Abstract
Considerable effort has been put into targeting tumors through therapeutic vaccination using dendritic cell-, DNA-, protein-, or peptide-based vaccines. Purified peptides and proteins are generally not immunogenic and need to be administered with an adjuvant that will trigger an appropriate immune response. Safe adjuvants that favor induction of tumor reactive CD8+ T cells with the capacity to directly kill tumor cells are therefore a high priority. We have previously reported on the effect and mechanism of a cationic adjuvant formulation, CAF01, which incorporates synthetic mycobacterial cord factor and primes protective Th1, Th17, and antibody responses in animal models of bacterial, viral, and parasitic infections. The CAF01 adjuvant is currently in clinical trial. Using CAF01 as a backbone, we recently demonstrated that incorporating the TLR3 ligand polyinosinic/polycytidylic acid [poly(I:C)] primes CD8+ T cells specific to the SIINFEKL epitope of the model antigen ovalbumin. In the present study, we demonstrate that CAF01/poly(I:C), termed cationic adjuvant formulation 05 or CAF05, can induce CD8+ T cells that efficiently lyse target cells and significantly reduce tumor growth in two different mouse tumor models: lung B16-OVA melanoma expressing ovalbumin and the self-antigen TRP2, and subcutaneous TC-1 tumors expressing the human papillomavirus-16 protein E7.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-011-1156-6) contains supplementary material, which is available to authorized users.
Keywords: Adjuvant, CTL, Vaccine, Mouse tumor, CAF01, CAF05
Introduction
Highly purified protein or peptides used for modern subunit vaccines are generally not immunogenic, and formulation with adjuvants that stimulate innate immunity and facilitate an adaptive response toward the vaccine and pathogen is critical for the success of vaccination [1]. Very few adjuvants have been approved for use in man, and currently, only aluminium compounds and aluminium hydroxide in combination with the TLR4 ligand MPL (adjuvant AS04) are licensed in the US, whereas also influenza virosomes and the emulsion adjuvants MF59, AS03, and AF03 are approved in Europe. The commercially available vaccines including these adjuvants exert their function through promoting Th2 and antibody responses. While this strategy has worked well against diseases where neutralizing antibodies can block the invading microorganisms, it is generally not thought to suffice when vaccinating against intracellular pathogens or cancer. Studies in mouse models [2] and humans [3, 4] have shown that tumor-specific interferon-γ (IFN-γ) producing CD4+ T cell (Th1) and CD8+ T cell (Tc1) immunity is essential for a reduction in tumor growth and good disease prognosis. Also, in the case of human papilloma virus (HPV)-induced cancer, an inverse relationship between cervical intraepithelial neoplasia and a Tc1 response against HPV proteins E6 and E7 is described [5]. Thus, finding safe adjuvants that promote cellular immunity is of great importance for developing vaccines and immune therapies against a range of infectious diseases and cancer.
We have previously reported on the effect of the cationic liposome adjuvant CAF01 that in several models of infectious diseases primes protective humoral, Th1 and IL-17 producing CD4+ T cell (Th17) responses [6–9]. CAF01, which is currently in clinical trial (eudraCT 2008-006003-23), enhances antigen uptake by antigen-presenting cells [10] and mediates activation of these cells through interaction of the synthetic mycobacterial cord factor Trehalose dibehenate (TDB) with the innate C-type lectin receptor Mincle [11, 12]. Using CAF01 as a backbone, we have explored the use of additional immune stimulants with the focus of increasing the CTL-inducing capacity of the vaccine. We recently reported on a formulation of CAF01 incorporating the TLR3 ligand polyinosinic/polycytidylic acid [poly(I:C)] that yields a high percentage of specific CD8+ T cells without inducing an acute inflammatory response as observed after injection of poly(I:C) alone [13]. Here, we test such a formulation of CAF01 and poly(I:C), termed CAF05, in two different tumor models. CAF05 adjuvated vaccines based on HPV-16 protein E7, the model antigen hen egg ovalbumin (OVA) or the self-antigen tyrosinase-related protein 2 (TRP2) were found to induce high frequencies of antigen-specific cytotoxic CD8+ T cells and significantly reduce tumor growth in mouse models using an E7-expressing skin tumor and an OVA/TRP2-expressing lung tumor.
Materials and methods
Cell lines
The murine HPV-16 E7-expressing tumor cell line TC-1 was purchased by the American Type Culture Collection (Manassas, VA) and grown as recommended. The ovalbumin-expressing murine melanoma cell line B16-OVA was a kind gift from Dr. Natalia Martin-Orozco and Dr. Chen Dong.
Vaccine components
The cationic liposome adjuvant CAF01 (Statens Serum Institut, Copenhagen, Denmark) consists of 50 μg of the glycolipid trehalose 6,6′-dibehenate incorporated in 250 μg of dimethyldioctadecyl ammonium per dose formulated as described previously [7]. Addition of poly(I:C) (50 μg/dose) (Sigma-Aldrich, St. Louis, MO) to CAF01 was done stepwise in a manner to avoid aggregation of the CAF05 adjuvant. When used on its own, poly(I:C) was administered in 10 mM Tris–HCl (pH 7.4) at 50 μg/dose. IFA (Sigma-Aldrich) was used at 0.1 ml per dose and formulated with antigen and 50 μg of poly(I:C) per dose in 10 mM Tris–HCl buffer as described by the supplier. Montanide ISA 720 (Seppic, France) was used at 0.14 ml per dose and formulated with 50 μg/dose of poly(I:C) in 10 mM Tris–HCl buffer as described by the supplier. Ovalbumin (Sigma-Aldrich) and HPV16-E7 (produced in-house) were diluted in 10 mM Tris–HCl buffer and used at 5 μg or 50 μg/dose. TRP2 peptide (Genscript, Piscataway, NJ) stocks were dissolved in dimethylformamide and diluted in 10 mM Tris buffer for vaccines (12.5 μg of each of TRP2176–190 and TRP2180–188 per dose). Total vaccine volume was 0.2 ml, and for intraperitoneal immunization, vaccines were made isotonic prior to injection.
