Abstract
Prostate cancer is a major cause of death in older men, and bone metastasis is the primary cause of morbidity and mortality in prostate cancer. Prostate is an abundant source of nerve growth factor (NGF) that is secreted by malignant epithelial cells and utilized as an important autocrine factor for growth and metastasis. We previously showed that intravenous gammaglobulin (IVIg) contains natural antibodies against NGF, which inhibit growth and differentiation of the NGF-dependent cell line PC-12. In the present study, we examined the effects of these natural antibodies on in vitro migration or metastasis of two prostate cancer cell lines namely DU-145 and PC-3. Cancer cell migration was assessed using these cell lines in the upper chambers of Matrigel invasion chambers. The effects of IVIg and affinity-purified anti-NGF antibodies on cell migration through membrane into the lower chamber were assessed in dose/response experiments by a colorimetric method. Affinity-purified natural IgG anti-NGF antibody inhibited DU-145 migration by 38% (p = 0.01) and PC-3 migration by 25% (p = 0.02); whereas, a monoclonal anti-NGF antibody inhibited DU-145 migration by 40% (p = 0.01) and PC-3 migration by 37% (p = 0.02), at the same concentration. When IVIg was depleted of NGF-specific IgG by affinity chromatography, there was no significant inhibition of migration of the DU-145 and PC-3 cells at a concentration of 1 mg/well. Removal of the NGF-specific antibody from the IVIg was also demonstrated by a lack of effect on PC-12 cell differentiation. Therefore, IVIg is able to inhibit the migration of prostate cancer cell lines, through Matrigel chambers in vitro, only when the natural NGF-specific antibodies actively are present in IVIg.
Keywords: Anti-NGF effects, IVIg, NGF, Prostate cancer cell invasion
Introduction
The incidence of prostate cancer rose dramatically in the latter half of the twentieth century and is currently the most prevalent cancer diagnosed in men in North America and Western Europe [1]. It is also the cancer resulting in the second highest mortality rate in this group [1]. Series of factors are responsible for initiation, promotion and progression of this disease [2, 3]. Role of paracrine and autocrine growth factors in the development of both normal prostate and prostate cancer is becoming clearer, and these factors are probably responsible for both the promotion and progression of prostate cancer [4–9] and the growth factors include neurotrophins and particularly nerve growth factor.
Besides having growth and differentiation effects in many organ systems, including the nervous system and the immune system, NGF is known to play a major role in the development and metastatic spread of several cancers, including breast cancer, malignant melanoma, prostate cancer and squamous cell carcinoma of the esophagus [10–17].
The prostate is one of the most abundant sources of NGF outside the nervous system. In the normal prostate, most of the NGF is localized to stromal cells, but in prostate cancer, NGF is synthesized and secreted by malignant epithelial cells [18, 19]. Hence malignant transformation of prostate epithelial tumor cells may be the result of the acquisition of autocrine expression of NGF. In vitro, neurotrophins and their receptors are clearly linked to prostate cancer cell invasion [20–27].
We have previously demonstrated that natural antibodies to NGF exist in commercial batches of IVIg and these antibodies are biologically functional, inhibiting growth and differentiation of NGF-dependent cell lines such as PC-12 [29]. In vitro studies of NGF actions on prostate cancer cell lines have shown that these cells have an increased capacity for migration through Matrigel-coated invasion chambers in response to neurotrophins [28].
Evidence from in vivo studies of mice have shown that monoclonal anti-NGF antibodies can reduce rates of metastasis and bone pain in prostate cancer models; and there is a preliminary evidence suggesting a beneficial effect of IVIg on metastasis of certain cancer types in humans [30–33]. Suggested mechanisms for this effect include an increase in apoptosis in the cells treated with IVIg, an increase in the expression of p53, pRb, and p21 genes and Fas, and an increase in the secretion of IL-12, an NK cell activator [34].
In the present study, we examined the effects of IVIg and affinity-purified NGF-specific IgG antibody from IVIg on the migration of prostate cancer cell lines DU-145 and PC-3 through cell migration chambers. It was shown that IVIg has the capacity to reduce prostate cancer cell migration and that is due to NGF-specific antibodies in IVIg.
Materials and methods
Cell culture
The human prostate cancer cell lines, DU-145 and PC-3, were obtained from the American Type Culture Collection. The DU-145 was propagated in Eagle MEM medium with 2 mM l-glutamine, 1.5 g/L sodium bicarbonate, 0.1 mM non-essential amino acids, and 1.0 mM sodium pyruvate with 10% fetal bovine serum. PC-3 was cultured in Ham’s F12K medium with 2 mM l-glutamine, 1.5 g/L sodium bicarbonate with 10% fetal bovine serum. Both the cell lines were maintained on T-25 flasks and were incubated at 37°C in a 5% CO2 atmosphere.
Source of IVIg
Immune gammaglobulin (IVIg) was obtained from Bayer Corporation, Pharmaceutical Division, Elkhart, IN, USA.
Purification of anti-NGF antibodies from IVIg
Anti-NGF antibodies were purified from IVIg using Pierce AminoLink® affinity columns prepared according to the manufacturer’s instructions (Pierce Biotechnology Inc., Rockford, IL, USA).
