Abstract
The efficacy of immunotherapy in cancer patients is influenced by differences in their immune status. An evaluation of immunocompetence before therapy may help to predict therapeutic success and guide the selection of appropriate regimens. We assessed the preexisting cellular immunity against prostate-specific antigen (PSA) in untreated prostate cancer patients and healthy controls through measurement of the phenotype and function of CD8+ T cells. Our data show that the majority of healthy men possess functional PSA-specific CD8+ T cells in contrast to cancer patients, where <50 % showed a CD8+ T cell response. PSA146–154-specific CD8+ T cells of these patients had a higher expression of the activation marker CD38 and the exhaustion marker Tim-3, indicating that PSA-specific cells are exhausted. The heterogeneity of the CD8+ T cell response against PSA in prostate cancer patients may influence their response to therapy and is a factor to be taken into account while designing and selecting treatment regimens.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1752-y) contains supplementary material, which is available to authorized users.
Keywords: PSA, Antigen-specific T cells, Prostate cancer, Exhaustion
Introduction
Prostate-specific antigen (PSA) is almost exclusively expressed by prostatic epithelial cells and secreted into the lumen of the prostate gland. Healthy men have minimal quantities of PSA in the peripheral blood, and its elevation results from the disruption of the prostatic epithelium during prostatitis, hyperplasia, and/or prostate cancer (PCa). Apart from its diagnostic and post-treatment monitoring value, PSA has been considered as a target antigen for immunotherapies against PCa due to its restricted expression in the prostatic epithelium, potentially reducing the risk of on-target/off-tumor toxicity, a major side effect of current immunotherapies [1, 2]. Importantly, PSA-derived peptides can be recognized by the human CD8+ T cell receptor repertoire, as demonstrated by the detection of PSA-specific IFNγ-secreting CD8+ T cells in PCa patients and healthy donors (HD) [3]. Therefore, different therapeutic vaccines based on PSA and other prostatic antigens have been investigated but showed only limited efficacy in clinical settings, with the exception of Sipuleucel-T and Prostvac® [4–7]. The efficacy of new immunotherapeutic strategies against cancer is highly variable due to the diversity of patients and tumors. Patients with low levels of tumor-infiltrating lymphocytes [8, 9] or with high activity of immunosuppressive mechanisms [10] often have a poor prognosis and might not benefit from immunotherapy regimens such as therapeutic vaccinations and immunological checkpoint blockade (αPD-1, αPD-L1, and αCTLA-4) [11, 12]. Consequently, a better understanding of the patient’s immune status may allow an individual design of appropriate treatment regimens to increase therapeutic response rates [13–16].
We hypothesized that differences in the immune status of PCa patients, particularly in their preexisting tumor-specific T cells, exist and could be used in the future as predictive immune signatures before immunotherapies. Therefore, the aim of this work was to investigate how PSA-specific CD8+ T cell responses differ quantitatively and qualitatively between PCa patients and age-matched HD.
Flow cytometric analysis of PSA-specific CD8+ T cells using a MHC class I multimer and intracellular cytokine staining after in vitro activation revealed that both PCa patients and healthy controls had PSA-specific CD8+ T cells. Nevertheless, PCa patients could be stratified into those who displayed a cytokine response after in vitro stimulation with PSA peptides and those who did not. Furthermore, the high percentage of cells expressing the exhaustion and activation markers Tim-3 and CD38 among PSA-specific CD8+ T cells may explain why these T cells fail to produce cytokines in some PCa patients. These data can lead to a better selection of the appropriate immunotherapeutic strategy according to each patient’s immunological status.
Materials and methods
Human samples and preparation
This study was conducted in accordance with the Declaration of Helsinki. Human blood was obtained, after provision of informed consent, from 20 healthy volunteers (mean age 59, range 51–79) and 32 PCa patients (mean age 66, range 49–75) before prostatectomy with a Gleason score of 7 or higher. Serum PSA level analysis was carried out in a certified clinical laboratory (Charité, Berlin) using a commercially available electrochemiluminescence immunoassay. Peripheral blood mononuclear cells (PBMCs) were separated from heparinized whole blood using Ficoll–Hypaque (PAA) gradient and were cultured in RPMI 1640 medium (Gibco) supplemented with 100 U/ml penicillin, 0.1 mg/ml streptomycin, 0.3 mg/ml glutamine, and 10 % inactivated human AB serum (PAA). All experiments followed protocols approved by the local authorities.
