Abstract
Antigen-specific immunotherapy was studied in a multi-institutional phase 1/2 study by combining decitabine (DAC) followed by an autologous dendritic cell (DC)/MAGE-A1, MAGE-A3 and NY-ESO-1 peptide vaccine in children with relapsed/refractory solid tumors. Patients aged 2.5–15 years with relapsed neuroblastoma, Ewing’s sarcoma, osteosarcoma and rhabdomyosarcoma were eligible to receive DAC followed by DC pulsed with overlapping peptides derived from full-length MAGE-A1, MAGE-A3 and NY-ESO-1. The primary endpoints were to assess the feasibility and tolerability of this regimen. Each of four cycles consisted of week 1: DAC 10 mg/m2/day for 5 days and weeks 2 and 3: DC vaccine once weekly. Fifteen patients were enrolled in the study, of which 10 were evaluable. Generation of DC was highly feasible for all enrolled patients. The treatment regimen was generally well tolerated, with the major toxicity being DAC-related myelosuppression in 5/10 patients. Six of nine patients developed a response to MAGE-A1, MAGE-A3 or NY-ESO-1 peptides post-vaccine. Due to limitations in number of cells available for analysis, controls infected with a virus encoding relevant genes have not been performed. Objective responses were documented in 1/10 patients who had a complete response. Of the two patients who had no evidence of disease at the time of treatment, one remains disease-free 2 years post-therapy, while the other experienced a relapse 10 months post-therapy. The chemoimmunotherapy approach using DAC/DC-CT vaccine is feasible, well tolerated and results in antitumor activity in some patients. Future trials to maximize the likelihood of T cell responses post-vaccine are warranted.
Keywords: Cancer germline genes, Decitabine, Dendritic cell vaccine, Immunotherapy, Neuroblastoma, Sarcoma
Introduction
Children with relapsed or therapy-refractory malignant solid tumors have few treatment options with curative potential, making the investigation of novel approaches, such as immunotherapy of interest. The success of cellular immunotherapy depends upon the recognition of tumor cells by antigen-specific cytotoxic T lymphocytes (CTL). However, cancer cells tend to downregulate tumor-specific antigens and MHC molecules, thereby evading immune recognition and limiting the therapeutic potential of this strategy [1].
The cancer germline genes (CGGs) MAGE-A1, MAGE-A3 and NY-ESO-1 have a restricted pattern of expression, limited to male germline cells, placenta, as well as a number of solid tumors, including neuroblastoma, rhabdomyosarcoma (RMS), osteogenic sarcoma (OS) and Ewing’s sarcoma (ES) [2–5]. Previous studies have reported the expression of MAGE-A1 (44 %), MAGE-A3 (21 %) and NY-ESO-1 (30–82 %) in patients with neuroblastoma [6]. Jacobs and group detected the expression of several CGGs in pediatric tumors, with MAGE-A1 and NY-ESO-1 detected on 25 % of RMS cell lines and 89 % of OS cell lines, and MAGE-A3 on 42 % of RMS cell lines and 100 % of OS cell lines [7]. ES has been shown to express CGGs such as XAGE and LIPI, but there are little data on the expression of MAGE-A1, MAGE-A3 and NY-ESO-1 in these tumors [8, 9]. Demethylating agents such as decitabine (5-aza-2′-deoxycytidine, DAC) have been shown to upregulate the expression of CGGs on tumor cell lines, sensitizing them to killing by MAGE-A1-, MAGE-A3- and NY-ESO-1-specific CTL [10–14]. We have previously demonstrated that MAGE-A1, MAGE-A3 and NY-ESO-1 tumor antigens are upregulated in neuroblastoma cells after exposure to pharmacologic doses of DAC, with increased susceptibility to tumor antigen-specific CTL-mediated killing [15]. We have also shown that these antigens are upregulated in ES, RMS and OS cell lines following exposure to DAC. DAC has also been shown to increase the expression of MHC class I and II molecules as well as co-stimulatory molecule ICAM-1 in some tumor types [16].
Previous studies in adults have demonstrated the safety of tumor antigen vaccines for recurrent malignant solid tumors, using either whole CT antigen proteins or individual human leukocyte antigen (HLA)-restricted epitopes [17, 18]. The expansion of tumor antigen-specific CTL post-vaccination was demonstrated in these and other studies, with the development of antigen-specific immunity correlating with clinical responses. Odunsi and group recently reported the combined use of DAC and an NY-ESO-1-specific vaccine with doxorubicin in 12 patients with ovarian cancer, with the majority of subjects developing NY-ESO-1-specific T cell and antibody responses [19]. Our preliminary studies have confirmed that DAC treatment enhances the expression of MAGE-A1, MAGE-A3 and NY-ESO-1 tumor antigens and HLA molecules on neuroblastoma and sarcoma cells and increases susceptibility to killing by tumor antigen-specific CTL [15]. Therefore, we implemented a clinical trial targeting tumor antigens in these patients. This phase 1/2 study was conducted in children with relapsed, therapy-refractory neuroblastoma and sarcoma using low-dose DAC to facilitate epigenetic upregulation of CGGs expression on tumor cells followed by a dendritic cell (DC)/NY-ESO-1, MAGE-A1, MAGE-A3 peptide vaccine. The aims of the study were (1) to evaluate the tolerability of DAC when used with a DC/MAGE-A1, MAGE-A3 and NY-ESO-1 peptide vaccine, (2) to study the feasibility of generating DC for vaccine and (3) to determine the immunologic and clinical outcomes of patients treated with this regimen. This study is registered with ClinicalTrials.gov, NCT01241162.
