Abstract
Oropharyngeal cancer (OPC) is a type of squamous cell head and neck cancer that is often associated with human papillomavirus (HPV) infection, suggesting the potential for immunotherapeutic targeting of HPV antigens. This study aimed to determine the effect of radical therapy on HPV-specific T cells and other immune parameters in 20 OPC patients, as a prelude to future immunotherapy studies. HPV DNA could be detected in 9/12 available tissue samples (8/9 HPV+ samples were also p16+). HPV-specific T cell responses against HPV16 E6 and E7 peptides were detected by enzyme-linked immunoSPOT in 10/13 and 8/13 evaluable patients, respectively, but did not appear to correlate with HPV status. Post-treatment, both HPV E6 and E7 T cell responses were decreased (4/13 and 2/13 patients, respectively). These reductions in T cell response could not be explained by a concurrent decrease in memory T cells whose absolute numbers were relatively unaffected by radical therapy (27,975 vs. 25,661/105 PBMC) despite a significant decrease in overall lymphocyte counts (1.74 vs. 0.69 × 109/L). Instead, there were significant increases in regulatory T cells (3.7 vs. 6.8 %) and a population of myeloid-derived suppressor cells (CD14−HLA-DR−CD15hi, 12.38 vs. 21.92 %). This suggests that immunosuppression may contribute to the reduction in HPV-specific T cell responses post-treatment, although study of larger patient cohorts will be required to test whether this affects clinical outcome. Overall these findings suggest that HPV-targeted immunotherapy in post-therapy OPC patients will require multiple strategies to boost T cell immunity and to overcome the influence of immunosuppressive cells.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1488-5) contains supplementary material, which is available to authorized users.
Keywords: OPC, HPV, T cells, Immunosuppression
Introduction
The increased incidence of oropharyngeal cancer (OPC), a squamous cell carcinoma affecting the tonsils, tongue base and soft palate, has been described as an epidemic [1]. In 2009, 1,346 patients were diagnosed with OPC in the UK and incidence rates are still rising [1, 2].
Human papilloma virus (HPV) is an oncogenic DNA virus that is commonly associated with cancers of the cervix, anus, vulva and penis and is now widely accepted as a key aetiological agent in the development of OPC [3, 4]. HPV has two major oncoproteins, E6 and E7, which functionally inactivate the tumour suppressor proteins p53 (leading to loss of p53-dependent apoptosis) and retinoblastoma (resulting in loss of cell cycle control), respectively, leading to malignant transformation [5].
Despite its oncogenic potential, it is well documented that HPV+ OPC has a better prognosis than HPV− OPC [6, 7]. HPV+ tumours respond better to induction chemotherapy and chemoradiotherapy compared to HPV− tumours and are associated with longer overall survival. One likely explanation for this is immune involvement, triggered by the presence of ‘non-self’ HPV antigens, E6 and E7. There is indeed strong evidence that immune parameters are a key-determining factor in disease outcome. Pre-clinical models have shown that chemoradiotherapy induces tumour regression preferentially in immune competent mice [8]. Furthermore, limited immune cell infiltration of tumours is associated with decreased overall survival and increased locoregional recurrences in head and neck cancer (HNC) patients [9], and HPV+ tumours often show strong T cell infiltration compared to HPV− tumours [10, 11]. Immune engagement with HPV is also demonstrated by the presence of functional HPV-specific T cells and circulating antibodies to HPV16 in patients [10, 12, 13].
These observations strengthen the argument that there is a role for T cell-mediated immunity in HPV+ OPC. However, such studies focus on patients at time of diagnosis, prior to treatment. Despite much interest in immunotherapeutic vaccine development for the treatment for HPV+ OPC [14–16], little is known about changes in HPV-specific immune responses and immune cell phenotype in OPC patients undergoing radical (potentially curative) treatment. This is particularly important as there is no consensus on whether curative therapy in HNC patients favours or hinders anti-tumour immunity [17]. One study has looked at the differences in intratumoural immune profiling pre- and post-chemoradiotherapy and reported that immunosuppressive regulatory T cells (Tregs) were depleted to a greater extent than cytotoxic T cells (CTL) and this shift towards a more cytotoxic response was associated with better disease free survival [1, 18]. However, antigen specificity was not determined.
Immunosuppressive cells of relevance to HNC are Tregs and myeloid-derived suppressor cells (MDSC). Tregs are correlated with poor prognosis in HNC patients [2, 19, 20]. They have been shown to inhibit T cell function via mechanisms including the induction of T cell apoptosis via Fas/FasL pathway [3, 4, 21] and ATP hydrolysis, generating immunosuppressive adenosine [5, 22]. MDSC are a heterogeneous population of myeloid cells with diverse tumorigenic and immunosuppressive mechanisms [6, 7, 23]. Their induction, expansion and function have been linked to various inflammatory cytokines and growth factors, some of which have been found in higher levels in the serum of HNC patients with progressive disease [8, 24]. In HNC patients and mouse models, MDSC were shown to suppress T cell function via release of reactive oxygen intermediates, nitric oxide and arginine depletion [9, 25, 26]. They have also been implicated in the induction and expansion of Tregs in cancer patients [10, 11, 27]. Elucidating their frequency post-therapy is also particularly useful for vaccine design as both have been described as significant barriers to immunotherapy [1, 10, 12, 13, 28].
This study addresses, for the first time, treatment-induced changes in HPV-specific T cell responses as well as frequencies of pro-inflammatory memory T cells and immunosuppressive cell populations in the peripheral blood of OPC patients.
Materials and methods
Patient profile
Ethical approval was gained from the South East Wales Research Ethics Committee, and this study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
A total of 20 patients, diagnosed with OPC between 18/10/2010 and 07/10/2011 at the University Hospital of Wales, Cardiff (10 patients) and the Royal Gwent Hospital, Newport (10 patients) were recruited. The mean age of patients was 56 years, and the male–female ratio was 4:1. While patients were not selected on the basis of the stage of their disease, all 20 patients had stage IVa or IVb disease with no distant metastatic disease.