PSA-specific stimulation, intracellular cytokine staining, and analysis
A total of 1.5–5 × 106 PBMCs were stimulated (or left unstimulated as negative control) for 6 h at 37 °C with a peptide pool spanning the whole human PSA protein (15mers with 11 amino acid overlaps, JPT Peptide Technologies) in the presence of brefeldin A (Sigma-Aldrich), αCD28 (CD28.2, BD), and αCD107a antibody (H4A3, Biolegend). Intracellular cytokine staining was performed with following antibodies: αIFNγ (B27, BD); αCD40L (24–31, Biolegend); αCD3 (OKT3, eBioscience); αCD4 (RPA-T4, BD); and αCD8 (SK1, Biolegend) for 30 min at 4 °C after fixation and permeabilization of cells with FACS-lysing and FACS-perm2 solutions (BD) according to the manufacturer’s protocol. All conjugates were titrated to determine their optimal dilution. To avoid Fc receptor binding, cells were stained in the presence of 1 mg/ml Beriglobin (Sanofi-Aventis). The cells were subsequently acquired on a LSRII flow cytometer (BD) and analyzed with FlowJo software (Tree Star). The response index was calculated by dividing the frequency of IFNγ+, CD107a+, or CD40L+ in the PSA-stimulated sample by the background frequency in the unstimulated sample of the same donor. Donors with a CD8+ T cell response index higher than 3 in IFNγ and/or CD107a were classified as responder.
Dextramer staining and analysis
A total of 1.5–5 × 106 PBMCs from HLA-A*0201+ PCa patients and HD were incubated for 10 min at room temperature with the APC-coupled PSA146–154 MHC class I dextramer (Immudex) in a total volume of 50 µl. Subsequently, the following antibodies were added: αCD38 (HIT2, Biolegend); αTim-3 (344823, R&D Systems); αCD8 (RPA-T8, BD); αCD3 (OKT3, eBioscience); and αCD4 (RPA-T4, BD), and the samples were incubated for another 20 min at 37 °C. Dextramer and antibodies were centrifuged for 10 min at 18,000g before use to prevent unspecific staining by protein aggregates. The cells were subsequently acquired on a LSRII flow cytometer (BD) and analyzed with FlowJo software (Tree Star).
Tim-3 blockade in PBMC stimulation culture
PBMC stimulation with antigen and Tim-3 blockade was performed as described previously [17]. Briefly, 2.5 × 106 PBMCs from six additional PCa patients were cultured for 7 days in culture medium containing 50 IU/ml rhIL-2 (Miltenyi Biotec), 1 µg/ml PSA peptide pool (JPT Peptide Technologies), and 10 µg/ml αTim-3 blocking antibody (2E2, Biolegend) or isotype control (Mouse IgG1, Biolegend). Medium was exchanged every 2–3 days. On day 7, cells were restimulated with the PSA peptide pool and stained intracellularly as described above.
Statistics
Statistics were performed in the Prism software (GraphPad). The two-tailed Mann–Whitney nonparametric test was applied for direct comparison of the groups. The Fisher’s exact test was used to compare responder ratios between PCa patients and HD. The Wilcoxon signed-rank test was applied in the Tim-3 blockade experiment.