Column construction
Construction method of the affinity column was followed from the Pierce kit instructions protocol. Briefly, 1 mg of murine 2.5S, β-subunit (BD-Biosciences) was dissolved in 2 mL of coupling buffer. A reductant solution was prepared separately by adding 0.5 mL of NaOH to 32 mg of AminoLink reductant. The column was equilibrated with 5 mL of coupling buffer and 2 mL of protein solution and followed by 200 μL reductant. The 2 mL column was agitated end-over-end overnight at 4°C on a rotary mixer (DiaMed). At the end of the incubation period the column was washed with 5 mL of coupling buffer. Following washing, the remaining active sites on the column gel were blocked with the addition of 2 mL of quenching buffer and a further 200 μL of AminoLink reductant solution. The column was then rotated for further 30 min at room temperature. After incubation the column was washed with 15–20 mL of AminoLink wash solution and this was followed by a wash with 4 mL phosphate buffered saline with 0.05% sodium azide. The column was stored at 4°C until required.
Purification of anti-NGF antibodies
Anti-NGF antibodies were purified from Bayer Gamimune N, 10%. The pH of the Gamimune was neutralized by dialysis against PBS for 48 h at 4°C using a sterile Spectro/Por DispoDialyzer MW cutoff 10 kDa prior to purification. The AminoLink column was equilibrated with 6 mL of PBS just prior to use. A measure of 2 mL of Gamimune was applied to the column (200 mg total protein) and allowed to enter the gel bed. The column was then placed on a rotary agitator (DiaMed) for 1 h at room temperature. After 1 h, the column was washed with 12 mL of sample buffer. The bound protein (anti-NGF antibody) was eluted from the column with 100 mM pH 2.5 glycine buffer. Samples measuring 1 mL each were collected and neutralized with 50 μL of 1 M Tris buffer, pH 9.0. Samples of each fraction were monitored by absorbance at 280 nm and the total protein concentration was determined using 260/280 absorbance method. The active samples having OD > 0.01 were pooled and dialyzed against sterile distilled H2O in a sterile Spectro/Por DispoDialyzer, MW cutoff 10 kDa. After 24 h, the samples were recovered from the dialysis tube and dried in a Thermo Savant UVS400 Speed Vac. The resulting product was reconstituted at the desired concentration in PBS and sterilized using a 1-mL Millipore filter (pore size 0.20 μm). Approximately 25 μg of affinity-purified anti-NGF antibody was obtained from 200 mg of IVIg. Several passes and elutions were required in the depletion experiments.
Purification of control antibody (anti-tetanus toxin antibody)
Antibodies against tetanus toxoid were recovered from IVIg using a similar technique as described above. A measure of 1 mg tetanus toxoid (Wyeth, Fort Dodge, IA) was bound to the column as described previously. Using the prepared column, 2 mL of Bayer Gamimune N, 10%, was applied to the column and incubated as described above. The elution, isolation and preparation of anti-tetanus antibodies were also experimented as described above for anti-NGF antibodies.
Matrigel invasion chambers
Cell migration rates were determined using the 6-well BD BioCoat Matrigel Invasion Chamber.
Preparation and utilization of Matrigel invasion chambers
Chamber wells were rehydrated using 2 mL bicarbonate based media (Eagles MEM media for DU-145 and Ham’s F12 K for PC-3) and were incubated for 2 h at 37°C, 5% CO2. After the rehydration period, the media was gently removed from the chamber using a 2-mL pipette and then 1.25 × 105 cells were suspended in 2 mL of the appropriate media (without serum) and gently layered into the top chamber. The test antibody was added to the top chamber at the appropriate concentration. In experiments using affinity-purified anti-NGF antibodies, an equivalent amount of the affinity-purified anti-TT antibodies were used as controls. The concentrations of IVIg or affinity-purified anti-NGF antibody were based upon a competitive binding assay described previously [28] in which IVIg or affinity-purified anti-NGF was shown to inhibit a polyvalent sheep anti-NGF antibody binding to NGF. Purified anti-TT antibody had no effect in this assay. Concentrations were chosen based on their efficiency to inhibit binding by 50% or more. A measure of 2.5 mL of appropriate media with serum was added to the lower chamber as a chemo attractant. The chambers were incubated at 37°C with 5% CO2 for 48 h.
Removal of non-invading cells
Non-invading cells present at the top chamber were removed from the ECM membrane with a cotton swab. The top membrane was washed with PBS and the loosened cells were removed with a pipette. This process was repeated again. Once the non-migrating cells were removed completely, the membrane insert was first placed in 50% methanol/water mixture for 2 min and then immersed in Wright–Giemsa stain (Sigma–Aldrich) for 5 min. After staining, they were washed in water for 30 s and then the membrane was removed from the insert by a scalpel [35, 36].
Assessing cell migration
In initial experiments, cell migration was assessed by counting the number of stained cells present in ten HPFs; but subsequently it was assessed by colorimetric technique as the distribution of the migrating cells is uneven. The membrane with the fixed and stained migrating cells was allowed to air dry and it was then immersed in 400 μL of a 10% acetic acid/PBS and 200 μL of the dye solution was transferred to a 96-well plate for colorimetric reading of OD at 560 nm [35, 36]. Experiments were performed in triplicate.
Nerve growth factor bioassay
This was carried out by adding increasing quantities of NGF to the developing cultures of PC-12 cells, as described previously [29]. Standard curve concentrations were 0, 25, 50 and 100 ng/mL. Cells were seeded at 2 × 104 cells/mL in 2 mL of the appropriate media, in Collagen PRO COAT six well plates (Sigma). Cultures were incubated with 2 mg of IVIg and the same concentration of IVIg that is depleted of NGF-specific antibody by affinity chromatography. To assess differentiation, a PC-12 cell was scored if the dendrite was 2 or more cell diameters in length. Totally 200 cells were counted in three random fields.
