Abstract
MLAA-34 is a newly identified monocytic leukemia-associated antigen. Previous data indicated that MLAA-34 might be a novel anti-apoptosis factor related closely to carcinogenesis or progression of acute monocytic leukemia. The over-expression of MLAA-34 is intuitively expected to be associated with unfavorable clinical features in acute myeloid leukemia. However, there have been no clinical studies about the prognostic relevance of MLAA-34 expression in human malignancies. This study was done to investigate the clinical relevance of the expression of MLAA-34 in de novo acute myeloid leukemia. In 126 patients with de novo acute myeloid leukemia, the level of MLAA-34 expression and protein expression ratio were determined by using quantitative reverse transcriptase-PCR and western blot, respectively. The results were analyzed with respect to the patients’ clinical features and treatment outcomes. Both MLAA-34 expression rates and expression levels were found to be higher in patients with the French–American–British classification subtype M5, and the expression levels were also higher in patients with a leukocyte number of ≥20 × 109/L and patients with extramedullary disease. In addition, MLAA-34 over-expression (≥median expression) was associated with an unfavorable day 7 response to induction chemotherapy and also associated with a poor survival rate. In multivariate analysis, high MLAA-34 levels was independently associated with a poorer relapse-free survival and overall survival in AML patients. In conclusion, our data indicate that MLAA-34 may be used as a prognostic marker for treatment decision-making in acute monocytic leukemia through validation by further studies.
Keywords: MLAA-34, Acute monocytic leukemia, Real-time PCR, Prognosis
Introduction
AML is a common acute leukemia in adult. With intensive induction therapy, complete remission (CR) rates of between 65 and 75% are achieved in younger patients (<60 years). However, more than 50% of these patients will relapse, leading to an overall survival rate of only 30–40% after 5 years. Results are more unfavorable in patients older than 60 years [1–3]. Given the prognosis of AML patients, there is a fervent need for novel therapies that could reduce the risk of relapse after chemotherapy or stem cell transplantation by eliminating residual leukemic cells. Immunotherapeutic approaches targeting leukemia-associated antigens to prevent relapse may represent a promising novel treatment option to improve the outcomes of AML patients. Leukemia-associated antigens (LAAs) have been shown to induce specific T-cell immune responses in leukemia patients, thus representing potential target structures for specific immunotherapies. Targeted immunotherapies require the identification and characterization of appropriate antigen structures. So far, there are various peptides derived from leukemia-associated antigens which are under clinical investigation for AML patients. These antigens include the following: FLT3 [4], WT1 [5], Survivin [6], RHAMM [7], PRAME [8], BCL-2 [9], and so on, as the most interesting LAAs in the SEREX screening with sera from AML patients. In contrast to the other subtypes of the AML, only a few leukemia-associated antigens have been characterized in patients of AML-M5, a distinct subtype of acute myeloid leukemia with characteristic clinical features. Clinically, the disease is associated with hyperleukocytosis [10], extramedullary involvement [11], and coagulation abnormalities [12]. Identification of immunogenic leukemia-associated antigens as target structures is mandatory for specific immunotherapy of AML-M5.
MLAA-34 is one of the novel identified leukemia-associated antigens. We have applied the method of serologic analysis of recombinant cDNA expression library (SEREX) on acute monocytic leukemia to identify monocytic leukemia-associated antigens (MLAA). With this approach, MLAA-34 has been identified as a novel gene, which exclusively reacts with sera from allogeneic leukemia patients but not with normal donor sera [13]. With RNA interference technology, the function of MLAA-34 has verified that the down-regulation of its expression significantly suppresses the proliferation of U937 cells in vitro and increases the spontaneous apoptosis of these leukemia cells [14]. Apoptosis is an active biological mechanism that leads to programmed cell deaths. The failure of apoptosis may result in the development of a wide variety of diseases, including cancers. Moreover, the up-regulation of antiapoptotic proteins would certainly be advantageous for tumor survival [15–19].
The pattern of MLAA-34 expression in acute myeloid leukemia, especially in acute monocytic leukemia, remains unclear. There are no reports regarding its expression in acute monocytic leukemia, especially with real-time PCR, which can be used to determine the levels of mRNA expression and allows researchers to examine the expression patterns of a large number of genes at the RNA level. It will be possible to refine current prognosis-based stratification systems if specific patterns of gene expression can be correlated with the clinical features in AML-M5. The purposes of this study were to investigate MLAA-34 expression at diagnosis of acute monocytic leukemia and to find whether there was a correlation between MLAA-34 expression levels and the known prognostic parameters, and the clinical outcomes. The results revealed a strong correlation between MLAA-34 over-expression and the unfavorable clinical features in AML-M5. This is the first clinical study demonstrating the prognostic implication of MLAA-34 expression in malignancies.
Materials and methods
Patients and sample collection
Peripheral blood mononuclear cells (PBMCs, n = 36) and bone marrow (BM) cells (n = 90) of 126 patients with newly diagnosed de novo AML were obtained before the initiation of chemotherapy, after patients had given informed consent. The diagnosis of AML and its subtype was determined according to the French–American–British (FAB) classification based on a morphologic assessment of the Wright–Giemsa-stained smears of the bone marrow aspirates along with special stains and immunophenotyping by flow cytometry. Laboratory investigation included conventional and molecular cytogenetic analysis. The characteristics and subtype distribution of patients are shown in Table 1. Serial bone marrow samples were obtained at diagnosis prior to treatment, post-therapy and at relapse, as part of the routine assessment. Peripheral blood was used from patients with blast counts >80% when bone marrow samples were unavailable. In total, 146 bone marrow samples collected at diagnosis and during follow-ups, and the peripheral blood auto grafts of three patients which were collected after G-CSF mobilization were analyzed. Follow-ups were conducted on 35 patients with AML-M5 and 72 patients with other subtype of AML, 23 of 35 patients with M5 were serially monitored for approximately 7 times (range 4–11 times) during the follow-up (median 11 months, range 4–17.5 months), 12 of 35 patients with M5 and other non-M5 patients were only tested at the time of their diagnosis, and hematological relapse. Bone marrow samples were obtained from 34 healthy donors for hematopoietic stem cell transplantation as control, as well as from 126 patients with acute leukemia in different clinical stages enrolled in the department of hematology of the Second Affiliated Hospital of Xi’an Jiaotong University, from January 2008 to January 2010. All samples were collected with the informed consent of the participants. The studies were approved by the ethics committees of School of Medicine, Xi’an Jiaotong University, and were conducted in accordance with the Declaration of Helsinki.
Table 1.