Results
To analyze PSA-specific cytokine production by T cells in PCa patients and HD, we used a 15mer overlapping peptide pool of PSA to stimulate PBMCs, independent of their HLA haplotypes, and measured the reactive expression of the cytokine IFNγ and the degranulation marker CD107a on CD8+ T cells and the activation marker CD40L on CD4+ T cells [18]. Except for one patient, no CD40L-expressing CD4+ T cells were detected among CD4+ T cells upon PSA stimulation (supplementary Fig. 1). However, we detected IFNγ- and/or CD107a-expressing cells among the CD8+ T cell population (Fig. 1a). The frequencies of CD107a+ CD8+ T cells after background subtraction ranged from 0.00 to 1.37 % (n = 32, 95 % CI 0.04–0.26) in PCa patients and from 0.00 to 0.48 % (n = 20, 95 % CI 0.04–0.17) in HD. The frequencies of IFNγ+ CD8+ T cells ranged from 0.00 to 1.18 % (n = 32, 95 % CI 0.01–0.19) in PCa patients and from 0.00 to 0.32 % (n = 20, 95 % CI 0.04–0.12) in HD (Fig. 1b). Notably, frequencies of IFNγ+ cells were significantly higher in HD as compared to PCa patients, even though some PCa patients showed high frequencies of IFNγ- or CD107a-expressing CD8+ T cells (Fig. 1a, b). Due to this discrepancy in the IFNγ and CD107a frequencies of PCa patients, we classified them into responders (r-PCa) and non-responders (n-PCa) by introducing a response index (as described in Materials and Methods). Donors with a response index higher than 3 in IFNγ and/or CD107a were classified as responder (Fig. 2a, b). By this way, no predetermined frequency threshold falsely classified donors with low PSA response as non-responders, but still considered normal sample deviation in the individual stimulated and unstimulated samples. Therefore, we classified 46.8 % of PCa patients and 80 % of HD as responders who had functional cytokine-producing PSA-specific CD8+ T cells (Fig. 2b). Importantly, the frequency of responders among HD was significantly higher than among PCa patients (P < 0.05, Fisher’s exact test). These results suggest that n-PCa patients either lack PSA-specific CD8+ T cells or the cells have become dysfunctional. PSA itself has been proposed to have immunosuppressive properties [19], and the abnormal levels of PSA might drive the PSA-specific CD8+ T cells into an exhausted or anergic state [20]. Therefore, we asked whether the CD8+ T cell response in PCa patients was associated with their serum PSA levels. We observed a tendency of PCa patients, who had serum PSA values lower than 10 ng/ml, to have higher frequencies of IFNγ- or CD107a-producing PSA-specific CD8+ T cells as patients with more than 10 ng/ml serum PSA (Fig. 2c). However, we could not observe a significant difference in PSA serum levels of r-PCa and n-PCa patients.
Fig. 1.
Effector functions of PSA-specific CD8+ T cells in PCa patients and HD. a PBMCs from PCa patients and HD were stimulated with a PSA peptide pool. The dot plots show intracellular IFNγ and CD107a staining of CD3+CD4−CD8+-gated lymphocytes from four representative PCa patients each with a distinct expression pattern (#31 IFNγ and CD107a; #36 only IFNγ; #23 only CD107a; #21 no cytokines). b Summary of the frequencies of IFNγ+ and CD107a+ CD8+ T cells after PSA stimulation from PCa patients (n = 32) and HD (n = 20). Line indicates median. *P < 0.05, two-tailed Mann–Whitney test
Fig. 2.
Functional PSA-specific CD8+ T cells are less frequently detected in PCa patients than in HD. a The response index was calculated by dividing the frequency of IFNγ+ or CD107a+ cells in the stimulated sample by the corresponding background frequency in the unstimulated sample from the same donor. Line indicates median. *P < 0.05, two-tailed Mann–Whitney test. b The response index for IFNγ+ and CD107a+ in PCa patients and HD was plotted against each other. A response index ≥3 in either one parameter defined a responsive donor. The pie graphs summarize the data and present frequencies of responders and non-responders in each donor group. c The frequencies of IFNγ+ or CD107a+ CD8+ T cells were plotted against the serum concentration of PSA in PCa patients before prostatectomy
