Abstract
Natural killer (NK) cell activation is strictly regulated to ensure that healthy cells are preserved, but tumour-transformed or virus-infected cells are recognized and eliminated. To carry out this selective killing, NK cells have an ample repertoire of receptors on their surface. Signalling by inhibitory and activating receptors by interaction with their ligands will determine whether the NK cell becomes activated and kills the target cell. Here, we show reduced expression of NKp46, NKp30, DNAM-1, CD244 and CD94/NKG2C activating receptors on NK cells from acute myeloid leukaemia patients. This reduction may be induced by chronic exposure to their ligands on leukaemic blasts. The analysis of ligands for NK cell-activating receptors showed that leukaemic blasts from the majority of patients express ligands for NK cell-activating receptors. DNAM-1 ligands are frequently expressed on blasts, whereas the expression of the NKG2D ligand MICA/B is found in half of the patients and CD48, a ligand for CD244, in only one-fourth of the patients. The decreased expression of NK cell-activating receptors and/or the heterogeneous expression of ligands for major receptors on leukaemic blasts can lead to an inadequate tumour immunosurveillance by NK cells. A better knowledge of the activating receptor repertoire on NK cells and their putative ligands on blasts together with the possibility to modulate their expression will open new possibilities for the use of NK cells in immunotherapy against leukaemia.
Keywords: NK cells, NCR, DNAM-1, NKG2D, Leukaemia, PIVAC 10
Introduction
Natural killer (NK) cells were originally defined as lymphocytes able to kill tumour cells and virus-infected cells. They were named ‘natural killers’ because of their ability to spontaneously kill tumour cells without the requirement of prior sensitization. Unlike T and B lymphocytes, NK cells do not rearrange their receptor genes somatically, but are dependent on a wide array of germline-encoded inhibitory and activating NK cell receptors that are capable of recognizing major histocompatibility complex (MHC) class I and class I-like molecules, as well as other ligands [8, 23]. Although NK cells are considered part of the innate immune response, it has been recently described in murine models that NK cells can exhibit some characteristics normally associated to adaptive immunity as their ability to mount an enhanced secondary recall response [61]. In addition, NK cell contribution to the initiation of adaptive immune responses is underlined by their crosstalk with dendritic cells (DCs) promoting the maturation of DCs with high capacity to induce T helper type-1 (Th1) and cytotoxic T lymphocyte (CTL) responses [31, 76]. Thus, due to their immunoregulatory effects on adaptive responses, NK cells are considered a bridge between innate and adaptive immunity [74].
Human NK cells comprise 10–15% of all peripheral blood lymphocytes and can be defined phenotypically by the surface expression of CD56 and/or CD16 and the lack of expression of CD3 [23]. Two distinct subpopulations of human NK cells can be distinguished by CD56 surface density expression. CD56dim NK cells constitute the majority (90%) of peripheral blood NK cells, express higher levels of CD16 (FcγRIII) and possess greater cytotoxic capacity than its counterpart the CD56bright NK cell subset that represent the minority of peripheral blood NK cells that produce larger amounts of cytokines. Although it is not entirely clear what factors drive NK cell development, there are evidences supporting that CD56bright subset represent a more immature developmental stage than CD56dim NK cells [15]. A third subset, defined as CD56− CD16+ NK cells, has been identified that represents a small percentage of total NK cells in the blood in healthy individuals and expresses many NK cell receptors including KIRs, CD94/NKG2A and NKG2D. CD56− CD16+ NK cells possess, although to a reduced extent compared with CD56+ NK cells, the capacity to kill target cells and produce cytokines [2]. Expansion of CD56− CD16+ NK cells has been described in several situations as chronic viral infections (HIV and hepatitis C virus) and following allogeneic hematopoietic cell transplantation and in cord blood [2, 37, 64, 72].
Mature NK cells have the ability to kill a variety of tumour cells spontaneously while sparing normal cells. Thus, their activation must be strictly regulated to avoid the destruction of healthy tissues and to assess an effective response to virus-infected or tumour-transformed cells. NK cells have the capacity to detect and respond to the lack or the down-regulation of MHC class I expression (Karre ‘missing self’ hypothesis) [34]. Several processes are involved in NK cell education and tolerance including receptor repertoire formation. Thus, NK cells acquire an ample repertoire of inhibitory and activating receptors during development. NK cells become tolerant towards self based on their interactions with MHC class I molecules [1, 32]. NK cell recognition of altered MHC class I expression on target cells is due to the expression of inhibitory receptors specific for MHC class I and MHC class I-like molecules.
Two hypotheses were proposed to explain the observation that the generation of functional NK cells is based in the recognition of MHC class I molecules. In the ‘licensing model’, it was suggested an instructive mode of action for MHC class I molecules in the acquisition of NK cell function. In the ‘disarming model’, it was suggested that in the absence of MHC class I, NK cells may be chronically stimulated, resulting in NK cells that are hyporesponsive. Recent studies demonstrating that chronic interaction of an activating receptor in vivo may induce NK cell tolerance that is independent of MHC class I-specific inhibitory receptors support the ‘disarming’ model [32, 65].
Once activated, NK cells exert their function by direct cytolysis of infected or tumour cells and by secretion of several cytokines. If not restrained by inhibitory receptors recognizing MHC class I molecules on the surface of self cells, NK cells are able to kill normal, healthy cells provided that they express ligands for activating receptors [46]. Some of the activating receptors are specific for non-self (e.g. viral) or stress-induced self-ligands. This allows the specific detection of potentially harmful cells that have to be eliminated [29].
As downregulation of MHC class I molecules is an immunoescape mechanism frequently used by tumours and virus-infected cells to avoid CD8 T cell-mediated lysis, NK cells play a significant role in the immunosurveillance of different tumour types and in the immune response against viral infections. The integration of activating and inhibitory signals dictates whether or not the NK cells exert their cytotoxic function on the target cell. Thus, signals transmitted through activating receptors are frequently restrained by signals transmitted through inhibitory receptors [8, 73].
Here, we review current knowledge on the role of human NK cells in the defence against cancer focusing on acute myeloid leukaemia (AML). The ability of NK cells to destroy leukaemic cells as demonstrated by hematopoietic stem cell transplantation suggests that NK cells are involved in the immunosurveillance of leukaemia. Despite recent progress in the treatment of AML, its prognosis remains frequently poor. NK cell-based immunotherapy may offer an alternative as demonstrated in hematopoietic stem cell transplantation; however, further studies are needed to increase our knowledge of NK cells and to modulate their receptor repertoire to increase the expression of activating receptors in order to counteract inhibitory receptor-mediated signalling and to overcome leukaemia immunoescape mechanisms to avoid NK cell recognition [35, 56].
In this work, we overview the main alterations described on NK cell receptors and in their putative ligands in blasts from AML patients and discuss how these changes can lead to an inadequate function of NK cells allowing malignant cells to escape from NK cell immunosurveillance.
NK cell recognition of acute myeloid leukaemia
AML is a life-threatening haematological malignancy with considerable phenotypic and genotypic heterogeneity characterized by the acquisition of somatic mutations in normal hematopoietic stem cells. These alterations induce differentiation arrest and/or excessive proliferation of abnormal leukaemic cells or blasts and impair hematopoietic differentiation [30].