HPV-16 E7—recombinant expression and purification
The gene encoding the HPV-16 E7 protein was amplified from chromosomal DNA R1 from TC-1 cells (ATCC, Manassas, VA) using the oligonucleotides ggggacaagtttgtacaaaaaagcaggcttaATGCATGGAGATACACCTACATT and ggggaccactttgtacaagaaagctgggtcTTATGGTTTCTGAGAACAGATGG (gene-specific sequences are in upper case). For amplification, the iProof® polymerase (Bio-Rad Life Science, Hercules, CA) and supplied mastermix were used in a 25 μl reaction together with 10 μM of each oligonucleotide and 5 ng template DNA. After an initial denaturation at 98°C for 30 s, the conditions for the 30-cycle PCR reaction were 98°C for 10 s and 72°C for 60 s followed by one final extension at 72°C for 8 min. The resulting 355 bp DNA fragment was purified and inserted in the expression vector pDest17 by Gateway cloning according to the manufacture’s instruction (Invitrogen, Denmark). After transformation into E. coli Bl21-AI, a 6 l culture was grown at 37°C to a density of OD600 ~0.5 at which point E7 expression was induced for 3 h by adding 0.2% arabinose. After pelleting, bacteria were resuspended and lysed in B-PER (Thermo Scientific, UK) and inclusion bodies purified and washed 3 times in 20 mM Tris–HCl (pH 8.0), 0.1 M NaCl, 1 mM EDTA, and 0.1 deoxycholic acid before being dissolved in 0.01 M Tris–HCl (pH 8.0), 0.1 M NaH2PO4 (pH 8.0), and 8 M urea. Dissolved protein was applied to a TALON® His-Tag purification column, and bound protein washed 3 times by alternating between 3 column volumes (CV) 50 mM NaH2PO4 (pH 8.0), 3 M urea and 3 CV 60% isopropanol, 10 mM Tris–HCl (pH 8.0), 3 M urea. Bound protein was eluted using a 20 CV linear gradient from 0 to 150 mM imidazole in the initial binding buffer. Selected fractions were pooled, dialyzed against 10 mM Tris–HCl (pH8.0), 3 M urea, and applied to an anion exchange column (HiTrapQ®, GE Healthcare Bio-Sciences, Denmark). After washing with 5 CV 10 mM Tris–HCl (pH8.0), 3 M urea bound protein was eluted using a 20 CV linear salt gradient from 0 to 500 mM NaCl. Selected fractions were pooled and dialyzed against 10 mM Tris–HCl (pH 7.4). The concentration of the purified E7 protein was 0.5 mg/ml, the final yield 5 mg, and the purity of the protein was >99%.
Mouse vaccinations
All experiments were performed according to the Danish Ministry of Justice and Animal Protection Committees and in compliance with European Community Directive 86/609. Six- to 8-week-old female C57BL/6J (Harlan Scandinavia, Denmark) were used for all immunization studies. The mice were vaccinated 3 times at 2-week intervals for immunization studies and prophylactic tumor vaccine studies, and for therapeutic vaccination, mice received three vaccinations at 3–4 day intervals starting when tumors were palpable (day 9–11) with additional booster vaccines at a later time point as described in the individual figures. Vaccines were administered intraperitoneally in a total volume of 0.2 ml. The body temperature of the mice was measured using an IPTT-300 implant, a wireless WRS-6007 Smart Probe, and DASHost software (Bio Medic Data Systems, Inc., Seaford, DE) before vaccination (t = 0) and at regular intervals after vaccination. Implants were injected subcutaneously in the neck and given a unique identification number for each mouse.
Mouse tumor challenge
Subcutaneous tumors were established using HPV-16 E7-expressing TC-1 cells. On the day of TC-1 inoculation, cells were harvested with trypsin/EDTA, washed twice with PBS, and resuspended in ice-cold PBS. Mice were injected intradermally on the right flank with 5 × 104 cells in 50 μl PBS buffer. Tumor growth was monitored 2–3 times a week, and mice with tumors exceeding 200 mm2 were euthanized. Mice with ulcerating tumors and mice that displayed signs of excessive pain and discomfort were also euthanized. Lung tumors were established by intravenous injection of 5 × 105 OVA and TRP2 expressing B16-OVA melanoma cells in PBS. Mice were euthanized no later than 16 days after injection of B16-OVA tumor cells, and the number of tumor foci was counted manually. Some lungs had too many tumors to be counted precisely and were given a number of 300 foci, which were the maximum number of foci that could be clearly distinguished in these experiments. Consequently, a nonparametric test was used for statistical testing of tumor burden.
Vaccine-induced responses
For vaccine-induced immune responses, blood was drawn via the facial vein or by periorbital puncture, and PBMCs were purified by Lympholyte (Cedarlane, Canada) centrifugation. Spleens were disrupted, and single cells suspensions were prepared as described previously [14]. Lungs were perfused with PBS and Heparin (50 IE/ml) (LEO pharmaceuticals, Denmark) and digested with Collagenase D (Roche, Germany) before the separation of lymphocytes and tissue on a Lympholyte (Cedarlane, Canada) gradient by centrifugation. An acute systemic reaction to vaccination was evaluated by analyzing serum levels of selected inflammatory markers after i.p. vaccination using a Meso Scale Discovery assay and a mouse IL-6 ELISA kit (eBioscience, CA) following the manufacturer’s instructions. For recall responses analyzed by ELISA, cells were restimulated with full length recombinant E7 or ovalbumin proteins or peptides SIINFEKL (OVA257–264), RAHYNIVTF (E749–57), or SVYDFFVWL (TRP2180–188) at 5 μg/ml. All peptides were from Genscript NJ. PMA (0.25 μg/ml) + Ionomycin (1 μg/ml), and media control samples were included as positive and negative controls, respectively. Cells were incubated for approximately 72 h, and supernatants were harvested and analyzed for cytokines by ELISA as described previously [6]. For recall responses analyzed by FACS, cells were restimulated with the same antigens as above for 1 h in the presence of 1 μg/ml of anti-CD28 and anti-CD49d (BD Biosciences, CA), then added Brefeldin A 10 μg/ml (Sigma-Aldrich) and Golgistop 3.5 μl/ml media (BD Biosciences, San Jose, CA) and incubated a further 6 h at 37°C. The cells were surface-stained with anti-CD4, CD8 and CD44, or CD62L antibodies, permeabilized using the cytofix/cytoperm kit and stained intracellularly using anti-IFN-γ, TNF-α, IL-2, or IL-17 antibodies (all reagents from BD Biosciences). Antigen-specific CD8+ T cells were stained with pentamers specific for TCRs recognizing H-2Kb: SIINFEKL or H-2Db: RAHYNIVTF or H-2Kb: SVYDFFVWL (Proimmune, UK) as recommended by the manufacturer. The cells were acquired on a FACS Canto cytometer (BD Biosciences) and analyzed using FACS Diva v.6.1.3 (BD Biosciences) and FlowJo v.8.8.6 (Treestar, Ashland, OR) software.
Meso scale discovery
To measure acute systemic reaction, the level of selected pro-inflammatory cytokines was measured in plasma 2 h after a single i.p. administration of adjuvant. The cytokine levels were determined by the multiplex electrochemiluminescence assay from MSD (Meso Scale Discovery, Gaithersburg, MD). To this end, undiluted plasma samples were applied to an ultra-sensitive mouse pro-inflammatory 7-plex kit, and the assay performed according to the manufacturer’s instructions. The plates were read on a Sector Imager 2400 system, and calculation of cytokine concentrations was subsequently determined by 4-parameter logistic non-linear regression analysis of the standard curve.