All patients were treated radically; 15 patients received radical chemoradiotherapy (CRT) to a total dose of 66 Gy in 30 daily fractions; 1 patient had concurrent EGFR monoclonal antibody (cetuximab) and radiotherapy to the same dose; 4 patients received primary surgical treatment with post-operative radiotherapy of 60 Gy in 30 daily fractions, with concurrent chemotherapy in 2 patients. Healthy individuals were included as controls for flow cytometry.
Isolation of peripheral blood mononuclear cells (PBMC)
Venepuncture (with informed consent) of 50 ml of blood was performed at two time points, prior to commencing treatment and median 9 weeks and 3 days (range 6 weeks 4 days to 16 weeks 5 days) after completion. At each time point, a full blood count (FBC) was determined. Blood was transferred into heparinised tubes and immediately transported at room temperature to the laboratory for separation. PBMC were isolated by density gradient centrifugation on Histopaque (Sigma, UK). Cells were cryopreserved at a cell density of 3–5 × 106 cells/vial in 1 ml freezing media comprising 40 % complete RPMI 1640 media (supplemented with 2 mM glutamine, 100 μg/ml streptomycin, 100 U/ml penicillin and 10 mM HEPES; Invitrogen, UK), 50 % foetal calf serum (Invitrogen, UK) and 10 % DMSO (Sigma, UK). PBMC from patient and healthy blood were cryopreserved and only assayed once all longitudinal samples had been collected.
Enzyme-linked immunoSPOT (ELISPOT) assay
The ELISPOT protocol was adapted from Smith et al. [2, 14–16, 29]. PBMC samples pre- and post-therapy from each patient were tested in the same experiment. PBMC (2 × 106) were seeded in a 24-well plate (BD Biosciences, UK) before addition of 15mer HPV16 E6 (25 peptides) or E7 peptide pool (15 peptides), to a final concentration of 5 μg/ml. A positive peptide pool (PPP; 5 μg/ml; 18 peptides) containing peptides from influenza, human cytomegalovirus, Epstein–Barr virus and tetanus was used as a positive control. The peptide sequences used are detailed in a table in Online Resource 1. Following a three-day incubation, 20 IU/ml IL-2 (Novartis, UK) was added for a further 3 days. Then following a further day without IL-2, PBMC were harvested for setting up the ELISPOT assay.
A Multiscreen 96-well plate (Millipore, UK) was coated with IFN-γ capture antibody (Mabtech, Sweden), and 1 × 105 cells/well were seeded. As a negative control, cells were incubated either alone or with 1 × 105 autologous PBMC (used to calculate background). The test wells were incubated with autologous PBMC loaded with either E6, E7 or PPP peptides (5 μg/ml). A mitogen mix was also included, as a positive control for T cell integrity, comprising PHA (6 μg/ml), PMA (150 ng/ml), ionomycin (2.25 mg/ml) and concanavalin A (60 μg/ml). Each treatment condition was set up in triplicate. The ELISPOT plates were incubated for 18–20 h at 37 °C and developed following the manufacturer’s instructions (Mabtech, Sweden). The spots were counted using an AID ELISPOT plate reader (AID-Diagnostika, Germany).
To determine peptide-specific T cell responses, a modified version of a previous method [3, 4, 17, 30] was used. The mean + 2SD of controls wells (T cells + PBMC) was subtracted from the mean of experimental wells (T cells + PBMC + peptide). A positive response was assigned if this exceeded 10 spots per 105 input cells [5, 30]. Out of the 20 patients assayed, results from 13 patients were technically evaluable (clinical details summarised in Table 1). A supplemental data table (Online Resource 2) details the mean spot counts and standard deviations obtained from each test condition.
Table 1.
HPV status, clinical details and HPV-specific T cell responses of OPC patients in this study
| Patienta | p16b | HPV DNAc | Neoadjuvant Chemothxd | Definitive Platinum-based CRT | RT + Cetuximab (EGFR) | Primary Surgery Post-op RT | E6 or E7 T cell Resp.e |
|---|---|---|---|---|---|---|---|
| 2 | + | HPV16 | 0 | 1 | 0 | 0 | + |
| 3 | + | HPV16 | PF | 0 | 1 | 0 | + |
| 4 | + | HPV16 | TPF | 1 | 0 | 0 | + |
| 5 | + | HPV16 | TPF | 1 | 0 | 0 | + |
| 6 | + | HPV16 | 0 | 0 | 0 | 1 | + |
| 7 | + | NT | 0 | 1 | 0 | 0 | + |
| 8 | + | HPV16 | 0 | 0 | 0 | 1 | – |
| 9 | + | HPV16 | TPF | 1 | 0 | 0 | + |
| 16 | – | No HPV | TPF | 1 | 0 | 0 | + |
| 17 | – | No HPV | PF | 0 | 1 | 0 | + |
| 18 | + | No HPV | TPF | 1 | 0 | 0 | + |
| 19 | + | HPV16 | TPF | 0 | 1 | 0 | + |
| 20 | + | HPV16 | 0 | 1 | 0 | 0 | + |
aOnly typing results for patients tested for immune responses are shown. There was adequate biopsy material for a total of 17 patients; 13/17 tested positive for HPV DNA with 12/13 also testing positive for p16 expression
bExpression of p16 Ink4A as detected by immunohistochemistry
cPresence of HPV DNA in tumour tissue as detected by PCR; NT = not tested due to inadequate sample
dTPF = chemotherapy with docetaxel (T), cisplatin (P) and 5 fluorouracil (F), CRT = concurrent platinum-based chemotherapy and radiotherapy
ePeptide-specific response as detected by IFNγ ELISPOT, pre-therapy
Immunohistochemistry (IHC)
Immunohistochemistry for p16Ink4A, a surrogate marker for ‘biologically active’ HPV [3, 6, 7], was carried out by pathology departments at referring hospitals (University Hospital of Wales, Cardiff and Royal Gwent Hospital, Newport).