Materials and methods
Eligibility
Children between 12 months and 18 years of age with relapsed high-risk or therapy-refractory neuroblastoma, OS, ES or RMS were eligible for enrollment. Patients were required to have adequate bone marrow function (absolute neutrophil count, ANC: ≥500/µl; platelet count: ≥75,000/µl), renal function: creatinine clearance or radioisotope glomerular filtration rate (GFR) ≥70 ml/min/1.73 m2 or a serum creatinine based on the Schwartz formula for estimating GFR [20], liver function: total bilirubin ≤1.5 × normal for age, and alanine aminotransferase [ALT (SGPT)] and aspartate aminotransferase [AST (SGOT)] ≤3 × normal for age and normal cardiac function (ejection fraction >55 % by echocardiogram (ECG) or radionuclide MUGA evaluation or fractional shortening ≥28 %) and a Lansky performance scale of over 70. Patients must have received treatment with standard therapy for their disease, and neuroblastoma patients were to be at least 6 months from autologous stem cell transplant. Patients were excluded if they had autoimmune disease, hypersensitivity to DAC, imiquimod or any vaccine component or were receiving concurrent systemic steroid therapy. This multi-institutional study was approved by the Institutional Review Boards of the University of Louisville, Penn State University, Dana-Farber Cancer Institute, and by the FDA (IND 13973). Written informed consent from parents or guardians and assent (as appropriate) were obtained according to local institutional guidelines.
Study design
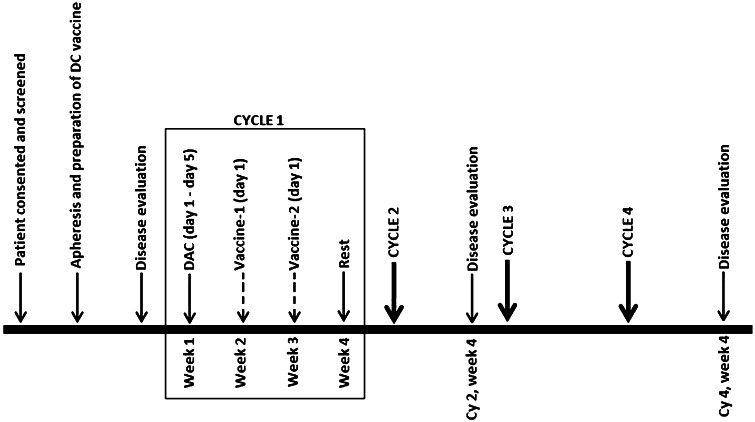
The treatment schema is shown in Fig. 1. Blood from eligible patients was collected either by phlebotomy or by apheresis for dendritic cell (DC) vaccine preparation. Prior to therapy, disease status was determined using CT/PET/MIBG imaging and/or bone marrow aspirates to evaluate previous known sites of tumor. The treatment regimen (entirely outpatient) included 4 cycles, each consisting of DAC 10 mg/m2/day intravenously for 5 days, followed by two weekly vaccinations. A platelet count ≥75,000/µl and ANC ≥ 500/µl was set as the criteria for DAC and vaccine administration. Tolerability of the first 2 cycles of therapy was defined as the ability to receive both cycles of DAC at ≥50 % dosing and 3 of the 4 planned vaccinations. The number of DC administered was based on patient weight (<20 kg, 20–40 kg and >40 kg) and ranged from 3 to 10 × 106 cells. The topical Toll-like-receptor (TLR) agonist imiquimod was used at the site of vaccination as an adjuvant. Peripheral blood was collected weekly for assessment of antigen-specific immune responses from the start of therapy (Cycle1-week1 (C1W1) of the study).
Fig. 1.
Treatment schema. Peripheral blood was collected weekly (on day 1) beginning from Cycle1-week1 (C1W1) to assess antigen-specific immune responses. C1W1 represents the baseline response
Toxicity
Toxicities were analyzed based on the NCI Common Terminology Criteria for Adverse Events (CTCAE) (version 4.0). We defined hematologic dose-limiting toxicity (DLT) as any non-hematologic toxicity ≥grade 3. Since DAC was expected to cause some degree of myelosuppression in most patients, this by itself was not considered dose limiting for subsequent cycles. If ANC was <500/µl or platelet count <25,000/µl at 4 weeks from the beginning of DAC, patients received a 50 % DAC dose reduction when counts recovered (ANC > 1000/µl, platelets >75,000/µl). If at the start of a cycle ANC or platelet criteria were not met, the cycle was delayed until these criteria were met. If not met within 2 weeks, the decision was made to administer granulocyte colony-stimulating factor (GCSF).
Response assessment
Disease status was evaluated at the end of cycles 2 and 4. Tumor responses defined as complete or partial remission, stable disease and disease progression were judged by CT/PET/MIBG scans. Complete response was defined as resolution of all radiographic evidence of tumor and partial response as 50 % or greater reduction in the size of all tumors. Stable disease was defined as <50 % reduction in tumor without progression of any single lesion after cycles 2 and 4 and progressive disease as the appearance of new lesions at any time point, >50 % increase in tumor size from pre-treatment scans, or up to 25 % increase in tumor size compared with nadir measurements from two consecutive observations at least 4 weeks apart. Patients who demonstrated a complete or partial response or stable disease after 2 cycles received 2 more cycles of therapy, and those with a similar response after the fourth cycle were eligible for an additional 2 cycles. Patients who developed new lesions at 2 or 4 months after starting treatment, or an increase in the size of existing lesions at 4 months after starting the regimen received no further study-related treatment.