Statistical analysis
Results are presented as means ± SD and significance is determined by ANOVA and by Kruskal–Wallis one way Analysis of Variance.
Results
Effects of IVIg on prostate cancer cell migration in vitro
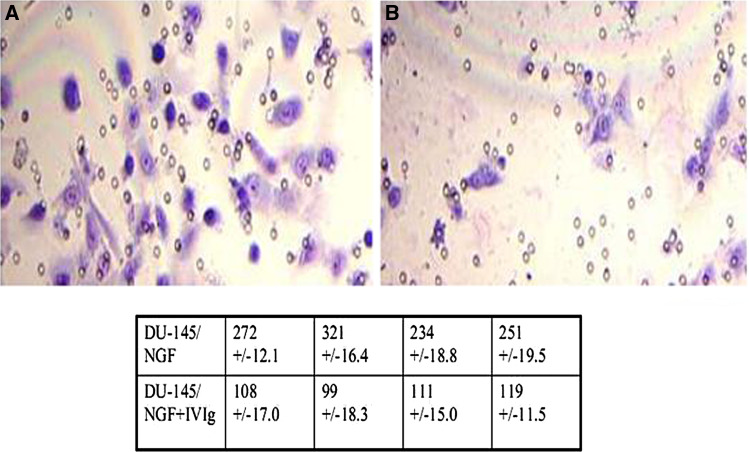
Using an ECM invasion chamber and the NGF-responsive human prostate cell line DU 145, we initially showed that IVIg inhibits the migration of DU145 through the ECM membrane, resulting in 59.15 ± 7.4% inhibition using IVIg at a concentration of 7.7 mg/chamber (Fig. 1).
Fig. 1.
Giemsa stained membranes from ECM invasion chambers using the NFG-responsive human prostate cancer cell line DU145 are shown. Micrograph a shows migration in the absence of added IVIg at 7.7 mg/chamber, while b shows migration in the presence of IVIg. Table shows the number of cells in 10 HPF in the absence (upper) and presence (lower) of IVIg, with resultant inhibition of 59.15 ± 7.4%
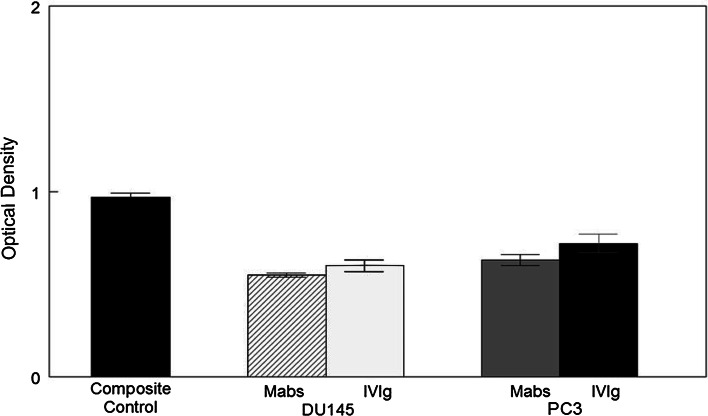
We then examined the effects of IVIg on in vitro migration of two prostate cell lines, DU-145 and PC-3, in dose/response experiments from 500 μg IVIg/chamber to 2 mg/chamber. Migration was significantly inhibited at all three concentrations of IVIg, DU-145 being more affected than PC-3, although not significantly. Inhibition was statistically significant at p = 0.05 (500 μg) and p = 0.01 (1, 2 mg, respectively; Fig. 2).
Fig. 2.
The effects of IVIg on cell migration of DU145 and PC3 are shown. Fine shading represents DU145 and course shading PC3. A measure of 500 μg for both the cell types shows significant inhibition (p = 0.05) over control; 1.0 mg gives a significant value of p = 0.01 for DU145 and p = 0.05 for PC3. At 2.0 mg both the cell types show a significant difference of p = 0.01 over control migration which was in media alone
Effects of affinity-purified anti-NGF on prostate cancer cell migration
In order to demonstrate that the inhibition of DU-145 and PC-3 was due to NGF-specific antibodies in the IVIg, these antibodies were isolated by affinity column purification. In Fig. 3 the effects of these purified antibodies at 50, 100 and 250 ng per chamber on the migration of DU-145 and PC-3 cells are shown. Again DU-145 was more sensitive to the antibody than PC-3 and inhibition of migration was significant at p = 0.01 for DU-145 at 100 ng and p = 0.05 for PC-3. At 250 ng, inhibition of both cell lines was significant at p = 0.01. In these experiments, controls contained an equal amount of affinity-purified anti-tetanus toxoid antibody.
Fig. 3.