Polymerase chain reaction-enzyme-linked immunoassay (PCR-EIA)
Sectioning was performed with appropriate precautions to prevent inter-block DNA contamination (e.g. thorough cleaning of microtome, use of fresh blades). DNA was extracted from 2 × 10 μm sections of FFPE biopsies using the Qiagen FFPE Kit (Qiagen, Hilden, Germany). To control for contamination during sectioning, sections were cut from a blank paraffin block and processed in parallel with the tumour sections. Adequacy of extracted DNA was assessed by PCR for the human β-globin gene as previously described [8, 31].
HPV testing was performed by GP5 +/GP6 + PCR-ELISA [9, 32]. A two-tier method was applied with initial screening by PCR-ELISA using cocktails of high-risk or low-risk HPV type-specific probes, followed by a second PCR-ELISA of all high-risk-positive samples with 14 individual probes for HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68. PCR cycling conditions were 94 °C 4 min then 40 cycles of 94 °C 30 s, 40 °C 90 s, 72 °C 60 s followed by 72 °C for 4 min. Positive (CaSki HPV16 positive cell line DNA) and negative (water) controls were included.
Flow cytometry
Sufficient PBMC numbers were available from 16 of the 20 patients for phenotyping. Cells (4 × 105) were labelled with fluorochrome-conjugated antibodies to identify memory T cells (CD3, CD4, CD8, CD69, CD28 and CD45RA), MDSC (CD33, HLA-DR, CD124, CD15, CD14 and CD11b) and Tregs (CD3, CD4, CD25, Foxp3 and Ki67). All antibodies were purchased from Ebioscience (UK). Cells were stained in a staining buffer comprising of PBS supplemented with 2 % FBS and 1 mM EDTA. Cells stained for T cell and MDSC phenotypes were incubated with antibodies on ice for 40 min. For Treg staining, cells were first stained for CD3, CD4, and CD25 on ice for 40 min and following washing, cells were stained for intracellular Foxp3, using the Foxp3 staining kit according to the manufacturer’s instructions (Ebioscience). Analysis was carried out on a BD FACSCanto flow cytometer using FACSDiva software.
Statistics
Statistical significance of changes in HPV-specific T cell responses and immune cell phenotype, pre- and post-treatment, was determined with a paired t test and 95 % confidence interval, using GraphPad Prism software.
Results
HPV-specific T cell responses were diminished post-treatment in the majority of patients
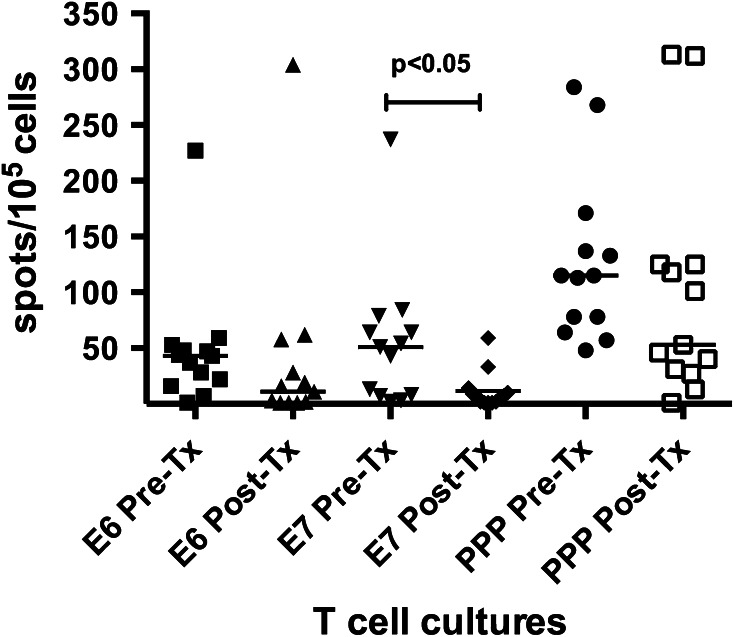
Overall, T cell responses to HPV16 E6 and E7 peptides were detected pre-treatment in 77 % (10/13) and 62 % (8/13) of patients, respectively. For evaluated patients as a whole, there was a trend towards decreased ELISPOT responses (Fig. 1), with a significant decrease in E7 peptide responses (p < 0.05, Fig. 1). More detailed breakdown of the responses showed that the number of patients with post-treatment T cell responses to E6 and E7 peptides had decreased: E6 peptide responses fell from 77 % (10/13) pre-treatment to 31 % (4/13) post-treatment and E7 responses fell from 62 % (8/13) pre-treatment to 15 % (2/13) post-treatment (Online Resource 2).
Fig. 1.
Scatter plot of all evaluable patients tested for HPV16 E6 and E7, and PPP-peptide-specific T cell responses by IFN-γ ELISPOT. Each dot represents a mean spot count for the replicate test wells (T cells + PBMC + peptide) after subtraction of the background (T cells + PBMC). The horizontal bars indicate the means for all patients (n = 13)
Recall antigen (PPP) responses were higher in magnitude compared to HPV16 responses pre- and post-treatment, but more variable between patients (Fig. 1, Online Resource 2). Some patients (2/13) did not have demonstrable PPP responses due to high background responses. There was a trend towards lower magnitude PPP responses (Fig. 1) post-treatment; however, the number of positive responses (11/13) remained the same pre- and post-treatment (Online Resource 2). Representative T cell responses against HPV16 peptides and PPP are shown graphically in supplemental data (Online Resource 3).
HPV-specific T cell responses did not correlate with tumour HPV status
To determine whether the ELISPOT data correlated with HPV status, we assessed HPV positivity in two ways; p16 overexpression as a surrogate marker detected by IHC and presence of HPV DNA as detected by GP5 +/6 + PCR-EIA [30].
Data summarising HPV status are shown in Table 1. Eighty-five percentage (11/13) of patient tumours overexpressed p16 by IHC. Only 12/13 patients had adequate DNA for HPV typing by GP5+/6 + PCR. In 75 % (9/12) of these patients, HPV DNA was detected in the tumour. In 92 % (11/12) of dually evaluable tumour blocks, p16 expression and HPV DNA were concordant, however, in patient 18 only positivity for p16 was observed.