The expression rates and expression level of MLAA-34 with respect to the clinical characteristics at diagnosis
| Clinical characteristics | No. of patients | Expression rates of MLAA-34, No. (%) | P value | Expression level of MLAA-34 | P value |
|---|---|---|---|---|---|
| Status | |||||
| Donors | 34 | 1 (2.8) | 0.070 | 0.195 | <0.001 (vs. M5) |
| CR | 72 | 5 (6.9) | 0.604 | <0.001 (vs. M5) | |
| Gender | |||||
| Female | 48 | 16 (33.3) | 0.062 | 31.85 | 0.390 |
| Male | 78 | 21 (26.9) | 39.36 | ||
| Age | |||||
| ≥60 | 25 | 8 (32.0) | 0.138 | 43.25 | 0.23 |
| <60 | 101 | 15 (14.9) | 41.32 | ||
| Leukocyte (109/L) | |||||
| ≥20 | 32 | 10 (31.2) | 0.024 | 115.20 | 0.001 |
| <20 | 94 | 15 (15.9) | 53.25 | ||
| Extramedullary disease | |||||
| Absent | 101 | 16 (15.8) | 0.006 | 70.30 | 0.026 |
| Present | 25 | 9 (36.0) | 104.45 | ||
| Cytogenetics | |||||
| Favorable | 76 | 16 (18.4) | 0.165 | 35.56 | 0.063 |
| Others | 50 | 8 (16.0) | 48.15 | ||
| FAB classification | |||||
| M5 | 35 | 29 (82.9) | <0.001 | 465.3 | <0.001 |
| No M5 subtype | 91 | 22 (24.1) | 118.36 | ||
| FLT3 length mutation | |||||
| Absent | 94 | 25 (26.5) | 0.123 | 89.05 | 0.056 |
| Present | 18 | 6 (33.3) | 106.30 | ||
| Not examined | 14 | 4 (28.5) | 65.32 | ||
| Induction of remission | |||||
| Yes | 72 | 7 (9.7) | 0.115 | 20.35 | 0.315 |
| No | 46 | 16 (34.7) | 345.68 | ||
| Not evaluable* | 8 | ||||
The expression levels are presented as the median values. Differences in the level of MLAA-34 were analyzed using the Mann–Whitney U test. Differences in MLAA-34 expression rates were analyzed using the chi-square test
* Eight patients died from toxicity during induction chemotherapy
Protocols and therapies
All patients with AML other than acute promyelocytic leukemia were treated with MAE regimen as induction therapy. MAE regimen consisted of mitoxantrone (MIT), 8 mg/m2 intravenously on days 1–3, cytosine arabinoside (Ara-c), 100 mg/m2/day continuous infusion on days 1–7, and etoposide (VP16), 100 mg/m2 intravenously on days 1–3. Discontinuation of the chemotherapy was allowed in patients who experienced sepsis with unstable vital signs before the completion of the induction regimen if at least 7 days of induction chemotherapy had been provided. If complete remission (CR) was not achieved after the primary induction chemotherapy and regimen, an additional course of induction chemotherapy with HA regimen (homoharringtonine (HHT) and Ara-c) was given. Once CR had been achieved, patients with an appropriate stem cell donor received consolidation chemotherapy until the hematopoietic stem cell transplantation. An entire course of consolidation chemotherapy was given to patients without an appropriate stem cell donor. Patients with AML-M3 were treated according to acute promyelocytic leukemia’s protocols [20, 21].
Response definition
Complete remission either after the first or second cycle of induction chemotherapy was defined as CR if <5% blasts were present in bone marrow and with concomitant evidence of erythropoiesis, granulopoiesis, and megakaryopoiesis. Granulocytes and platelets in peripheral blood should be at least 1.5 × 109/L and 100 × 109/L, respectively. Relapse was defined as marrow infiltration by >5% blasts in previous morphologic normal bone marrow.
RNA isolation, cDNA synthesis, and RT–PCR
Mononuclear cells were isolated from bone marrow samples or peripheral blood by density centrifugation on Ficoll-Hypaque. Total RNA was extracted from 1 × 106 MNC by Trizol (Gibco-BRL, Gaitherburg, MD). RNA quality was assessed visually by conformation of intact 28 and 18 s ribosomal band following agarose gel electrophoresis and ethidium bromide staining. The cDNA was synthesized using first Strand cDNA synthesis kit (Takara, Dalian, China). Briefly, 5 μl (1–2 μg) of RNA was RT into cDNA in a 20-μl reaction for 10 min at 25°C and 60 min at 42°C using 25 mg/L random hexamer, 20 U of RNase inhibitor, 25 μl dNTP, and 200 U of MMLV-RT according to the manufacturer’s instructions.
One microgram of total RNA was reverse transcribed with SuperScript III reverse transcriptase (Invitrogen Corporation, Carlsbad, CA) and an oligo (dT) 20 primer (Invitrogen). The resulting cDNAs were used as template to amplify MLAA-34 transcripts in a 50-μl reaction containing 0.4 μM forward primer 5′-TCTGTGTGGAACGAACGACAA-3′ and reverse prime 5′-TGACACTCATAGCTGACCTGCA-3′. These primers were specific for human MLAA-34. Included in the PCR were as follows: 1.5 mM MgCl2, 200 μM of each dNTP, and 2.5 U of GoldTaq polymerase in supplied buffer from Applied Biosystems (Foster City, CA). The 7-min initial denaturation at 95°C was followed by 35 cycles of PCR, each consisting of 30 s denaturation at 94°C, 30 s annealing at 64°C, and 1 min extension at 72°C. The PCR was finished after a further 10-min extension at 72°C. Under similar conditions, β-actin was amplified as an internal control for each human cDNA with the forward primer 5′-AGC CAC ATC GCT CAG ACA CC-3′ and reverse primer 5′-GTA CTC AGC GCC AGCATCG-3′.
Real-time quantitative PCR assay
TaqMan-based RT–PCR technology was used. PCR and fluorescence measurements were performed on an ABI PRISM7300 real-time PCR system (PE Applied Biosystems, USA). β-actin was selected as a control gene to compensate for the variations in quality and quantity of the RNA and cDNA. Primers and probe for MLAA34 were designed with Primer Express Software Version 2.0. The sequences of MLAA-34 and β-actin were follows: 5′-AAG CCG GAG AAC CTG AAA CTC-3′ forward primer: 5′-TGA GGA CTG GCC ACA AAC AC-3′ reverse primer probe: 5′-FAM-TGA TGA ACC TCC TTCGGGATAAAAG-TAMRA-3′. Primers and probes for β-actin were as follows: forward primer: 5′-TCC TTC CTG GGT ATG GAATC-3′, reverse primer: 5′-GCACTGTGTTGGCATAGAGG-3′ probe: 5′-FAM CGG ATG TCA ACG TCA CACACTTCATGA-TAMRA-3′. The PCR mixture contained 10 μL of 2× TaqMan Universal PCR Master Mix (PE Applied Biosystems, USA), 300 nM of each primer, 200 nM of probe (100 nM for MLAA-34), and 2 μL of cDNA in a total volume of 20 μL. All PCRs were performed under the following conditions: 93°C for 2 min, then 93°C for 30 s, 55°C 1 min followed by 40 cycles. Each PCR run included a negative control (H2O), a positive control, and a set of serial dilutions of PMD18-T plasmids. The assays were validated on serially diluted cDNA from U937 cells (100–10−6). The lowest amounts of U937 cDNA which showed good linear correlations with Ct values for MLAA-34 and β-actin were 1 × 10−4 and 2 × 10−6 dilutions, respectively. The reproducible sensitivities were 2 × 10−5 and 1 × 10−6 dilutions, and both Ct values were around 37. The levels of MLAA-34 in unknown samples were calculated as a ratio of MLAA-34 to β-actin. The levels of MLAA-34 and β-actin mRNA expression were quantified with the standard curves generated from known serial dilutions of the standard RNA obtained from U937 cells by assuming a linear relationship between the first cycle number, at which the fluorescence signal significantly increased Ct values and the logarithm of the starting quantity. A negative control without a template was included in each experiment. Data were presented as 2-ΔΔ Ct, where Ct was the threshold cycle and ΔCt was the Ct value of target amplification minus that of reference amplification [22].