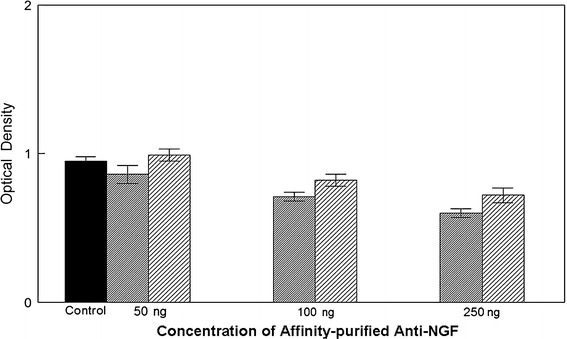
This figure shows the effects of anti-NGF antibodies isolated from IVIg. With a treatment of 50 ng anti-NGF there was no significant difference between the control (affinity-purified anti-tetanus toxoid antibody) and the migration of both the cell types. With a treatment concentration of 100 ng, DU145 migration inhibition was significant at p = 0.01 and PC3 showed a significant difference over control to a level of p = 0.05. When the treatment levels were increased to 250 ng, both differed from control with a significance at p = 0.01. Controls contained an equal concentration of anti-TT antibody to that of anti-NGF antibody in the test wells
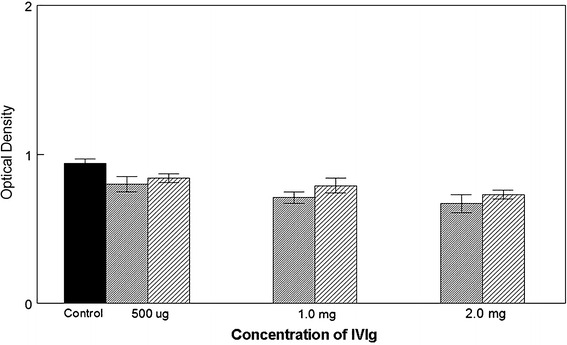
Effects of depletion of anti-NGF antibodies on the inhibition of migration by IVIg
To demonstrate that the inhibition of DU-145 and PC-3 cells was due to the NGF-specific antibodies in IVIg, we attempted to deplete IVIg of the anti-NGF antibodies by repeated passage through an NGF-affinity column. As shown in Fig. 4, the IVIg depleted of NGF-specific IgG no longer caused significant inhibition of migration of DU-145 and PC-3 cells at 500 μg and 1 mg per chamber, but there was still inhibition at 2 mg per chamber (p = 0.05). To show that IVIg did not inhibit migration of DU-145 or PC-3 cells non-specifically, the migration of these cells in the presence of 1 mg of IVIg and 1 mg of anti-NGF depleted IVIg was assessed. There was no significant difference between migration in the presence of depleted IVIg compared to its absence (p = 0.323 for DU-145 and p = 1.000 for PC-3).
Fig. 4.
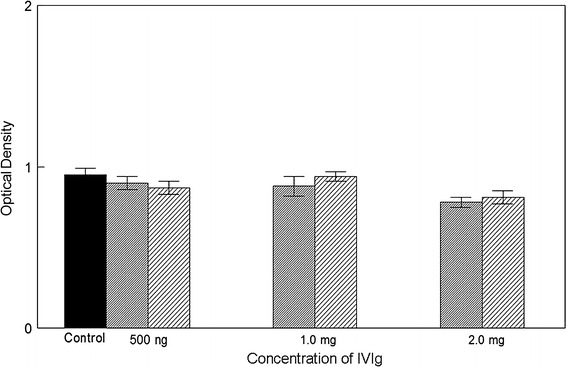
The results of the addition of IVIg depleted of anti-NGF are shown. The fine shading represents DU145 and the course shading PC3. At doses of 500 ng and 1.0 mg there was no significant difference in migration for either cell line. With a treatment of 2.0 mg both cell lines showed a significant difference to controls (medium alone) at a level of p = 0.05
We used PC-12 cells in a bio-detection assay to demonstrate the depletion of anti-NGF antibodies from IVIg, by its effect upon NGF-induced differentiation [29]. In Fig. 5, the effects of NGF on differentiation of PC-12 cells in a dose/response experiment are shown. The inhibitory effects of IVIg on differentiation are compared with the same concentration of IVIg after the depletion of anti-NGF antibody, but the latter failed to inhibit PC-12 cell differentiation at all concentrations tested.
Fig. 5.
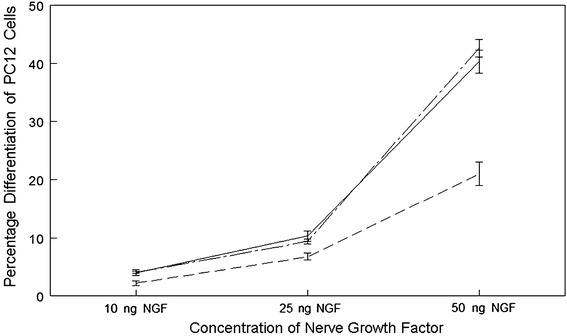
The figure represents the data from the PC12 based bio-detection assay. The solid line shows the results from PC12 cells treated with NGF but no anti-NGF. The dashed line represents PC12 cells treated with NGF and 2 mg of non-depleted IVIg. The intermittent line represents PC12 cells, again treated with 2 mg NGF, and with IVIg depleted of anti-NGF
Comparison of the effects of affinity-purified human anti-NGF with murine monoclonal anti-NGF antibody
We then compared the effects of a mouse monoclonal anti-NGF antibody (Clone AS18, IgG1, Exalpha Biologicals, Shirley, MA, USA) with the affinity-purified human anti-NGF antibodies used at the same concentration. In these experiments, control wells contain the same concentration of affinity-purified anti-tetanus toxoid antibody or medium alone. Both Murine monoclonal anti-NGF and human anti-NGF antibodies inhibited migration of DU-145 and PC-3 cells and there was no significant difference between the two antibodies at the concentrations used, as shown in Fig. 6. Anti-tetanus toxoid antibody had no effect upon migration.
Fig. 6.