Preparation and administration of vaccine
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient centrifugation of peripheral blood or apheresis products. Cells were seeded at 1 × 107 cells/2 ml/well into 6-well plates (Corning) in CellGenix™ DC medium and incubated for 2 h to collect adherent monocytes. The adherent blood monocytes were cultured 5 days in CellGenix™ DC medium supplemented with human recombinant granulocyte macrophage colony-stimulating factor (GM-CSF; 1000 U/ml, Bayer) and interleukin 4 (IL-4; 10 ng/ml; R&D Systems). Immature DC were matured for 48 h in the presence of 10 ng/ml of TNF-α, 10 ng/ml of IL-1β, 10 ng/ml of IL-6 (R&D Systems) and 1 µg/ml of PGE2 (Sigma); 75 × 106 mature DC in 3 ml DC medium were equally divided into three tubes and were pulsed with 5 µg/ml of MAGE-A1, MAGE-A3 and NY-ESO-1 overlapping peptide mixes, consisting of pooled, 11 amino acid overlapping 15mers derived from the full-length protein (JPT Peptide Technologies). Lyophilized peptide mix was reconstituted in DMSO, and an aliquot was sent for quality assurance testing [testing for bacterial, fungal contamination (by culture) and endotoxin levels]. After 2 h of incubation at 37 °C and gentle mixing at every 30 min, dendritic cells from the three tubes were combined, washed and cryopreserved in multiple aliquots of appropriate size for each vaccine. A sample of cell suspension (containing a 1 % cell suspension) from the final wash was sent for endotoxin testing. Release criteria for the final vaccine product were ≥70 % viable cells, expression of mature DC surface markers CD80 (≥70 %), CD83 (≥50 %) and CD86 (≥70 %), absence of bacterial, fungal (by culture) and mycoplasma contamination (by PCR and culture) with endotoxin levels <3 EU/kg. On the day of vaccination, one vial of vaccine was thawed, washed and resuspended in normal saline (Baxter) containing 1 % human serum albumin (Talecris). Based on the weight of the patient, 3–10 × 106 cells in 0.5 ml were drawn into a tuberculin syringe labeled with two patient identifiers, and delivered directly to the study physician. Due to venous access issues, DCs were generated from a cryopreserved autologous GCSF-mobilized peripheral blood stem cell product in one patient (subject-03). The DC met criteria based on the expression of CD80, CD83 and CD86 and also viability. However, due to theoretical concerns about the predominant generation of DC2 over DC1-type cells in GCSF-mobilized product along with the report that DC2 polarize T cells toward Th2 type, GCSF-mobilized peripheral blood stem cell products were not used for the generation of DC in other patients under this study [22].
Serological analyses against MAGE-A1, MAGE-A3 and NY-ESO-1 peptide antigens
Patient serum was archived at weekly intervals pre- and post-vaccine and sent to Serametrix (Carlsbad, CA) for analysis of seroreactivity to MAGE-A1, MAGE-A3 and NY-ESO-1 peptide antigens. Serum samples were recorded as positive for IgG antibody if the averaged data point at the 1:100 dilution was greater than or equal to three times the background. The level of background was previously determined from seronegative samples from healthy donors.
Detection and quantification of antigen-specific CD8+ and CD4+ T cell responses to MAGE-A1, MAGE-A3 and NY-ESO-1 overlapping peptide mix
PBMCs obtained from patients at time points (as indicated in Fig. 2) were stimulated individually with MAGE-A1, MAGE-A3 and NY-ESO-1 peptide mixes. After 24 h, antigen-specific CD137-expressing CD8 and CD4 T cells were analyzed by flow cytometry as described previously with slight modifications [21]. Briefly, 1 × 106 cells were stained with antibodies for surface markers after peptide mix stimulation, and the non-stimulated cells served as a control. The following directly conjugated antibodies were used: PE-CD137, APC-Cy7-CD4 (BD PharMingen), Qdot705-CD8 and Qdot-655-CD3 (Invitrogen). FITC-conjugated CD19, CD14 and CD56 (BD PharMingen) were added to create a ‘dump channel’ so that only CD4+/CD8+CD3+CD137+ cells were quantified. The cells were acquired using an LSR II (BD Biosciences) and analyzed using FACS Diva software (BD Biosciences). The frequency of T cells was calculated as the number of antigen-specific CD3+CD4+CD137+ or CD3+CD8+CD137+ cells in 106 cells. Development of an antibody or a T cell response to the vaccine was defined as either a new onset or a twofold increase in the level of antibodies or the number of MAGE-A1-, MAGE-A3- and NY-ESO-1-specific CD137+ T cells over baseline levels. The criteria for employing baseline CD137 responses as a reference point are based on a previous study in patients with acute myeloid leukemia in which MAGE-specific T cell responses were compared against baseline samples obtained at the start of therapy. [23].