Western blotting
Western blotting was carried out using cell lysates derived from the Ficoll separation-generated mononuclear fraction of bone marrow samples or peripheral blood of patients and normal individuals. Isolated leukemic cells or cell lines were washed in PBS, and total proteins were extracted with RIPA buffer (25 mM Tris–HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS. Pierce, Rockford, IL) for 15 min on ice. Sample concentrations were determined with a BCA protein assay reagent kit (Pierce, Rockford, IL), 30 μg of total proteins was subjected to SDS–PAGE and transferred to nitrocellulose membrane (Amersham Bioscience, Sunnyvale, CA), and the membrane was blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 1 h at room temperature. Each gel run included a sample of U937 cells as a positive control. After blotting, membranes were probed with specific anti-MLAA34 primary Ab [Mouse monoclonal to CAB39L (Santa Cruz Biotechnology, USA)] at a 1:2000 dilution in Tris-buffered saline (TBS) and 0.1% Tween 20 containing 5% bovine serum albumin overnight at 4°C. To verify the presence and quality of the protein (specifically, lack of degradation) in each sample, we also included an anti–β-actin Ab (Santa Cruz) at a 1:200 dilution. The secondary Ab was Goat anti-mouse IgG-HRP (1:500; Santa Cruz). Proteins were visualized by using the enhanced chemiluminescence technique (Amersham Pharmacia Biotech). The protein levels of MLAA-34 and β-actin from the same patient were qualified by using Quantity One 4.2.2 Software (Bio-Rad, Hercules, CA), and the ratio of MLAA-34 to β-actin was then defined as the MLAA-34 protein level.
Statistical analysis
The Fisher’s exact test was used to make comparisons of baseline clinical variables between groups for categoric data; the nonparametric Mann–Whitney U test was applied for continuous variables. The significance of difference in relative expression value of MLAA-34 mRNA and protein expression ratio between two patient groups were determined by using Mann–Whitney U test. Spearman correlation and logistic regression were adopted to determine the correlation of MLAA-34 mRNA relative expression value and dichotomized level with clinical data, respectively. The differences in MLAA-34 expression with respect to the clinical factors at diagnosis [i.e. gender, age, leukocyte count, the presence or absence of extramedullary disease, French–American–British (FAB) classification [23], and structural cytogenetic abnormalities] and the treatment outcome (day 7 response to induction chemotherapy and induction of remission with the primary induction chemotherapy regimen) were analyzed using a Mann–Whitney U test. The expression levels are presented as median values. The patients were categorized into two groups according to the levels of MLAA-34 expression (≥median versus <median). The proportions of relapsed patients in the two groups of patients were compared by using a Pearson Chi-square test. The probability of relapse-free survival (RFS) and overall survival (OS) rates along with SE were estimated with the Kaplan–Meier method. An event was defined as a disease relapse or treatment-related death. The differences in the survival rates according to the MLAA-34 expression levels (≥median versus <median) were compared by using a log-rank test. Cox proportional hazards models were constructed for RFS and OS. All of above programs were executed with SPSS statistical software version 16.0 or by Graphpad Prism 5.0 (Graphpad Software, San Diego, CA, USA). P < 0.05 was accepted as statistically significant.
Results
MLAA-34 transcript level in NBM and normal peripheral blood
MLAA-34 levels were either extremely low or undetectable in the control samples. The mean MLAA-34 relative expression detected in the control group was 1.02E-03 (range 3.27E-03, 1.35E-01). Samples were considered positive for MLAA-34 expression if their expression level was one log higher than the mean level of expression reported for the control group. Samples were considered as MLAA-34 over-expression if their expression level was at least three standard deviations above the mean level of expression reported for the normal group.
Both expression levels and expression rates of MLAA-34 were markedly up-expression in AML-M5
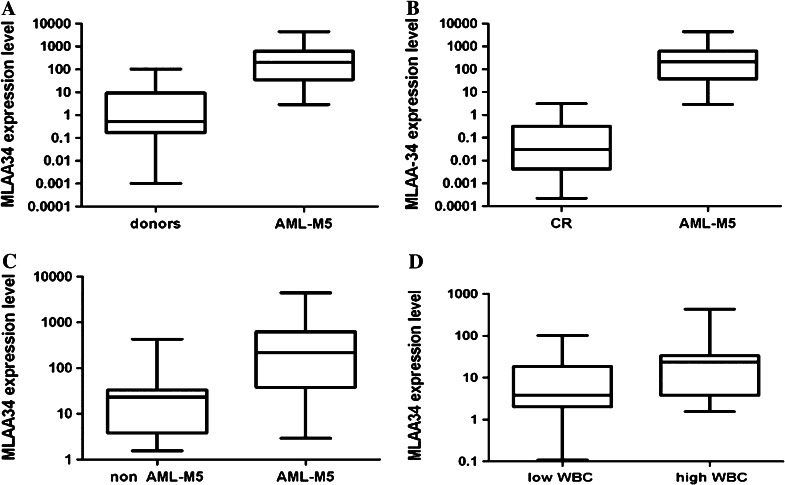
Relative quantification of the transcripts of the MLAA-34 gene using quantitative real-time RT–PCR revealed a striking increase in AML-M5 patients who were in disease progression (newly diagnosed, refractory/relapsed), in comparison with healthy donors and AML patients in complete remission (CR) (P < 0.001, Fig. 1a, b). There was no significant difference between normal control and CR groups (P = 0.065). We also observed a more increasing up-regulation of MLAA-34 mRNA expression in refractory/relapsed patients, compared with newly diagnosed patients (P < 0.001). With regard to the distribution profile of MLAA-34 expression among different subtypes of AML-M5 and other FAB subtypes of AML, no difference was observed (P = 0.057). However, high expression of MLAA-34 was more prominent in M5 subtype than others in AML patients (P < 0.001, Fig. 1c). We did not identify any significant distinction in MLAA-34 expression when grouping the patients by gender (P = 0.390) or age (P = 0.230). Patients were divided into two groups according to their peripheral blood WBC count, and notably those with high values of WBC count (≥20 × 109/L) had a greater increase in MLAA-34 expression than those with WBC count <20 × 109/L (P = 0.001, Fig. 1d). Also, the bone marrow blast percentages were analyzed for correlation between MLAA-34 transcript levels, and no evident difference was found for this factor. The expression rates of MLAA-34 with respect to the common prognostic factors are presented in Table 1. The expression rates of MLAA-34 were higher in patients with AML-M5, compared with the other subtype AML patients (P < 0.001, Fig. 2). Of note, the expression rate of MLAA-34 was higher in patients with AML-M4 than in patients with other subtype of AML. There was no correlation between MLAA-34 protein levels and MLAA-34 mRNA expression levels (r = 0.273, P = 0.192). However, there was a significant correlation between the MLAA-34 protein level and the WBC count (r = 0.712, P = 0.002). Therefore, MLAA-34 protein level probably depends on the total mass of leukemic cells over-expressing MLAA-34 gene.