A comparison between the effects of mab anti-NGF antibodies and anti-NGF antibodies isolated from IVIg compared to control chambers, containing 250 ng anti-TT7 antibody per chamber. Both antibodies were tested at 250 ng per chamber. There was no significant difference between the two different anti-NGF antibodies and levels of migration inhibition, compared to anti-TT control antibody
Discussion
The value of intravenous immunoglobulin (IVIg) in the treatment of antibody deficiencies has been recognized for over 25 years [37]. IVIg has also been used to treat certain autoimmune and inflammatory disorders such as thrombocytopenia, Kawasaki’s disease, Guillain–Barré syndrome and Myasthenia gravis [38–41]. Recent preliminary investigations suggest that IVIg may also assist in lowering the metastatic rate of some cancers, such as melanoma, breast cancer and prostate cancer [34]. Except in the case of replacement therapy, the mechanisms of action of IVIg are still undetermined, but have been suggested to include interference with the complement and cytokine networks, modulation of Fc receptors and idiotype networks and effects upon T cell, B cell, NK cell and antigen-presenting cell functions [39].
In a previous paper we had demonstrated the presence of biologically active anti-NGF antibodies in IVIg affecting the growth and differentiation of the NGF-dependent pheochromocytoma cell line PC-12 [29]. Three different lots from two manufacturers contained anti-NGF antibodies that varied between lots by about 25%. Since a number of other malignant cell types including prostate cancer, breast cancer and melanoma appear to make use of NGF as an autocrine growth factor, one possible explanation for a beneficial effect of IVIg in lowering metastatic rate in such cancers might be via these NGF-specific antibodies [16, 17]. We therefore examined the effects of IVIg and affinity-purified NGF-specific natural IgG antibodies from IVIg on the in vitro migration of prostate cancer cells.
Using two prostate cancer cell lines, DU-145 and PC-3 in Matrigel invasion chambers, it was shown that IVIg has the capacity to inhibit the migration rates of these two cancer cell lines. To demonstrate that it is the anti-NGF antibody component of IVIg, which is responsible for the inhibition of cell migration through the chamber, anti-NGF antibodies were isolated from IVIg by affinity chromatography and the effect of these antibodies on prostate cancer cell in vitro migration was assessed. The results clearly show that the purified anti-NGF antibody from IVIg does inhibit the migration process. In addition, IVIg that had been depleted of anti-NGF antibody, on the basis of the Matrigel chamber assay, had no effect upon migration of the DU-145 and PC-3 cells at a concentration of 1 mg/well, although residual activity remained at 2 mg/well.
The activity of the affinity-purified antibody was then compared to that resulting from the use of a Murine monoclonal anti-NGF antibody. The differences found between the monoclonal anti-NGF and the anti-NGF isolated from IVIg were not significantly different in terms of their effects upon in vitro migration of the two prostate cancer cell lines; whereas an affinity-purified anti-tetanus toxoid antibody did not affect migration when compared to control media.
To ascertain, if all biologically active NGF-specific antibodies had been removed, an in vitro NGF-induced differentiation assay using the pheochromocytoma cell line PC-12 was performed. The IVIg depleted of anti-NGF antibody had no effect upon NGF-induced differentiation of PC-12 cells. However, after anti-NGF depletion, IVIg did retain some capacity to reduce cell migration rates of DU-145 and PC-3 at the highest concentration of the IVIg used. This difference is probably a reflection of the sensitivity of the two assays. The PC-12 cells do not produce NGF by themselves but require NGF in order to differentiate, whereas the two prostate cancer cell lines have been shown to produce an NGF-like molecule which they can utilize in an autocrine fashion [18, 19].
Overall, these results have demonstrated that anti-NGF antibody in IVIg is responsible in significant part for the observed effect of IVIg on prostate cancer cell migration in vitro. This anti-cytokine effect of natural NGF-specific antibodies from IVIg may explain some of the beneficial effects of IVIg on cancer metastasis.
Within prostate epithelial cells, NGF is localized to secretory vesicles in the cytoplasm [18–20]. This NGF is able to induce phosphorylation of the high affinity NGF receptor TrkA and stimulate anchorage-independent growth of prostate tumor cells [23, 24]. NGF also increases the formation of satellite tumors, both contiguous and non-contiguous to the primary tumor mass [26]. The formation of satellite tumors of prostate cells is suppressed by the expression of the low affinity NGF receptor p75NTR, a member of the TNF receptor family [26]. There is evidence for malignancy of prostate cancer being accompanied by an increase in TrkA receptor signaling, with a reduction of p75NTR expression and loss of androgen responsiveness [42, 43, 45–47]. Sigala et al. [46] have shown by reverse transcriptase PCR that both DU-145 and PC-3 cells express TrkA receptors but virtually they lack p75NTR. Exposure of these cells to exogenous NGF results in a marked increased of p75 NTR mRNA with no change in TrkA expression. Prolonged exposure of DU-145 and PC-3 cells to NGF for several days results in a reduction of invasiveness [47], which is in contrast to the findings of Geldorf et al. [23] and Djakiew et al. [48] who found that NGF enhanced in vitro metastatic potential of these cell lines. It has been shown that in DU-145 and PC-3 NGF acts via the 140 kDa TrkA receptor and its effect is inhibited by kinase inhibitor K252a, which also inhibits the action of autocrine NGF, an effect not reversed by human recombinant NGF, and results in accumulation of these cells in G0/G1 [49].
