Abstract
Background
Since antitumor immune reactions between tumors and intratumoral immunocytes have been verified in several human tumors, immunological therapeutic strategies must be considered to obtain the proper efficacy of tumor shrinkage under these conditions. Human leukocyte antigen (HLA) class I expression in cancer cells and degree of infiltration of regulatory T cells (Tregs) in the stroma have been regarded as important markers of antitumor immune reactions in the context of independent immunological mechanisms. In the current study, we investigated HLA class I expression and Treg cells infiltration in gastric cancer and discussed the clinical implications of this combinatory analysis in gastric cancer.
Patients and methods
A total of 141 gastric cancer patients who received R0 gastrectomy at Kagoshima University Hospital were studied. Immunohistochemically, in 141 gastric cancer patients, HLA class I expression and Treg cell infiltration in cancerous tissue were evaluated using HLA class I (EMR8-5) and forkhead box p3 (FOXP3) monoclonal antibodies. The correlation between clinical factors and tumor-infiltrating Treg cells was analyzed.
Results
HLA class I expression was positively associated with depth of tumor invasion (P < 0.05). Infiltration of Foxp3-positive cells did not correlate with any clinicopathological markers. HLA class I expression had no association with Treg cell infiltration (r = 0.04). A better postoperative outcome was associated with fewer numbers of Treg infiltration (P = 0.034). A combination of HLA and Treg analysis may lead to a more accurate prediction of postoperative outcome (P = 0.02).
Conclusions
Two different antitumor immunological markers, Treg infiltration and HLA class I expression, affected clinicopathological factors in gastric cancer by different mechanisms. Thus, an immunological combination of HLA class I expression and Treg cell infiltration may more accurately predict postoperative outcome. Immunological balance needs to be restored after evaluation of each immunological deficit in gastric cancer.
Keywords: Gastric cancer, Regulatory T cells, HLA class I, Antitumor activity, Immunosuppressant, Postoperative outcome
Introduction
Gastric cancer is a common malignant disease and is a major cause of cancer-related death in eastern Asia, especially Japan [1]. When gastric cancer patients are treated at an early stage, they show a favorable outcome after endoscopic or surgical treatment [2–4]. However, some patients show a poor outcome even after receiving curative surgery [5]. This may be due to the aggressiveness of the tumor cells, or genetic mutations and aberrant expression of adhesion molecules, which have also been shown to accelerate tumor growth and metastatic potential. On the other hand, several mechanisms allowing tumor escape from the host immune system have been observed [6–8]. It has been determined that the risk of recurrence partly depends on the local immune response. Regulatory T cells (Treg) are a subpopulation of helper T cells that contribute to cancer-related immunosuppression [9, 10]. Treg cells were found to be significantly increased not only in the peripheral blood, but also in the tumor stroma of cancer patients. Forkhead box p3 (Foxp3) is a good marker for discriminating Tregs from other lymphocytes [11]. Treg cell infiltration into the tumor stroma has been reported in ovarian [12], hepatocellular [13], renal cell [14], and gastric cancers [15] and detailed in clinicopathological features.
Since the cancer-stroma immunocyte relationship has been detailed, a strong correlation between cytotoxic T cells and human leukocyte antigen (HLA) class I expression in tumor cells has been identified that eliminates tumor cells and maintains antitumor immunological reactions in the host. We previously reported on the clinical impact of HLA class I expression in gastric cancer [16], and other researchers have found an immune-evasion mechanism from host cytotoxic T-lymphocytes (CTL) through loss of HLA molecules on tumor cells in several cancers [17–20]. The clinical definition of HLA class I expression is under debate, and there have been few studies on the analysis of these two immunological markers, namely Treg cell infiltration and HLA class I expression, in gastrointestinal cancer. Yet, these markers may be important for predicting the responsiveness of the antitumor immune defense system. Thus, in the current study, we tried to simultaneously evaluate HLA class I expression and stromal Treg infiltration in gastric cancer, and we discussed their clinical implications in gastric cancer.