When linking HPV status with the ELISPOT data (Table 1), we found that 67 % (8/12) of patients who had detectable HPV-specific T cell responses also had HPV+ tumours; however, three patients with HPV− tumours also displayed such responses. This suggests that a correlation is unlikely between HPV status of the tumour and T cell responses to HPV antigens in these patients.
Lymphocyte, but not monocyte, counts decrease following treatment
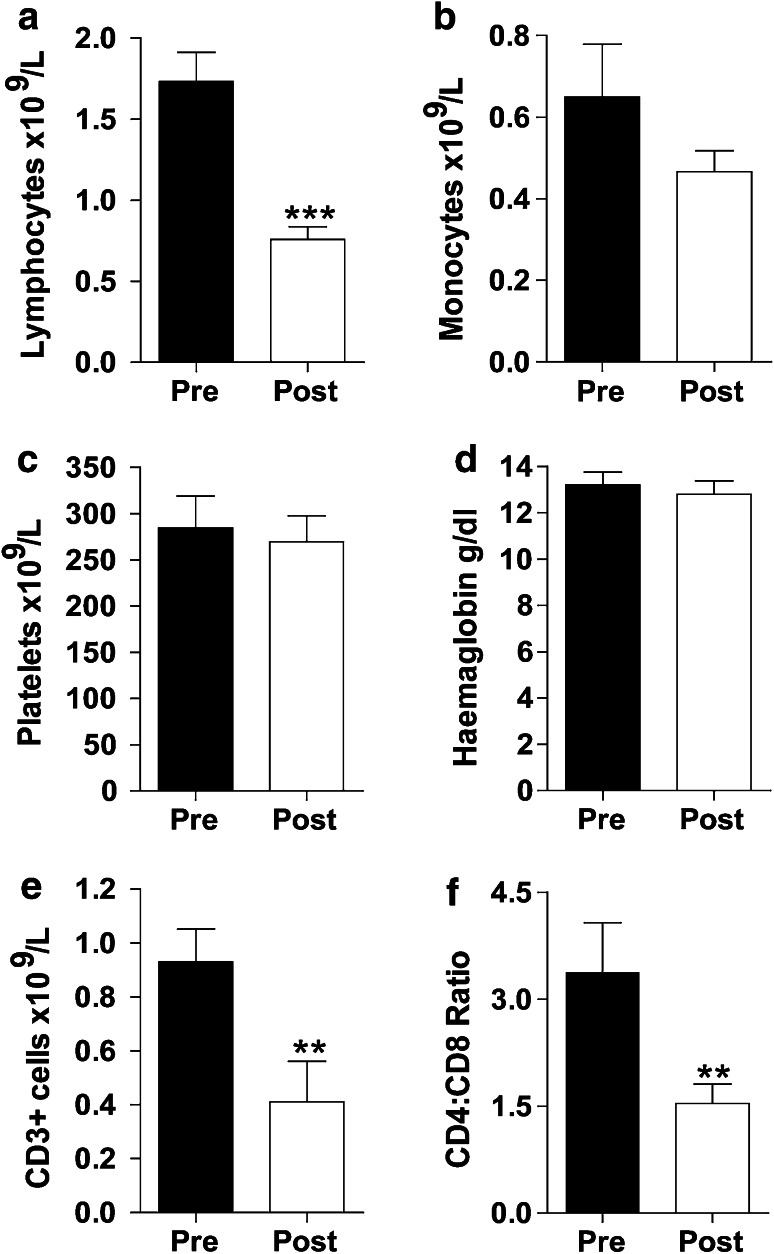
Radical therapy is commonly associated with lymphopaenia. As we were assessing T cell responses in these patients, it was important to determine treatment-related haematological changes. Total lymphocyte count was significantly reduced post-treatment (1.7 vs. 0.7 × 109/L, p < 0.001; Fig. 2a), while monocyte numbers (0.4 vs. 0.6 × 109/L; Fig. 2b), platelet counts (282 vs. 259 × 109/L; Fig. 2c) and haemoglobin (13.6 vs. 12.5 g/dl; Fig. 2d) remained more stable.
Fig. 2.
Changes in haematological parameters in OPC patients following radical therapy. The graphs show changes in a lymphocyte counts, b monocyte counts, c platelet counts, d haemoglobin levels, e CD3+ T cell numbers and f CD4:CD8 ratios both pre- and post-treatment. The lymphocyte, monocyte, platelet counts and haemoglobin levels were taken from the routine FBC in the clinic. The CD3+ T cell count was calculated using the CD3+ T cell frequencies (determined by flow cytometry) as a proportion of the lymphocytes. The CD4:CD8 ratios were calculated using the frequencies of CD4+ and CD8+ (single positives) within the CD3+ T cell population. Each data set represents n = 16 patients
In accordance with reduced lymphocyte numbers, the number of CD3+ T cells was also significantly reduced post-treatment (0.9 × 109/L vs. 0.4 × 109/L, p < 0.005; Fig. 2e). The CD4:CD8 ratio (within CD3+ T cells) pre-treatment was similar to that seen in healthy controls (3.58, n = 4). However, the CD4:CD8 ratios were significantly decreased by more than 50 % post-treatment (3.4 vs. 1.5, p < 0.005; Fig. 2f). Based on this analysis of absolute cell numbers, both CD4+ and CD8+ T cell numbers were decreased post-treatment; however, there was a larger decrease in CD4+ T cells relative to CD8+ T cells (3.3-fold vs. 1.4-fold decrease, data not shown).