In vivo cytolytic assay
To evaluate the functional capacity of the vaccine-induced CD8+ T cell response, splenocytes from naïve mice were stained with 10 or 1 μM CFSE (Invitrogen) for 10 min at 37 C, washed, and the 1 μM CFSE population of splenocytes were then pulsed with minimal epitopes SIINFEKL (for the evaluation of OVA vaccines) or RAHYNIVTF (for the evaluation of E7 vaccines) (10 μg/ml) for 90 min at 37°C. The 10 μM CFSE population was left unpulsed. CFSE-high and -low cells were mixed and injected i.v. in recipient mice that had previously been vaccinated i.p. 3 times at 2-week intervals with saline, Ag alone, Ag + CAF01, or Ag + CAF05 (the Ag being either OVA or E7 protein). 4.5 × 106 cells of each of the CFSE-high and CFSE-low cell populations were injected into recipient mice. Eighteen h after injection of CFSE-stained cells, recipient mice were killed, spleens processed, and the frequency of CFSE-high and -low cells were determined by flow cytometry. Specific lysis was calculated using the formula: specific lysis = 100 – [((% pulsed immunised/% unpulsed immunised)/(% pulsed naïve/% unpulsed naïve)) × 100].
Statistical analysis
The data were analyzed using GraphPad Prism 5.01 for Windows (GraphPad Software, San Diego, CA). Frequencies of pentamer positive cells, specific lysis, and ELISA IFN-γ responses were analyzed using ANOVA w/Tukey’s posttest, the number of lung tumor foci was analyzed using the nonparametric Kruskall–Wallis w/Dunn’s posttest, skin tumor size was analyzed using ANOVA w/Tukey’s posttest (on individual days), and survival was analyzed using the log-rank test. Mouse body temperature and weight data were evaluated by two-way ANOVA.
Results
CAF05 primes cytotoxic CD8+ T cells against OVA and HPV-16 E7
In order to assess the functional capability of CD8+ T cells raised by CAF05 vaccination, we studied the lysis of antigen-pulsed target cells in vivo following vaccination.
Groups of three mice were vaccinated with either OVA or E7 protein in CAF05 3 times at 2-week intervals. Antigen-specific CD8+ T cells were identified 1 week after the 2nd vaccine in pooled blood using H-2Kb-SIINFEKL pentamers (for OVA-vaccinated mice) or H-2Db-RAHYNIVTF pentamers (for E7-vaccinated mice). OVA + CAF05 vaccination induced 16.4% antigen-specific CD8+ T cells (Fig. 1a, top panel) and E7 + CAF05 vaccination raised 13.9% antigen-specific CD8+ T cells (Fig. 1b, top panel). Only a very low percentage of CD8+ T cells in antigen and antigen + CAF01 control mice were pentamer positive. Vaccination with antigen + soluble poly(I:C) was previously observed not to induce CTL immunity [13], and we confirmed this observation in a separate experiment (Supplementary Fig. 1). The cytolytic potential of the CAF05-induced CD8+ T cells was assayed 2 weeks after the third vaccination. SIINFEKL peptide-pulsed (for OVA vaccinated) or RAHYNIVTF peptide-pulsed (for E7 vaccinated) (CFSE-low) and non-peptide-pulsed (CFSE-high) splenocytes from naïve donors were transferred into vaccinated mice. Eighteen h after cell transfer, spleens were harvested from recipient mice and CFSE-high and -low cells were detected by flow cytometry. Specific lysis of antigen-pulsed splenocytes was markedly higher in OVA + CAF05 vaccinated (86.23 ± 4.07%) (Mean ± SE) than in OVA or OVA + CAF01-vaccinated mice (Fig. 1a, lower panel and graph), and a similar level of cytotoxicity was obtained with E7 + CAF05 vaccine (83.15 ± 7.8%) (Fig. 1b, lower panel and graph).
Fig. 1.
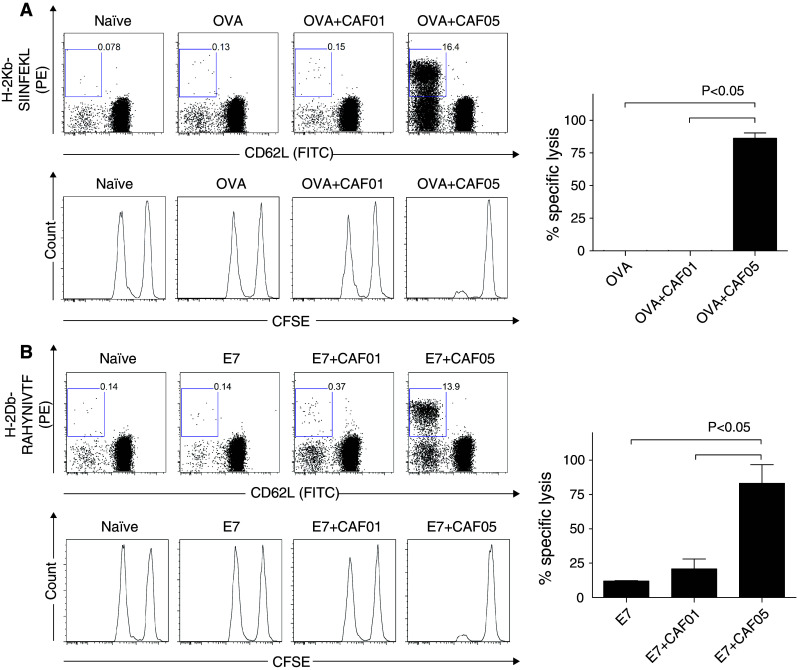
CAF05 induces cytotoxic T-lymphocytes. Groups of three C57BL/6 mice were vaccinated i.p. 3 times at 2-week intervals with (a) OVA- or (b) E7-based vaccines. CFSE-stained OVA256–264 and E749–57 peptide-pulsed target cells were transferred 2 weeks after the third vaccine, and spleens were harvested 18 h after transfer to determine in vivo lysis of target cells. Top panels show representative dot plots of pentamer positive CD62L negative PBMCs (initially gated for CD19−/CD4−/CD8+) 1 week after the second vaccine. Middle panels are representative histograms of CFSE-positive target cells (left peak peptide-pulsed, right peak non-pulsed), and graphs show specific lysis (mean + SE). P value is ANOVA w/Tukey’s multiple comparison posttest. Data are the representative of two experiments
CAF05 adjuvated vaccine protect against B16-OVA lung tumor and TC-1 skin tumor
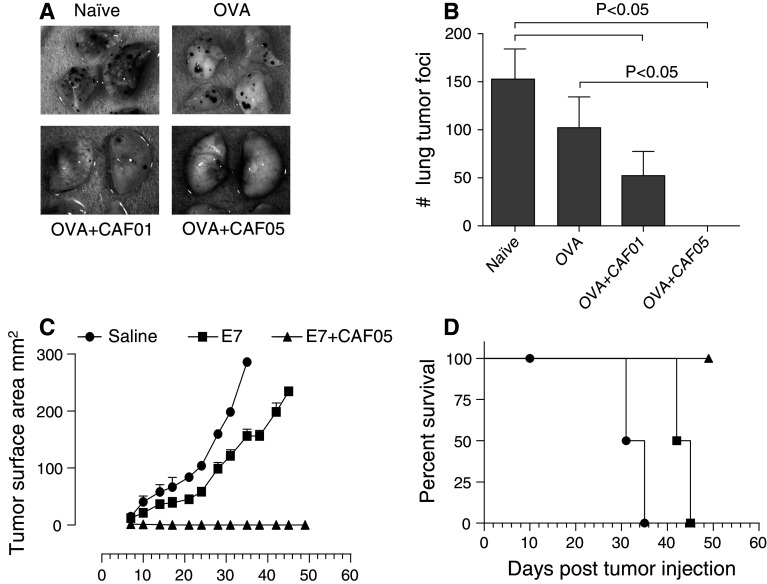