Materials and methods
A total of 141 consecutive gastric cancer patients, who received R0 resection at Kagoshima University Hospital between 2001 and 2005, were enrolled in the present study. The patient group comprised 92 men and 36 women, ranging in age from 31 to 82 years (mean 61 years). The male-to-female ratio was 120:41. Sixty-five, 78, and 21 patients received total, distal, and proximal gastrectomy, respectively. No patients received preoperative chemotherapy. All patients underwent R0 resection with more than D1 lymph node dissection. The study was approved by the Institutional Review Board of Kagoshima University School of Medicine. Clinical factors were assessed by the Japanese Classification of Gastric Carcinoma [21].
Immunohistochemistry
Intratumoral Treg cell infiltration and cancerous HLA class I expression were assessed according to previous reports [13, 16], and prepared specimens were visualized by avidin biotin complex (ABC) immunohistochemistry. Namely, paraffin-embedded sections (4 μm), including tumor nests, were obtained from the 141 gastric cancer patients, deparaffinized, and soaked in PBS prior to immunohistochemical analysis. Sections were treated with 3% H2O2 for 30 min in order to block endogenous tissue peroxidase, followed by treatment with bovine serum for 30 min in order to reduce nonspecific binding. HLA class I monoclonal antibody (mouse monoclonal EMR8-5, Hokudo Japan) and FOXP3 (Ancell DAKO Japan) antibody were diluted to 1:200 and 1:500, respectively, and incubated with the sections for 2 h at room temperature. EMR 8-5 is a novel monoclonal anti-pan HLA class I heavy chain antibody reacting with paraffin-embedded sections. Before treatment with primary antibody, sections underwent antigen retrieval with 1% citrate buffer with 120 atoms of steam. Sections were rinsed in PBS and visualized by standard techniques for labeled avidin–biotin immuno-peroxidase staining. Then, HLA class I and Foxp3 were visualized using the DAB Substrate Kit. The slides were briefly counterstained with hematoxylin and aqueously mounted.
Evaluation of Treg cell infiltration and HLA class I positivity in gastric cancer
The positivity of cancerous HLA class I and Foxp3-positive cells were identified in the cell membrane (Fig. 1) and nucleus (Fig. 2), respectively. HLA positivity and degree of infiltrating Foxp3-positive cells were evaluated according to previous reports [13, 16]. Namely, the positivity of HLA class I was calculated in 10 representative high-power fields (400×) not only in each tumor nest, but also at the invasive front of the tumor. If the percentage of HLA class I positivity exceeded 10%, the cases were regarded as HLA class I-positive. The absolute number of Foxp3-positive lymphocytes was counted in 10 high-power fields (HPFs) at 400× and averaged. Group with high and low numbers of Foxp3-positive cell infiltration were distinguished based on the average Foxp3-positive cell infiltration; patients with more than 10/HPF-positive cells were regarded as a Foxp3-positive group. All immunostained slides were evaluated by two independent observers (SI and AT) who were unaware of the clinical data or disease outcome. Clinicopathological factors were described according to the general rules of gastric cancer in Japan [21].
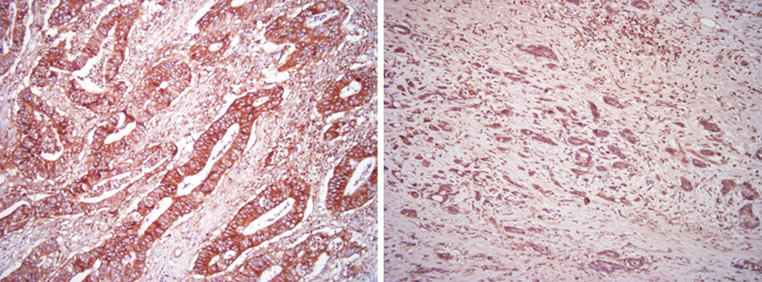
Fig. 1.
HLA class I positivity in gastric cancer (right differentiated adenocarcinoma, left undifferentiated adenocarcinoma). HLA class I positivity was identified in the membrane of cancer cells and in infiltrating stromal cells
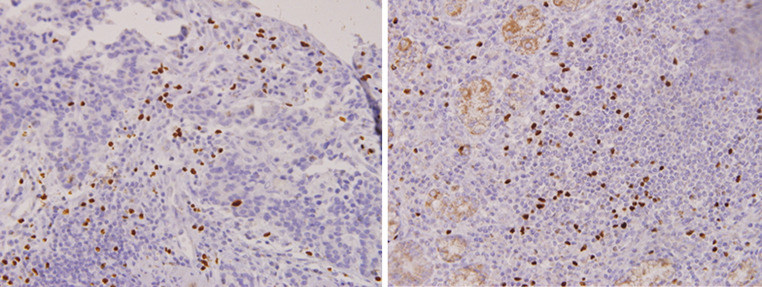
Fig. 2.