Fig. 1.
Differential expression of MLAA-34 mRNA was illustrated in box plots between a de novo AML-M5 patients and healthy, b de novo AML-M5 patients and CR patients, c de novo AML-M5 patients and other subtypes of no AML-M5 patients, and d de novo AML-M5 patients with high leukocyte count and those with low leukocyte count. The value was presented as relative quantification (RQ) and expressed as median
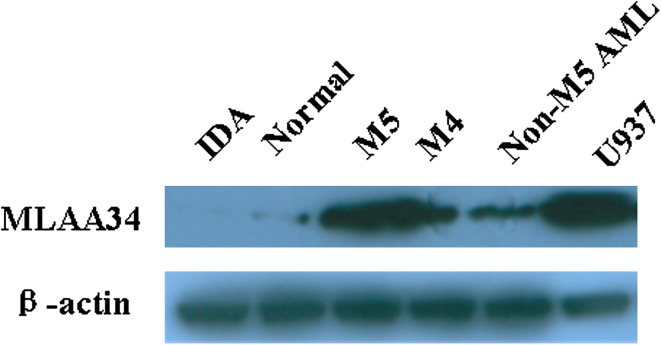
Fig. 2.
Representative Western blot for MLAA-34 protein expression. Blots were probed with an anti-MLAA-34 antibody and also probed for β-actin, IDA (iron deficient anemia) sample is a MLAA-34-negative control, and human acute monocytic leukemia cell line U937 serves as a MLAA-34-positive control, lane normal, normal individuals
Relevance of MLAA-34 expression levels to the unfavorable clinical features at diagnosis
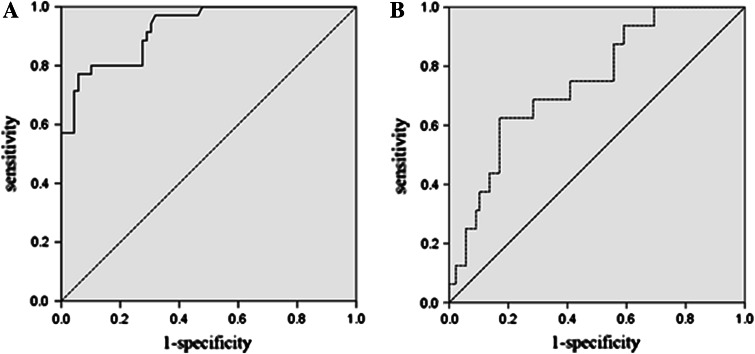
Clinical characteristics of the patients at diagnosis are presented in Table 1. MLAA-34 over-expression was found to be associated with the unfavorable clinical features at diagnosis. The level of MLAA-34 expression was higher in patients with a leukocyte number of ≥20 × 109/L (P = 0.001), patients with an extramedullary disease (P = 0.0083), and patients with an FAB M5 (P < 0.001) than in those with a leukocyte number of <20 × 109/L, those without extramedullary disease, and those with an FAB subtype other than M5. There was no difference in the level of MLAA-34 expression between M3 and other subtypes. The level of MLAA-34 expression was higher in patients with unfavorable cytogenetic abnormalities defined by the absence of t (8; 21), t (15; 17), and inv (16), albeit without a statistical significance. The level of MLAA-34 expression was higher in patients with 11q23 abnormalities or FLT3 gene length mutations than in patients lacking those genetic aberrations (median 2.14E+01 versus 3.10E+00 and 1.85E+01 versus 2.89E+00, respectively), which was not statistically significant. The percentages of AML leukemic cells expressing MLAA-34 ranged from 0 to 100%. Based on the 35% cutoff value from the ROC analysis (Fig. 3), 57 patients (45%) were defined as MLAA-34-postive and 69 patients (55%) as MLAA-34-negative, respectively. The expression rate also was higher in patients with AML-M5 than other subtypes of AML (P < 0.01). MLAA-34 expression rates were also higher in patients with WBC count ≥20 × 109/L (P = 0.024) and patients with extramedullary disease (P = 0.006) than in those patients with WBC count <20 × 109/L and patients without extramedullary disease. There was no difference in the MLAA-34 expression rate with respect to the other impact factors, such as age, sex, cytogenetic abnormalities, or FLT3 gene length mutations.
Fig. 3.