We initially tested the efficacy of prophylactic vaccination with OVA- or E7-based vaccines in two different murine tumor models: Lung B16-OVA melanoma and skin TC-1 tumor. Mice previously vaccinated with OVA alone or OVA administered in CAF01 or CAF05 were given an i.v. injection with B16-OVA tumor cells, and 16 days after the tumor challenge, the mice were euthanized and the lungs analyzed. The frequency of OVA-specific CD8+ T cells in the lungs of OVA + CAF05-vaccinated mice reached very high levels (40.38 ± 3.54%) (Supplementary Fig. 2A and 2B), and significant quantities of IFN-γ were measured in supernatants from lung lymphocytes ex vivo restimulated with OVA257–264 (Supplementary Fig. 2C). Antigen-specific CTL’s were also detected in the lungs of OVA + CAF01-vaccinated mice but at significantly lower levels (Supplementary Fig. 2B), and no IFN-γ production was detected upon restimulation (Supplementary Fig. 2C). The prominent CD8+ T cell response in the OVA + CAF05 group correlated with the levels of tumor burden as all 11 mice remained tumor free (P < 0.05) (Fig. 2b, representative photos Fig. 2a). Although OVA + CAF01-vaccinated mice had significantly fewer tumors than non-vaccinated, the CAF01 adjuvant was less effective compared to CAF05 with 8 mice out of 12 developing tumors. All non-treated (N = 11) or OVA-alone vaccinated (N = 12) developed lung tumors.
Fig. 2.
Prophylactic vaccination with CAF05 adjuvated vaccine protects against B16-OVA lung tumor and TC-1 skin tumor. Mice were vaccinated i.p. 3 times at 2-week intervals and received an i.v. challenge with 5 × 105 B16-OVA cells or an intradermal challenge with 5 × 104 TC-1 cells 2 weeks after the last vaccination. a Representative photos of lungs taken 16 days after B16-OVA tumor challenge and (b) average + SEM B16-OVA tumor load in vaccinated mice 16 days after challenge, lungs with too many tumors to count were defined as 300, P value is Kruskal–Wallis nonparametric test, N = 11–12, graph depicts pooled data from two independent experiments. c TC-1 tumor size measured at regular intervals after challenge, there was a significant (P < 0.05) difference in tumor size between saline and E7 vaccinated from day 17 onwards and between saline and E7 + CAF05 from day 7 onwards, ANOVA w/Tukey’s posttest (individual days), N = 3–4. d Mice in Fig. 2c were euthanized when a humane end point was met, log-rank test of survival data: saline versus E7 + CAF05 P = 0.0018, E7 versus E7 + CAF05 P = 0.008, E7 versus saline P = 0.018. There was a non-tumor-related death in the saline group at day 10
Similarly, in the TC-1 model, the mice received three vaccinations prior to an intradermal TC-1 challenge and tumor growth was monitored for 49 days. The non-treated mice all developed tumors that reached the humane end point as early as day 31 (Fig. 2c), and mice vaccinated with E7 alone also developed tumors although the tumor growth in this group was delayed resulting in significantly smaller tumors on day 17–31 and prolonged survival compared to untreated (Fig. 2d). In the E7 + CAF05-vaccinated group of mice, only 2 out of 4 mice developed transiently palpable tumors (Fig. 2c), and by day 14, all the mice in this group were tumor free and survived throughout the experiment (Fig. 2d).
Therapeutic vaccination with CAF05 reduces tumor burden
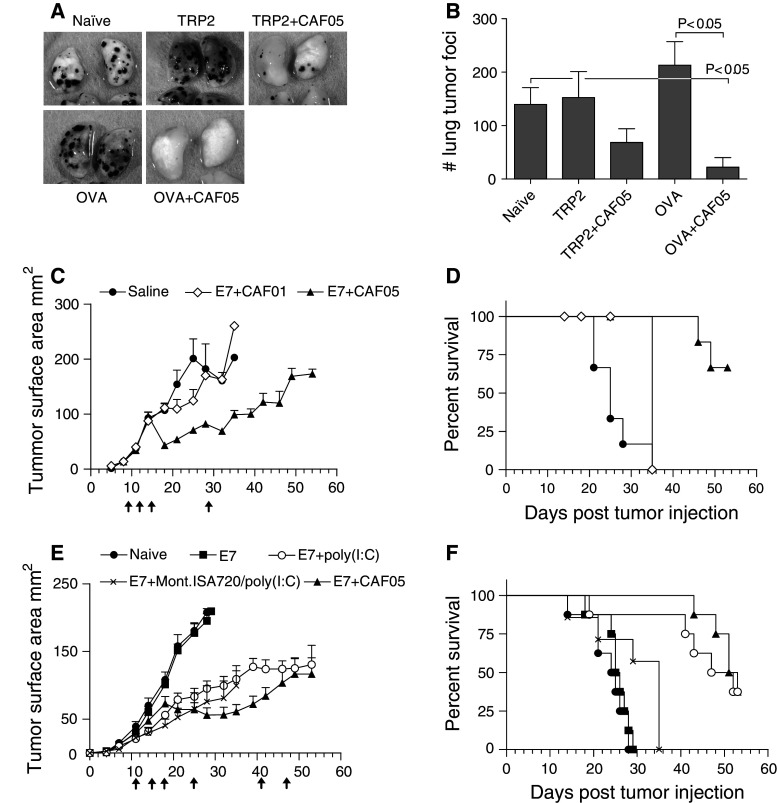
The therapeutic effect of CAF05 adjuvated vaccines was subsequently tested in the two models. In the B16-OVA model, mice received OVA or self-antigen TRP2-based vaccines at day 2, 6, and 10 relative to the day of challenge. In the lungs of OVA + CAF05-vaccinated mice, the number of tumors (22 ± 18) were significantly lower than observed in untreated mice (139 ± 31) or OVA-alone vaccinated (213 ± 44), whereas the reduction in tumor burden observed in TRP2 + CAF05-vaccinated mice (68 ± 25) was not significant when compared to untreated or TRP2-alone vaccinated mice (152 ± 48) (Fig. 3b and representative photos in Fig. 3a). Vaccine-specific CTLs were also found to accumulate in the lungs of OVA + CAF05 therapeutically vaccinated mice (7.47 ± 2.22%, mean ± SE) (Supplementary Fig. 3A) as well as TRP2 + CAF05-vaccinated mice (1.63 ± 0.23%) (Supplementary Fig. 3B) and ex vivo restimulation with OVA257–264 (Supplementary Fig. 3C) or TRP2180–188 (Supplementary Fig. 3D) resulted in significant IFN-γ, TNF-α, and IL-2 production by lung CD8+/CD44+ T cells.