Foxp3-positive cells infiltrated cancerous stroma (right) and lymph follicles (left). Foxp3 positivity was identified in the nucleus of lymphocytes
Statistical analysis
Statistical analysis of clinical features between the HLA and Foxp3 groups was performed by the χ2 test. Association between degree of Foxp3-positive cell infiltration and the degree of HLA class I positivity was compared by Pearson’s correlation coefficient. Survival curves were produced using the Kaplan–Meier method, and statistical significance was calculated using the generalized Wilcoxon method. Multivariate analysis was performed to determine prognostic factors. A P value <0.05 was considered significant.
Results
Distribution of HLA class I and Treg cells in gastric cancer
HLA class I was partially identified in the cell membrane of cancer cells and infiltrating cells in the stroma (Fig. 1). Treg cells were identified as Foxp3-positive cells in cancerous stroma. Foxp3 positivity was found in the nucleus of infiltrating lymphocytes (Fig. 2).
Evaluation of HLA class I positivity and Treg cell infiltration in the cancer nest
HLA class I positivity was estimated from 0 to 100% (average 56%), and Foxp3-positive cell infiltration ranged from 0 to 112 (average 12 ± 10). According to a previous evaluation of HLA class I expression and Treg cell infiltration, 141 patients were divided into two groups (89 in the HLA class I-positive group and 52 in the HLA class I-negative group; 65 in the high Treg group and 76 in the low Treg group).
Clinicopathological features of HLA class I-positive or Treg infiltration in gastric cancer
We selected seven significant clinical factors in gastric cancer, as shown in Table 1. Among these factors, only depth of tumor invasion was negatively associated with HLA class I expression (P < 0.05). Foxp3-positive cell infiltration did not correlate with any clinicopathological markers, and other clinical factors did not associate with HLA expression and Treg cells infiltration (Table 1).
Table 1.
Association between clinicopathological features and HLA class I, and Foxp3-positive cell infiltration in 141 gastric cancer
| Factors | HLA class I | Foxp3 cell infiltration | ||||
|---|---|---|---|---|---|---|
| Positive (n = 89) | Negative (n = 52) | P value | Many (n = 65) | Few (n = 76) | P value | |