The p75 NGF receptor is a death receptor [42–44] and in the absence of ligand, p75NTR-dependent cell cycle arrest occurs via the intrinsic mitochondrial pathway, with apoptosis and nuclear fragmentation. However, NGF can function as a survival factor for p75NTR-expressing neuroblastoma cells [45]. Neutralization of the ligand for p75NTR and TrkA has been demonstrated to be of value in experimental models of prostate cancer, where anti-NGF antibodies result in significant reduction of prostate cancer xenografts and reduction in early and late bone cancer pain-related behavior [17, 28, 30, 31, 45]. More recently, it has been proposed that NGF may act in other ways than via TrkA and p75NTR receptors in prostate cancer cell lines, by an effect upon voltage-gated sodium channel expression, upregulating these enhancers of cell motility [50–52].
The metastatic process is inherently inefficient with growth at the secondary site which is a major rate-limiting step. Hence, by preventing dissemination to the distant site or impairing growth, the use of NGF-specific antibody could potentially improve clinical outcomes. And the existence of natural NGF-specific antibodies in normal plasma raises the issue of a natural immunity to prostate cancer occurring in healthy individuals. Although the effects of natural NGF antibodies are not prominent in vitro, their in vivo effects on the growth and spread of sparse metastatic cancer cells may be significant.
Acknowledgments
Funding for this work was provided by Bayer Inc. Canada.
Abbreviations
- ECM
Extracellular matrix
- IL-12
Interleukin-12
- IVIg
Intravenous gammaglobulin
- MEM
Minimal essential medium
- NGF
Nerve growth factor
- NTR
Neurotrophin receptor
- OD
Optical density
- TrKA
Tyrosine kinase A
Footnotes
Author Keith E. Lewis is deceased.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Culig Z, Hobisch A, Cronauer MV, et al. Regulation of prostatic growth and function by peptide growth factors. Prostate. 1996;28(6):392–405. doi: 10.1002/(SICI)1097-0045(199606)28:6<392::AID-PROS9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 3.Bonfil RD, Chinni S, Fridman R, Kim HR, Cher ML. Proteases, growth factors, chemokines, and the microenvironment in prostate cancer bone metastasis. Urol Oncol. 2007;25(5):407–411. doi: 10.1016/j.urolonc.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Jacob K, Webber M, Benayahu D, Kleinman HK. Osteonectin promotes prostate cancer cell migration and invasion: a possible mechanism for metastasis to bone. Cancer Res. 1999;59(17):4453–4457. [PubMed] [Google Scholar]
- 5.Angelucci A, Festuccia C, D’Andrea G, Teti A, Bologna M. Osteopontin modulates prostate carcinoma invasive capacity through RGD-dependent upregulation of plasminogen activators. Biol Chem. 2002;383(1):229–234. doi: 10.1515/BC.2002.024. [DOI] [PubMed] [Google Scholar]
- 6.Hellawell GO, Turner GD, Davies DR, Poulsom R, Brewster SF, Macaulay VM. Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res. 2002;62(10):2942–2950. [PubMed] [Google Scholar]
- 7.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 8.Chinni SR, Sivalogan S, Dong Z, Filho JC, Deng X, Bonfil RD, Cher ML. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate. 2006;66(1):32–48. doi: 10.1002/pros.20318. [DOI] [PubMed] [Google Scholar]
- 9.Luo Y, He DL, Ning L, et al. Over-expression of hypoxia-inducible factor-1alpha increases the invasive potency of LNCaP cells in vitro. BJU Int. 2006;98(6):1315–1319. doi: 10.1111/j.1464-410X.2006.06480.x. [DOI] [PubMed] [Google Scholar]
- 10.Lambiase A, Micera A, Sgrulletta R, Bonini S, Bonini S. Nerve growth factor and the immune system: old and new concepts in the cross-talk between immune and resident cells during pathophysiological conditions. Curr Opin Allergy Clin Immunol. 2004;4(5):425–430. doi: 10.1097/00130832-200410000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Tometten M, Blois S, Arck PC. Nerve growth factor in reproductive biology: link between the immune, endocrine and nervous system? Chem Immunol Allergy. 2005;89:135–148. doi: 10.1159/000087962. [DOI] [PubMed] [Google Scholar]
- 12.Nockher WA, Renz H. Neurotrophins in allergic diseases: from neuronal growth factors to intercellular signaling molecules. J Allergy Clin Immunol. 2006;117(3):583–589. doi: 10.1016/j.jaci.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 13.Nockher WA, Renz H. Neurotrophins and asthma: novel insight into neuroimmune interaction. J Allergy Clin Immunol. 2006;117(1):67–71. doi: 10.1016/j.jaci.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 14.Linker R, Gold R, Luhder F. Function of neurotrophic factors beyond the nervous system: inflammation and autoimmune demyelination. Crit Rev Immunol. 2009;29(1):43–68. doi: 10.1615/critrevimmunol.v29.i1.20. [DOI] [PubMed] [Google Scholar]