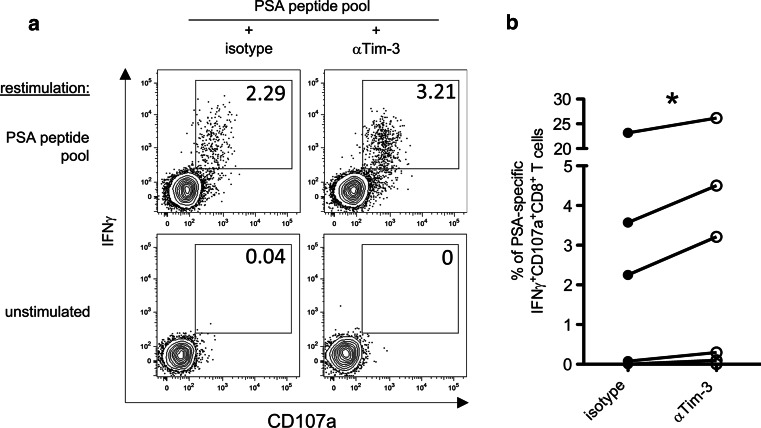
In parallel to the analysis of the functional quality of PSA-specific T cells, we employed the MHC class I dextramer technology to directly assess specific CD8+ T cells against one defined HLA-A2-restricted PSA epitope (PSA146–154, sequence KLQCVDLHV) in HLA-A2+ PCa patients (n = 15) and HD (n = 11). The measured frequencies of PSA146–154 dextramer+ CD8+ T cells in donors of both groups were in the range of 0–0.7 % (Fig. 3a, b). Interestingly, the group of n-PCa patients had the highest frequencies of PSA146–154-specific CD8+ T cells. To pursue the question whether the cause of the observed T cell dysfunction in n-PCa patients was due to chronic activation of the cells, we analyzed the activation marker CD38 and the recently defined exhaustion marker Tim-3 [17, 21–23] on polyclonal and PSA146–154 dextramer+ CD8+ T cells. In the polyclonal CD8+ T cell population, no significant difference could be observed in the frequencies of CD38+ Tim-3+ CD8+ T cells among r-PCa, n-PCa, and HD (Fig. 4a, b). In contrast, PSA146–154 dextramer+ CD8+ T cells of n-PCa patients contained significantly higher percentages of CD38+ Tim-3+ cells compared to r-PCa patients and HD (Fig. 4a, c), suggesting that the unresponsiveness of PSA-specific CD8+ T cells in n-PCa was due to exhaustion. We next assessed the impact of Tim-3 expression on the function of PSA-specific CD8+ T cells. PBMCs from another six PCa patients (supplementary Table 1) were cultured for 7 days with PSA peptide pool and an αTim-3 blocking antibody or IgG isotype control. After 7 days, cells were restimulated for 6 h with PSA peptides, and cytokine production and degranulation were evaluated. We observed a significant increase in the frequency of CD107a+IFNγ+CD8+ T cells after incubation with PSA peptides and αTim-3 antibody, as compared to incubation with PSA peptides and IgG isotype control (Fig. 5a, b). Together, these data suggest a relevant role for Tim-3 in the dysfunction of PSA-specific CD8+ T cells observed in PCa patients.
Fig. 3.
PSA146–154-specific CD8+ T cells can be detected in PCa patients and HD via MHC class I multimer staining. a PBMCs from HLA-A2+ PCa patients and HD were stained with the PSA146–154 MHC class I dextramer and phenotypic markers. Dot plots show a representative dextramer staining for each donor group, and numbers indicate frequency of PSA146–154-specific cells among CD8+ T cells. b Summary of the PSA+146–154 cell frequencies in the CD8+ T cell populations. r-PCa (responder PCa patients, n = 10); n-PCa (non-responder PCa patients, n = 5); HD (healthy donors, n = 11). Line indicates median. *P < 0.05, two-tailed Mann–Whitney test
Fig. 4.
PSA146–154-specific CD8+ T cells in n-PCa patients display an exhausted phenotype. a PBMCs from HLA-A2+ PCa patients and HD were stained with the PSA146–154 MHC class I dextramer and phenotypic markers. Representative data from all three donor groups show the staining of CD38 and Tim-3. Upper row shows contour plots for polyclonal CD8+ T cells; numbers indicate frequency of CD38+Tim-3+ cells. Lower row shows overlaid plots of polyclonal CD8+ T cells (shaded gray) and PSA146–154-specific CD8+ T cells (dots); numbers indicate frequencies of CD38+Tim-3+ cells among the PSA-specific population. b Summary of the frequencies of CD38+Tim-3+ cells among the polyclonal CD8+ T cell population. Line indicates median. c Summary of the frequencies of CD38+Tim-3+ cells among PSA146–154-specific CD8+ T cells. Line indicates median. *P < 0.05, two-tailed Mann–Whitney test
Fig. 5.