| Age | 64 | 61 | n.s. | 62 | 63 | n.s. |
| Sex | ||||||
| Male | 66 | 33 | n.s. | 42 | 57 | n.s. |
| Female | 23 | 21 | 23 | 19 | ||
| T | ||||||
| T1 | 41 | 18 | 0.02 | 22 | 38 | n.s. |
| T2 | 31 | 15 | 25 | 21 | ||
| T3 | 17 | 18 | 18 | 17 | ||
| N factor | ||||||
| NO | 55 | 26 | n.s. | 32 | 49 | n.s. |
| N1 | 16 | 10 | 12 | 14 | ||
| N2 | 18 | 16 | 21 | 13 | ||
| Ly factor | ||||||
| Yes | 54 | 32 | n.s. | 45 | 41 | n.s. |
| No | 35 | 20 | 20 | 35 | ||
| V factor | ||||||
| Yes | 35 | 17 | n.s. | 30 | 22 | n.s |
| No | 54 | 35 | 35 | 54 | ||
| Histology | ||||||
| Diff | 57 | 27 | n.s. | 35 | 49 | n.s |
| Undiff | 32 | 25 | 30 | 27 | ||
| Foxp3 cell | ||||||
| Many | 44 | 21 | n.s. | |||
| Few | 45 | 31 | ||||
N factor depth of invasion, Ly factor lymphatic invasion, V factor venous invasion, Diff differentiated adenocarcinoma, Undiff undifferentiated adenocarcinoma
Correlation between HLA class I positivity and Treg infiltration in gastric cancer
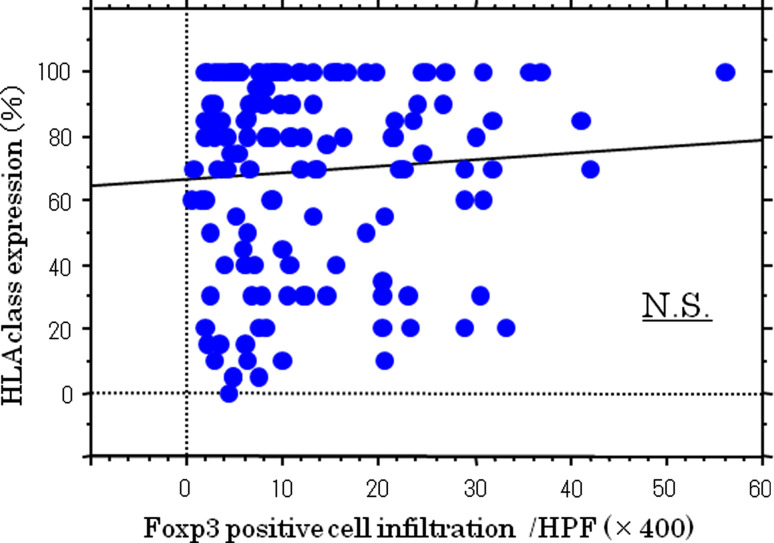
The data in HLA class I and Foxp3 positivity were plotted, and the correlation between HLA class I expression and Treg cell infiltration was analyzed. HLA class I expression did not correlate with Treg cell infiltration (r = 0.04 P = 0.1; Fig. 3).
Fig. 3.
Correlation between HLA class I positivity and Foxp3 cell infiltration. There was no significant correlation between HLA class I positivity and Foxp3 cell infiltration (r = 0.04, P = 0.1)
Survival analyses of HLA class I-positive and/or Treg cell infiltration in gastric cancer patients
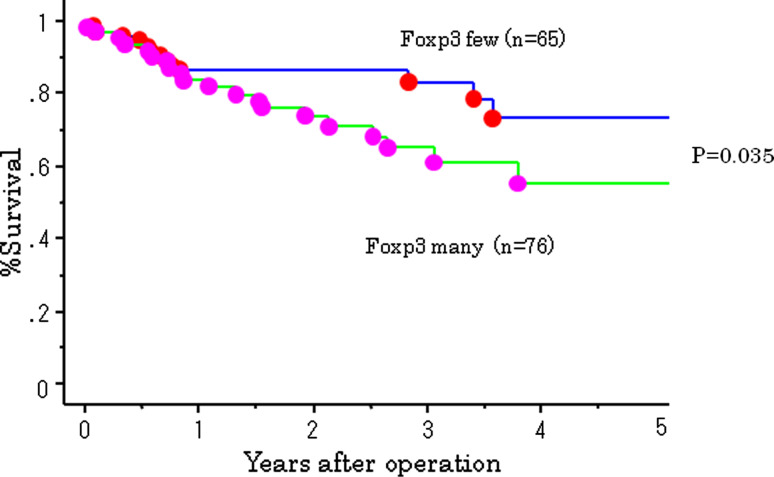
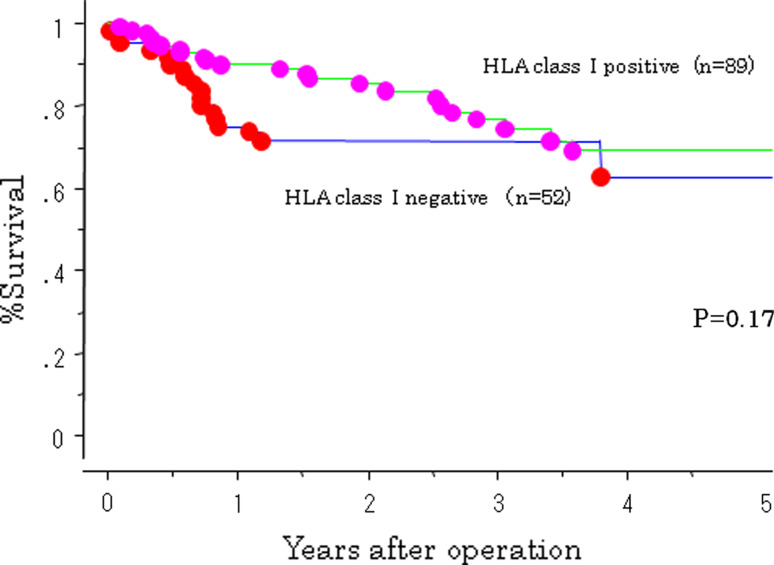
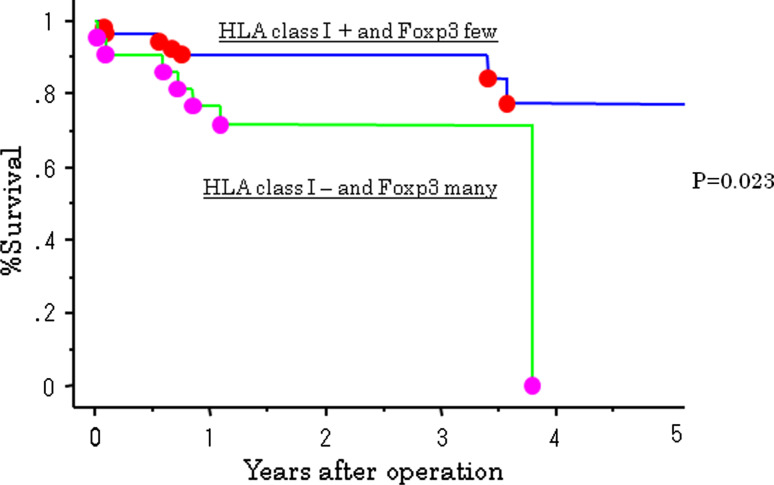
The 5-year survival rate of patients in the high Treg cell group was 53%, which was significantly less than patients in the low Treg cell group. A better postoperative outcome was associated with a lower number of Treg cell infiltration (P = 0.035; Fig. 4). The HLA class I-positive group tended to have a better postoperative outcome; however, there were no significant differences between HLA class I positivity and survival (Fig. 5). A combination of HLA and Treg cell infiltration was analyzed. Patients who were HLA class I-positive and had a low number of Treg cell infiltration had a significantly better outcome than patients who were HLA-negative and had high levels of Treg cell infiltration (P = 0.02; Fig. 6).