a ROC curve analysis of the different MLAA-34 levels with expression-positive patients and expression-negative patients. The area under the curve was 0.925. The best cutoff point was at approximately 1.63E+01. b ROC curve analysis of the different MLAA-34 protein ratios with expression-positive patients and expression-negative patients. The area under the curve was 0.738. The best cutoff point was at about 35%
MLAA-34 transcript levels during follow-ups and early detection of relapse
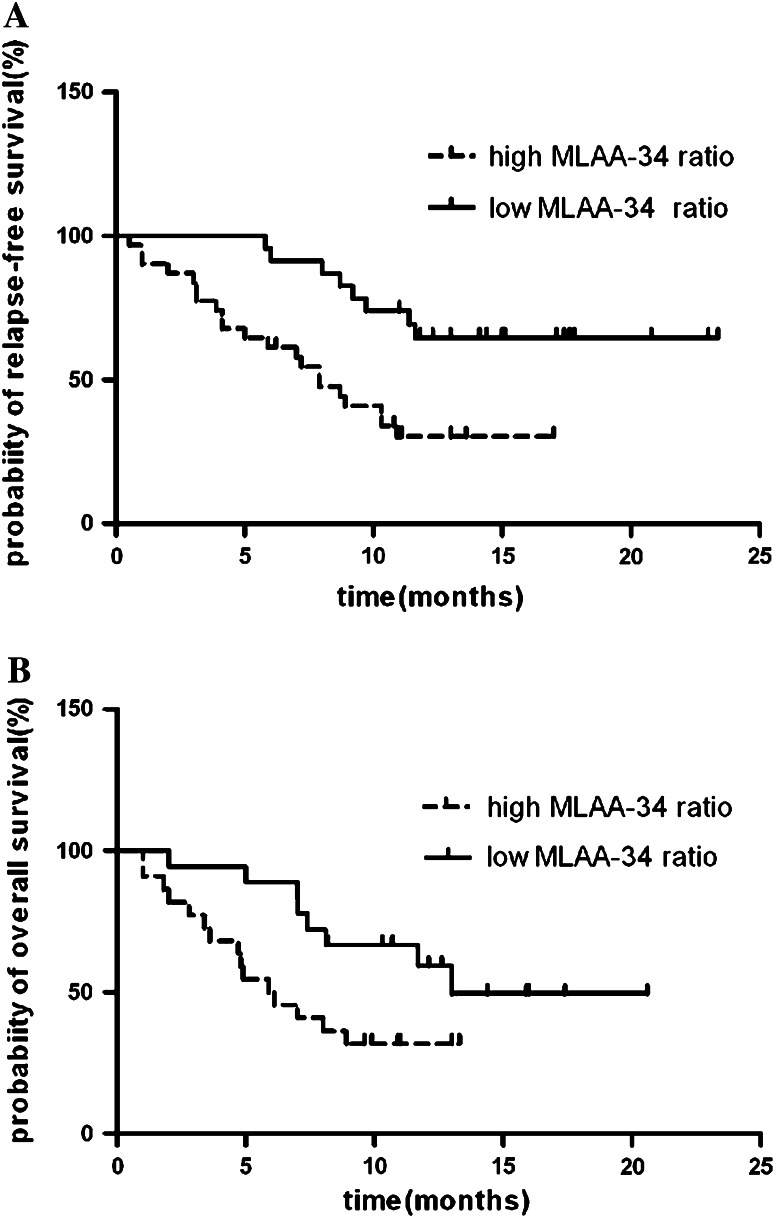
The level of MLAA-34 was analyzed during the follow-ups at various time points, usually in the interval of once every 3 months. Serially monitored values of individual cases showed marked heterogeneity of pattern in MLAA-34 transcript expression level and protein expression ratio. To define cutoff values for MLAA-34 protein and mRNA expression that could be used to discriminate the patients of the CR and in refractory/relapsed, ROC analyses were performed (Fig. 3). ROC analyses yielded cutoff values of 35% and 1.63E+01 for MLAA-34 protein and mRNA expression, respectively. With these discrimination values, RFS was adopted as an indicator for disease progression to perform Kaplan–Meier analyses. The median relapse-free period was shorter in patients with high MLAA-34 protein expression than in those with low MLAA-34 protein expression (25.3% versus 53.2% at 2 years, P = 0.002, log-rank test; Fig. 4a). Moreover, the results showed that patients with high level MLAA-34 expression had a significantly shorter OS (Fig. 4b; Table 3) than those with low MLAA-34 expression. To investigate whether MLAA-34 level was an independent predictor of remission duration or survival, the univariate and multivariate Cox model analyses were performed. The previously identified prognostic factors included were age, WBC counts, extramedullary disease, FLT3 mutation, and MLAA-34 level. Significant predictor variables in univariate analyses included WBC count, extramedullary disease, and FLT3 mutation, along with the MLAA-34 terms (Table 2).
Fig. 4.
Impact of MLAA-34 expression on survival. Kaplan–Meier analyses of a relapse-free survival (RFS) and b overall survival (OS) showing a significantly inferior outcome for patients with high MLAA-34 expression compared with patients with low MLAA-34 expression. Patients undergoing stem cell transplantation as consolidation treatment were excluded
Table 3.
Multivariate analysis of RFS and OS in AML patients
| Variable | RFS | OS | ||
|---|---|---|---|---|
| P | R R (95% CI) | P | R R (95% CI) | |
| Age (year) | ||||
| <60 versus ≥60 | 0.6 | 0.98 (0.69–1.38) | 0.55 | 1.12 (0.78–1.60) |
| WBC (×109/L) | ||||
| <20 versus ≥20 | 0.52 | 0.79 (0.34–1.87) | 0.78 | 1.03 (0.54–1.84) |
| Extramedullary disease | ||||
| Absent versus present | 0.004 | 2.32 (0.98–5.46) | 0.008 | 2.40 (1.26–4.58) |
| FLT3 mutation | ||||
| Absent versus present | 0.308 | 1.36 (1.40–2.86) | 0.313 | 1.53 (0.62–3.82) |
| MLAA-34 level | ||||
| Low versus high | 0.002 | 1.82 (1.25–2.68) | 0.002 | 1.85 (1.26–2.73) |
RFS relapse-free survival; OS overall survival; AML acute myeloid leukemia; RR relative risk; CI confidence interval
Table 2.
Univariate analysis of age, WBC, extramedullary disease, FLT3 mutation, and MLAA-34 level for RFS and OS in AML patients
| Variable | P a | |
|---|---|---|
| RFS | OS | |
| Age (year) | ||
| <60 versus ≥60 | 0.06 | 0.08 |
| WBC (×109/L) | ||
| <20 versus ≥20 | <0.01 | <0.01 |
| Extramedullary disease | ||
| Absent versus present | 0.004 | 0.006 |
| FLT3 mutation | ||
| Absent versus present | 0.026 | 0.032 |
| MLAA-34 level | ||
| Low versus high | <0.01 | <0.01 |
WBC white blood cell; RFS relapse-free survival; OS overall survival; AML acute myeloid leukemia
aLog-rank test
Correlation between MLAA-34 expression with time and the first treatment and overall survival
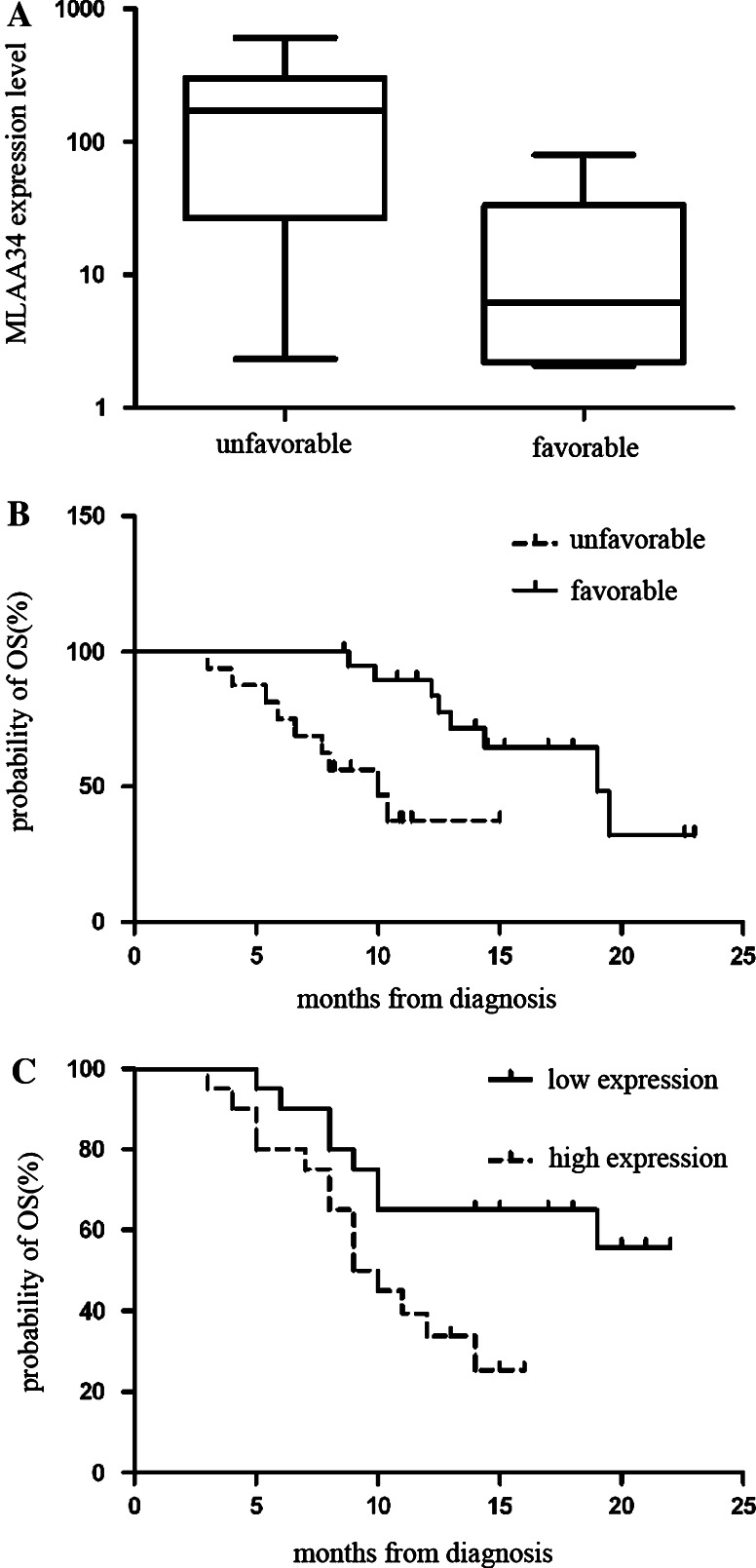