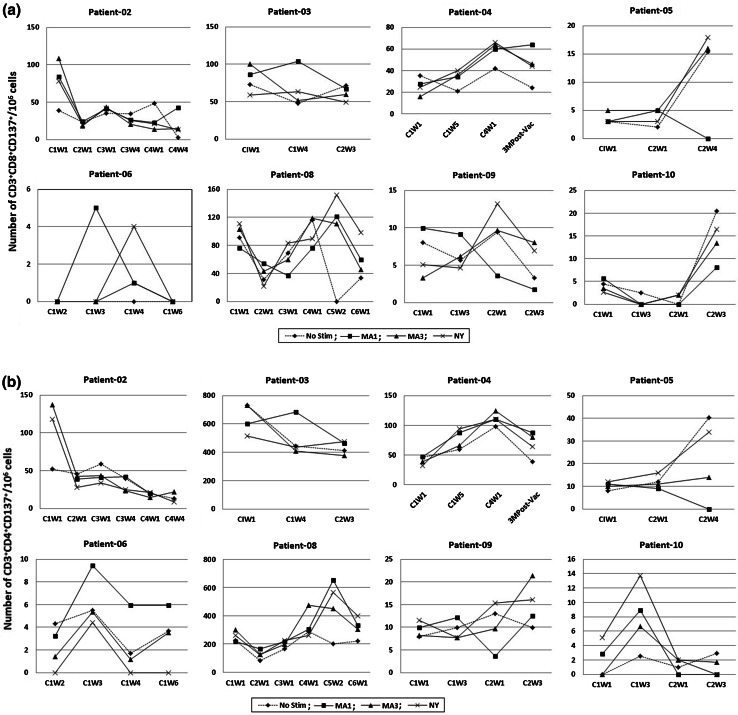
Fig. 2.
Antigen-specific T cell responses to the vaccine. Peripheral blood mononuclear cells were archived at various time points (as indicated on x-axis), stimulated for 24 h with MAGE-A1, MAGE-A3 and NY-ESO-1 peptide mixes and analyzed for the presence of CD137+ antigen-specific CD8+ (a) and CD4+ (b) T cells by flow cytometry. C1W1 indicates Cycle1-week1 of therapy. a CD8 T cell response, b CD4 T cell response
Results
Patient characteristics
Fifteen patients were enrolled on the study between February 2011 and July 2013, with a median age of 7.25 years (range 2.5–15 years). Ten patients had a diagnosis of relapsed neuroblastoma, two each with Ewing’s sarcoma and osteosarcoma, and one with rhabdomyosarcoma. All of the patients had received several courses of multi-agent chemotherapy and/or radiation for their relapsed disease (Table 1). Ten of 15 enrolled patients received vaccine therapy and hence were fully evaluable for toxicity. Five patients (2 = NB, 2 = OS and 1 = ES) did not receive therapy due to further progression between the time of enrollment and the start of therapy.
Table 1.
Clinical characteristics of patients who received DAC/DC vaccine therapy
| No. | Agea (years) | Diagnosis | Prior to study enrollment | Treatment for relapse (number of cycles) | Site of relapse/extent of disease at treatment | Therapy status | Toxicity (grade 4 myelotoxicity and other toxicity) | Clinical outcome | |
|---|---|---|---|---|---|---|---|---|---|
| No. of SCT received | Anti-GD2 therapy received | ||||||||
| 01 | 5.5 | NB | 1 | Yes | Topotecan, cyclophosphamide (3 cycles) | Bone marrow; chemotherapy resistant | Completed 3 cycles |
Grade 4 myelotoxicity at cycle 3, week 4 Elevated alkaline phosphatase during cycle 3, weeks 3–5 |
Complete response, 3.5 years post-vaccine [21] |
| 02 | 3 | NB | 1 | No | Radiotherapy, temozolomide/irinotecan (5 cycles) | Left maxillary sinus, radiation and chemotherapy resistant | Completed 4 cycles | None | Tumor shrinkage after 2 cycles followed by progression |
| 03 | 4.5 | NB | 1 | No | Whole brain radiation, temozolomide/irinotecan | CNS | Completed 3 cycles | None | Progression |
| 04 | 2.5 | NB | 1 | Yes |
Radiation to right humerus 2 courses of Topotecan–Vincristine–Cyclophosphamide |
Right proximal humerus NED at time of treatment |
Completed 4 cycles | Gr 3 neutropenia—delay in cycles 2 and 4b | Relapsed 10 months post-therapy |
| 05 | 6.5 | NB | 1 | No | Never achieved remission; multiple sites of disease progression; therapeutic MIBG and stem cell rescue | Lung nodules, paraspinal disease, bone and bone marrow progressive disease | Completed 2 cycles | None | Progression |
| 06 | 4 | NB | 1 | Yes | Resection, radiation, temozolomide, irinotecan and intra-Ommaya anti-GD2 | Posterior Right and Frontal lobe NED at time of treatment | Completed 1 cycle | Grade 2 skin hypersensitivity reaction post-vaccine Developed grade 2 erythema multiforme post-vaccine | Patient remains disease-free at 2 years post-vaccine |
| 07 | 6 | NB | 1 | Yes | Cyclophosphamide, topotecan (5 cycles), 2 cycles of therapeutic MIBG |
Bone marrow and multiple skeletal sites Disease at treatment: no gross evidence of disease |
Completed 1 cycle | Grade 3 increased ALT | Progression |
| 08 | 8.5 | NB | 2 | Yes | COG ADVL 0918 (Irinotecan, temozolomide, temsirolimus)/6 cycles | Lower lumbar vertebral body | Completed 6 cycles | Grade 4 neutropenia at cycle 2 and cycle 4b | Progression |
| 09 | 14 | ES | 0 | No | Three separate relapses; focal radiation; vincristine, temozolomide, irinotecan (multiple cycles) | femur, sacrum, chest wall, lung and liver | Completed 2 cycles | Grade 4 neutropenia at cycle 2 | Progression |
| 10 | 14 | RMS | 0 | No | Three pulmonary relapses, resection, vincristine, adriamycin, cyclophosphamide, etoposide, ifosfamide, radiation therapy, sorafenib | Multiple lung nodules, progression at the start of treatment. | Completed 2 cycles | Grade 4 neutropenia—at cycle 2b | Progression |
SCT stem cell transplant, NED no evidence of disease, NB neuroblastoma, ES Ewing’s sarcoma, RMS rhabdomyosarcoma
aAge at the time of enrollment
bDelayed start of 1–2 cycles
Feasibility
The DC vaccine was successfully prepared for all 15 patients, proving the feasibility of this approach. Vaccine preparation, qualification and product release was complete in 4–6 weeks after PBMCs were received. The median time from enrollment to therapy was 2 months (range 1–5.5 months) with variations due to disease status and/or need for additional therapy prior to study treatment and patient choice.
