Abstract
The discovery that antibody blockade of the T cell co-inhibitory receptor cytotoxic T lymphocyte-associated protein 4 (CTLA-4) can restore tumor immunity against many murine transplantable tumors leading to complete rejection of established cancer forever changed the field of immunotherapy. In more robust murine models as well as human cancer, however, CTLA-4 blockade alone can slow tumor growth and extend patient survival, but is rarely curative. Subsequent studies have revealed a large family of T cell immune checkpoint receptors which tumors engage to shield themselves from host immunity. As with CTLA-4, blockade of one of these additional inhibitory receptors, programmed death 1, has led to remarkable therapeutic responses against tumors of multiple lineages. Checkpoint monotherapy has demonstrated that durable, immune-mediated cures of established metastatic cancers are possible, yet the percentage of patients experiencing these outcomes remains low due to both redundant mechanisms of immune suppression in the tumor and limiting toxicity associated with some therapies. Thus, extending the curative potential of immunotherapy to a larger percentage of patients with a broader spectrum of malignancies will likely require combinations of co-inhibitory blockade and co-stimulatory activation designed to peel back multiple layers of tumor immune suppression while at the same time minimizing immune-mediated toxicity. As over a dozen T cell immune checkpoints and an additional dozen more co-stimulatory receptors have now been described, the challenge before us is to identify the most advantageous combinations of these agents based on the knowledge of their underlying biology and preclinical studies in murine tumor models.
Keywords: CIMT 2014, CTLA-4, 4-1BB, PD-1, Immunotherapy, Checkpoint
Introduction
The concept of immune therapy can be traced as far back as Dr. William Coley’s attempts to treat tumors through injection of a mixture of bacteria known as “Coley’s Toxins” in the 1890s. Not long after, Ehrlich first proposed the theory of immune surveillance in a 1,909 manuscript describing nascent transformed cells arise continuously in our bodies and that the immune system scans for and eradicates these transformed cells before they are manifested clinically [1]. These early insights were largely lost until Doherty and Zinkernagel [2] described the capacity of the T cell receptor to recognize peptides derived from foreign proteins displayed on host major histocompatibility complex (MHC) molecules and thus discriminate self from non-self. With the accompanying realization that tumors constituted a substantially altered form of “self,” the field of tumor immunology re-emerged to answer the question of why the immune system failed to recognize and reject cancer. A variety of peptide and viral vaccines were developed which could elicit elevated frequencies of T cells specific for tumor antigens, yet these approaches yielded few, if any, substantive clinical responses [3]. Schreiber and colleagues elegantly demonstrated that failure of tumor immunity was not due to lack of tumor-specific T cells or to systemic immune exhaustion by showing that mice bearing tumor expressing a foreign antigen could rapidly reject skin grafts which differed only in expression of that same antigen [4].
The landscape of tumor immunotherapy was forever changed when Krummel and Allison [5] described how the T cell co-stimulatory receptor CTLA-4 actually functioned as a potent inhibitor of T cell responses when activated by the same B7 molecules which were initially required for productive T cell activation by binding to CD28. Strikingly, they showed that antibody blockade of CTLA-4 restored tumor immunity and, alone, could promote T cell-mediated rejection of some pre-established transplantable murine tumors [6]. These critical findings illustrated that the major barrier to immune-mediated tumor rejection was, in fact, local tumor immune suppression and provided a paradigm whereby mechanisms of T cell dysfunction in the tumor microenvironment could be identified, targeted, and remedied.