Fig. 3.
Therapeutic vaccination with CAF05 adjuvated vaccine reduces tumor growth. a Mice received an i.v. challenge with 5 × 105 B16-OVA cells and were vaccinated i.p. at day 2, 6, and 10 relative to the day of challenge, photos are the representative of lungs taken 16 days after challenge. b Average + SEM number of lung tumors 16 days after challenge, lungs with too many tumors to count were defined as 300, P value is Kruskal–Wallis nonparametric test, N = 16 (naïve) or N = 8 (vaccine groups). c Mice were challenged intradermally with 5 × 104 TC-1 cells, vaccinated i.p. on day 9, 12, 15, and 29 (arrows) relative to tumor challenge, and tumor sizes was recorded for 54 days; there was a significant (P < 0.05) difference in tumor size between E7 + CAF05-vaccinated and naïve mice from day 18 onwards and between E7 + CAF01-vaccinated mice and naïve on day 25 only, P < 0.05, ANOVA w/Tukey’s posttest (on individual days), N = 8 all groups. The mean tumor size fluctuates at late time point in some groups due to euthanization of mice with tumors >200 mm2. d Mice in Fig. 3c were euthanized when a humane end point was met, log-rank test of survival data: saline versus E7 + CAF05 P < 0.0001, E7 + CAF01 versus E7 + CAF05 P = 0.0047, saline versus E7 + CAF01 P = 0.0082. There were some non-humane end point-related deaths during bleeding of mice on day 14, 18, and 25. e TC-1 tumor growth (5 × 104 TC-1 cells given intradermally) of mice vaccinated i.p. on day 11, 15, 18, 25, 41, and 47 (arrows); there was a significant (P < 0.05) difference in tumor size between naïve and E7 + poly(I:C) and naïve and E7 + Mont.ISA720/poly(I:C) from day 18 onwards and between naïve and E7 + CAF05 from day 21 onwards, ANOVA w/Tukey’s posttest (on individual days), N = 7–8. f Mice in Fig. 3e were euthanized when a humane end point was met, log-rank test of survival data: E7 + CAF05 versus naïve or E7 P < 0.0001, E7 + CAF05 versus E7 + Mont.ISA720/poly(I:C) P = 0.0002, E7 + poly(I:C) versus naïve P = 0.0012, E7 + poly(I:C) versus E7 + Mont.ISA720/poly(I:C) P = 0.0032. The remaining four mice in the E7 + Mont.ISA720/poly(I:C) group on day 35 were euthanized due to excessive pain caused by vaccine-induced inflammation of the peritoneum
Therapeutic vaccination in the TC-1 skin model was started on day 9 when tumors had reached a clearly palpable size, and booster vaccines were given on day 12, 15, and 29. While saline-vaccinated mice reached the humane end point as early as 21 days after TC-1 injection (Fig. 3c), the tumors of E7 + CAF05-vaccinated mice were significantly reduced following vaccination and remained smaller than the saline control mice from day 18 to 32, with only 2 out of 6 mice meeting the humane end point during the experimental period of 54 days (Fig. 3d). Vaccination with E7 + CAF01 initially slowed the tumor growth resulting in a significantly smaller tumor load on day 25, but no difference in survival compared to saline was observed.
Experimental therapeutic subunit vaccines against cancer are most frequently formulated as water-in-oil emulsions, and in a separate experiment, we compared the effect of E7 + CAF05 vaccine to Montanide ISA 720/poly(I:C) adjuvated vaccine as well as E7 alone and E7 + soluble poly(I:C). Mice received vaccinations on day 11, 15, 18, 25, 41, and 47 relative to the day of tumor challenge. E7 + CAF05 vaccination was confirmed to reduce tumor burden significantly compared to untreated and antigen-alone vaccinated mice (Fig. 3e). The tumor growth rate was also reduced in E7 + poly(I:C) and E7 + Mont.ISA720/poly(I:C)-vaccinated mice resulting in a significantly smaller tumor load compared to untreated from day 18 (Fig. 3e). However, the four mice remaining in the Mont.ISA720/poly(I:C) group on day 35 had to be euthanized due to vaccine-induced inflammation of the peritoneum (Fig. 3f).
In conclusion, CAF05 demonstrated to induce potent tumor reactive CTL immunity with a protective effect in different prophylactic and therapeutic tumor models.