- 15.Abram M, Wegmann M, Fokuhl V, et al. Nerve growth factor and neurotrophin-3 mediate survival of pulmonary plasma cells during the allergic airway inflammation. J Immunol. 2009;182(8):4705–4712. doi: 10.4049/jimmunol.0802814. [DOI] [PubMed] [Google Scholar]
- 16.Krüttgen A, Schneider I, Weis J. The dark side of the NGF family: neurotrophins in neoplasias. Brain Pathol. 2006;16(4):304–310. doi: 10.1111/j.1750-3639.2006.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papatsoris AG, Liolitsa D, Deliveliotis C. Manipulation of the nerve growth factor network in prostate cancer. Expert Opin Investig Drugs. 2007;16(3):303–309. doi: 10.1517/13543784.16.3.303. [DOI] [PubMed] [Google Scholar]
- 18.Djakiew D, Delsite R, Pflug B, Wrathall J, Lynch JH, Onoda M. Regulation of growth by a nerve growth factor-like protein which modulates paracrine interactions between a neoplastic epithelial cell line and stromal cells of the human prostate. Cancer Res. 1991;51(12):3304–3310. [PubMed] [Google Scholar]
- 19.Delsite R, Djakiew D. Characterization of nerve growth factor precursor protein expression by human prostate stromal cells: a role in selective neurotrophin stimulation of prostate epithelial cell growth. Prostate. 1999;41(1):39–48. doi: 10.1002/(SICI)1097-0045(19990915)41:1<39::AID-PROS6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Dalal R, Djakiew D. Molecular characterization of neurotrophin expression and the corresponding tropomyosin receptor kinases (trks) in epithelial and stromal cells of the human prostate. Mol Cell Endocrinol. 1997;134(1):15–22. doi: 10.1016/S0303-7207(97)00165-2. [DOI] [PubMed] [Google Scholar]
- 21.Pflug BR, Dionne C, Kaplan DR, Lynch J, Djakiew D. Expression of a Trk high affinity nerve growth factor receptor in the human prostate. Endocrinology. 1995;136(1):262–268. doi: 10.1210/en.136.1.262. [DOI] [PubMed] [Google Scholar]
- 22.Pflug BR, Onoda M, Lynch JH, Djakiew D. Reduced expression of the low affinity nerve growth factor receptor in benign and malignant human prostate tissue and loss of expression in four human metastatic prostate tumor cell lines. Cancer Res. 1992;52(19):5403–5406. [PubMed] [Google Scholar]
- 23.Geldof AA, De Kleijn MA, Rao BR, Newling DW. Nerve growth factor stimulates in vitro invasive capacity of DU145 human prostatic cancer cells. J Cancer Res Clin Oncol. 1997;123(2):107–112. doi: 10.1007/BF01269888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walch ET, Marchetti D. Role of neurotrophins and neurotrophins receptors in the in vitro invasion and heparanase production of human prostate cancer cells. Clin Exp Metastasis. 1999;17(4):307–314. doi: 10.1023/A:1006652605568. [DOI] [PubMed] [Google Scholar]
- 25.Krygier S, Djakiew D. Molecular characterization of the loss of p75(NTR) expression in human prostate tumor cells. Mol Carcinog. 2001;31(1):46–55. doi: 10.1002/mc.1038. [DOI] [PubMed] [Google Scholar]
- 26.Krygier S, Djakiew D. Neurotrophin receptor p75(NTR) suppresses growth and nerve growth factor-mediated metastasis of human prostate cancer cells. Int J Cancer. 2002;98(1):1–7. doi: 10.1002/ijc.10160. [DOI] [PubMed] [Google Scholar]
- 27.Festuccia C, Muzi P, Gravina GL, et al. Tyrosine kinase inhibitor CEP-701 blocks the NTRK1/NGF receptor and limits the invasive capability of prostate cancer cells in vitro. Int J Oncol. 2007;30(1):193–200. [PubMed] [Google Scholar]
- 28.Warrington RJ, Lewis KE. Biologically active anti-nerve growth factor antibodies in commercial intravenous gammaglobulin. J Autoimmun. 2007;28(1):24–29. doi: 10.1016/j.jaut.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Halvorson KG, Kubota K, Sevcik MA, et al. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res. 2005;65(20):9426–9435. doi: 10.1158/0008-5472.CAN-05-0826. [DOI] [PubMed] [Google Scholar]
- 30.Miknyoczki SJ, Wan W, Chang H, et al. The neurotrophin–trk receptor axes are critical for the growth, progression of human prostatic carcinoma, pancreatic ductal adenocarcinoma xenografts in nude mice. Clin Cancer Res. 2002;8(6):1924–1931. [PubMed] [Google Scholar]
- 31.Schachter J, Katz U, Mahrer A, et al. Efficacy and safety of intravenous immunoglobulin in patients with metastatic melanoma. Ann N Y Acad Sci. 2007;1110:305–314. doi: 10.1196/annals.1423.032. [DOI] [PubMed] [Google Scholar]
- 32.Damianovich M, Solomon AS, Blank M, Shoenfeld Y. Attenuation of colon carcinoma tumor spread by intravenous immunoglobulin. Ann N Y Acad Sci. 2007;1110:567–577. doi: 10.1196/annals.1423.061. [DOI] [PubMed] [Google Scholar]
- 33.Fishman P, Bar-Yehuda S, Shoenfeld Y. IVIg to prevent tumor metastases. Int J Oncol. 2002;21(4):875–880. [PubMed] [Google Scholar]
- 34.Muir D, Sukhu L, Johnson J, Lahorra MA, Maria BL. Quantitative methods for scoring cell migration and invasion in filter-based assays. Anal Biochem. 1993;215(1):104–109. doi: 10.1006/abio.1993.1561. [DOI] [PubMed] [Google Scholar]