Several evidences support that NK cells play an important role in the control and clearance of leukaemic cells [16, 70], and it has been shown that NK cell activity correlates positively with the relapse-free survival in AML patients [16, 24]. The participation of NK cells in leukaemia clearance is highlighted by the results obtained from allogeneic hematopoietic stem cell transplantation studies. Thus, when patients and donors were mismatched for human leucocyte antigen (HLA)-specific inhibitory killer cell immunoglobulin-like (KIR) receptors, the risk for relapse was virtually null compared with perfect KIR matching between donors and recipients [24, 53, 67], indicating that in these cases, killing of leukaemic blasts by NK cells is inhibited by the interaction of the KIR inhibitory receptor with their ligands.
However, rapid disease progression and the high incidence of relapses after treatment with high-dose chemotherapy or after transplantation of allogeneic hematopoietic stem cells indicate that leukaemic blasts can escape NK cell recognition [24, 44]. Thus, impaired NK cell function has been described in AML patients [16, 62] probably due to the decreased expression of several activating receptors on the NK cells [16, 24, 57, 62] or to the low levels of ligands for major activating receptors on leukaemic blasts [44].
Activating receptors on NK cells from AML patients and expression of their ligands on AML blasts
NK cell recognition and elimination of leukaemia cells depends on a tune balance between activating and inhibitory signals transmitted through receptors on NK cell surface. Although the interaction of inhibitory NK cell receptors with HLA class I molecules on leukaemic blasts may be sufficient to control NK cell activation, the presence of high levels of ligands for activating receptors can counterbalance this inhibition [70]. Thus, cumulative evidences support that an important tumour escape mechanism from NK cell surveillance is the absence of ligands of activating receptors on AML blasts [44] and/or the downregulation of activating receptors on NK cells from these patients [16, 24, 57, 62].
NK cells require specific activating signals to exert their effector functions. Activating signals are mediated by a wide array of receptors including, among others, members of the C lectin-like family as NKG2D and members of the immunoglobulin superfamily as the natural cytotoxicity receptors (NCRs) that include NKp30, NKp46 and NKp44, and the DNAX accessory molecule-1 (DNAM-1, also known as CD226). Other activating receptors are CD244 (2B4) and the activating forms of KIR and CD94/NKG2C [10, 23].
Natural cytotoxicity receptors (NCRs) and their ligands in AML patients
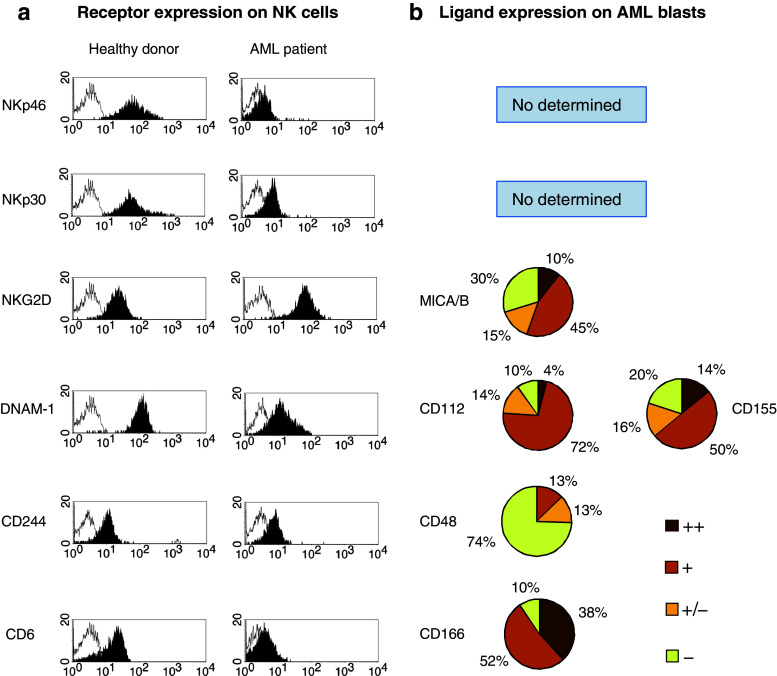
NCRs are major triggering receptors involved in the elimination of tumour and virus-infected cells by NK cells. NCRs are type I transmembrane glycoproteins that belong to the immunoglobulin superfamily and comprise NKp30, NKp46 and NKp44. Whereas NKp30 and NKp46 are constitutively expressed on NK cells, NKp44 expression is induced after NK cell activation. It has been described that NK cells from most healthy donors display a NKp46brightNKp30bright (NCRbright) phenotype, whereas NK cells from AML patients had an increase of NKp46dullNKp30dull (NCRdull) or a NCRdiscordant phenotype (NKp46dullNKp30bright or NKp46brightNKp30dull) [16, 24, 57].
Although several viral ligands of these NCRs have been described (e.g. hemagglutinin of influenza virus), relatively little is known about the cellular targets of these receptors. It has been shown that NKp30, NKp46 and NKp44 recognize different microdomains on heparan sulphate on cancer cells [28] and that human leucocyte antigen B-associated transcript 3 (BAT3) [52] and B7-H6, a member of the B7 family, [6] are tumour cell ligands for NKp30. NKp30 and NKp46 associate to CD3ζ or FcεRIγ molecules containing immunoreceptor tyrosine-based activation motifs (ITAMs) that mediate the transduction of the triggering signal and NKp44 associates with the ITAM-containing adapter protein DAP12 [8].
Among NK cell-activating receptors, the role of NCRs in the recognition and killing of leukaemia cells has been underlined. Indirect evidences support that these receptors are involved in the elimination of AML blasts by NK cells in vivo. In addition, in vitro cytotoxicity assays have demonstrated that blocking of NKp30 and NKp46 reduces the lysis of AML blasts by allogeneic NK cells [16]. The NCRdull phenotype in AML patients is correlated with weak cytolytic activity against autologous leukaemic cells and lower survival rates [16, 24, 62]. It has also been shown that the downregulation of NKp46 and NKp30 is the consequence of direct contact between NKp30 and NKp46 on NK cells with their putative ligands on leukaemic blasts [24].
The cellular ligands for NCRs on AML blasts have not been yet identified [44, 70]. Using soluble NCRs for the measure of ligands for NKp30, NKp46 and NKp44, it was shown that blasts from 10 out of 12 AML patients analysed showed low binding to the soluble receptors. It was also observed that NCR ligand expression increases during hematopoietic cell differentiation suggesting that its expression on blasts may be also related to the AML subtype, although a larger number of patients should be studied in order to confirm this hypothesis [44].
The prevalence of the NCRdull phenotype of NK cells in AML patients [16, 24, 44, 57] (Fig. 1a) and the in vitro results showing downregulation of NKp30 and NKp46 after co-culture of NK cells with blasts ([16, 24, 57] and Sanchez-Correa unpublished data) suggest that interactions between NCRs and their ligands occur in vivo.
Fig. 1.