Relative increase in memory T cell frequencies in patients post-treatment
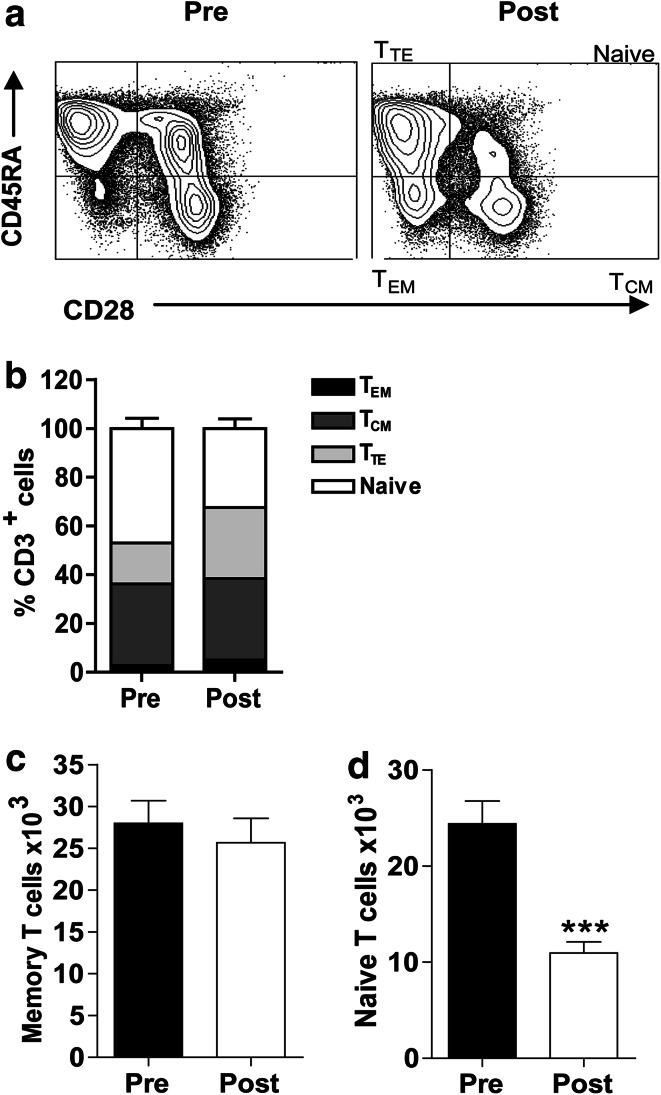
To investigate potential mechanisms for the decrease in HPV T cell responses post-treatment, we determined the effects of radical therapy on the frequencies of memory T cells, namely central memory (TCM; CD28+CD45RA−), effector memory (TEM; CD28−CD45RA−) and terminal effector (TTE; CD28−CD45RA+) memory cells [10, 11, 33]. The contour plots used to identify these subsets among CD3+ T cells are illustrated in Fig. 3a.
Fig. 3.
The effects of radical therapy on memory T cells. a A representative contour plot demonstrating the quadrants used to determine memory T cell subsets and naïve T cells within the CD3+ population. Changes in the frequencies of memory and naïve T cells (b) and in the absolute numbers of memory (c) and naïve (d) T cells, calculated as a proportion of 1x105 total PBMC pre- and post-treatment. Each data set represents n = 16 patients
The frequencies of all three CD3+ memory subsets increased post-treatment, of which, TEM and TTE cell frequencies were significantly increased (2.7 vs. 5.1 %, p < 0.05 and 16.8 vs. 29 % p < 0.05, respectively, Fig. 3b). Conversely, naïve T cell frequencies were significantly reduced (47 vs. 32.5 %, p < 0.0001). This demonstrates a preferential survival or expansion of memory T cells after treatment (Fig. 3b).
In order to determine whether the absolute number of memory T cells was reduced post-treatment, accompanying the decrease in CD3+ T cell frequencies, we calculated absolute numbers of the combined (TCM, TEM and TTE) CD3+ memory and naïve T cells as a proportion of 1 × 105 total PBMC (as seeded in the ELISPOT wells). Although a slight decrease in absolute memory T cell numbers was observed post-treatment, this was not statistically significant (27,975 vs. 25,661, Fig. 3c). There was, however, a very significant reduction in absolute naïve T cell numbers (24,385 vs. 10,966, p < 0.0001, Fig. 3d). This demonstrates that the naïve T cells were preferentially eliminated or matured by the radical treatment. Taken together, our results suggest that changes in memory T cell numbers/frequencies were not the primary reason for the observed decrease in E6- and E7-specific T cell frequencies after treatment.
Immunosuppressive cells were also expanded in patients post-treatment
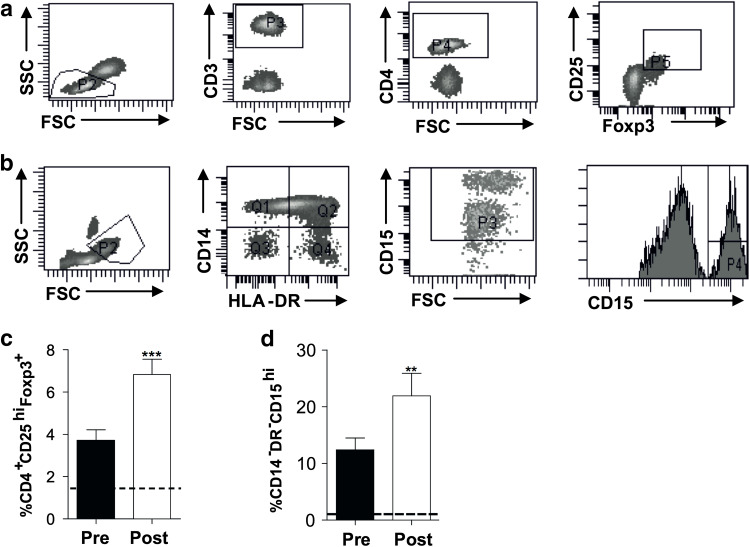
In order to determine whether reduced T cell responses post-treatment could result from tumour-associated immunosuppression, we assessed treatment-related differences in the frequencies of Tregs (CD4+CD25hiFoxp3+) and granulocytic MDSC (defined here as CD14−HLA-DR−CD15hi). Gating strategies for Tregs and MDSCs are shown in Fig. 4a, b.
Fig. 4.