The median follow-up duration among 107 live patients was 12.5 months (range 4–22 months). The leukemia relapsed in 32 patients, and the treatment-related mortality was observed in 8 patients. The overall survival rates and relapse-free survival rates (±95% CI) in all 107 patients were 64.1 ± 7.4 and 56.3 ± 7.1%, respectively. MLAA-34 high expression was associated with an unfavorable early response to induction chemotherapy. The level of MLAA-34 expression was higher in patients with an unfavorable day 7 response (defined as >5% leukemic blasts on the bone marrow aspirate on day 7, the presence of a leukemic cell cluster on the bone marrow tissue section on day 7, or a persistence of circulating leukemic blasts in the peripheral blood on day 7) than in those with a favorable day 7 response (absence of an unfavorable response, P = 0.035; Fig. 5a). The overall survival of the cohort patients was analyzed as a function of MLAA-34 expression. The OS rate was higher in patients with a favorable day 7 response than in those with an unfavorable response (75.7 ± 8.9% versus 35.0 ± 15.4%, P = 0.002, Fig. 5b). The level of MLAA-34 expression was higher in patients who did not achieve CR after chemotherapy with the primary induction regimen than those who did; however, the difference was not significant. The relapse of disease was more frequent in patients with MLAA-34 high expression (15 of 32) than those without (17 of 75, P = 0.090). Similarly, the OS rate was lower in patients with MLAA-34 high expression than in those without (48.3 ± 11.2% versus 78.7 ± 8.5%, P = 0.020; Fig. 5c). In multivariate analysis with a Cox regression model, the only independent variables associated with a poor RFS and OS were extramedullary disease (P = 0.004; P = 0.008) and the high MLAA-34 expression level (P = 0.002). Thus, the over-expression of MLAA-34 in patients at diagnosis was an independent prognostic factor in this cohort of AML patients (Table 3).
Fig. 5.
a The level of MLAA-34 expression was higher in patients with an unfavorable day 7 response than in those with a favorable day 7 response. b the probability of OS rate was higher in patients with a favorable day 7 response than in those with an unfavorable response. c the probability of OS rate was lower in patients with high expression of MLAA-34 than in those without it
Discussion
MLAA-34 (GenBank no: AY288977) antigen was a newly identified acute monocytic leukemia-associated antigen, located on human chromosome 13q2. Our previous researches show that the down-regulation of MLAA-34 expression significantly suppresses the proliferation of U937 cells in vitro and increases the spontaneous apoptosis of these leukemia cells, indicating that MLAA-34 might be a novel anti-apoptotic factor related closely to carcinogenesis or progression of acute monocytic leukemia [14]. The resistance of tumor cells to apoptosis may pose serious clinical problems and may be associated with high-risk features at diagnosis as well as a poorer response to various treatments, such as chemotherapy and radiotherapy. A variety of anti-apoptotic proteins expressed in different tumors and their expression levels may be related to the unfavorable features at diagnosis and/or a poor response to treatment. However, the clinical relevance of these biologic regulators remains largely elusive, and particularly, little is known about MLAA-34 in this respect. From this context, this study was done to investigate the possible correlation between MLAA-34 expression and the clinical features in de novo AML-M5.
In this study, MLAA-34 up-expression was found to be associated with initial clinical characteristics at diagnosis. For example, the level of MLAA-34 expression was higher in patients with a leukocyte count of ≥20 × 109/L and patients with extramedullary disease; these patients have a poorer RFS and OS than those patients with a leukocyte count < 20 × 109/L and patients without extramedullary disease. This suggests that MLAA-34 high expression is associated with the high-proliferative nature of leukemic blasts. Considering that the survival in patients with the FAB M5 subtype is very poor [24], it is notable that the level of MLAA-34 expression was also higher in these patients than in patients with the other FAB subtypes. In addition, the level of MLAA-34 expression in patients with favorable cytogenetics [t(8;21), t(15;17), and inv(16)] was lower, albeit without a statistical significance. Lastly, MLAA-34 up-expression was consistently associated with the lower OS rates (Table 3). Overall, MLAA-34 up-expression is associated with the unfavorable clinical features at diagnosis in AML.