Analysis of NK cell receptors and their ligands in AML patients. a Representative histograms of NKp46, NKp30, NKG2D, DNAM-1, CD244 and CD6 expression on NK cells from healthy donors (n = 47) and AML patients (n = 50). Open histograms represent isotype controls. NK cells were defined as CD3 − CD56 + cells within the lymphocyte gate. b Analysis of the percentage of AML patients expressing ligands for NK cell-activating receptors on their blasts. Surface expression was determined by flow cytometry using a FACScan and specific mAbs. Intensity of staining of MICA/B, CD112, CD155, CD48 and CD166 is shown (n = 50). Mean relative fluorescence intensity (MRFI) was calculated by dividing the mean fluorescent intensity (MFI) of the relevant mAb by the MFI of its isotype control. Negative < 1.5; weakly positive (±) >1.5; positive (+) >2 and strongly positive (++) >10. The following monoclonal antibodies were used: FITC-conjugated anti-CD56 (NCAM16.2), anti-CD45 (2D1), anti-CD33 (HIM3-4), anti-CD48 (TÜ145); anti-CD6 (M-T605); PE-conjugated anti-CD56 (MY31); anti-CD226 (DX11), anti-CD112 (R2.525), anti-CD155 (300907), anti-NKp46 (9E2/NKp46) anti-NKG2D (1D11); anti-MICA/B (6D4); anti-CD244 (2-69); anti-CD166 (3A6). PerCp-conjugated anti-CD3 (SK7), anti-CD45 (2D1) and anti-CD34 (8G12), all from BD Biosciences (San Jose, CA, USA). PE-conjugated anti-NKp30 (AF29-4D12) from Miltenyi Biotec (Bergisch Gladbach, Germany). Isotype-matched immunoglobulins were used as negative controls (BD Biosciences)
Up-regulation of NCR ligands on AML blasts by IFN-γ and several grow factors increases their susceptibility to NK cell-mediated cytotoxicity supporting the capacity of NCRs to elicit cytolytic responses against the leukaemic blasts [44] and suggest the possibility to modulate the susceptibility of AML blasts to NK cells by treatment with biological agents.
NKG2D and its ligands in AML patients
Human NKG2D is a type II transmembrane glycoprotein, containing C-type lectin-like receptor domains [19]. NKG2D is constitutively expressed on NK cells, CD8 T cells, γδ T cells and invariant NKT cells and can be found expressed under certain conditions on CD4+ T cells [9, 14, 51]. It is an activating receptor for NK cells, promoting both lysis of target cells and cytokine release even in the presence of inhibitory signals mediated by MHC-specific receptors. In contrast, in CD8 T cells, NKG2D is a co-stimulatory receptor requiring TCR-mediated stimulus for their activation. NKG2D forms homodimers and associates with adaptor molecules which stabilizes its surface expression and mediate the transduction of the triggering signal. Human NKG2D exclusively binds to DAP10, while mouse NKG2D can also associate with DAP12 [9, 75].
Ligands for NKG2D comprise the MHC class I chain-related gen A and B (MICA/B) and the UL-16 binding proteins 1-5 (ULBP1-5) [14]. Human NKG2D ligands can be up-regulated by stress conditions such as infections and tumour transformation. NKG2D engagement by its ligands activates NK cell functions and co-stimulates effector T cell responses [9]. It is of clinical relevance that the expression of NKG2D may be up-regulated by some cytokines such as IL-12 and IL-15 inducing an enhancement of NKG2D-mediated effector functions [9].
NKG2D-ligands are expressed by a wide range of tumour cell lines of different histotypes as melanoma, lymphoma or carcinoma cell lines. However, NKG2D ligands are not universally expressed on tumours of the same origin, and certain variability can be found as observed in melanoma cell lines [13, 14, 33, 41, 59]. Tumour editing by the immune system may also account for the variations on NKG2D ligand expression observed [38].
We have studied the expression of MICA/B on blasts from AML patients (n = 50). This ligand is expressed on AML blasts in 55% of the patients analysed (Fig. 1b), supporting previous results showing a high variability on the expression of NKG2D ligands on leukaemic blasts from AML patients. Thus, whereas in some reports, the expression of NKG2D ligands in blasts from AML patients is negative or very low [44, 50], others reveal a heterogeneous expression of NKG2D ligands, with MICA being more frequently expressed than other NKG2D ligands [18, 54]. It has been shown that NKG2D ligands are preferentially expressed in leukaemia of the M4 and M5 subtypes suggesting that these molecules are acquired at late maturation stages in the myeloid lineage differentiation process [18]. It is important to note that the levels of ULBPs on normal myeloid cells increase during hematopoietic cell differentiation and cell maturation arrest during malignant transformation may restrain ULBP expression [44].
In addition, high levels of soluble MICA [54], soluble ULBP1 [18] and soluble ULBP2 [75] have been found in sera from AML patients suggesting that the low surface expression of these ligands may be the consequence of their shedding. In different experimental models, it has been shown that the interaction of NKG2D with its ligands, either on the cell surface or shed by tumour cells, can downregulate NKG2D expression [9, 14, 27, 55].
Nevertheless, the expression of NKG2D on the surface of NK cells from leukaemic patients has been scarcely studied. We have not observed a decrease of NKG2D expression in NK cells from AML patients [57] in spite of the expression of MICA/B on leukaemic blasts in 55% of the patients (Fig. 1a, b). No correlation between MICA/B expression on blasts and NKG2D on NK cells was found (data not shown). A decreased expression of NKG2D has been described on peripheral blood NK cells from myelodysplastic syndrome patients that was not associated with either the expression of MICA/B in blasts or the increased serum levels of MICA/B [22], suggesting that additional mechanisms such as the secretion of immunomodulatory cytokines are implicated in NKG2D downregulation [70].
DNAM-1 and its ligands in AML patients
The triggering receptor DNAX accessory molecule-1 (DNAM-1) is a type I transmembrane glycoprotein belonging to the immunoglobulin superfamily. In humans, DNAM-1 is expressed on NK, T cells, monocytes, a subset of B cells and platelets. Stimulation of DNAM-1 by the interaction with its ligands CD155 (poliovirus receptor, PVR) and CD112 (Nectin-2) leads to NK cell activation and target cell lysis [5, 48, 63]. DNAM-1 ligands can be expressed on different types of tumours including leukaemia, melanoma, ovarian carcinoma and myeloma [11–13, 20, 41, 48, 57].
DNAM-1 participates in NK cell-mediated tumour immunosurveillance as demonstrated in vivo using a DNAM-1 knockout model [26]. Gilfillan et al. observed a reduced NK cell-mediated suppression of tumours in DNAM-1−/− mice, and it was suggested that NK cells require DNAM-1 for the elimination of tumour cells that are less susceptible to cytotoxicity due to the low expression of ligands for other NK cell-activating receptors [26]. In addition, DNAM-1 acts as a costimulatory molecule for CD8 T cells when non-professional antigen-presenting cells are used for stimulation [26]. DNAM-1 was also shown to be critical in NK cell recognition of ovarian carcinoma cells and blasts in myelodysplastic syndrome [11, 12].
The role of DNAM-1 on NK cell function against leukaemia is underlined by previous reports showing the consistent expression of CD112 and CD155 on AML blasts and its involvement in the lysis of blasts [50, 57]. In contrast to other tumour types, as melanoma [13] or ovarian carcinoma [12], the expression of CD155 was less frequent on leukaemic blasts than CD112 [57] (Fig. 1b).