Blockade of Tim-3 increases the frequency of PSA-specific CD107a+IFNγ+CD8+ T cells. PBMCs from PCa patients (n = 6) were cultured for 7 days in the presence of PSA peptide pool and αTim-3 blocking antibody or IgG isotype control. After 7 days, cells were restimulated for 6 h with PSA peptide pool and then analyzed for CD107a exposure and IFNγ expression on CD8+ T cells. a Dot plots gated on CD3+CD4−CD8+ T cells from a representative donor showing the frequencies of CD107a+IFNγ+ cells specific for PSA peptides after incubation with αTim-3 blocking antibody or IgG isotype control. b Summary of the frequencies of PSA-specific CD107a+IFNγ+CD8+ T cells after 7-day culture with PSA peptides and αTim-3 blocking antibody or IgG isotype control. *P < 0.05, Wilcoxon signed-rank test
Discussion
Here, we compared the T cell response against PSA in PCa patients before prostatectomy and age-matched healthy men. The obtained data suggest that the relative quantification of PSA-specific CD8+ T cells via MHC class I multimers is not sufficient to distinguish between PCa patients who have a functional tumor-specific T cell compartment and those who do not. In this respect, measurement of effector functions, such as cytokine secretion and degranulation capacity, and analysis of activation and exhaustion markers are necessary for a more thorough assessment of the patient’s CD8+ T cell response to PSA. Instead of the widely used exhaustion marker PD-1, we measured the co-inhibitory receptor Tim-3 that is frequently upregulated in antigen-specific CD4+ and CD8+ T cells during chronic viral infections and cancer and which strongly contributes to T cell exhaustion in these pathologies [17, 22, 24–28]. Through this approach, we found that the increased level of Tim-3 and CD38 expression in PSA-specific T cells, caused probably by continuous and inefficient antigen-dependent activation [20], correlated with unresponsiveness during ex vivo PSA stimulation and would most likely negatively affect the outcome of vaccination in these patients. However, it is still necessary to evaluate in an envisaged prospective study whether ex vivo effector CD8+ T cell responses against PSA can stratify immunotherapeutic efficacy.
Our findings are in agreement with previous reports that Tim-3 expression identifies functionally impaired antigen-specific CD8+ T cells in mice and men [17, 24–29]. Importantly, blockade of the Tim-3/galectin-9 pathway supports tumor rejection in preclinical murine models [24, 30] and restores the proliferation and cytokine production of exhausted NY-ESO-1-specific CD8+ T cells obtained from melanoma patients [17, 31, 32]. Interestingly, simultaneous blockade of the Tim-3/galectin-9 and PD-1/PD-L1 pathways in those studies resulted in a synergistic effect, indicating non-redundancy between these immunological modulators. PD-1 expression is as well frequently observed in prostate tumor-infiltrating lymphocytes [33, 34] and might therefore contribute to the observed T cell dysfunction; nonetheless, a clinical study performed with 17 castration-resistant PCa patients treated with an αPD-1 antagonistic antibody showed no objective response [35]. On the other hand, results of clinical trials with ipilimumab (a CTLA-4-blocking antibody) have been more promising [36]. Our observation that Tim-3 is associated with the exhaustion of PSA-specific CD8+ T cells encourages further studies using αTim-3 antagonistic antibodies as a treatment for PCa. It is very likely that combination therapies with multiple independent immunotherapeutic agents will surpass monotherapies in providing therapeutic benefit for cancer patients [37]. Thus, understanding the influence of different immunological pathways leading to T cell dysfunction in each cancer entity and developing therapeutic targets accordingly will further increase the efficacy of immunotherapies.
In line with other studies, we also detected PSA-specific CD8+ T cells in elderly HD [4, 38–40]. Surprisingly, these donors had mostly antigen-experienced memory T cells that responded accordingly with IFNγ secretion and degranulation (surface CD107a) after a short (6 h) ex vivo stimulation. This finding implicates that thymic negative selection against the self-antigen PSA is not as stringent as expected and/or that peripheral tolerance in the elderly is less efficient due to immunological changes associated with aging [41–44].