The effects of radical therapy on immunosuppressive cells. The gating strategies used to identify a Tregs (CD3+CD4+CD25hiFoxp3+ cells within the lymphocyte gate) and b MDSC (CD14−HLA-DR− and CD15hi within the CD15+ population, to exclude lymphocytes within the monocyte gate). The frequencies of c Tregs and d MDSC were determined pre- and post-treatment. The dotted line indicates the frequency in healthy individuals. Each data set represents n = 16 patients
Following treatment, Treg frequency significantly increased by almost twofold (3.7 vs. 6.8 %, p < 0.0001; Fig. 4c). However, Treg proliferation, assessed by Ki67 expression, did not change (data not shown). We found that CD14−HLA-DR− cells overexpressing the carbohydrate adhesion molecule CD15 were greatly elevated in patients at baseline when compared to healthy individuals. Furthermore, these cells were significantly increased post-treatment (12.38 vs. 21.92 %, p < 0.005; Fig. 4d).
Our data demonstrate that Tregs and granulocytic MDSC (CD14−HLA-DR−CD15hi) were elevated in patients and increase in frequency post-treatment. Therefore, there are changes in the frequency of potentially immunosuppressive cells that may regulate T cell responses.
Discussion
This pilot study has shown that the frequency of systemic HPV16-specific T cells in the peripheral blood of 13 patients with OPC can fall dramatically following radical treatment. Furthermore, there was an expansion in the frequency of Tregs and MDSC post-treatment in 16 patients studied. While the patient numbers were small, the results suggest the potential for a more immunosuppressive environment in the periphery despite decreased tumour burden.
Functional HPV-specific T cells were detectable in the majority of OPC patients (92 %, 12/13). These cells were detectable in patients with both HPV+ (73 %, 8/11) and HPV− (100 %, 2/2) tumours. This study confirms previous findings detecting peripheral HPV-specific T cells prior to treatment in 48 % (10/21) of OPC patients [10]. In common with our study, they found that E6-specific T cells were more common than E7 (38 % E6 vs. 14 % E7 published; 77 % E6 vs. 62 % E7 this study) and that these T cells can be found in patients with HPV− tumours. Radical therapy decreased the numbers of patients for whom HPV E6- or E7-specific T cell responses could be detected (Online resource 2), although there were also patients for whom there was no significant change in HPV-specific T cell response post-therapy (e.g. HPV16 E6 for patient 9, Online Resource 2 and 3). The small number of patients studied, and the heterogeneity of their clinical characteristics, made it difficult to establish any correlations between HPV-specific immunity and disease burden (Online Resource 4).
It may be that the detection of HPV-specific T cells in HPV− patients is simply evidence of a previous viral encounter. Indeed, HPV-specific memory T cells can be found in healthy individuals with no obvious HPV-associated diseases [10, 12, 13, 30]. The fact that tumour formation can proceed in the presence of demonstrable HPV-specific T cell responses highlights the uncertainty that remains in understanding the role of the immune system in patients with HPV+ OPC. However, it should be noted that systemic T cell responses against HPV are relatively weak when compared to T cell responses against other tumour antigens, but can be readily boosted by vaccination for clinical benefit, e.g. in HPV-associated anogenital neoplasia [14–16, 34]. Alternatively, the inability of T cells to control disease may be a product of systemic elimination, attributed to spontaneous apoptosis in vivo, of tumour-reactive T cells, which has been described in HNC patients [17, 35–37]. This may be exacerbated by radical therapies involving chemoradiotherapy.
It has been argued that the favourable prognosis observed in HPV+ OPC vs. HPV− OPC may be due to favourable immune responses directed against non-self viral antigens. In support of this, recent studies have shown that there is a correlation between tumour-infiltrating CD4+ [18, 38] and CD8+ [19, 20, 39, 40] T cells and favourable prognosis. HPV16-specific T cells can be grown from tumour biopsies [10, 21], suggesting that at least some of the infiltrating T cells are HPV-specific.
The frequency of systemic HPV-specific T cell responses was diminished in patients post-treatment. These observations might be accounted for by decreased lymphocyte and CD3+ T cell counts post-treatment. However, accompanying this lymphopaenia was a redistribution of T cell subsets, for example, we have shown that the CD4:CD8 ratio decreases following therapy, which can be associated with improved overall survival in OPC patients [22, 41]. The decrease in this ratio was a reflection of a relative gain in CD8+ T cell numbers at the expense of CD4+ T cells, suggesting that CD4+ T cells might be more sensitive to the systemic effects of radical therapy. It is possible that global loss of CD4+ T cells may have affected the frequency of the HPV-specific T cells detected by ELISPOT. Such a global loss should also affect the detection of PPP-specific T cells, but there was no difference in the number of patients responding to PPP pre- and post-therapy.
The significant reduction in T cells was absorbed predominantly by the loss of naïve T cells, as the memory T cell fraction was significantly increased and only marginally decreased in absolute numbers. The increase in frequency of memory T cells relative to naïve T cells may be characteristic of the lymphopaenia induced by therapy, which may drive the differentiation of naïve T cells into memory T cells [23, 42]. Indeed, it has been proposed that lymphodepletion removes cellular elements that act as sinks for cytokines which in turn augments the activity of tumour-specific T cells [24, 43]. In HNC patients, chemoradiotherapy was also reported to induce antigen-specific T cell responses both in vitro [25, 26, 44] and ex vivo [27, 45], which may supplement the memory T cell pool.
Overall, in this relatively small group of patients, it was difficult to find any correlation between the results obtained from immune cell phenotyping and detection of HPV-specific T cell responses. The latter uses ELISPOT assays that are likely to be predominantly detecting CD4+ effector cells [29]. However, these assays also involve activation and culture of T cells in vitro. Multiple variables might influence the end result, including the survival of CD4+ T cells, the distribution and complexity of the CD4+ T cell subsets within each patient and the capacity of T cells to proliferate in vitro. For a deeper understanding of the role of HPV-specific T cells in disease, more direct methods that allow simultaneous phenotyping and quantitation of HPV-specific T cells ex vivo are required.