We have recently found a reduced expression of DNAM-1 on NK cells from AML patients when compared with healthy controls (Fig. 1a) that correlate with CD112 expression on leukaemic blasts [57]. In vitro experiments demonstrated that direct contact between DNAM-1 on NK cells and its ligands on blasts induces DNAM-1 downregulation [57].
CD244 and its ligands in AML patients
CD244 (2B4) is a type I transmembrane protein, member of the immunoglobulin superfamily, that belongs to the CD150 (signalling lymphocyte activation molecule, SLAM) subfamily within the CD2 family. CD244 was originally discovered on mouse NK cells and T cells displaying non-MHC-dependent cytotoxicity [66]. Human CD244 receptor has homology with murine CD244 and is expressed on human NK cells, subsets of CD8 T cells, γδT cells, monocytes, basophils and eosinophils [3]. The CD244 ligand, CD48 molecule is a glycosyl-phosphatidyl-inositol (GPI)-anchored cell-surface protein that also belongs to the CD2 family. CD48 has been found expressed on various hematopoietic cells, and its expression may be upregulated by viral infection. CD48 binds with high affinity to CD244. Soluble CD48 has been found elevated in serum from patients with lymphoid leukaemia, arthritis and acute Epstein-Barr virus (EBV) infection. It has been hypothesized that soluble CD48 is the consequence of cleaving of the GPI anchor after interaction with CD244 [3, 21].
In mice, two isoforms of CD244 have been identified that display different functions; the activating isoform contains a short intracytoplasmic tail and the inhibitory isoform that possesses a long cytoplasmic tail [66]. Human NK cells also express two isoforms of CD244 that differ in a small portion of the extracellular domain. The isoform A but not B was able to mediate NK cell cytotoxicity against target cells expressing CD48 [36].
CD244 signalling is mediated by its cytoplasmic immunoreceptor tyrosine-based switch motifs (ITSM). In humans, CD244 interactions with its ligand CD48 result in cytotoxicity and cytokine production [39]. CD244 can also enhance signals by other NK receptors such as NKp30 and NKp46 thereby acting as a costimulator [58].
We have observed that the expression of CD244 was decreased on NK cells from AML patients [57] (Fig. 1a). However, the analysis of CD48 showed that AML blasts from the majority of the patients did not express this molecule (Fig. 1b). These results suggest that other mechanisms rather than direct contact between CD244 on NK cells and CD48 on blasts may be responsible of the decrease of CD244. Nevertheless, we cannot exclude the presence of soluble CD48 in serum of these patients as responsible of CD244 downregulation.
CD6 and its ligand in AML patients
CD6 is a surface receptor belonging to the scavenger receptor cysteine-rich (SRCR) protein superfamily. It is expressed by T lymphocytes, thymocytes, a subset of B cells and NK cells. CD6 is expressed by most CD56dim cells and absent on CD56bright NK cells. Although the precise function of CD6 remains to be elucidated, several studies point towards a role for CD6 as a co-stimulatory molecule in T cell activation. With respect to CD6 function on NK cells, it has been shown that CD6 triggering did not result in degranulation but induced secretion of cytokines and chemokines [7].
The ligand for CD6, CD166 also known as activated leucocyte cell adhesion molecule (ALCAM) is a member of the immunoglobulin superfamily of proteins that it is expressed in many types of tumours as melanoma, prostate cancer, breast cancer, colorectal carcinoma, bladder cancer and oesophageal squamous cell carcinoma. CD166 can be also expressed on some subpopulations of leucocytes including B cells and monocytes, but its role in the immune system is as yet unclear. The expression of CD166 on tumours has been correlated with invasiveness and metastatic potential, and it is associated to poor prognosis [45]. CD166 mediates low affinity CD166-CD166 homotypic interactions and higher affinity interactions with CD6 [7]. We have found that the expression of CD6 is reduced on NK cells from AML patients and that AML blasts express its ligand CD166 in 90% of the patients analysed (Fig. 1).
MHC-specific NK cell receptors in AML patients
NK cell inhibitory receptors contain in their cytoplasmic tails one or more immunoreceptor tyrosine-based inhibitory motif (ITIM). NK cell inhibitory receptors can recognize ‘classical’ and ‘non-classical’ MHC class I proteins. Some of the NK inhibitory receptors can detect shared allelic determinants of MHC class I proteins, while others are able to recognize a broad spectrum of MHC class I proteins. The variegated pattern of expression of these receptors generates a NK cell repertoire that is unique to each human individual. As part of the NK cell education, acquisition of the receptor repertoire ensures a self-tolerant NK cell population able to discriminate different alleles of MHC class I molecules [46].
The two major families of MHC-specific inhibitory receptors identified in humans include receptors that belong to the Ig superfamily: the killer Ig-like receptors (KIR) and leucocyte Ig-like inhibitory receptors (LIR) and receptors that are members of the C-type lectin receptor superfamily as CD94/NKG2A [10, 23]. The analysis of KIR phenotype in leukaemic patients showed that the frequency of particular inhibitory KIRs in association with their putative HLA class I ligands was significantly increased in leukaemic patients compared to the controls supporting the hypothesis that leukaemic cells may have evolved an escape mechanism from immune surveillance based on the dominance of inhibitory over activating KIR signals [56, 68, 69].
CD94/NKG2 is a heterodimer expressed on NK cells and on subsets of T cells. This receptor varies in function as an inhibitory or activating receptor depending on which isoform of NKG2 is expressed. CD94/NKG2A heterodimer constitutes an inhibitory receptor that is expressed on the majority of human NK cells. CD94 can also form activating receptors when paired with NKG2C or NKG2E. Functional isoforms of NKG2A and NKG2E have been identified, known as NKG2B (inhibitory) and NKG2H (activating), respectively that are generated by alternative splicing [4].
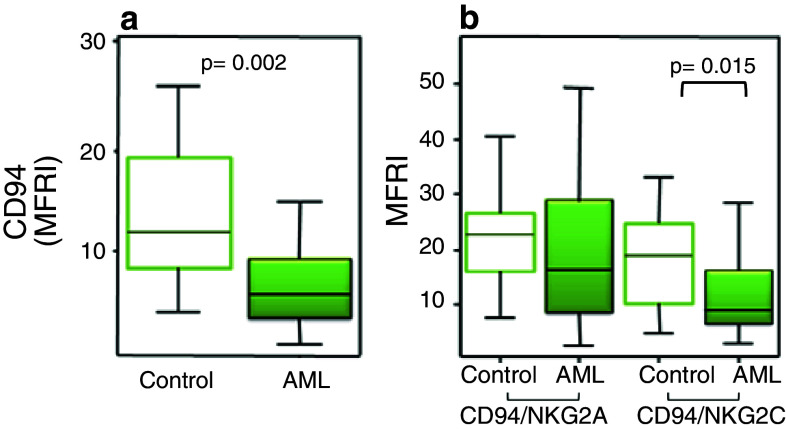
The ligand for CD94/NKG2 is HLA-E in humans and its homolog, Qa1 in mouse, which are non-classical class I molecules that bind leader peptides from other class I molecules. Most NK cells express the inhibitory isoform, and its engagement may lead to a complete block of cytotoxicity [4]. We have observed that AML patients have a low expression level of CD94 on NK cells (Fig. 2a) and that the activating form is also reduced when compared to healthy donors (Fig. 2b). These results confirm previous data showing the absence of NKG2C in four leukaemic patients [68]. Altogether, these results point out that in AML patients, the balance of inhibitory versus activating signals mediated by MHC-specific NK cell receptors is shifted to inhibition. A hypothetical model for the impact of KIR/ligand combinations on the immune response to melanoma has been proposed, where KIR/ligand pairs expected to result in a prevalence of inhibitory over activating signals might facilitate tumour development and dissemination [42].