- 35.Saito K, Oku T, Ata N, Miyashiro H, Hattori M, Saiki I. A modified and convenient method for assessing tumor cell invasion and migration and its application to screening inhibitors. Biol Pharm Bull. 1997;20(4):345–348. doi: 10.1248/bpb.20.345. [DOI] [PubMed] [Google Scholar]
- 36.Wood P. Primary antibody deficiency syndromes. Ann Clin Biochem. 2009;46(Pt 2):99–108. doi: 10.1258/acb.2008.008175. [DOI] [PubMed] [Google Scholar]
- 37.Darabi K, Abdel-Wahab O, Dzik WH. Current usage of intravenous immune globulin and the rationale behind it: the Massachusetts General Hospital data and a review of the literature. Transfusion. 2006;46(5):741–753. doi: 10.1111/j.1537-2995.2006.00792.x. [DOI] [PubMed] [Google Scholar]
- 38.Graff-Dubois S, Sibéril S, Elluru S, et al. Use of intravenous polyclonal immunoglobulins in autoimmune and inflammatory disorders. Transfus Clin Biol. 2007;14(1):63–68. doi: 10.1016/j.tracli.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Galeotti C, Maddur MS, Kazatchkine MD, Mouthon L, Kaveri SV. Mechanisms of action of IVIG in autoimmune and inflammatory disorders: Recent developments. Transfus Clin Biol. 2009;16(2):75–79. doi: 10.1016/j.tracli.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Sarti L, Falai T, Pinto F, Tendi E, Matà S. Intravenous immune globulin usage for neurological and neuromuscular disorders: an academic centre, 4 years experience. Neurol Sci. 2009;30(3):213–218. doi: 10.1007/s10072-009-0043-9. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Bauer JH, Li Y, et al. Characterization of a p75(NTR) apoptotic signaling pathway using a novel cellular model. J Biol Chem. 2001;276(36):33812–33820. doi: 10.1074/jbc.M010548200. [DOI] [PubMed] [Google Scholar]
- 42.Rabizadeh S, Bredesen DE. Ten years on: mediation of cell death by the common neurotrophin receptor p75(NTR) Cytokine Growth Factor Rev. 2003;14(3–4):225–239. doi: 10.1016/S1359-6101(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 43.Khwaja F, Tabassum A, Allen J, Djakiew D. The p75(NTR) tumor suppressor induces cell cycle arrest facilitating caspase mediated apoptosis in prostate tumor cells. Biochem Biophys Res Commun. 2006;341(4):1184–1192. doi: 10.1016/j.bbrc.2006.01.073. [DOI] [PubMed] [Google Scholar]
- 44.Giraud S, Lautrette C, Bessette B, Decourt C, Mathonnet M, Jauberteau MO. Modulation of Fas-induced apoptosis by p75 neurotrophin receptor in a human neuroblastoma cell line. Apoptosis. 2005;10(6):1271–1283. doi: 10.1007/s10495-005-2649-6. [DOI] [PubMed] [Google Scholar]
- 45.Weeraratna AT, Arnold JT, George DJ, DeMarzo A, Isaacs JT. Rational basis for Trk inhibition therapy for prostate cancer. Prostate. 2001;45(2):140–148. doi: 10.1002/1097-0045(20001001)45:2<140::AID-PROS8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 46.Sigala S, Tognazzi N, Rizzetti MC, Farzoni I, Missale C, Bonmassar E, Spano P. Nerve growth factor induces the re-expression of functional androgen receptors and p75(NGFR) in the androgen-insensitive prostate cancer cell line DU145. Eur J Endocrinol. 2002;147(3):407–415. doi: 10.1530/eje.0.1470407. [DOI] [PubMed] [Google Scholar]
- 47.Sigala S, Faraoni I, Botticini D, Paez-Pereda M, Missale C, Bonmassar E, Spano P. Suppression of telomerase, re-expression of KA11, and abrogation of tumorigenicity by nerve growth factor in Prostate Cancer cell lines. Clin Cancer Res. 1999;5:1211–1218. [PubMed] [Google Scholar]
- 48.Djakiew D, Pflug BR, Delsite R, Onoda M, Lynch JH, Arand G, Thompson EW. Chemotaxis and chemokinesis of human prostate tumor cell lines in response to human prostate stromal cell secretory products containing a nerve growth factor-like protein. Cancer Res. 1993;53(6):1416–1420. [PubMed] [Google Scholar]
- 49.Delsite R, Djakiew D. Anti-proliferative effect of the kinase inhibitor K252a on human prostatic carcinoma cell lines. J Androl. 1996;17(5):481–490. [PubMed] [Google Scholar]
- 50.Fraser FP, Salvador V, Manning EA, Mizal J, Alfun S, Raza M, Berridge RJ, Djamgoz MB. Contribution of functional voltage-gated Na+ channel expression to cell behaviors involved in the metastatic cascade in rat cancer. J Cell Physiol. 2003;195(3):479–487. doi: 10.1002/jcp.10312. [DOI] [PubMed] [Google Scholar]
- 51.Diss JK, Stewart D, Pani F, Walker MM, Patel A, Djamgoz MB. A potential novel marker for human prostate cancer: voltage-gated sodium channel expression in vivo. Prostate Cancer Prostatic Dis. 2005;8(3):266–273. doi: 10.1038/sj.pcan.4500796. [DOI] [PubMed] [Google Scholar]
- 52.Brackenbury WT, Djamgoz MB. Nerve growth factor enhances voltage-gated Nat channel activity and transwell migration in Mat-LyLu rat prostate cancer cell line. J Cell Physiol. 2007;210(3):602–608. doi: 10.1002/jcp.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]