Formulation of poly(I:C) with CAF01 reduces the acute inflammatory response to poly(I:C) and is superior to a poly(I:C) containing water-in-oil emulsion
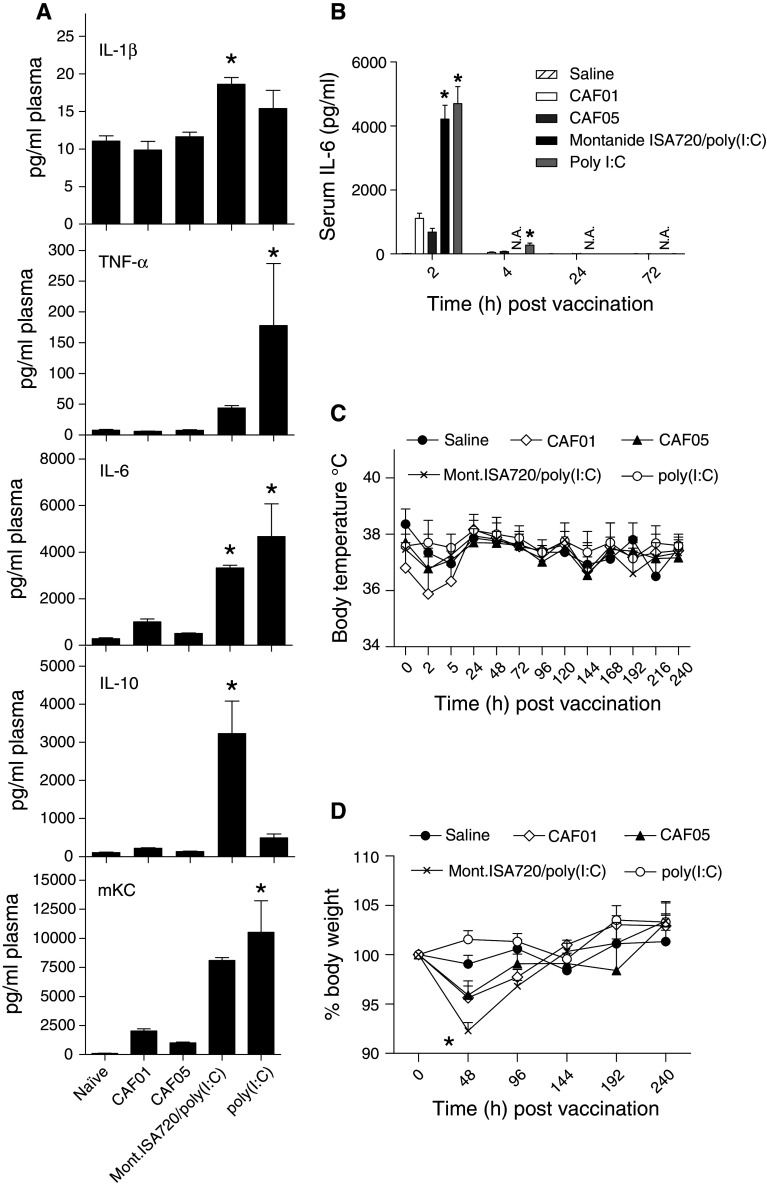
A limited vaccine-related inflammatory response is tolerable in most instances and especially when vaccinating therapeutically against serious disease such as cancer, but obviously great efforts are invested in making adjuvants as safe and tolerable as possible. Injection of adjuvants may lead to a broad activation of immune cells and thereby systemic adverse effects, and we therefore monitored blood levels of inflammation markers as well as body temperature and weight following vaccination with poly(I:C), CAF01, CAF05, and Montanide ISA720/poly(I:C). Serum levels of TNF-α, IL-6, and mKC were significantly elevated in mice 2 h after injection of poly(I:C) (Fig. 4a), whereas no significant increase was observed after injection of CAF05 or CAF01. Formulation of poly(I:C) with emulsion adjuvant Montanide ISA720 did not reduce serum levels of IL-6 or mKC but did significantly increase levels of IL-1β and IL-10. As the CAF adjuvants are known to form a depot at injection site [15], we investigated whether the systemic effects of poly(I:C) was delayed when delivered in CAF05, but no IL-6 was detected in serum samples taken at later time points (Fig. 4b). Also, body weight and temperature measured at regular intervals after vaccination did not indicate that CAF05 vaccination led to clinical discomfort. An initial small drop in body temperature was observed following vaccination in all groups (including saline) except the one receiving soluble poly(I:C) (Fig. 4c) and was not considered to reflect any discomfort to the mice. A significant decrease in body weight was observed 2 days after vaccination with Montanide ISA720/poly(I:C), whereas none of the other groups were significantly affected in terms of body mass (Fig. 4d). Thus, we conclude that by formulating poly(I:C) with CAF01 (i.e., CAF05) but not emulsion adjuvant Montanide ISA720, the systemic reaction to poly(I:C) is curtailed, thereby potentially reducing any poly(I:C)-related negative effects.
Fig. 4.
The systemic inflammatory response to poly(I:C) is reduced when formulated with CAF01. a Serum levels of pro-inflammatory markers IL-1β, IL-6, mKC, and TNF-α as well as IL-10 analyzed 2 h after vaccination by a Meso scale discovery assay. IL-12p70 and IFN-γ levels were also measured but did not differ significantly between groups and are not depicted. N = 4–5, *P < 0.05 versus naïve, and CAF05 vaccinated, ANOVA w/Tukey’s posttest. b Serum levels of IL-6 evaluated by ELISA at times indicated after i.p. injection, N = 9 at t = 2 and N = 4–5 at other time points, *P < 0.05 versus saline and CAF05 vaccinated, ANOVA w/Tukey’s posttest, NA not analyzed. c Body temperature was measured at times indicated during a 10-day period following vaccination using a chip implant, and we did not observe a significant decrease in temperature of vaccinated mice compared to saline injected mice, N = 5, two-way ANOVA. d Body weight was recorded every other day in a 10-day period following vaccination, N = 5, *P < 0.05 Mont.ISA720/poly(I:C) versus saline at 48 h, two-way ANOVA
Discussion
Safe Th1- and CTL-inducing adjuvants are an important step on the way to developing more efficient therapeutic vaccines against malignancies and infectious diseases. Currently, the adjuvant of choice for therapeutic cancer vaccine trials based on protein or peptides has most often been GM-CSF or mineral oil based emulsions; however, both of these strategies have several drawbacks. The adjuvant effect of GM-CSF has recently been questioned [16], and the emulsion adjuvants have been associated with undesirable local reactions [17, 18], and also primarily prime antibody responses in man [16]. Furthermore, in 2006, the formulation of the most frequently used emulsion adjuvant, incomplete Freunds adjuvant (IFA), was changed to include a plant-based oleic acid component that rendered the adjuvant less immunogenic [16]. Also, recent studies in mice indicate that IFA vaccines may be more tolerogenic than water-based vaccines and may not be optimal since vaccine-induced T cells are sequestered at the long-lasting vaccine depot instead of homing to the tumor [19]. However, for cancer vaccine purposes, the main problem with all of the currently available adjuvants has been their limited ability to induce CD8+ T cell responses.
CAF01 is an adjuvant that has been characterized in detail with regard to mechanism of action and efficacy in several animal disease models [6, 8] It is currently being evaluated in clinical trials of TB and HIV vaccines. In the present study, we demonstrate that a formulation of the TLR3 ligand poly(I:C) with CAF01 induces high frequencies of antigen-specific and biologically active CD8+ T cells in mice that effectively lyse target cells and reduce tumor burden in mice. Also, formulating poly(I:C) with CAF01 abrogated a systemic inflammatory response to poly(I:C) ([13], Fig. 4), whereas the oil-in-water emulsion did not reduce poly(I:C) toxicity and induced excessive inflammation in tumor bearing mice. Previously, it was generally assumed that a potent adjuvant effect was dependent on widespread DC activation, but nonetheless, a recent study showed that CAF01-based vaccination leads to an exquisite targeting and activation of a minute proportion of DCs. The same intrinsic property was observed with the cationic peptide adjuvant IC31®, and despite the relatively small number of activated DC (<0.3% of CD11c+ in draining lymph node), both of these adjuvants were shown to induce strong IFN-γ responses in animal models [20, 21] and in human clinical trials ([22] and personal communication, Ingrid Kromann). In addition, both of these adjuvants had a favorable toxicology profile with no systemic reactions [23]. Similarly, we speculate that electrostatic interaction between poly(I:C) and CAF01 prevents poly(I:C) from being released upon injection, but instead is taken up by relatively few immune cells as a complex of lipid, innate receptor ligand TDB, poly(I:C), and antigen. Whether such targeted activation of a limited number of dendritic cells with poly(I:C) is occurring by co-formulating in CAF01 is currently being investigated.