Fig. 2.
Analysis of CD94, NKG2A and NKG2C on NK cells from AML patients. The expression of these receptors was analysed by flow cytometry on NK cells from healthy donors (n = 47) (white boxes) and AML patients (n = 50) (coloured boxes) for the expression of CD94 (a) and NKG2A and NKG2C within CD94+ NK cells (b). The following monoclonal antibodies were used: FITC-conjugated anti-CD94 (HP-3D9); PerCp-conjugated anti-CD3 (SK7); PE-conjugated anti-NKG2A (131411) and anti-NKG2C (134591) from R&D Systems (Minneapolis, USA). Isotype-matched immunoglobulins were used as negative controls (BD Biosciences). The lower boundary of the box indicates the 25th percentile and the upper boundary the 75th percentile. Bars above and below the box indicate the 90th and 10th percentiles. The line within the box marks the median
In contrast with solid tumours as melanoma that frequently show altered expression of HLA class I molecules [40], complete loss of HLA class I expression on AML blasts is infrequent [70, 71]. On the contrary, partial downregulation of one or more HLA class I alleles on leukaemic cells was observed in 68.4% of the AML samples analysed [71]. Because T cell-mediated selective pressure that facilitates the expansion of malignant cells with HLA class I molecule defects may require long periods of time, the usually short time between malignant transformation and diagnosis in leukaemia in comparison with solid tumours may explain the low frequency of complete loss of HLA class I molecules observed in leukaemia [70, 71].
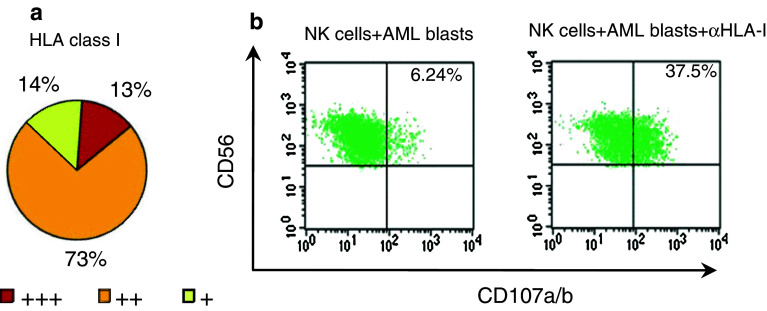
We have performed the analysis of HLA class I expression on blasts from 50 AML patients. Our results showed expression of HLA class I molecules on leukaemic blasts from most AML patients (Fig. 3a) although frequently the level of expression was lower as compared with normal leucocytes from the same patient.
Fig. 3.
HLA class I molecule is expressed on AML blasts and may protect them from NK cell-mediated lysis. a Percentage of AML patients expressing HLA class I molecules on leukaemic blasts. MRFI was calculated as indicated in Fig. 1 (n = 50). b Representative FACS plots showing CD107a/b expression on NK cells from a healthy young donor after activation with AML blasts in the presence (right) or absence (left) of anti-HLA class I (αHLA-I) monoclonal antibody (clone B9.12.1, Beckman Coulter)
In most instances, the level of expression of HLA class I molecules on leukaemic blasts is sufficient to inhibit NK cell function and the disruption of the interaction between HLA class I and the inhibitory receptors expressed on NK cells increase NK cell cytotoxicity against leukaemic blasts. Therefore, in the presence of antibodies against HLA class I molecules, NK cells obtained from healthy donors were able to kill leukaemic blasts [16]. We have analysed the anti-tumour activity of NK cells from healthy donors against leukaemic blasts by measuring the expression of the lysosome-associated membrane proteins CD107a and CD107b as markers of degranulation. We have observed that the expression of HLA class I molecules on AML blasts inhibits NK cell degranulation that is reverted by the addition of antibodies against HLA class I molecules that significantly increased the percentage of CD107a/b+ NK cells (Fig. 3b). In contrast to NK cells from healthy donors, NK cells from AML patients were not able to kill autologous AML blasts, and lysis was not significantly incremented in the presence of anti-HLA class I antibodies, suggesting that resistance to lysis is the result of the decreased expression of major activating receptors on NK cells from AML patients [16].
The expression of HLA class I molecules on AML blasts may protect them from NK cell-mediated lysis and explain why the incompatibility between the recipient’s HLA class I antigens and the KIR repertoire expressed by the donor NK cells shows beneficial anti-tumour effects in hematopoietic transplantation of patients with AML [43, 53, 67]. The analysis of HLA class I allele expression and the expression level of ligands for NK cell-activating receptors on leukaemic cells together with the expression of inhibitory and activating receptors on NK cells may allow the selection of the most appropriate donors [70, 71].
Ageing, AML and NK cells
Age-associated alterations of NK cells have been observed including diminished lytic efficiency of individual NK cells and impaired production of cytokines and chemokines. Thus, infection risk and mortality in elderly individuals seem to be strongly correlated with NK cell activity that may also compromise NK cell-driven adaptive immune responses in the elderly [17, 25, 47, 60]. AML is generally considered a disease of older adults as more than half of AML patients are over age 65 at diagnosis. Ageing implies many adverse features as the refractoriness of the disease or the frailty of this population that as a whole contribute to a low rate of survival [30].
We have recently reported that NK cells from young AML patients have a reduced expression of NCRs and DNAM-1 receptors [57]. This NK cell phenotype resembles that found in healthy elderly individuals supporting that NK cells from young AML patients are immunosenescent cells probably as consequence of chronic stimulation with activating ligands on leukaemic blasts. Further analysis of young and elderly AML patients in remission is required to better understand the role of age-associated alterations in the survival of elderly AML patients.
Concluding remarks and future prospects
Taken together, these studies show that the leukaemic blasts of most AML patients express ligands able to trigger NK cell activation. Antibody-mediated blocking of NK cell-activating receptors has revealed a dominant role for NCRs and DNAM-1 [50]. NKG2D signalling may also contribute to AML cell recognition by NK cells provided they express enough levels of NKG2D ligand [49, 54]. However, leukaemic blasts show frequently low susceptibility to autologous NK cells that may be explained in part by the decreased expression of several NK cell-activating receptors (e.g. NKp46, NKp30, DNAM-1, CD244) observed in AML patients [16, 22, 24, 57, 62, 70]. Myeloid maturation arrest at early stages of blood cell differentiation may explain the low expression of ULBPs and NCR ligands on the majority of AML patients [44]. In addition, in vivo selection of tumour cells with low levels of ligands for activating receptors or the shedding of these ligands may also contribute to the immunoescape from NK cell recognition allowing disease progression.