Tolerability
Of the 10 patients who received study therapy, three completed all 4 cycles, and two completed 3 cycles. Five patients received ≤2 cycles only, four due to disease progression and one due to a vaccine reaction (Table 1). The median number of cycles received was 2.5 (range 1–6). DAC was on the whole well tolerated with the major toxicity being neutropenia. Major dose-limiting toxicity included reversible myelosuppression (ANC < 500/µl), managed with dose reductions in DAC and the use of GCSF. Five of ten subjects experienced transient myelosuppression (4 subjects had grade 4 myelosuppression (ANC < 500), and one subject had grade 3 myelosuppression) three of whom received growth factor support and two experienced treatment delays. Four patients developed fever, and none of these episodes were related to neutropenia. One patient (patient-07) was noted to have elevated ALT levels (grade 3), at the first week of DAC, which was thought to possibly be related to DAC or to prior chemotherapy treatment. Patient-06 developed urticaria multiforme with fever, mild hypotension and a generalized urticarial rash after the first dose of vaccine. This reaction was deemed to be possibly related to the vaccine although interestingly, this patient had a history of a transfusion hypersensitivity reaction following stem cell transplant. No further vaccine courses were administered to this patient.
Response
Of the 10 patients who received therapy, seven patients (5 = NB, 1 = ES, 1 = RMS) progressed during therapy and one (NB) relapsed 10 months after the last vaccine (Table 1). One patient (patient-06) who had a history of two intracerebral relapses following treatment for stage 4 neuroblastoma continues to remain disease-free 2 years from his last vaccine. One patient (patient-01) had a complete response, and we have recently reported the results of this regimen in this patient, a 5.5-year-old child with chemotherapy-refractory neuroblastoma isolated to the bone marrow [21]. This patient is currently in complete remission 3.5 years following his last vaccine (Table 1).
Immune response to the vaccine
We investigated the number of T cells responding to MAGE-A1, MAGE-A3 and NY-ESO-1 peptide mix in the peripheral blood of patients pre- and post-vaccination based on the expression of CD137 after 24-h stimulation with respective antigens. Due to limitations in the numbers of cells available for analysis, particularly following the use of chemotherapy, cytofluorometry for the activation marker CD137 is a practical method to assess numbers of antigen-specific T cells [23]. CD137 is a marker that is uniformly upregulated 24 h after stimulation on all responding cells irrespective of their differentiation stage and is therefore used to identify antigen-specific T cells [24, 25]. The time points studied for immune response varied in different patients based on sample availability, and these data are presented in Fig. 2. The DAC/DC vaccine strategy induced a positive T cell response to MAGE-A1, MAGE-A3 or NY-ESO-1-overlappping peptide mixes in six of nine patients evaluated (Table 2). We have previously reported that patient-01 had an increase in the number of MAGE-A3-peptide mix-specific CD4+ and CD8+ T cells post-vaccine [21]. Similarly, patient-06 demonstrated an increase in the number of CD3+CD4+CD137+ and CD3+CD8+CD137+ T cells (Fig. 2). In this patient, while a CD8+ T cell response was seen against both MAGE-A1 and NY-ESO-1 peptide mix, a CD4+ T cell response was seen against MAGE-A1 alone. Due to limitations in the numbers of cells available for analysis, controls with target cells expressing the relevant genes have not been performed. We cannot therefore exclude that the immune response is directed against impurities contained in the synthetic peptide batch. Vaccine-induced T cell responses can be measured based on CD137 expression [23]; nevertheless, T cell responses to MAGE-A1, MAGE-A3 and NY-ESO-1 peptide mix from our vaccine were weak. Patient-04 developed a CD8+ T cell response but no CD4+ T cell response, while patient-08, patients-09 and patients-10 developed a CD4+ T cell response and no CD8+ T cell response. Three of nine patients did not develop either CD4+ or CD8+ T cell responses. Although patient-02 had preexisting CT antigen-specific CD4+ and CD8+ T cells responding to peptide mix, no increase was seen post-vaccination (Table 2; Fig. 2). However, in patient-10 the number of preexisting MAGE-A1-, MAGE-A3- and NY-ESO-1-specific CD4+ T cells responding to peptide mix increased (>twofold) by week 3 of the first cycle. None of the patients evaluated developed an antibody response against any of the antigens post-vaccination.
Table 2.