Fig. 4.
Survival curves of 141 gastric cancer patients according to Foxp3-positive cell infiltration. Foxp3 many group showed significantly poorer outcome than Foxp3 little group (P = 0.033)
Fig. 5.
Survival curves of 141 gastric cancer patients according to a combination of HLA class I expression and Foxp3 cell infiltration. HLA class I (+) and Foxp3 little infiltration patients showed better outcome than HLA class I (−) and Foxp3 many infiltration significantly (P = 0.02)
Fig. 6.
Survival curves of 141 gastric cancer according to combination of HLA class I and Foxp3 cell infiltration
Discussion
It has been reported that multiplying different antitumor immunological markers can more correctly evaluate behaviors of cancers. Surprisingly, Gallon et al. [22] showed that multiplying immunological markers is a better prognostic marker that exceeds TNM classification. In the current study, we selected two immunological markers, HLA class I expression and Treg cell infiltration, which function by different immunological mechanisms. By considering each immunological mechanism underlying HLA class I expression and Treg cell infiltration, we speculated that these two markers may act independently, and we did not identify a significant correlation between these two factors in the present study.
HLA class I expression on tumor cells is believed to be essential for recognizing antigens on antigen-presenting cells [23, 24]. For immunotherapy-induced antitumor immune responses, HLA class I expression on tumor cells is one of the key molecules that recognize autologous tumors by tumor-specific cytotoxic T cells. In the current study, HLA class I-positive gastric cancer patients tended to have a better postoperative outcome. However, we could not show a definitively better postoperative outcome in HLA class I-positive gastric cancer patients, as has been shown for patients with bladder [25] and esophageal cancer [26]. It has been demonstrated that HLA class I expression on tumor cells altered exogenous stimulation such as gastritis and Helicobacter pylori [16]. Therefore, HLA class I expression in gastric cancer cannot directly evaluate the ability of antigen presentation for antitumor antigen-specific CTL in gastric cancer.