The early assessment of response to therapy represents an in vivo assessment of chemosensitivity and may be a powerful tool to delineate the prognosis in individual patients [25]. In adult de novo acute monocytic leukemia, the failure of achieving blast clearance from the bone marrow aspirates after 1 or 2 weeks of remission induction chemotherapy [26] or the persistence of circulating blasts after 1 week of multiagent chemotherapy [27] indicates a poor prognosis. Although it is likely that the initial response to induction chemotherapy may also be predictive of the outcomes of AML, but there are currently limited data available to draw any conclusion. Some studies have evaluated the response to induction chemotherapy by assessing the degree of residual leukemic infiltration in the bone marrow after 6 or 14 days of chemotherapy [26, 28–30]. In this study, the early response to induction chemotherapy was evaluated on day 7, and the unfavorable day 7 response was strongly associated with a poorer OS. Here again, it is worth noting that MLAA-34 over-expression was associated with an unfavorable day 7 response. In addition, the level of MLAA-34 expression was higher in patients who did not achieve CR after primary induction chemotherapy than in those who did, although the difference was not statistically significant. Collectively, it suggests that MLAA-34 over-expression is associated with delayed blast clearance from the bone marrow or peripheral blood, and the resistance of blasts to apoptotic stimuli provided by the chemotherapeutic agents eventually leads to a poorer RFS. Over the last decade, a complex network of proapoptotic and antiapoptotic proteins, which strictly regulate the apoptosis pathways, have been revealed. In particular, a group of proteins known as the inhibitor of apoptosis proteins (IAP) were identified [31–34]. The IAP family proteins inhibit the apoptosis induced by a variety of stimuli, and therefore, their high expression is expected to be associated with the unfavorable clinical features in a variety of malignancies, including AML. However, the clinical significance of IAP over-expression in acute leukemia is not completely consistent with what was expected from previous in vitro studies. For example, IAP over-expression was not always associated with the unfavorable clinical features in acute leukemia [35]. Furthermore, it was recently reported that the high expression of Livin, also a member of IAP family proteins, is an independent favorable prognostic factor in childhood acute lymphoblastic leukemia [36]. This suggests that the role of IAP in leukemogenesis or in the maintenance of leukemic cells might be different from what has been previously recognized. However, the findings in this study show that the clinical significance of MLAA-34 expression is consistent with what has been previously recognized in in vitro studies.
This is the first report demonstrating that MLAA-34 over-expression is associated with unfavorable clinical features at diagnosis and a poorer treatment outcome in de novo AML-M5. Through validation by further studies, MLAA-34 expression may be used as a prognostic marker in AML-M5. Studies involving a larger group of patients with different malignancies, particularly with respect to other apoptosis-related molecules, will be needed to reveal the pathophysiologic and clinical relevance of MLAA-34 expression in human cancers.
Acknowledgments
This work was supported by the National Natural Sciences Foundation of China. (No. 30971284).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Giles FJ, Keating A, Goldstone AH, Avivi I, Willman CL, Kantarjian HM. Acute myeloid leukemia. Hematology. 2002;2002(1):73–110. doi: 10.1182/asheducation-2002.1.73. [DOI] [PubMed] [Google Scholar]
- 2.Estey E. Acute myeloid leukaemia. Lancet. 2006;368(9550):1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 3.Schaich M, Schlenk RF, Al-Ali HK, Dohner H, Ganser A, Heil G, Illmer T, Krahl R, Krauter J, Sauerland C, Buchner T, Ehninger G. Prognosis of acute myeloid leukemia patients up to 60 years of age exhibiting trisomy 8 within a non-complex karyotype: individual patient data-based meta-analysis of the German acute myeloid leukemia intergroup. Haematologica. 2007;92(6):763–770. doi: 10.3324/haematol.11100. [DOI] [PubMed] [Google Scholar]
- 4.Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, Walker H, Wheatley K, Bowen DT, Burnett AK, Goldstone AH, Linch DC. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom medical research council AML 10 and 12 trials. Blood. 2001;98(6):1752–1759. doi: 10.1182/blood.V98.6.1752. [DOI] [PubMed] [Google Scholar]
- 5.Madden S, Cook D, Morris J, Gashler A, Sukhatme V, Rauscher F., III Transcriptional repression mediated by the WT1 Wilms tumor gene product. Science. 1991;253(5027):1550–1553. doi: 10.1126/science.1654597. [DOI] [PubMed] [Google Scholar]
- 6.Ito T, Shiraki K, Sugimoto K, Yamanaka T, Fujikawa K, Ito M, Takase K, Moriyama M, Kawano H, Hayashida M, Nakano T, Suzuki A. Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology. 2000;31(5):1080–1085. doi: 10.1053/he.2000.6496. [DOI] [PubMed] [Google Scholar]
- 7.Greiner J, Li L, Ringhoffer M, Barth TFE, Giannopoulos K, Guillaume P, Ritter G, Wiesneth M, Dohner H, Schmitt M. Identification and characterization of epitopes of the receptor for hyaluronic acid-mediated motility (RHAMM/CD168) recognized by CD8+ T cells of HLA-A2-positive patients with acute myeloid leukemia. Blood. 2005;106(3):938–945. doi: 10.1182/blood-2004-12-4787. [DOI] [PubMed] [Google Scholar]
- 8.Epping MT, Wang L, Edel MJ, Carlée L, Hernandez M, Bernards R. The human tumor antigen PRAME is a dominant repressor of retinoic acid. Recept Signal. 2005;122(6):835–847. doi: 10.1016/j.cell.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Greiner J, Dohner H, Schmitt M. Cancer vaccines for patients with acute myeloid leukemia—definition of leukemia-associated antigens and current clinical protocols targeting these antigens. Haematologica. 2006;91(12):1653–1661. [PubMed] [Google Scholar]
- 10.Porcu P, Cripe LD, Ng EW, Bhatia S, Danielson CM, Orazi A, McCarthy LJ. Hyperleukocytic leukemias and leukostasis: a review of pathophysiology, Clinical presentation and management. Leuk Lymphoma. 2000;39(1):1–18. doi: 10.3109/10428190009053534. [DOI] [PubMed] [Google Scholar]
- 11.Caligiuri MA, Schichman SA, Strout MP, Mrozek K, Baer MR, Frankel SR, Barcos M, Herzig GP, Croce CM, Bloomfield CD. Molecular rearrangement of the ALL-1 gene in acute myeloid leukemia without cytogenetic evidence of 11q23 chromosomal translocations. Cancer Res. 1994;54(2):370–373. [PubMed] [Google Scholar]