In summary, our data show that PSA-specific CD8+ T cells can be detected in most elderly men; however, in around 50 % of PCa patients and 20 % of healthy individuals, these cells are non-functional with respect to potent antitumor effector functions (IFNγ secretion and degranulation). Based on the increased surface expression of CD38 and Tim-3, we suggest that the unresponsiveness is associated with T cell exhaustion, probably caused by chronic activation and other immunosuppressive mechanisms. Our findings are encouraging with regard to the appropriate individual selection of different immunotherapies with the aim to boost and/or reconvert CD8+ T cell antitumor immunity into a functional state.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank Beate Möwes and the BCRT Flow Cytometry Lab for expert technical help. This work was supported by the German Research Foundation (DFG) Sonderforschungsbereich Transregio 36 (SFB TR36) and Th 806/5-1, and a flexible funds grant from the Berlin-Brandenburg Center for Regenerative Therapies/Federal Ministry of Education and Research (BCRT/BMBF).
Abbreviations
- CTLA-4
Cytotoxic T lymphocyte antigen 4
- HD
Healthy donors
- HLA
Human leukocyte antigen
- MHC
Major histocompatibility complex
- PBMCs
Peripheral blood mononuclear cells
- PCa
Prostate cancer
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed death-ligand 1
- PSA
Prostate-specific antigen
- Tim-3
T cell immunoglobulin and mucin domain 3
Compliance with ethical standards
Conflict of interest
The authors have no conflicting financial interests.
Footnotes
Alberto Sada Japp and M. Alper Kursunel as well as Andreas Thiel and Marco Frentsch have contributed equally to this work.
References
- 1.Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa G, Bossi G, Vuidepot A, Powlesland AS, Legg A, Adams KJ, Bennett AD, Pumphrey NJ, Williams DD, Binder-Scholl G, Kulikovskaya I, Levine BL, Riley JL, Varela-Rohena A, Stadtmauer EA, Rapoport AP, Linette GP, June CH, Hassan NJ, Kalos M, Jakobsen BK. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med. 2013;5(197):197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM, Cimino PJ, Binder-Scholl GK, Smethurst DP, Gerry AB, Pumphrey NJ, Bennett AD, Brewer JE, Dukes J, Harper J, Tayton-Martin HK, Jakobsen BK, Hassan NJ, Kalos M, June CH. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122(6):863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elkord E, Williams PE, Kynaston H, Rowbottom AW. Differential CTLs specific for prostate-specific antigen in healthy donors and patients with prostate cancer. Int Immunol. 2005;17(10):1315–1325. doi: 10.1093/intimm/dxh309. [DOI] [PubMed] [Google Scholar]
- 4.Hadaschik B, Su Y, Huter E, Ge Y, Hohenfellner M, Beckhove P. Antigen specific T-cell responses against tumor antigens are controlled by regulatory T cells in patients with prostate cancer. J Urol. 2012;187(4):1458–1465. doi: 10.1016/j.juro.2011.11.083. [DOI] [PubMed] [Google Scholar]
- 5.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Investigators IS. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 6.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(7):1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanda MG, Smith DC, Charles LG, Hwang C, Pienta KJ, Schlom J, Milenic D, Panicali D, Montie JE. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53(2):260–266. doi: 10.1016/S0090-4295(98)00539-1. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 9.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 10.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulloa-Montoya F, Louahed J, Dizier B, Gruselle O, Spiessens B, Lehmann FF, Suciu S, Kruit WH, Eggermont AM, Vansteenkiste J, Brichard VG. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol. 2013;31(19):2388–2395. doi: 10.1200/JCO.2012.44.3762. [DOI] [PubMed] [Google Scholar]
- 12.Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, Alaparthy S, Berman D, Jure-Kunkel M, Siemers NO, Jackson JR, Shahabi V. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61(7):1019–1031. doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galon J, Pages F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA. The immune score as a new possible approach for the classification of cancer. J Transl Med. 2012;10:1. doi: 10.1186/1479-5876-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angell H, Galon J. From the immune contexture to the immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25(2):261–267. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 16.Chang S, Kohrt H, Maecker HT. Monitoring the immune competence of cancer patients to predict outcome. Cancer Immunol Immunother. 2014;63(7):713–719. doi: 10.1007/s00262-014-1521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207(10):2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, Scheffold A, Thiel A. Direct access to CD4 + T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11(10):1118–1124. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy-Smith AG, McKenzie JL, Owen MC, Davidson PJ, Vuckovic S, Hart DN. Prostate specific antigen inhibits immune responses in vitro: a potential role in prostate cancer. J Urol. 2002;168(2):741–747. doi: 10.1016/S0022-5347(05)64738-6. [DOI] [PubMed] [Google Scholar]