It has been reported that Treg cell infiltration in the tumor nest is a good indicator of local immunosuppression in several cancers [27–31]. In the current study, we also found an inverse prognostic significance of Treg infiltration in itself without association of TNM factors. Treg infiltration seems to be able to solely function as a strong suppressive immunological marker in gastric cancer. Considering the mechanism of these markers, although inverse correlation was predicted, we found no correlation between Treg cell infiltration and HLA class I expression in cancerous tissue. The simultaneous evaluation of Treg cell infiltration and HLA class I expression in gastric cancer may have clinical merit in different aspects of immune function. In the current study, we showed that analyzing a combination of HLA class I expression and Treg cell infiltration more accurately evaluated patients’ outcome in gastric cancer. Kruijf et al. [20] simultaneously evaluated HLA class I expression and Treg cell infiltration in early breast cancer. This is the second report detailing the simultaneous evaluation of the combination of HLA class I expression and Treg cell infiltration. Kruijf et al. showed that both prognostic efficacy and chemotherapeutic efficacy could be predicted by these two immunological markers. Recently, the accumulation of tumor-infiltrating lymphocytes (TILs) closely correlated with chemotherapeutic efficacy and patient survival [32, 33]. In this study, 141 gastric cancer patients did not receive preoperative chemotherapy. Upon the advent of new anticancer agents for gastric cancer, the degree of Treg cell infiltration may be a good indicator of responsiveness to chemotherapy.
Recently, Coe et al. [34] showed normalization of the immunosuppressive condition with anti-GITR mAb targeting Tregs. Cohen et al. [35] also demonstrated that anti-GITR mAbs deleted the number of Tregs as an immunotherapeutic strategy for melanoma. On the contrary, to normalize cytotoxic signaling from antigen-presenting cells to T cells in HLA class I-negative cancer cells, Ferris et al. [36] suggested that intralesional administration of IFN-gamma induced restoration of HLA class I expression on tumor cells. To the contrary, Garrido et al. [37] showed some HLA class I lesions were irreversible and did not respond to IFN treatment. Anyway, we must pay attention to the restoration of HLA class I expression in identifying loss of HLA class I of tumor cells. According to the deficit in antitumor mechanisms, we must change the treatment strategy to normalize the local immune response.
In conclusion, a combination of HLA class I expression and Treg cells infiltration may be a promising prognostic marker in gastric cancer. According to each immunological deviation, therapeutic strategies must be altered when patients are treated by immune therapy in the perioperative course.
References
- 1.Wong J, Jackson P. Gastric cancer surgery: an American perspective on the current options and standards. Curr Treat Options Oncol. 2011;12:72–84. doi: 10.1007/s11864-010-0136-y. [DOI] [PubMed] [Google Scholar]
- 2.Tanizawa Y, Terashima M. Lymph node dissection in the resection of gastric cancer: review of existing evidence. Gastric Cancer. 2010;13:137–148. doi: 10.1007/s10120-010-0560-5. [DOI] [PubMed] [Google Scholar]
- 3.Gotoda T, Iwasaki M, Kusano C, Seewald S, Oda I. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg. 2010;97:868–871. doi: 10.1002/bjs.7033. [DOI] [PubMed] [Google Scholar]
- 4.Nomura S, Kaminishi M. Surgical treatment of early gastric cancer. Dig Surg. 2007;24:96–100. doi: 10.1159/000101895. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi M, Okabayashi T, Sano T, Araki K. Metastatic bone cancer as a recurrence of early gastric cancer—characteristics and possible mechanisms. World J Gastroenterol. 2005;11:5587–5591. doi: 10.3748/wjg.v11.i36.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhutia SK, Mallick SK, Maiti TK. Tumour escape mechanisms and their therapeutic implications in combination tumour therapy. Cell Biol Int. 2010;34:553–563. doi: 10.1042/CBI20090206. [DOI] [PubMed] [Google Scholar]