Immunological and clinical response to the vaccine
| Patient no. | Antibody response | T cell response | Clinical response | |
|---|---|---|---|---|
| CD4 | CD8 | |||
| 1 | Not determined | Yes | Yes | Complete response [21] |
| 2 | No | No | No | Progression after a minor response |
| 3 | No | No | No | Disease progression |
| 4 | No | No | Yes | Relapsed 10 months post-therapy |
| 5 | Not determined | No | No | Disease progression |
| 6 | No | Yes | Yes | Remains disease-free 2 years post-vaccine |
| 7 | No | Not determined | Disease progression | |
| 8 | No | Yes | No | Disease progression |
| 9 | No | Yes | No | Disease progression |
| 10 | Not determined | Yes | No | Disease progression |
Discussion
We report the results of a phase I clinical trial that combines a demethylating agent known to upregulate the expression of cancer–testis antigens with a DC vaccine targeting the MAGE-A1, MAGE-A3 and NY-ESO-1 tumor antigens. This is the first study to combine DAC and a DC-based peptide vaccine for pediatric patients with relapsed neuroblastoma and sarcoma. CGGs have been detected on several childhood cancer cells, but their expression is highly heterogenous [19, 26]. Several clinical trials have detected clinical and immunologic response post-vaccination using tumor antigen vaccines in adult patients with relapsed malignant solid tumors [17, 18, 27, 28]. We have previously reported that DAC induces upregulation of MAGE-A1, MAGE-A3 and NY-ESO-1 expression in neuroblastoma and sarcoma cells and that antigen-specific CTL preferentially lyse DAC-treated tumor cells in vitro [15, 16]. These preclinical findings were translated in a phase I clinical trial targeting MAGE-A1, MAGE-A3 and NY-ESO-1 in children with relapsed or therapy-refractory neuroblastoma and sarcoma.
In general the regimen of DAC and a DC/MAGE-A1, MAGE-A3 and NY-ESO-1 peptide vaccination was well tolerated, with one vaccine-related toxic event and five patients experiencing DAC-related myelosuppression, an expected side effect of this agent. This trial enrolled relapsed patients with tumor burden ranging from minimal residual disease to therapy-refractory, bulky tumors. One patient who achieved a complete response and another who had no evidence of disease at the time of treatment, remain disease-free 3.5 and 2 years, respectively, post-therapy. Both of these patients demonstrated CD4+ and CD8+ T cell responses to one or more CGG peptides post-vaccination. The other patients who developed either CD4+ or CD8+ T cell responses experienced relapse, stable disease or progression. These data indicate that favorable patient outcomes were associated with the induction of both CD4+ and CD8+ T cell responses and that loss of either arm of the T cell response may be associated with early relapse/disease progression. Our studies indicate that the DC-MAGE-A1, MAGE-A3, NY-ESO-1 vaccine is associated with T cell responses to CGG overlapping peptides in up to two-thirds of patients. However, these responses were weak (<100 cells in 1 million cells, <0.01 %) as measured by the expression of CD137 on cells 24 h post-stimulation with MAGE-A1, MAGE-A3 and NY-ESO-1 peptide mixes. For measures of T cell responses, unstimulated cells were used as control. Further in vitro analysis (for example, intracellular staining for IFN-γ or ELISPOT assays post-stimulation) and inclusion of additional controls (cells stimulated with irrelevant peptide mix) were not possible due to limited availability of the samples.
The low number of antigen-specific cells seen in some patients could be attributed to compromised cellular immunity, as evidenced by the absence of preexisting T cell or antibody responses. Although DAC can upregulate the expression of CGGs, it also causes myelosuppression with a prolonged white blood cell nadir. Due to the immunocompromised nature of these patients, adjuvants are often added to immunotherapy regimens to facilitate antigen-presenting cell and immune effector cell function. Several cancer vaccine studies have used either exogenous GM-CSF or a cellular vaccine product that secretes GM-CSF to enhance Th1 immune effector cells [29, 30]. In addition to promoting Th1 immune responses to the vaccine, GM-CSF can also minimize chemotherapy-induced myelosuppression and can be added in future trials to overcome these limitations [31]. Studies in adult cancer patients have examined the feasibility of administering autologous, activated T cells prior to cancer vaccines; however, it is not clear whether these nonspecifically activated T cells are capable of developing immune responses against tumor antigens [32, 33].
In conclusion, the findings from this phase I trial indicate that a regimen consisting of DAC and a DC/MAGE-A1, MAGE-A3 and NY-ESO-1 peptide vaccine is feasible, generally well tolerated in children with relapsed neuroblastoma and sarcoma and elicits T cell responses in the majority of patients. The fact that patients who have achieved a ≥2-year period of progression-free survival had minimal disease burden at study entry suggests that these subjects might be ideal candidates for this therapy. Future studies will be done with a focus on improving and sustaining stronger antigen-specific immune responses post-vaccination. This could be accomplished by eliminating Tregs and choosing more potent adjuvants that can activate DC and promote a strong cellular immune response, such as Hiltonol (Poly-ICLC) [34]. The addition of GM-CSF could minimize leukopenia from DAC and to help facilitate antigen-presenting cell function. Another potential direction would be the development of strategies to expand tumor antigen-specific T cells from patient peripheral blood, with the goal of subsequent reinfusion of these cells following DAC.