Changes in the immunosuppressive milieu following treatment may also explain reduced frequency or function of HPV-specific T cells. HNC are well characterised for supporting an immunosuppressive microenvironment [46]. However, recent studies have suggested the situation may be more complex, for example, associations have been found between increased frequencies of tumour-infiltrating CD4+ T cells such as Tregs with either improved locoregional control [47] or increased survival [38], depending on the study. The T cells infiltrating HNC are phenotypically complex, so multiple populations have the potential to influence clinical outcome. For example, increased frequencies of activated CD4+CD69+ T cells [47] and CD4+PD1+ T cells are associated with improved overall survival [38]. PD-1, a molecule associated with immune exhaustion in chronic viral infection, has been found to be highly expressed on the majority of tumour-infiltrating CD8+ T cells in HPV+ OPC [48]. But, PD1+ T cells can consist of T cells with both activation and exhaustion phenotypes [38], emphasising the care needed in correlating T cell markers with clinical outcome.
It is not clear whether T cells with the same apparent phenotypic markers can have different functions depending on their location (tumour vs. periphery), and how this relates to clinical prognosis. We found significantly elevated frequencies of systemic Tregs post-treatment. A previous cross-sectional study similarly reported an expansion of peripheral Tregs in response to chemotherapy and showed that they persisted post-treatment, even in the absence of detectable disease [19]. Importantly, that study showed that the systemic Tregs in OPC patients had potent suppressive function. Further studies are required to resolve this apparent dichotomy between tumour-infiltrating and systemic Tregs in OPC.
We identified a population of CD14−HLA-DR−CD15hi granulocytic MDSC that were elevated in patients following radical therapy. MDSC have been shown to inhibit T cells responses to both antigen and mitogen stimulation, and their inhibition through maturation can restore in vitro T cell responses [26]. Although MDSC were first isolated from the tumours of HNC patients, peripheral MDSC have not been extensively studied in HNC. Increased frequencies of MDSC correlate with poorer clinical prognosis in several human cancers [28]; therefore, it is likely that such cells will mediate immunosuppression in OPC patients. It is also possible that the increase in Treg frequencies may be linked to induction by MDSC [27]. While we showed a clear increase in MDSC post-therapy using phenotypic markers, it will be important to test the function of these cells in future studies. Murine models suggest that the function and activity of MDSC may depend on their anatomical location [49], and this may also be the case for human MDSC.
Our study points towards a potential increase in immunosuppressive influences post-radical treatment. In order to effectively target HPV-infected tumour cells after treatment, an immune response of considerably greater magnitude than the natural immune response identified in this study is needed. As a result, we have instigated a phase I trial termed ‘REALISTIC’ where adjuvant vaccination with HPV16 E7 protein expressed by live recombinant listeria (ADXS11-001) will be given to OPC patients following standard therapy. This trial will investigate the safest dose of vaccine that will induce strong systemic HPV16 E7-specific T cell responses. The current study has suggested multiple additional immune cell markers that could be investigated alongside HPV-specific T cell responses, to provide a more complete picture of the immunomodulating effects of this vaccine.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Lynne Rich for helping recruit patients into this study, Dr Andrew Caley for providing clinical information, and Drs Ryan Wong and Claudia Nunes for their help with the ELISPOT. This study was funded by Velindre NHS Trusts Small Grants Scheme. The research of Stephen Man, Ned Powell and Mererid Evans was funded in part by Cancer Research Wales. Research time for Mererid Evans is funded through a National Institute for Social Care and Health Research AHSC award.
Conflict of interest
All the authors declare that they have no potential conflicts of interest in relation to this study.
Footnotes
Saly Al-Taei and Russell Banner have contributed equally to the study and are joined as first authors.
References
- 1.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous (2012) Oral Cancer Incidence Statistics. In: cancerresearchuk.org. http://www.cancerresearchuk.org/cancer-info/cancerstats/types/oral/incidence/uk-oral-cancer-incidence-statistics. Accessed 19 Aug 2013
- 3.Begum S, Cao D, Gillison M, et al. Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res. 2005;11:5694–5699. doi: 10.1158/1078-0432.CCR-05-0587. [DOI] [PubMed] [Google Scholar]
- 4.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 5.Munger K, Baldwin A, Edwards KM, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78:11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 8.Spanos WC, Nowicki P, Lee DW, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1137–1146. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 9.Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29:167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 10.Heusinkveld M, Goedemans R, Briet RJ, et al. Systemic and local human papillomavirus 16-specific T-cell immunity in patients with head and neck cancer. Int J Cancer. 2012;131:E74–E85. doi: 10.1002/ijc.26497. [DOI] [PubMed] [Google Scholar]
- 11.Kong CS, Narasimhan B, Cao H, et al. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2009;74:553–561. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreimer AR, Clifford GM, Snijders PJ, et al. HPV16 semiquantitative viral load and serologic biomarkers in oral and oropharyngeal squamous cell carcinomas. Int J Cancer. 2005;115:329–332. doi: 10.1002/ijc.20872. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann TK, Arsov C, Schirlau K, et al. T cells specific for HPV16 E7 epitopes in patients with squamous cell carcinoma of the oropharynx. Int J Cancer. 2006;118:1984–1991. doi: 10.1002/ijc.21565. [DOI] [PubMed] [Google Scholar]