NK cells can recognize both haematological tumours and solid tumours, but the receptors involved may be different since the expression of ligands varies in tumours of different origins. Moreover, variations in the expression of ligands are also observed among patients reflecting either different stages of tumour transformation or immune escape mechanisms to avoid NK cell recognition. Current insights into the mechanisms that regulate NK cell function suggest that it may be possible to develop novel NK cell-based immunotherapeutic strategies against cancer. However, considering the heterogeneity of NK cell receptors and their ligands, for an efficient use of NK cells in a clinical setting, the variations in the expression of ligands for activating receptors among AML patients should be considered to select functionally competent NK cells. Since downregulation of activating receptors has been observed in different types of tumours, new strategies based in the induction of these receptors on NK cells and/or their ligands on tumour cells may open new opportunities in the treatment of tumours.
Assessment of the NK cell phenotype and function in AML patients at diagnosis and after achieving complete remission will allow to identify new prognosis markers and to develop NK cell-based therapies leading to enhance NK cell elimination of AML blasts. In addition, new insights on the effect of age on NK cells and its relevance in elderly AML patients are necessary to implement novel therapies for this group of patients. Future studies on large patient cohorts and longer follow-up periods will be of interest to analyse patient survival and its relationship with the phenotype and functional capacity of NK cells. Finally, since combined therapies are frequently used for the treatment of AML patients, their effect on NK cell function should also be considered.
Acknowledgments
We apologize to our colleagues whose work was not cited due to space limitations. This work was supported by grants SAF2006-03687 and SAF2009-09711 (to RT) from the Ministry of Science and Innovation of Spain, FIS PI061320 and PS09/00723 (to R.S.) from Spanish Ministry of Health, PRI09A029 and grants to INPATT research group from Junta de Extremadura (GRU08077, GRU09156 and GRU10104) and from University of Extremadura, cofinanced by European Regional Development Fund (FEDER). BSC is a postdoctoral fellow supported by grant ACCIII/22 from University of Extremadura Spain, and SM is a predoctoral fellow supported by grant PRE07047 from Junta de Extremadura. We thank J.J. Gordillo and M.R. Gonzalez and for their technical assistance in cell culture and flow cytometry.
Footnotes
Beatriz Sanchez-Correa and Sara Morgado contributed equally to the manuscript.
This paper is a Focussed Research Review based on a presentation given at the Tenth International Conference on Progress in Vaccination against Cancer (PIVAC 10), held in St. Catharine’s College, Cambridge, UK, 27th–30th September 2010. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagne F, Ugolini S, Vivier E. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Bjorkstrom NK, Ljunggren HG, Sandberg JK. CD56 negative NK cells: origin, function, and role in chronic viral disease. Trends Immunol. 2010;31:401–406. doi: 10.1016/j.it.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Boles KS, Stepp SE, Bennett M, Kumar V, Mathew PA. 2B4 (CD244) and CS1: novel members of the CD2 subset of the immunoglobulin superfamily molecules expressed on natural killer cells and other leukocytes. Immunol Rev. 2001;181:234–249. doi: 10.1034/j.1600-065X.2001.1810120.x. [DOI] [PubMed] [Google Scholar]
- 4.Borrego F, Masilamani M, Marusina AI, Tang X, Coligan JE. The CD94/NKG2 family of receptors: from molecules and cells to clinical relevance. Immunol Res. 2006;35:263–278. doi: 10.1385/IR:35:3:263. [DOI] [PubMed] [Google Scholar]
- 5.Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N, Vitale M, Moretta L, Lopez M, Moretta A. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198:557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, Moretta A, West R, Xu W, Vivier E, Levin SD. The B7 family member B7–H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun M, Muller B, Ter MD, Raffegerst S, Simm B, Wilde S, Spranger S, Ellwart J, Mosetter B, Umansky L, Lerchl T, Schendel DJ, Falk CS (2010) The CD6 scavenger receptor is differentially expressed on a CD56 natural killer cell subpopulation and contributes to natural killer-derived cytokine and chemokine secretion. J Innate Immun E-pub ahead of print [DOI] [PubMed]
- 8.Bryceson YT, Long EO. Line of attack: NK cell specificity and integration of signals. Curr Opin Immunol. 2008;20:344–352. doi: 10.1016/j.coi.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess SJ, Maasho K, Masilamani M, Narayanan S, Borrego F, Coligan JE. The NKG2D receptor: immunobiology and clinical implications. Immunol Res. 2008;40:18–34. doi: 10.1007/s12026-007-0060-9. [DOI] [PubMed] [Google Scholar]
- 10.Campbell KS, Purdy AK. Structure/function of human killer cell immunoglobulin-like receptors: lessons from polymorphisms, evolution, crystal structures and mutations. Immunology. 2011;132:315–325. doi: 10.1111/j.1365-2567.2010.03398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlsten M, Baumann BC, Simonsson M, Jadersten M, Forsblom AM, Hammarstedt C, Bryceson YT, Ljunggren HG, Hellstrom-Lindberg E, Malmberg KJ. Reduced DNAM-1 expression on bone marrow NK cells associated with impaired killing of CD34+ blasts in myelodysplastic syndrome. Leukemia. 2010;24:1607–1616. doi: 10.1038/leu.2010.149. [DOI] [PubMed] [Google Scholar]
- 12.Carlsten M, Norell H, Bryceson YT, Poschke I, Schedvins K, Ljunggren HG, Kiessling R, Malmberg KJ. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J Immunol. 2009;183:4921–4930. doi: 10.4049/jimmunol.0901226. [DOI] [PubMed] [Google Scholar]
- 13.Casado JG, Pawelec G, Morgado S, Sanchez-Correa B, Delgado E, Gayoso I, Duran E, Solana R, Tarazona R. Expression of adhesion molecules and ligands for activating and costimulatory receptors involved in cell-mediated cytotoxicity in a large panel of human melanoma cell lines. Cancer Immunol Immunother. 2009;58:1517–1526. doi: 10.1007/s00262-009-0682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235:267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan A, Hong DL, Atzberger A, Kollnberger S, Filer AD, Buckley CD, McMichael A, Enver T, Bowness P. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J Immunol. 2007;179:89–94. doi: 10.4049/jimmunol.179.1.89. [DOI] [PubMed] [Google Scholar]
- 16.Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, Gastaut JA, Pende D, Olive D, Moretta A. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99:3661–3667. doi: 10.1182/blood.V99.10.3661. [DOI] [PubMed] [Google Scholar]