- 7.Kirkbride KC, Blobe GC. Inhibiting the TGF-beta signalling pathway as a means of cancer immunotherapy. Expert Opin Biol Ther. 2003;3:251–261. doi: 10.1517/14712598.3.2.251. [DOI] [PubMed] [Google Scholar]
- 8.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Higashi H, Iwashige H, Aridome K, Hokita S, Aikou T. CD3-zetachain expression of intratumoral lymphocytes is closely related to survival in gastric carcinoma patients. Cancer. 2002;94:1437–1442. doi: 10.1002/cncr.10346. [DOI] [PubMed] [Google Scholar]
- 9.Betts GJ, Clarke SL, Richards HE, Godkin AJ, Gallimore AM. Regulating the immune response to tumours. Adv Drug Deliv Rev. 2006;58:948–961. doi: 10.1016/j.addr.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Wang RF. Regulatory T cells and innate immune regulation in tumor immunity. Springer Semin Immunopathol. 2006;28:17–23. doi: 10.1007/s00281-006-0022-7. [DOI] [PubMed] [Google Scholar]
- 11.Chang X, Zheng P, Liu Y. FoxP3: a genetic link between immunodeficiency and autoimmune diseases. Autoimmun Rev. 2006;5:399–402. doi: 10.1016/j.autrev.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 13.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths RW, Elkord E, Gilham DE, et al. Frequency of regulatory T cells in renal cell carcinoma patients and investigation of correlation with survival. Cancer Immunol Immunother. 2007;56:1743–1753. doi: 10.1007/s00262-007-0318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 16.Ishigami S, Natsugoe S, Nakajo A, Arigami T, Kitazono M, Okumura H, Matsumoto M, Uchikado Y, Setoyama T, Sasaki K, Aikou T. HLA-class I expression in gastric cancer. J Surg Oncol. 2008;97:605–608. doi: 10.1002/jso.21029. [DOI] [PubMed] [Google Scholar]
- 17.Menon AG, Morreau H, Tollenaar RA, Alphenaar E, Puijenbroek M, Putter H, Janssen-Van Rhijn CM, Velde CJ, Fleuren GJ, Kuppen PJ. Down-regulation of HLA-A expression correlates with a better prognosis in colorectal cancer patients. Lab Invest. 2002;82:1725–1733. doi: 10.1097/01.lab.0000043124.75633.ed. [DOI] [PubMed] [Google Scholar]
- 18.Speetjens FM, de Bruin EC, Morreau H, Zeestraten EC, Putter H, van Krieken JH, van Buren MM, van Velzen M, Dekker-Ensink NG, van de Velde CJ, Kuppen PJ. Clinical impact of HLA class I expression in rectal cancer. Cancer Immunol Immunother. 2008;57:601–609. doi: 10.1007/s00262-007-0396-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaneko K, Ishigami S, Kijima Y, Funasako Y, Hirata M, Okumura H, Shinchi H, Koriyama C, Ueno S, Yoshinaka H, Natsugoe S. Clinical implication of HLA class I expression in breast cancer. BMC Cancer. 2011;11:454. doi: 10.1186/1471-2407-11-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Kruijf EM, van Nes JG, Sajet A, Tummers QR, Putter H, Osanto S, Speetjens FM, Smit VT, Liefers GJ, van de Velde CJ, Kuppen PJ. The predictive value of HLA class I tumor cell expression and presence of intratumoral Tregs for chemotherapy in patients with early breast cancer. Clin Cancer Res. 2010;16:1272–1280. doi: 10.1158/1078-0432.CCR-09-1844. [DOI] [PubMed] [Google Scholar]
- 21.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma—2nd English Edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/PL00011681. [DOI] [PubMed] [Google Scholar]
- 22.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 23.Seliger B. Novel insights into the molecular mechanisms of HLA class I abnormalities. Cancer Immunol Immunother. 2012;61:249–254. doi: 10.1007/s00262-011-1153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aptsiauri N, Cabrera T, Mendez R, Garcia-Lora A, Ruiz-Cabello F, Garrido F. Role of altered expression of HLA class I molecules in cancer progression. Adv Exp Med Biol. 2007;601:123–131. doi: 10.1007/978-0-387-72005-0_13. [DOI] [PubMed] [Google Scholar]
