 10.1038/leu.2012.113. [DOI] [PubMed] [Google Scholar]
- 18.Görgün GT, Whitehill G, Anderson JL, et al. Tumor promoting immune suppressive myeloid derived suppressor cells in multiple myeloma microenvironment. Blood. 2013;121:2975–2987. doi: 10.1182/blood-2012-08-448548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramachandran IR, Martner A, Pisklakova A, et al. Myeloid-derived suppressor cells regulate growth of multiple myeloma by inhibiting T cells in bone marrow. J Immunol. 2013;190:3815–3823. doi: 10.4049/jimmunol.1203373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu L, Payvandi F, Wu L, et al. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc Res. 2009;77:78–86. doi: 10.1016/j.mvr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Mitsiades N. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99:4525–4530. doi: 10.1182/blood.V99.12.4525. [DOI] [PubMed] [Google Scholar]
- 22.LeBlanc R, Hideshima T, Catley LP, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004;103:1787–1790. doi: 10.1182/blood-2003-02-0361. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, Li J, Ferguson GD, et al. Immunomodulatory drugs reorganize cytoskeleton by modulating Rho GTPases. Blood. 2009;114:338–345. doi: 10.1182/blood-2009-02-200543. [DOI] [PubMed] [Google Scholar]
- 24.Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343:305–309. doi: 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Girona A, Mendy D, Ito T, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26:2326–2335. doi: 10.1038/leu.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilgenhof S, Van Nuffel AMT, Corthals J, et al. Therapeutic vaccination with an autologous mRNA electroporated dendritic cell vaccine in patients with advanced melanoma. J Immunother. 2011;34:448–456. doi: 10.1097/CJI.0b013e31821dcb31. [DOI] [PubMed] [Google Scholar]
- 27.Van Nuffel AMT, Benteyn D, Wilgenhof S, et al. Intravenous and intradermal TriMix-dendritic cell therapy results in a broad T-cell response and durable tumor response in a chemorefractory stage IV-M1c melanoma patient. Cancer Immunol Immunother. 2012;61:1033–1043. doi: 10.1007/s00262-011-1176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonehill A, Van Nuffel AMT, Corthals J, et al. Single-step antigen loading and activation of dendritic cells by mRNA electroporation for the purpose of therapeutic vaccination in melanoma patients. Clin Cancer Res. 2009;15:3366–3375. doi: 10.1158/1078-0432.CCR-08-2982. [DOI] [PubMed] [Google Scholar]
- 29.Wilgenhof S, Pierret L, Corthals J, et al. Restoration of tumor equilibrium after immunotherapy for advanced melanoma: three illustrative cases. Melanoma Res. 2011;21:152–159. doi: 10.1097/CMR.0b013e328343ece0. [DOI] [PubMed] [Google Scholar]
- 30.De Keersmaecker B, Heirman C, Corthals J, et al. The combination of 4-1BBL and CD40L strongly enhances the capacity of dendritic cells to stimulate HIV-specific T cell responses. J Leukoc Biol. 2011;89:989–999. doi: 10.1189/jlb.0810466. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Götz M, Hofmann S, Greiner J. Immunogenic targets for specific immunotherapy in multiple myeloma. Clin Dev Immunol. 2012;2012:820394. doi: 10.1155/2012/820394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Keersmaecker B, Allard SD, Lacor P, et al. Expansion of polyfunctional HIV-specific T cells upon stimulation with mRNA electroporated dendritic cells in the presence of immunomodulatory drugs. J Virol. 2012;86:9351–9360. doi: 10.1128/JVI.00472-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan J, Gnjatic S, Li H, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci USA. 2008;105:20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aranda F, Llopiz D, Díaz-Valdés N, et al. Adjuvant combination and antigen targeting as a strategy to induce polyfunctional and high-avidity T-cell responses against poorly immunogenic tumors. Cancer Res. 2011;71:3214–3224. doi: 10.1158/0008-5472.CAN-10-3259. [DOI] [PubMed] [Google Scholar]
- 35.Ding Z-C, Huang L, Blazar BR, et al. Polyfunctional CD4+ T cells are essential for eradicating advanced B-cell lymphoma after chemotherapy. Blood. 2012;120:2229–2239. doi: 10.1182/blood-2011-12-398321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galustian C, Meyer B, Labarthe M-C, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58:1033–1045. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasquez-Dunddel D, Pan F, Zeng Q, et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest. 2013;123:1580–1589. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin A, Cai W, Pan T, et al. Expansion of monocytic myeloid-derived suppressor cells dampens T cell function in HIV-1-seropositive individuals. J Virol. 2013;87:1477–1490. doi: 10.1128/JVI.01759-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mougiakakos D, Jitschin R, von Bahr L, et al. Immunosuppressive CD14+ HLA-DRlow/neg IDO+ myeloid cells in patients following allogeneic hematopoietic stem cell transplantation. Leukemia. 2012;27:377–388. doi: 10.1038/leu.2012.215. [DOI] [PubMed] [Google Scholar]
- 40.Poschke I, Mougiakakos D, Hansson J, et al. Immature immunosuppressive CD14+ HLA-DR−/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 41.Kotsakis A, Harasymczuk M, Schilling B, et al. Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J Immunol Methods. 2012;381:14–22. doi: 10.1016/j.jim.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schilling B, Sucker A, Griewank K, et al. Vemurafenib reverses immunosuppression by myeloid derived suppressor cells. Int J Cancer. 2013;133:1653–1663. doi: 10.1002/ijc.28168. [DOI] [PubMed] [Google Scholar]
- 43.Duffy A, Zhao F, Haile L, et al. Comparative analysis of monocytic and granulocytic myeloid-derived suppressor cell subsets in patients with gastrointestinal malignancies. Cancer Immunol Immunother. 2013;62:299–307. doi: 10.1007/s00262-012-1332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonehill A, Tuyaerts S, Van Nuffel AMT, et al. Enhancing the T-cell stimulatory capacity of human dendritic cells by co-electroporation with CD40L, CD70 and constitutively active TLR4 encoding mRNA. Mol Ther. 2008;16:1170–1180. doi: 10.1038/mt.2008.77. [DOI] [PubMed] [Google Scholar]
- 46.Wilgenhof S, Van Nuffel AMT, Benteyn D, et al. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann Oncol. 2013;24:2686–2693. doi: 10.1093/annonc/mdt245. [DOI] [PubMed] [Google Scholar]
- 47.Maecker B, Anderson KS, von Bergwelt-Baildon MS, et al. Viral antigen-specific CD8+ T-cell responses are impaired in multiple myeloma. Br J Haematol. 2003;121:842–848. doi: 10.1046/j.1365-2141.2003.04375.x. [DOI] [PubMed] [Google Scholar]
- 48.Sakamaki I, Kwak LW, Cha S-C, et al. Lenalidomide enhances the protective effect of a therapeutic vaccine and reverses immune suppression in mice bearing established lymphomas. Leukemia. 2014;28:329–337. doi: 10.1038/leu.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen N, Lau H, Kong L, et al. Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J Clin Pharmacol. 2007;47:1466–1475. doi: 10.1177/0091270007309563. [DOI] [PubMed] [Google Scholar]
- 50.Richter J, Neparidze N, Zhang L, et al. Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma. Blood. 2013;121:423–430. doi: 10.1182/blood-2012-06-435503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.