Poly(I:C) and the immunomodulator in CAF01, TDB, are two distinct pathogen-associated molecular patterns (PAMPs) signalling through TLR3 and the C-type lectin receptor Mincle, respectively. It is at this point not clear if immune cells are stimulated through both the TLR-3/TRIF and the Mincle/CARD9 pathway following CAF05 uptake, or if concomitant stimulation of these pathways is necessary for CAF05 activity. Previously, a synergistic effect of multiple innate receptor ligations has been described, but these studies have been restricted to analyzing combinations of TLR ligands only [24]. Investigating possible synergistic effects on DCs in vitro of TLR ligands and C-type lectin receptor ligands such as TDB is currently an ongoing research activity in our laboratory. In addition to the immunomodulatory effect, TDB also has a very important role in stabilizing DDA liposomes. Many cationic liposomes, including those prepared from DDA, are physically unstable and tend to aggregate upon dispersion in water rendering such formulations unacceptable for clinical use. An effective stabilizing method is to incorporate a glycolipid with the ability to interact with the surrounding water through hydrogen bonds. This hydration of individual liposomes results in a formulation with stable particles for more than 1 year when stored at 4°C [7]. In contrast, poly(I:C) does not have the same ability to stabilize DDA liposomes, and formulations of DDA/poly(I:C) were in our hands highly heterogeneous and unsuitable for evaluation in mice. Hence, no comparison between CAF05 and DDA/poly(I:C) has been performed in this study rendering it difficult to assess in vivo synergies between poly(I:C) and TDB.
Interestingly, we also observed an accumulation (albeit nonsignificant) of OVA-specific CD8+ T cells in the lungs of B16-OVA tumor-challenged mice vaccinated with OVA + CAF01 (Supplementary Fig. 2), a reduction in lung tumor load of OVA + CAF01-vaccinated mice (Fig. 2) and a limited protective effect of E7 + CAF01 vaccination in the skin tumor model (Fig. 3). We have previously demonstrated that CAF01 induces Th1 and Th17 immunity when administered s.c. in mice, and it is our experience that i.p. vaccination with CAF01 is inferior to s.c. vaccination for the induction of Th1 and Th17 immunity. In the present study, mice were vaccinated i.p., and though we could not detect a vaccine-specific Th1 or Th17 response in the lungs of B16-OVA-challenged mice on the day of killing (data not shown), it is possible that a transient CAF01-induced tumor-specific Th1 and Th17 response could have facilitated an endogenous anti-tumor CD8+ T cell response as described previously [25].
The CD8+ T cell inducing effect of CAF05 was found to be highly dependent on immunization route, with subcutaneous vaccine not inducing a strong CD8+ T cell response (data not shown). While the precise mechanism underlying this observation is currently unknown, initial studies have shown that i.p. vaccination with CAF05 leads to increased expression of type I interferons potentially functioning as a licensing signal for cross-priming [26]. It remains to be investigated if the observed route dependency is mouse specific but none of the less i.p. vaccination remains of clinical relevance for life-threatening conditions like cancer and has previously been tolerated well in man [27, 28]. It is also worth noting that the vaccine-induced reduction in TC-1 tumor size could not be sustained by late booster vaccinations (Fig. 3e). We have previously demonstrated that prophylactic vaccination with a CAF01/poly(I:C) formulation induces CTL memory [13], but it is possible that vaccine-induced CTLs become exhausted by the prolonged exposure to antigen by the tumor in a therapeutic vaccine setting, or alternatively that suppressive cells in the tumor microenvironment has a negative effect on CTL activity.
In conclusion, we demonstrate that formulation of poly(I:C) with CAF01 is a potent adjuvant that induces CTL immunity against foreign and self-antigens and reduces tumor burden in mice without causing a poly(I:C)-induced systemic inflammatory response. Our finding as well as previous studies by Fujimura et al. [29] and Zaks et al. [30] thus suggest that liposomal adjuvants incorporating TLR ligands are indeed very interesting adjuvants with a potential application in cancer vaccines. Immune-suppressive cells in the tumor microenvironment [31] may inhibit any vaccine-induced anti-tumor CTL responses, and additional therapies such as anti-CTLA4 [32], anti-IL-10 [33], anti-IL-13 [34], anti-TGF-β [35], radiotherapy, or chemotherapy [36] are likely to be needed in order to facilitate immune-mediated tumor killing. However, developing safe adjuvants that induce CD8+ T cell immunity is an important step, and we are currently working on optimizing CAF05 and other liposomal adjuvants for use in humans.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr. Natalia Martin Orozco and Dr. Chen Dong for kindly providing the B16-OVA cell line. Also, we thank veterinarian Jesper Juhl Hansen for expert technical assistance. The work presented in this paper was supported by The Danish National Advanced Technology Foundation.
Conflict of interest
The authors are employed by Statens Serum Institute, a nonprofit government research facility of which the CAF adjuvants are proprietary products.