- 14.De Costa AM, Young MR. Immunotherapy for head and neck cancer: advances and deficiencies. Anticancer Drugs. 2011;22:674–681. doi: 10.1097/CAD.0b013e328340fd18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson HC, Leibowitz MS, Lopez-Albaitero A, Ferris RL. Immunotherapy for head and neck cancer. Oral Oncol. 2009;45:747–751. doi: 10.1016/j.oraloncology.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rapidis AD, Wolf GT. Immunotherapy of head and neck cancer: current and future considerations. J Oncol. 2009;2009:346345. doi: 10.1155/2009/346345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen CT, Judd NP, Bui JD, Uppaluri R. The clinical implications of antitumor immunity in head and neck cancer. Laryngoscope. 2012;122:144–157. doi: 10.1002/lary.21913. [DOI] [PubMed] [Google Scholar]
- 18.Distel L, Buttner M. Radiochemotherapy fosters a favorable pattern of inflammatory cells in head and neck tumors. Oncoimmunology. 2012;1:982–983. doi: 10.4161/onci.20200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strauss L, Bergmann C, Gooding W, et al. The frequency and suppressor function of CD4+ CD25 highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6301–6311. doi: 10.1158/1078-0432.CCR-07-1403. [DOI] [PubMed] [Google Scholar]
- 20.Schaefer C, Kim GG, Albers A, et al. Characteristics of CD4+ CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer. 2005;92:913–920. doi: 10.1038/sj.bjc.6602407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strauss L, Bergmann C, Whiteside TL. Human circulating CD4+ CD25highFoxp3+ regulatory T cells kill autologous CD8+ but not CD4+ responder cells by Fas-mediated apoptosis. J Immunol. 2009;182:1469–1480. doi: 10.4049/jimmunol.182.3.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandapathil M, Szczepanski MJ, Szajnik M, et al. Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin Cancer Res. 2009;15:6348–6357. doi: 10.1158/1078-0432.CCR-09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sevko A, Umansky V. Myeloid-derived suppressor cells interact with tumors in terms of myelopoiesis, tumorigenesis and immunosuppression: thick as thieves. J Cancer. 2013;4:3–11. doi: 10.7150/jca.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byers LA, Holsinger FC, Kies MS, et al. Serum signature of hypoxia-regulated factors is associated with progression after induction therapy in head and neck squamous cell cancer. Mol Cancer Ther. 2010;9:1755–1763. doi: 10.1158/1535-7163.MCT-09-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandau S, Trellakis S, Bruderek K, et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol. 2011;89:311–317. doi: 10.1189/jlb.0310162. [DOI] [PubMed] [Google Scholar]
- 26.Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoechst B, Ormandy LA, Ballmaier M, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Vasievich EA, Huang L. The suppressive tumor microenvironment: a challenge in cancer immunotherapy. Mol Pharm. 2011;8:635–641. doi: 10.1021/mp1004228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith KL, Tristram A, Gallagher KM, et al. Epitope specificity and longevity of a vaccine-induced human T cell response against HPV18. Int Immunol. 2005;17:167–176. doi: 10.1093/intimm/dxh197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welters MJ, de Jong A, van den Eeden SJ, et al. Frequent display of human papillomavirus type 16 E6-specific memory t-Helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 2003;63:636–641. [PubMed] [Google Scholar]
- 31.Hibbitts S, Rieck GC, Hart K, et al. Human papillomavirus infection: an anonymous prevalence study in South Wales, UK. Br J Cancer. 2006;95:226–232. doi: 10.1038/sj.bjc.6603245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobs MV, Snijders PJ, van den Brule AJ, et al. A general primer GP5+/GP6(+)-mediated PCR-enzyme immunoassay method for rapid detection of 14 high-risk and 6 low-risk human papillomavirus genotypes in cervical scrapings. J Clin Microbiol. 1997;35:791–795. doi: 10.1128/jcm.35.3.791-795.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weng NP, Araki Y, Subedi K. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nat Rev Immunol. 2012;12:306–315. doi: 10.1038/nri3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenter GG, Welters MJP, Valentijn ARPM, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 35.Kuss I, Donnenberg AD, Gooding W, Whiteside TL. Effector CD8+CD45RO-CD27-T cells have signalling defects in patients with squamous cell carcinoma of the head and neck. Br J Cancer. 2003;88:223–230. doi: 10.1038/sj.bjc.6600694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albers AE, Schaefer C, Visus C, et al. Spontaneous apoptosis of tumor-specific tetramer +CD8+ T lymphocytes in the peripheral circulation of patients with head and neck cancer. Head Neck. 2009;31:773–781. doi: 10.1002/hed.21031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffmann TK, Dworacki G, Tsukihiro T, et al. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553–2562. [PubMed] [Google Scholar]
- 38.Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 39.Jung AC, Guihard S, Krugell S, et al. CD8-alpha T-cell infiltration in human papillomavirus-related oropharyngeal carcinoma correlates with improved patient prognosis. Int J Cancer. 2012;132:E26–E36. doi: 10.1002/ijc.27776. [DOI] [PubMed] [Google Scholar]
- 40.Ward M, Mellows T, Riley C, et al. OP027. Oral Oncol. 2013;49:S14. doi: 10.1016/j.oraloncology.2013.03.035. [DOI] [Google Scholar]
- 41.Wansom D, Light E, Worden F, et al. Correlation of cellular immunity with human papillomavirus 16 status and outcome in patients with advanced oropharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2010;136:1267–1273. doi: 10.1001/archoto.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choo DK, Murali-Krishna K, Anita R, Ahmed R. Homeostatic turnover of virus-specific memory CD8 T cells occurs stochastically and is independent of CD4 T cell help. J Immunol. 2010;185:3436–3444. doi: 10.4049/jimmunol.1001421. [DOI] [PubMed] [Google Scholar]
- 43.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gelbard A, Garnett CT, Abrams SI, et al. Combination chemotherapy and radiation of human squamous cell carcinoma of the head and neck augments CTL-mediated lysis. Clin Cancer Res. 2006;12:1897–1905. doi: 10.1158/1078-0432.CCR-05-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki Y, Mimura K, Yoshimoto Y, et al. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res. 2012;72:3967–3976. doi: 10.1158/0008-5472.CAN-12-0851. [DOI] [PubMed] [Google Scholar]
- 46.Duray A, Demoulin S, Hubert P, et al. Immune suppression in head and neck cancers: a review. Clin Dev Immunol. 2010;2010:701657. doi: 10.1155/2010/701657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Badoual C. Prognostic value of tumor-infiltrating CD4 + T-Cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 48.Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corzo CA, Condamine T, Lu L, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.