Acknowledgments
We thank the clinical research staff at the Dana-Farber Cancer Institute (DFCI), Kosair Charities Pediatric Clinical Research Unit and Penn State Children’s Hospital. The work was supported by funds from Hyundai Hope on Wheels, Solving Kids’ Cancer, The Andrew McDonough B + Foundation, the Pierce Phillips Charity and the Dana-Farber Cancer Institute (DFCI) Neuroblastoma Fund. We thank all the patients enrolled in this trial and their families for their participation in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- ALT
Alanine aminotransferase
- ANC
Absolute neutrophil count
- AST
Aspartate aminotransferase
- CGGs
Cancer germline genes
- CTCAE
Common terminology criteria for adverse events
- CTL
Cytotoxic T lymphocytes
- C1W1
Cycle1-week1
- DAC
Decitabine
- DC
Dendritic cell
- ECG
Echocardiogram
- ES
Ewing’s sarcoma
- GCSF
Granulocyte colony-stimulating factor
- GFR
Glomerular filtration rate
- GM-CSF
Granulocyte macrophage colony-stimulating factor
- HLA
Human leukocyte antigen
- NB
Neuroblastoma
- OS
Osteogenic sarcoma
- PBMCs
Peripheral blood mononuclear cells
- RMS
Rhabdomyosarcoma
Contributor Information
Rani E. George, Phone: +1 617 632 5281, Email: Rani_George@dfci.harvard.edu
Kenneth G. Lucas, Phone: +1 502 852 0043, Email: k0luca01@louisville.edu, Email: kenneth.lucas@louisville.edu
References
- 1.Igne FH, Krammer PH. Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukoc Biol. 2002;71(6):907–920. [PubMed] [Google Scholar]
- 2.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4:1. [PubMed] [Google Scholar]
- 3.Pollack SM, Loggers ET, Rodler ET, Yee C, Jones RL. Immune-based therapies for sarcoma. Sarcoma. 2011;2011:438940. doi: 10.1155/2011/438940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SY, Obata Y, Yoshida M, Stockert E, Williamson B, Jungbluth AA, et al. Immunomic analysis of human sarcoma. Proc Natl Acad Sci USA. 2003;100(5):2651–2656. doi: 10.1073/pnas.0437972100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholaou T, Ebert L, Davis ID, Robson N, Klein O, Maraskovsky E, et al. Directions in the immune targeting of cancer: lessons learned from the cancer-testis Ag NY-ESO-1. Immunol Cell Biol. 2006;84(3):303–317. doi: 10.1111/j.1440-1711.2006.01446.x. [DOI] [PubMed] [Google Scholar]
- 6.Wolfl M, Jungbluth AA, Garrido F, Cabrera T, Meyen-Southard S, Spitz R, et al. Expression of MHC class I, MHC class II, and cancer germline antigens in neuroblastoma. Cancer Immunol Immunother. 2005;54(4):400–406. doi: 10.1007/s00262-004-0603-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs JF, Brasseur F, Hulsbergen-van de Kaa CA, van de Rakt MW, Figdor CG, Adema GJ, et al. Cancer-germline gene expression in pediatric solid tumors using quantitative real-time PCR. Int J Cancer. 2007;120(1):67–74. doi: 10.1002/ijc.22118. [DOI] [PubMed] [Google Scholar]
- 8.Liu XF, Helman LJ, Yeung C, Bera TK, Lee B, Pastan I. XAGE-1, a new gene that is frequently expressed in Ewing’s sarcoma. Cancer Res. 2000;60(17):4752–4755. [PubMed] [Google Scholar]
- 9.Foell JL, Hesse M, Volkmer I, Schmiedel BJ, Neumann I, Staege MS. Membrane-associated phospholipase A1 beta (LIPI) Is an Ewing tumour-associated cancer/testis antigen. Pediatr Blood Cancer. 2008;51(2):228–234. doi: 10.1002/pbc.21602. [DOI] [PubMed] [Google Scholar]
- 10.Weber J, Salgaller M, Samid D, Johnson B, Herlyn M, Lassam N, et al. Expression of the MAGE-1 tumor antigen is up-regulated by the demethylating agent 5-aza-2′-deoxycytidine. Cancer Res. 1994;54(7):1766–1771. [PubMed] [Google Scholar]
- 11.Adair SJ, Hogan KT. Treatment of ovarian cancer cell lines with 5-aza-2′-deoxycytidine upregulates the expression of cancer-testis antigens and class I major histocompatibility complex-encoded molecules. Cancer Immunol Immunother. 2009;58(4):589–601. doi: 10.1007/s00262-008-0582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George RE, Lahti JM, Adamson PC, Zhu K, Finkelstein D, Ingle AM, et al. Phase I study of decitabine with doxorubicin and cyclophosphamide in children with neuroblastoma and other solid tumors: a Children’s Oncology Group study. Pediatr Blood Cancer. 2010;55(4):629–638. doi: 10.1002/pbc.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H, Hoshino K, Sanchez-Gonzalez B, Kantarjian H, Garcia-Manero G. Antileukemia activity of the combination of 5-aza-2′-deoxycytidine with valproic acid. Leuk Res. 2005;29(7):739–748. doi: 10.1016/j.leukres.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 14.Issa JP, Gharibyan V, Cortes J, Jelinek J, Morris G, Verstovsek S, et al. Phase II study of low-dose decitabine in patients with chronic myelogenous leukemia resistant to imatinib mesylate. J Clin Oncol. 2005;23(17):3948–3956. doi: 10.1200/JCO.2005.11.981. [DOI] [PubMed] [Google Scholar]