Subsequent studies of the regulation of T cell responses both within and without the tumor microenvironment led to the identification of large families of both T cell co-stimulatory receptors and T cell co-inhibitory, or immune checkpoint, receptors. Programmed death 1 (PD-1) was the second T cell checkpoint receptor discovered after CTLA-4 and, when engaged by its ligands PD-L1 and PD-L2, was found to potently inhibit T cell effector function and expansion [7]. PD-1, CTLA-4, and their ligands are homologous molecules belonging to the B7 superfamily. While CTLA-4 can be engaged by B7 molecules expressed by myeloid cells in the tumor stroma, PD-L1 was found to be expressed by both stromal cells and, in many cases, the tumor cells themselves. As with CTLA-4, antibody blockade of either PD-1 or PD-L1 promotes T cell-mediated tumor rejection. The CTLA-4-blocking antibody ipilimumab received FDA approval for treatment of metastatic melanoma in 2011, while the first PD-1-blocking antibody, pembrolizumab, received approval for the same indication in late 2014. Numerous additional T cell immune checkpoints have been identified in the intervening time since the discovery of CTLA-4 (Fig. 1). Antibodies which block the activity of these novel immune checkpoints are in development or being tested for their capacity to restore tumor immunity alone and in combination.
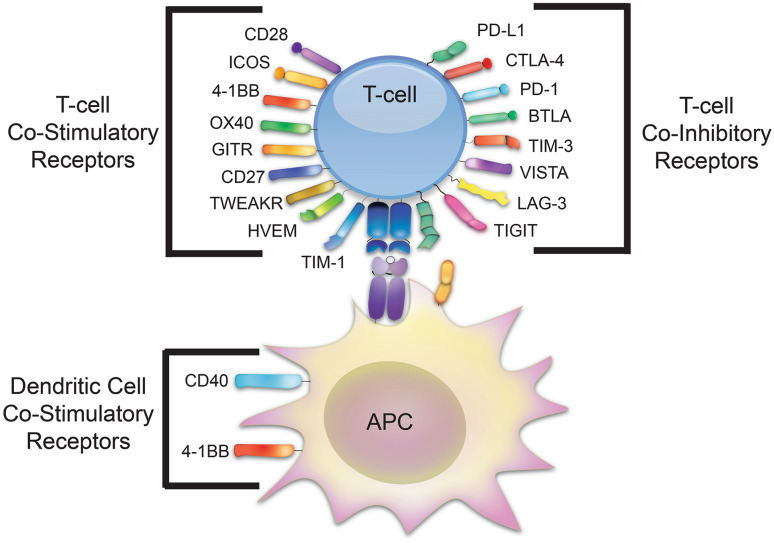
Fig. 1.
T cell co-stimulatory and co-inhibitory receptors. Shown are the families of T cell co-stimulatory and immune checkpoint receptors as well as those which affect dendritic cells responsible for T cell activation
While blocking the engagement of co-inhibitory receptors on T cells can protect them from attenuation in the tumor microenvironment, an alternative approach to restoring tumor immunity involves activating T cell co-stimulatory receptors using agonist antibodies. Not long after the therapeutic potential of CTLA-4 blockade was first described, Melero et al. [8] demonstrated that antibodies which activated the T cell co-stimulatory receptor 4-1BB enhanced proliferation, survival, and effector function of CD8 T cells and enhanced anti-tumor immunity. Shortly thereafter, Weinberg [9] described a similar potential of antibodies which activate the OX-40 receptor for tumor immunotherapy. OX-40 and 4-1BB belong to the TNF receptor family which includes multiple T cell co-stimulatory receptors which have been targeted with agonist antibodies including GITR, CD40, CD27, HVEM, LIGHT, APRIL, and TWEAK [10, 11]. The B7 superfamily also includes the T cell co-stimulatory molecules CD28 and inducible T cell co-stimulator (ICOS) [12, 13]. CD28 is a poor target for immunotherapy due to its expression on naïve T cells; however, ICOS is induced following T cell activation and can promote rejection of established tumors when activated.