- 17.Derhovanessian E, Solana R, Larbi A, Pawelec G. Immunity, ageing and cancer. Immun Ageing. 2008;5:11. doi: 10.1186/1742-4933-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diermayr S, Himmelreich H, Durovic B, Mathys-Schneeberger A, Siegler U, Langenkamp U, Hofsteenge J, Gratwohl A, Tichelli A, Paluszewska M, Wiktor-Jedrzejczak W, Kalberer CP, Wodnar-Filipowicz A. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK-cell lines with single KIR-HLA class I specificities. Blood. 2008;111:1428–1436. doi: 10.1182/blood-2007-07-101311. [DOI] [PubMed] [Google Scholar]
- 19.Eagle RA, Trowsdale J. Promiscuity and the single receptor: NKG2D. Nat Rev Immunol. 2007;7:737–744. doi: 10.1038/nri2144. [DOI] [PubMed] [Google Scholar]
- 20.El-Sherbiny YM, Meade JL, Holmes TD, McGonagle D, Mackie SL, Morgan AW, Cook G, Feyler S, Richards SJ, Davies FE, Morgan GJ, Cook GP. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 2007;67:8444–8449. doi: 10.1158/0008-5472.CAN-06-4230. [DOI] [PubMed] [Google Scholar]
- 21.Elishmereni M, Levi-Schaffer F. CD48: a co-stimulatory receptor of immunity. Int J Biochem Cell Biol. 2011;43:25–28. doi: 10.1016/j.biocel.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Epling-Burnette PK, Bai F, Painter JS, Rollison DE, Salih HR, Krusch M, Zou J, Ku E, Zhong B, Boulware D, Moscinski L, Wei S, Djeu JY, List AF. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood. 2007;109:4816–4824. doi: 10.1182/blood-2006-07-035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006;20:123–137. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, Costello RT. Deficient expression of NCR in NK cells from acute myeloid leukemia: Evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood. 2007;109:323–330. doi: 10.1182/blood-2005-08-027979. [DOI] [PubMed] [Google Scholar]
- 25.Gayoso I, Sanchez-Correa B, Campos C, Alonso C, Pera A, Casado JG, Morgado S, Tarazona R, Solana R (2011) Immunosenescence of human natural killer cells. J Innate Immun E-pub ahead of print [DOI] [PubMed]
- 26.Gilfillan S, Chan CJ, Cella M, Haynes NM, Rapaport AS, Boles KS, Andrews DM, Smyth MJ, Colonna M. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J Exp Med. 2008;205:2965–2973. doi: 10.1084/jem.20081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 28.Hecht ML, Rosental B, Horlacher T, Hershkovitz O, De Paz JL, Noti C, Schauer S, Porgador A, Seeberger PH. Natural cytotoxicity receptors NKp30, NKp44 and NKp46 bind to different heparan sulfate/heparin sequences. J Proteome Res. 2009;8:712–720. doi: 10.1021/pr800747c. [DOI] [PubMed] [Google Scholar]
- 29.Held W, Kijima M, Angelov GS, Bessoles S. The function of natural killer cells: education, reminders and some good memories. Curr Opin Immunol. 2011;23:228–233. doi: 10.1016/j.coi.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Jabbour EJ, Estey E, Kantarjian HM. Adult acute myeloid leukemia. Mayo Clin Proc. 2006;81:247–260. doi: 10.4065/81.2.247. [DOI] [PubMed] [Google Scholar]
- 31.Kalinski P, Mailliard RB, Giermasz A, Zeh HJ, Basse P, Bartlett DL, Kirkwood JM, Lotze MT, Herberman RB. Natural killer-dendritic cell cross-talk in cancer immunotherapy. Expert Opin Biol Ther. 2005;5:1303–1315. doi: 10.1517/14712598.5.10.1303. [DOI] [PubMed] [Google Scholar]
- 32.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 33.Lakshmikanth T, Burke S, Ali TH, Kimpfler S, Ursini F, Ruggeri L, Capanni M, Umansky V, Paschen A, Sucker A, Pende D, Groh V, Biassoni R, Hoglund P, Kato M, Shibuya K, Schadendorf D, Anichini A, Ferrone S, Velardi A, Karre K, Shibuya A, Carbone E, Colucci F. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest. 2009;119:1251–1263. doi: 10.1172/JCI36022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ljunggren HG, Karre K. In search of the ‘missing self: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-S. [DOI] [PubMed] [Google Scholar]
- 35.Malmberg KJ, Bryceson YT, Carlsten M, Andersson S, Bjorklund A, Bjorkstrom NK, Baumann BC, Fauriat C, Alici E, Dilber MS, Ljunggren HG. NK cell-mediated targeting of human cancer and possibilities for new means of immunotherapy. Cancer Immunol Immunother. 2008;57:1541–1552. doi: 10.1007/s00262-008-0492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathew SO, Rao KK, Kim JR, Bambard ND, Mathew PA. Functional role of human NK cell receptor 2B4 (CD244) isoforms. Eur J Immunol. 2009;39:1632–1641. doi: 10.1002/eji.200838733. [DOI] [PubMed] [Google Scholar]
- 37.Mavilio D, Lombardo G, Benjamin J, Kim D, Follman D, Marcenaro E, O’Shea MA, Kinter A, Kovacs C, Moretta A, Fauci AS. Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci USA. 2005;102:2886–2891. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGilvray RW, Eagle RA, Watson NF, Al-Attar A, Ball G, Jafferji I, Trowsdale J, Durrant LG. NKG2D ligand expression in human colorectal cancer reveals associations with prognosis and evidence for immunoediting. Clin Cancer Res. 2009;15:6993–7002. doi: 10.1158/1078-0432.CCR-09-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNerney ME, Lee KM, Kumar V. 2B4 (CD244) is a non-MHC binding receptor with multiple functions on natural killer cells and CD8+ T cells. Mol Immunol. 2005;42:489–494. doi: 10.1016/j.molimm.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 40.Mendez R, Aptsiauri N, Del CA, Maleno I, Cabrera T, Ruiz-Cabello F, Garrido F, Garcia-Lora A. HLA and melanoma: multiple alterations in HLA class I and II expression in human melanoma cell lines from ESTDAB cell bank. Cancer Immunol Immunother. 2009;58:1507–1515. doi: 10.1007/s00262-009-0701-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgado S, Sanchez-Correa B, Casado JG, Duran E, Gayoso I, Labella F, Solana R, Tarazona R (2011) NK cell recognition and killing of melanoma cells is controlled by multiple activating receptor-ligand interactions. J Innate Immun E-pub ahead of print [DOI] [PubMed]
- 42.Naumova E, Mihaylova A, Ivanova M, Mihailova S. Impact of KIR/HLA ligand combinations on immune responses in malignant melanoma. Cancer Immunol Immunother. 2007;56:95–100. doi: 10.1007/s00262-006-0151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen S, Beziat V, Roos-Weil D, Vieillard V (2011) Role of natural killer cells in hematopoietic stem cell transplantation: myth or reality? J Innate Immun E-pub ahead of print [DOI] [PubMed]
- 44.Nowbakht P, Ionescu MC, Rohner A, Kalberer CP, Rossy E, Mori L, Cosman D, De LG, Wodnar-Filipowicz A. Ligands for natural killer cell-activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood. 2005;105:3615–3622. doi: 10.1182/blood-2004-07-2585. [DOI] [PubMed] [Google Scholar]