- 15.Bao L, Dunham K, Lucas K. MAGE-A1, MAGE-A3, and NY-ESO-1 can be upregulated on neuroblastoma cells to facilitate cytotoxic T lymphocyte-mediated tumor cell killing. Cancer Immunol Immunother. 2011;60(9):1299–1307. doi: 10.1007/s00262-011-1037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnadas DK, Bao L, Bai F, Chencheri SC, Lucas K. Decitabine facilitates immune recognition of sarcoma cells by upregulating CT antigens, MHC molecules, and ICAM-1. Tumour Biol. 2014;35(6):5753–5762. doi: 10.1007/s13277-014-1764-9. [DOI] [PubMed] [Google Scholar]
- 17.Chianese-Bullock KA, Pressley J, Garbee C, Hibbitts S, Murphy C, Yamshchikov G, et al. MAGE-A1-, MAGE-A10-, and gp100-derived peptides are immunogenic when combined with granulocyte-macrophage colony-stimulating factor and montanide ISA-51 adjuvant and administered as part of a multipeptide vaccine for melanoma. J Immunol. 2005;174(5):3080–3086. doi: 10.4049/jimmunol.174.5.3080. [DOI] [PubMed] [Google Scholar]
- 18.Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, Chen Q, et al. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci USA. 2004;101(29):10697–10702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odunsi K, Matsuzaki J, James SR, Mhawech-Fauceglia P, Tsuji T, Miller A, et al. Epigenetic potentiation of NY-ESO-1 vaccine therapy in human ovarian cancer. Cancer Immunol Res. 2014;2(1):37–49. doi: 10.1158/2326-6066.CIR-13-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz GJ, Gauthier B. A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr. 1985;106(3):522–526. doi: 10.1016/S0022-3476(85)80697-1. [DOI] [PubMed] [Google Scholar]
- 21.Krishnadas DK, Shapiro T, Lucas K. Complete remission following decitabine/dendritic cell vaccine for relapsed neuroblastoma. Pediatrics. 2013;131(1):e336–e341. doi: 10.1542/peds.2012-0376. [DOI] [PubMed] [Google Scholar]
- 22.Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood. 2000;95(8):2484–2490. [PubMed] [Google Scholar]
- 23.Goodyear O, Agathanggelou A, Novitzky-Basso I, Siddique S, McSkeane T, Ryan G, et al. Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood. 2010;116(11):1908–1918. doi: 10.1182/blood-2009-11-249474. [DOI] [PubMed] [Google Scholar]
- 24.Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M, et al. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110(1):201–210. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Y, Chen L. CD137 as a biomarker for tumor-reactive T cells: finding gold in the desert. Clin Cancer Res. 2014;20(1):3–5. doi: 10.1158/1078-0432.CCR-13-2573. [DOI] [PubMed] [Google Scholar]
- 26.Akers SN, Odunsi K, Karpf AR. Regulation of cancer germline antigen gene expression: implications for cancer immunotherapy. Future Oncol. 2010;6(5):717–732. doi: 10.2217/fon.10.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackensen A, Herbst B, Chen JL, Köhler G, Noppen C, Herr W, Spagnoli GC, et al. Phase I study in melanoma patients of a vaccine with peptide-pulsed dendritic cells generated in vitro from CD34(+) hematopoietic progenitor cells. Int J Cancer. 2000;86(3):385–392. doi: 10.1002/(SICI)1097-0215(20000501)86:3<385::AID-IJC13>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 28.Bender A, Karbach J, Neumann A, Jäger D, Al-Batran SE, Atmaca A, Weidmann E, et al. LUD 00-009: phase 1 study of intensive course immunization with NY-ESO-1 peptides in HLA-A2 positive patients with NY-ESO-1-expressing cancer. Cancer Immun. 2007;7:16. [PMC free article] [PubMed] [Google Scholar]
- 29.Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, Levi J, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor–secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27(35):5911–5918. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King IL, Kroenke MA, Segal BM. GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J Exp Med. 2010;207(5):953–961. doi: 10.1084/jem.20091844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vadhan-Raj S, Broxmeyer HE, Hittelman WN, Papadopoulos NE, Chawla SP, Fenoglio C, et al. Abrogating chemotherapy-induced myelosuppression by recombinant granulocyte-macrophage colony-stimulating factor in patients with sarcoma: protection at the progenitor cell level. J Clin Oncol. 1992;10(8):1266–1277. doi: 10.1200/JCO.1992.10.8.1266. [DOI] [PubMed] [Google Scholar]
- 32.Rapoport AP, Aqui NA, Stadtmauer EA, Vogl DT, Fang HB, Cai L, et al. Combination immunotherapy using adoptive T-cell transfer and tumor antigen vaccination on the basis of hTERT and survivin after ASCT for myeloma. Blood. 2011;117(3):788–797. doi: 10.1182/blood-2010-08-299396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rapoport AP, Stadtmauer EA, Aqui N, Vogl D, Chew A, Fang HB, et al. Rapid immune recovery and graft-versus-host disease-like engraftment syndrome following adoptive transfer of costimulated autologous T cells. Clin Cancer Res. 2009;15(13):4499–4507. doi: 10.1158/1078-0432.CCR-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabbatini P, Tsuji T, Ferran L, Ritter E, Sedrak C, Tuballes K, et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res. 2012;18(23):6497–6508. doi: 10.1158/1078-0432.CCR-12-2189. [DOI] [PubMed] [Google Scholar]