- 20.Van Parijs L, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280(5361):243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 21.Terhorst C, van Agthoven A, LeClair K, Snow P, Reinherz E, Schlossman S. Biochemical studies of the human thymocyte cell-surface antigens T6, T9 and T10. Cell. 1981;23(3):771–780. doi: 10.1016/0092-8674(81)90441-4. [DOI] [PubMed] [Google Scholar]
- 22.Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, Rufer N, Speiser DE. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 24.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205(12):2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. Tim-3 expression on PD-1 + HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120(12):4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang ZZ, Grote DM, Ziesmer SC, Niki T, Hirashima M, Novak AJ, Witzig TE, Ansell SM. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J Clin Invest. 2012;122(4):1271–1282. doi: 10.1172/JCI59806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afanasiev OK, Yelistratova L, Miller N, Nagase K, Paulson K, Iyer JG, Ibrani D, Koelle DM, Nghiem P. Merkel polyomavirus-specific T cells fluctuate with merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markers. Clin Cancer Res. 2013;19(19):5351–5360. doi: 10.1158/1078-0432.CCR-13-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2010;107(33):14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71(10):3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 31.Fourcade J, Sun Z, Pagliano O, Chauvin JM, Sander C, Janjic B, Tarhini AA, Tawbi HA, Kirkwood JM, Moschos S, Wang H, Guillaume P, Luescher IF, Krieg A, Anderson AC, Kuchroo VK, Zarour HM. PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8(+) T cells induced by melanoma vaccines. Cancer Res. 2014;74(4):1045–1055. doi: 10.1158/0008-5472.CAN-13-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V, Zarour HM. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72(4):887–896. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+ Prostate. 2009;69(15):1694–1703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ebelt K, Babaryka G, Frankenberger B, Stief CG, Eisenmenger W, Kirchner T, Schendel DJ, Noessner E. Prostate cancer lesions are surrounded by FOXP3+ , PD-1+ and B7-H1+ lymphocyte clusters. Eur J Cancer. 2009;45(9):1664–1672. doi: 10.1016/j.ejca.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwek SS, Cha E, Fong L. Unmasking the immune recognition of prostate cancer with CTLA4 blockade. Nat Rev Cancer. 2012;12(4):289–297. doi: 10.1038/nrc3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue BH, Zhang Y, Sosman JA, Peace DJ. Induction of human cytotoxic T lymphocytes specific for prostate-specific antigen. Prostate. 1997;30(2):73–78. doi: 10.1002/(SICI)1097-0045(19970201)30:2<73::AID-PROS1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 39.Perambakam SM, Srivastava R, Peace DJ. Distinct cytokine patterns exist in peripheral blood mononuclear cell cultures of patients with prostate cancer. Clin Immunol. 2005;117(1):94–99. doi: 10.1016/j.clim.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Corman JM, Sercarz EE, Nanda NK. Recognition of prostate-specific antigenic peptide determinants by human CD4 and CD8 T cells. Clin Exp Immunol. 1998;114(2):166–172. doi: 10.1046/j.1365-2249.1998.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martins PN, Tullius SG, Markmann JF. Immunosenescence and immune response in organ transplantation. Int Rev Immunol. 2014;33(3):162–173. doi: 10.3109/08830185.2013.829469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.do Canto FB, Lima Junior C, Teixeira IA, Bellio M, Nobrega A, Fucs R. Susceptibility of neonatal T cells and adult thymocytes to peripheral tolerance to allogeneic stimuli. Immunology. 2008;125(3):387–396. doi: 10.1111/j.1365-2567.2008.02855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev. 2006;5(2):136–139. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Yu W, Jiang N, Ebert PJ, Kidd BA, Muller S, Lund PJ, Juang J, Adachi K, Tse T, Birnbaum ME, Newell EW, Wilson DM, Grotenbreg GM, Valitutti S, Quake SR, Davis MM. Clonal deletion prunes but does not eliminate self-specific alphabeta CD8(+) T lymphocytes. Immunity. 2015;42(5):929–941. doi: 10.1016/j.immuni.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.