- 45.Ofori-Acquah SF, King JA. Activated leukocyte cell adhesion molecule: a new paradox in cancer. Transl Res. 2008;151:122–128. doi: 10.1016/j.trsl.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell. 2010;142:847–856. doi: 10.1016/j.cell.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pawelec G, Solana R. Are cancer and ageing different sides of the same coin? Conference on Cancer and Ageing. EMBO Rep. 2008;9:234–238. doi: 10.1038/embor.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pende D, Bottino C, Castriconi R, Cantoni C, Marcenaro S, Rivera P, Spaggiari GM, Dondero A, Carnemolla B, Reymond N, Mingari MC, Lopez M, Moretta L, Moretta A. PVR (CD155) and Nectin-2 (CD112) as ligands of the human DNAM-1 (CD226) activating receptor: involvement in tumor cell lysis. Mol Immunol. 2005;42:463–469. doi: 10.1016/j.molimm.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 49.Pende D, Cantoni C, Rivera P, Vitale M, Castriconi R, Marcenaro S, Nanni M, Biassoni R, Bottino C, Moretta A, Moretta L. Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur J Immunol. 2001;31:1076–1086. doi: 10.1002/1521-4141(200104)31:4<1076::AID-IMMU1076>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 50.Pende D, Spaggiari GM, Marcenaro S, Martini S, Rivera P, Capobianco A, Falco M, Lanino E, Pierri I, Zambello R, Bacigalupo A, Mingari MC, Moretta A, Moretta L. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112) Blood. 2005;105:2066–2073. doi: 10.1182/blood-2004-09-3548. [DOI] [PubMed] [Google Scholar]
- 51.Peralbo E, DelaRosa O, Gayoso I, Pita ML, Tarazona R, Solana R. Decreased frequency and proliferative response of invariant Valpha24Vbeta11 natural killer T (iNKT) cells in healthy elderly. Biogerontology. 2006;7:483–492. doi: 10.1007/s10522-006-9063-5. [DOI] [PubMed] [Google Scholar]
- 52.von Pogge SE, Simhadri VR, von Tresckow B, Sasse S, Reiners KS, Hansen HP, Rothe A, Boll B, Simhadri VL, Borchmann P, McKinnon PJ, Hallek M, Engert A. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27:965–974. doi: 10.1016/j.immuni.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 53.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 54.Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, Steinle A. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102:1389–1396. doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- 55.Salih HR, Holdenrieder S, Steinle A. Soluble NKG2D ligands: prevalence, release, and functional impact. Front Biosci. 2008;13:3448–3456. doi: 10.2741/2939. [DOI] [PubMed] [Google Scholar]
- 56.Sanchez CJ, Le TT, Boehrer A, Knoblauch B, Imbert J, Olive D, Costello RT. Natural killer cells and malignant haemopathies: a model for the interaction of cancer with innate immunity. Cancer Immunol Immunother. 2011;60:1–13. doi: 10.1007/s00262-010-0898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanchez-Correa B, Gayoso I, Bergua JM, Casado JG, Morgado S, Solana R, Tarazona R (2011) Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol Cell Biol E-pub ahead of print [DOI] [PubMed]
- 58.Sivori S, Parolini S, Falco M, Marcenaro E, Biassoni R, Bottino C, Moretta L, Moretta A. 2B4 functions as a co-receptor in human NK cell activation. Eur J Immunol. 2000;30:787–793. doi: 10.1002/1521-4141(200003)30:3<787::AID-IMMU787>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 59.Solana R, Casado JG, Delgado E, DelaRosa O, Marin J, Duran E, Pawelec G, Tarazona R. Lymphocyte activation in response to melanoma: interaction of NK-associated receptors and their ligands. Cancer Immunol Immunother. 2007;56:101–109. doi: 10.1007/s00262-006-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solana R, Pawelec G, Tarazona R. Aging and innate immunity. Immunity. 2006;24:491–494. doi: 10.1016/j.immuni.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szczepanski MJ, Szajnik M, Welsh A, Foon KA, Whiteside TL, Boyiadzis M. Interleukin-15 enhances natural killer cell cytotoxicity in patients with acute myeloid leukemia by upregulating the activating NK cell receptors. Cancer Immunol Immunother. 2010;59:73–79. doi: 10.1007/s00262-009-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tahara-Hanaoka S, Shibuya K, Kai H, Miyamoto A, Morikawa Y, Ohkochi N, Honda S, Shibuya A. Tumor rejection by the poliovirus receptor family ligands of the DNAM-1 (CD226) receptor. Blood. 2006;107:1491–1496. doi: 10.1182/blood-2005-04-1684. [DOI] [PubMed] [Google Scholar]
- 64.Tarazona R, Casado JG, DelaRosa O, Torre-Cisneros J, Villanueva JL, Sanchez B, Galiani MD, Gonzalez R, Solana R, Pena J. Selective depletion of CD56(dim) NK cell subsets and maintenance of CD56(bright) NK cells in treatment-naive HIV-1-seropositive individuals. J Clin Immunol. 2002;22:176–183. doi: 10.1023/A:1015476114409. [DOI] [PubMed] [Google Scholar]
- 65.Tripathy SK, Keyel PA, Yang L, Pingel JT, Cheng TP, Schneeberger A, Yokoyama WM. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J Exp Med. 2008;205:1829–1841. doi: 10.1084/jem.20072446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vaidya SV, Mathew PA. Of mice and men: different functions of the murine and human 2B4 (CD244) receptor on NK cells. Immunol Lett. 2006;105:180–184. doi: 10.1016/j.imlet.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 67.Velardi A, Ruggeri L, Moretta A, Moretta L. NK cells: a lesson from mismatched hematopoietic transplantation. Trends Immunol. 2002;23:438–444. doi: 10.1016/S1471-4906(02)02284-6. [DOI] [PubMed] [Google Scholar]
- 68.Verheyden S, Bernier M, Demanet C. Identification of natural killer cell receptor phenotypes associated with leukemia. Leukemia. 2004;18:2002–2007. doi: 10.1038/sj.leu.2403525. [DOI] [PubMed] [Google Scholar]
- 69.Verheyden S, Demanet C. Susceptibility to myeloid and lymphoid leukemia is mediated by distinct inhibitory KIR-HLA ligand interactions. Leukemia. 2006;20:1437–1438. doi: 10.1038/sj.leu.2404279. [DOI] [PubMed] [Google Scholar]
- 70.Verheyden S, Demanet C. NK cell receptors and their ligands in leukemia. Leukemia. 2008;22:249–257. doi: 10.1038/sj.leu.2405040. [DOI] [PubMed] [Google Scholar]
- 71.Verheyden S, Ferrone S, Mulder A, Claas FH, Schots R, De Moerloose B, Benoit Y, Demanet C. Role of the inhibitory KIR ligand HLA-Bw4 and HLA-C expression levels in the recognition of leukemic cells by natural Killer cells. Cancer Immunol Immunother. 2009;58:855–865. doi: 10.1007/s00262-008-0601-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Verneris MR, Miller JS. The phenotypic and functional characteristics of umbilical cord blood and peripheral blood natural killer cells. Br J Haematol. 2009;147:185–191. doi: 10.1111/j.1365-2141.2009.07768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 74.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 76.Wehner R, Dietze K, Bachmann M, Schmitz M (2011) The bidirectional crosstalk between human dendritic cells and natural killer cells. J Innate Immun E-pub ahead of print [DOI] [PubMed]