- 12.PaydaŞ S, Zorludemir S, ŞAhin B. Vasculitis and leukemia. Leuk Lymphoma. 2000;40(1–2):105–112. doi: 10.3109/10428190009054886. [DOI] [PubMed] [Google Scholar]
- 13.Gang C, Wanggang Z, Xingmei C, Fuyang L, Xinping L, Libo Y. Serological identification of immunogenic antigens in acute monocytic leukemia. Leuk Res. 2005;29(5):503–509. doi: 10.1016/j.leukres.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Zhang P-Y, Zhang W-G, He A-L, Wang J-L, Li W-B. Identification and functional characterization of the novel acute monocytic leukemia associated antigen MLAA-34. Cancer Immunol Immunother. 2009;58(2):281–290. doi: 10.1007/s00262-008-0552-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anscher MS. Targeting the TGF-{beta}1 pathway to prevent normal tissue injury after cancer therapy. Oncologist. 2010;15(4):350–359. doi: 10.1634/theoncologist.2009-S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamm I, Kornblau S, Segall H, Krajewski S, Welsh K, Kitada S, Scudiero D, Tudor G, Qui Y, Monks A. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000;6(5):1796. [PubMed] [Google Scholar]
- 17.Herr I, Debatin K-M. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98(9):2603–2614. doi: 10.1182/blood.V98.9.2603. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki H, Altieri DC, Lu C-D, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998;58(22):5071–5074. [PubMed] [Google Scholar]
- 19.Kawasaki H, Toyoda M, Shinohara H, Okuda J, Watanabe I, Yamamoto T, Tanaka K, Tenjo T, Tanigawa N. Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer. 2001;91(11):2026–2032. doi: 10.1002/1097-0142(20010601)91:11<2026::AID-CNCR1228>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Niu C, Yan H, Yu T, Sun H-P, Liu J-X, Li X-S, Wu W, Zhang F-Q, Chen Y, Zhou L, Li J-M, Zeng X-Y, Yang R-RO, Yuan M-M, Ren M-Y, Gu F-Y, Cao Q, Gu B-W, Su X-Y, Chen G-Q, Xiong S-M, Zhang T-d, Waxman S, Wang Z-Y, Chen Z, Hu J, Shen Z-X, Chen S-J. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94(10):3315–3324. [PubMed] [Google Scholar]
- 21.Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, Pettenati MJ, Patil SR, Rao KW, Watson MS, Koduru PRK, Moore JO, Stone RM, Mayer RJ, Feldman EJ, Davey FR, Schiffer CA, Larson RA, Bloomfield CD. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from cancer and leukemia group B (CALGB 8461) Blood. 2002;100(13):4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta]CT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein S, Brunetto V, Davey F, Wurster-Hill D, Mayer R, Stone R, Schiffer C, Bloomfield C. Acute myeloid leukemia-type chemotherapy for newly diagnosed patients without antecedent cytopenias having myelodysplastic syndrome as defined by French–American–British criteria: a cancer and leukemia group B study. J Clin Oncol. 1996;14(9):2486–2494. doi: 10.1200/JCO.1996.14.9.2486. [DOI] [PubMed] [Google Scholar]
- 24.Athale UH, Razzouk BI, Raimondi SC, Tong X, Behm FG, Head DR, Srivastava DK, Rubnitz JE, Bowman L, Pui C-H, Ribeiro RC. Biology and outcome of childhood acute megakaryoblastic leukemia: a single institution’s experience. Blood. 2001;97(12):3727–3732. doi: 10.1182/blood.V97.12.3727. [DOI] [PubMed] [Google Scholar]
- 25.San Miguel JF, Vidriales MB, Lopez-Berges C, Diaz-Mediavilla J, Gutierrez N, Canizo C, Ramos F, Calmuntia MJ, Perez JJ, Gonzalez M, Orfao A. Early immunophenotypical evaluation of minimal residual disease in acute myeloid leukemia identifies different patient risk groups and may contribute to postinduction treatment stratification. Blood. 2001;98(6):1746–1751. doi: 10.1182/blood.V98.6.1746. [DOI] [PubMed] [Google Scholar]
- 26.Kern W, Haferlach T, Schoch C, Loffler H, Gassmann W, Heinecke A, Sauerland MC, Berdel W, Buchner T, Hiddemann W. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML cooperative group (AMLCG) 1992 trial. Blood. 2003;101(1):64–70. doi: 10.1182/blood-2002-02-0532. [DOI] [PubMed] [Google Scholar]
- 27.Gajjar A, Ribeiro R, Hancock M, Rivera G, Mahmoud H, Sandlund J, Crist W, Pui C. Persistence of circulating blasts after 1 week of multiagent chemotherapy confers a poor prognosis in childhood acute lymphoblastic leukemia. Blood. 1995;86(4):1292–1295. [PubMed] [Google Scholar]
- 28.Browman G, Preisler H, Raza A, Syracuse K, Azarnia N, Benger A, Chervenick P, D’Arrigo P, Doeblin T, Goldberg J, Gottlieb A, Grunwald H, Kirshner J, Larson R, Meyer R, Miller K, Priore R, Stein M, Vogler WR, Walker I, Wilson WEC, Barcos M. Use of the day 6 bone marrow to alter remission induction therapy in patients with acute myeloid leukaemia: a leukemia intergroup study. Br J Haematol. 1989;71(4):493–497. doi: 10.1111/j.1365-2141.1989.tb06308.x. [DOI] [PubMed] [Google Scholar]
- 29.Peters WG, Willemze R, Zwaan FE, Colly LP. Day-6 bone marrow aspirate for the prediction of response to remission induction therapy for acute myelogenous leukaemia. Ann Hematol. 1988;57(2):91–95. doi: 10.1007/BF00319732. [DOI] [PubMed] [Google Scholar]
- 30.Liso V, Albano F, Pastore D, Carluccio P, Mele G, Lamacchia M, Mestice A, Specchia G. Bone marrow aspirate on the 14th day of induction treatment as a prognostic tool in de novo adult acute myeloid leukemia. Haematologica. 2000;85(12):1285–1290. [PubMed] [Google Scholar]
- 31.Yang YL, Li XM. The IAP family: endogenous caspase inhibitors with multiple biological activities. Cell Res. 2000;10(3):169–177. doi: 10.1038/sj.cr.7290046. [DOI] [PubMed] [Google Scholar]
- 32.Deveraux QL, Reed JC. IAP family proteins—suppressors of apoptosis. Genes Dev. 1999;13(3):239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 33.Nachmias B, Ashhab Y, Ben-Yehuda D. The inhibitor of apoptosis protein family (IAPs): an emerging therapeutic target in cancer. Semin Cancer Biol. 2004;14(4):231–243. doi: 10.1016/j.semcancer.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H-G, Wang J, Yang X, Hsu H-C, Mountz JD. Regulation of apoptosis proteins in cancer cells by ubiquitin. Oncogene. 2004;23(11):2009–2015. doi: 10.1038/sj.onc.1207373. [DOI] [PubMed] [Google Scholar]
- 35.Wrzesień-Kuś A, Smolewski P, Sobczak-Pluta A, Wierzbowska A, Robak T. The inhibitor of apoptosis protein family and its antagonists in acute leukemias. Apoptosis. 2004;9(6):705–715. doi: 10.1023/B:APPT.0000045788.61012.b2. [DOI] [PubMed] [Google Scholar]
- 36.Choi J, Hwang YK, Sung KW, Lee SH, Yoo KH, Jung HL, Koo HH, Kim H-J, Kang HJ, Shin HY, Ahn HS. Expression of Livin, an antiapoptotic protein, is an independent favorable prognostic factor in childhood acute lymphoblastic leukemia. Blood. 2007;109(2):471–477. doi: 10.1182/blood-2006-07-032557. [DOI] [PubMed] [Google Scholar]