References
- 1.Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82(5):488–496. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda H, Chamoto K, Tsuji T, Suzuki Y, Wakita D, Takeshima T, Nishimura T. The critical role of type-1 innate and acquired immunity in tumor immunotherapy. Cancer Sci. 2004;95(9):697–703. doi: 10.1111/j.1349-7006.2004.tb03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 4.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakagawa M, Stites DP, Farhat S, Sisler JR, Moss B, Kong F, Moscicki AB, Palefsky JM. Cytotoxic T lymphocyte responses to E6 and E7 proteins of human papillomavirus type 16: relationship to cervical intraepithelial neoplasia. J Infect Dis. 1997;175(4):927–931. doi: 10.1086/513992. [DOI] [PubMed] [Google Scholar]
- 6.Agger EM, Rosenkrands I, Hansen J, Brahimi K, Vandahl BS, Aagaard C, Werninghaus K, Kirschning C, Lang R, Christensen D, Theisen M, Follmann F, Andersen P. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PloS One. 2008;3(9):e3116. doi: 10.1371/journal.pone.0003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidsen J, Rosenkrands I, Christensen D, Vangala A, Kirby D, Perrie Y, Agger EM, Andersen P. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate): a novel adjuvant inducing both strong CMI and antibody responses. Biochim Biophys Acta. 2005;1718(1–2):22–31. doi: 10.1016/j.bbamem.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Hansen J, Jensen KT, Follmann F, Agger EM, Theisen M, Andersen P. Liposome delivery of Chlamydia muridarum major outer membrane protein primes a Th1 response that protects against genital chlamydial infection in a mouse model. J Infect Dis. 2008;198(5):758–767. doi: 10.1086/590670. [DOI] [PubMed] [Google Scholar]
- 9.Holten-Andersen L, Doherty TM, Korsholm KS, Andersen P. Combination of the cationic surfactant dimethyl dioctadecyl ammonium bromide and synthetic mycobacterial cord factor as an efficient adjuvant for tuberculosis subunit vaccines. Infect Immun. 2004;72(3):1608–1617. doi: 10.1128/IAI.72.3.1608-1617.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korsholm KS, Agger EM, Foged C, Christensen D, Dietrich J, Andersen CS, Geisler C, Andersen P. The adjuvant mechanism of cationic dimethyldioctadecylammonium liposomes. Immunology. 2007;121(2):216–226. doi: 10.1111/j.1365-2567.2007.02560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, Agger EM, Stenger S, Andersen P, Ruland J, Brown GD, Wells C, Lang R. Cutting edge: mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose–dibehenate. J Immunol. 2010;184(6):2756–2760. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werninghaus K, Babiak A, Gross O, Holscher C, Dietrich H, Agger EM, Mages J, Mocsai A, Schoenen H, Finger K, Nimmerjahn F, Brown GD, Kirschning C, Heit A, Andersen P, Wagner H, Ruland J, Lang R. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9-dependent innate immune activation. J Exp Med. 2009;206(1):89–97. doi: 10.1084/jem.20081445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nordly P, Rose F, Christensen D, Nielsen HM, Andersen P, Agger EM, Foged C. Immunity by formulation design: induction of high CD8+ T-cell responses by poly(I:C) incorporated into the CAF01 adjuvant via a double emulsion method. J Control Release. 2011;150(3):307–317. doi: 10.1016/j.jconrel.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Bennekov T, Dietrich J, Rosenkrands I, Stryhn A, Doherty TM, Andersen P. Alteration of epitope recognition pattern in Ag85B and ESAT-6 has a profound influence on vaccine-induced protection against Mycobacterium tuberculosis . Eur J Immunol. 2006;36(12):3346–3355. doi: 10.1002/eji.200636128. [DOI] [PubMed] [Google Scholar]
- 15.Henriksen-Lacey M, Bramwell VW, Christensen D, Agger EM, Andersen P, Perrie Y. Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J Control Release. 2010;142(2):180–186. doi: 10.1016/j.jconrel.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol Rev. 2011;239(1):27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham BS, McElrath MJ, Keefer MC, Rybczyk K, Berger D, Weinhold KJ, Ottinger J, Ferarri G, Montefiori DC, Stablein D, Smith C, Ginsberg R, Eldridge J, Duerr A, Fast P, Haynes BF. Immunization with cocktail of HIV-derived peptides in montanide ISA-51 is immunogenic, but causes sterile abscesses and unacceptable reactogenicity. PloS One. 2010;5(8):e11995. doi: 10.1371/journal.pone.0011995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, Fay MP, Narum D, Rausch K, Miles AP, Aebig J, Orcutt A, Muratova O, Song G, Lambert L, Zhu D, Miura K, Long C, Saul A, Miller LH, Durbin AP. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PloS One. 2008;3(7):e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hailemichael Y, Dai Z, Jaffarzad N, Rabinovich BA, Hwu P, Overwijk W. Peptide/IFA emulsions vaccines can form a sink and graveyard for tumor-specific CD8+ T cells. J Immunother. 2010;33(8):1. [Google Scholar]
- 20.Kamath AT, Rochat AF, Christensen D, Agger EM, Andersen P, Lambert PH, Siegrist CA. A liposome-based mycobacterial vaccine induces potent adult and neonatal multifunctional T cells through the exquisite targeting of dendritic cells. PloS One. 2009;4(6):e5771. doi: 10.1371/journal.pone.0005771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamath AT, Valenti MP, Rochat AF, Agger EM, Lingnau K, von Gabain A, Andersen P, Lambert PH, Siegrist CA. Protective anti-mycobacterial T cell responses through exquisite in vivo activation of vaccine-targeted dendritic cells. Eur J Immunol. 2008;38(5):1247–1256. doi: 10.1002/eji.200737889. [DOI] [PubMed] [Google Scholar]
- 22.van Dissel JT, Soonawala D, Joosten SA, Prins C, Arend SM, Bang P, Tingskov PN, Lingnau K, Nouta J, Hoff ST, Rosenkrands I, Kromann I, Ottenhoff TH, Doherty TM, Andersen P. Ag85B-ESAT-6 adjuvanted with IC31(R) promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in volunteers with previous BCG vaccination or tuberculosis infection. Vaccine. 2011;29(11):2100–2109. doi: 10.1016/j.vaccine.2010.12.135. [DOI] [PubMed] [Google Scholar]
- 23.Fomsgaard A, Karlsson I, Gram G, Schou C, Tang S, Bang P, Kromann I, Andersen P, Andreasen LV. Development and preclinical safety evaluation of a new therapeutic HIV-1 vaccine based on 18 T-cell minimal epitope peptides applying a novel cationic adjuvant CAF01. Vaccine. 2011;29(40):7067–7074. doi: 10.1016/j.vaccine.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Q, Egelston C, Gagnon S, Sui Y, Belyakov IM, Klinman DM, Berzofsky JA. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J Clin Invest. 2010;120(2):607–616. doi: 10.1172/JCI39293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Bon A, Tough DF. Type I interferon as a stimulus for cross-priming. Cytokine Growth Factor Rev. 2008;19(1):33–40. doi: 10.1016/j.cytogfr.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Lue C, van den Wall Bake AW, Prince SJ, Julian BA, Tseng ML, Radl J, Elson CO, Mestecky J. Intraperitoneal immunization of human subjects with tetanus toxoid induces specific antibody-secreting cells in the peritoneal cavity and in the circulation, but fails to elicit a secretory IgA response. Clin Exp Immunol. 1994;96(2):356–363. doi: 10.1111/j.1365-2249.1994.tb06567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA, Kaur JS, Haluska PJ, Jr, Aderca I, Zollman PJ, Sloan JA, Keeney G, Atherton PJ, Podratz KC, Dowdy SC, Stanhope CR, Wilson TO, Federspiel MJ, Peng KW, Russell SJ. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70(3):875–882. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujimura T, Nakagawa S, Ohtani T, Ito Y, Aiba S. Inhibitory effect of the polyinosinic-polycytidylic acid/cationic liposome on the progression of murine B16F10 melanoma. Eur J Immunol. 2006;36(12):3371–3380. doi: 10.1002/eji.200636053. [DOI] [PubMed] [Google Scholar]
- 30.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, Bosio C, Dow S. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176(12):7335–7345. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, Royal RE, Kammula U, Restifo NP, Hughes MS, Schwartzentruber D, Berman DM, Schwarz SL, Ngo LT, Mavroukakis SA, White DE, Steinberg SM. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175(9):6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 32.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206(8):1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 34.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1(6):515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 35.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 36.Andersen MH, Junker N, Ellebaek E, Svane IM, Thor Straten P. Therapeutic cancer vaccines in combination with conventional therapy. J Biomed Biotechnol. 2010;2010:237623. doi: 10.1155/2010/237623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.