- 25.Kitamura H, Torigoe T, Honma I, Sato E, Asanuma H, Hirohashi Y, Sato N, Tsukamoto T. Effect of human leukocyte antigen class I expression of tumor cells on outcome of intravesical instillation of bacillus calmette-guerin immunotherapy for bladder cancer. Clin Cancer Res. 2006;12:4641–4644. doi: 10.1158/1078-0432.CCR-06-0595. [DOI] [PubMed] [Google Scholar]
- 26.Mizukami Y, Kono K, Maruyama T, Watanabe M, Kawaguchi Y, Kamimura K, Fujii H. Downregulation of HLA Class I molecules in the tumour is associated with a poor prognosis in patients with oesophageal squamous cell carcinoma. Br J Cancer. 2008;99:1462–1467. doi: 10.1038/sj.bjc.6604715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasparoto TH, Souza Malaspina TS, Benevides L, Melo EJ, Costa MR, Damante JH, Ikoma MR, Garlet GP, Cavassani KA, da Silva JS, Campanelli AP. Patients with oral squamous cell carcinoma are characterized by increased frequency of suppressive regulatory T cells in the blood and tumor microenvironment. Cancer Immunol Immunother. 2010;59:819–828. doi: 10.1007/s00262-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokokawa J, Cereda V, Remondo C, Gulley JL, Arlen PM, Schlom J, Tsang KY. Enhanced functionality of CD4+ CD25(high)FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res. 2008;14:1032–1040. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 29.Hillen F, Baeten CI, van de Winkel A, Creytens D, van der Schaft DW, Winnepenninckx V, Griffioen AW. Leukocyte infiltration and tumor cell plasticity are parameters of aggressiveness in primary cutaneous melanoma. Cancer Immunol Immunother. 2008;57:97–106. doi: 10.1007/s00262-007-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Dongen M, Savage ND, Jordanova ES, Briaire-de Bruijn IH, Walburg KV, Ottenhoff TH, Hogendoorn PC, van der Burg SH, Gelderblom H, van Hall T. Anti-inflammatory M2 type macrophages characterize metastasized and tyrosine kinase inhibitor-treated gastrointestinal stromal tumors. Int J Cancer. 2010;127:899–909. doi: 10.1002/ijc.25113. [DOI] [PubMed] [Google Scholar]
- 31.Pölcher M, Braun M, Friedrichs N, Rudlowski C, Bercht E, Fimmers R, Sauerwald A, Keyver-Paik MD, Kübler K, Büttner R, Kuhn WC, Hernando JJ. Foxp3(+) cell infiltration and granzyme B(+)/Foxp3(+) cell ratio are associated with outcome in neoadjuvant chemotherapy-treated ovarian carcinoma. Cancer Immunol Immunother. 2010;59:909–919. doi: 10.1007/s00262-010-0817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ono M, Tsuda H, Shimizu C, Yamamoto S, Shibata T, Yamamoto H, Hirata T, Yonemori K, Ando M, Tamura K, Katsumata N, Kinoshita T, Takiguchi Y, Tanzawa H, Fujiwara Y (2011) Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat [DOI] [PubMed]
- 33.Halama N, Michel S, Kloor M, Zoernig I, Benner A, Spille A, Pommerencke T, von Knebel DM, Folprecht G, Luber B, Feyen N, Martens UM, Beckhove P, Gnjatic S, Schirmacher P, Herpel E, Weitz J, Grabe N, Jaeger D. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71:5670–5677. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 34.Coe D, Begom S, Addey C, White M, Dyson J, Chai JG. Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1367–1377. doi: 10.1007/s00262-010-0866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen AD, Schaer DA, Liu C, Li Y, Hirschhorn-Cymmerman D, Kim SC, Diab A, Rizzuto G, Duan F, Perales MA, Merghoub T, Houghton AN, Wolchok JD. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS ONE. 2010;5:e10436. doi: 10.1371/journal.pone.0010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferris RL, Hunt JL, Ferrone S. Human leukocyte antigen (HLA) class I defects in head and neck cancer: molecular mechanisms and clinical significance. Immunol Res. 2005;33:113–133. doi: 10.1385/IR:33:2:113. [DOI] [PubMed] [Google Scholar]
- 37.Garrido F, Cabrera T, Aptsiauri N. “Hard” and “soft” lesions underlying the HLA class I alterations in cancer cells: implications for immunotherapy. Int J Cancer. 2010;127:249–256. doi: 10.1002/ijc.25270. [DOI] [PubMed] [Google Scholar]