Clearly, the evolutionary pressure to control T cell immunity and avoid immunity has led to the expansion of multiple families of co-stimulatory and co-inhibitory receptors which act to fine-tune immune responses. As tumors evolve under immune pressure, they evolve mechanisms to locally inactivate T cells through aberrant engagement of these immune checkpoints. Also, to reduce their immune visibility they surround themselves with suppressive stromal barriers devoid of T cell co-stimulatory signals. Numerous checkpoint-blocking and co-stimulatory agonist antibodies have already made their way into the clinic (Table 1). In addition, advanced preclinical programs across multiple companies worldwide promise additional agonist antibodies targeting OX-40, 4-1BB, CD40, GITR, ICOS, and LIGHT, as well as new co-inhibitory blockers aimed at TIM-3, LAG-3, CTLA-4, PD-1, PD-L1, TIGIT, and VISTA. The challenge has become to identify the most potent combinations which are capable of maximizing therapeutic benefit while minimizing immune-related adverse events (IRAE). Here we present examples of how understanding of the unique underlying biology of these T cell-modulating agents informs the assembly of therapeutically optimal combination therapies.
Table 1.
T cell immune checkpoint modulating antibodies in the clinic
| Target Molecule | Drug | Company | Development stage |
|---|---|---|---|
| CTLA-4 | Ipilimumab | Bristol-Myers Squibb | FDA approved |
| Tremelimumab | Medimmune/Astrazeneca | Phase III trial | |
| PD-1 | Pembrolizumab | Merck | FDA approved |
| Nivolumab | Bristol-Myers Squibb | FDA approved | |
| AMP-514/MEDI0680 | Medimmune/Astrazeneca | Phase I trial | |
| PD-L1 | MPDL3280A | Genentech/Roche | Phase III trial |
| MEDI4736 | Medimmune/Astrazeneca | Phase III trial | |
| MSB0010718C | EMD Serono | Phase II trial | |
| BMS-936559 | Bristol-Myers Squibb | Phase I trial | |
| 4-1BB | Urelumab | Bristol-Myers Squibb | Phase I trial |
| PF-05082566 | Pfizer | Phase I trial | |
| OX-40 | MEDI6469 | Medimmune/Astrazeneca | Phase I trial |
| MEDI6383 (rOX40L) | Medimmune/Astrazeneca | Phase I trial | |
| MOXR0916 | Genentech/Roche | Phase I trial | |
| GITR | TRX518 | Tolerx | Phase I trial |
| CD27 | CDX-1127 | Celldex | Phase I trial |
| CD40 | CP-870,893 | Genentech/Roche | Phase I trial |
| LAG3 | BMS-986016 | Bristol-Myers Squibb | Phase I trial |
Combination blockade of the CTLA-4 and PD-1 pathways
Mice lacking CTLA-4 develop a rapidly progressive lymphoproliferative disorder which is fatal within the first month of life, emphasizing the indispensable role of CTLA-4 regulation in restraining T cell responses [14]. PD-1-deficient animals, in contrast, develop a lupus-like glomerulonephritis on the C57BL/6 background, but live beyond a year of age [15]. On the BALB/C background, they manifest antibody-mediated cardiomyopathy that claims their lives by 6 months of age [16]. The distinct autoimmune phenotypes resulting from loss of these immune checkpoints demonstrated that the pathways by which they restrict T cell responses are largely non-redundant.
As we examined the impact of CTLA-4 blockade on T cells infiltrating B16 melanoma tumors, we found that both the percentage of effector T cells expressing PD-1 and the per-cell expression level of PD-1 were significantly increased. When we performed the converse experiment, we found a similar upregulation of CTLA-4 on tumor-infiltrating T cells following antibody blockade of PD-1 [17]. These observations suggested that blockade of either of these T cell immune checkpoints alone might transiently restore tumor-specific T cell responses; however, if those T cells failed to rapidly eliminate the cancer, they might be re-tolerized through induction of the other co-inhibitor. We thus hypothesized that blockade of both CTLA-4 and PD-1 might be necessary to achieve immune-mediated rejection of aggressive, non-immunogenic tumors such as B16 melanoma.
Combined with an irradiated B16-Flt3 ligand (FVAX) vaccine, we found that blockade of either CTLA-4, PD-1, or PD-L1 could cure 10–25 % of mice of a moderate challenge of the highly aggressive B16-BL6 clone of melanoma. Combination blockade of CTLA-4 and PD-1 increased the frequency of tumor-free animals to 50 %, and triple co-inhibitory blockade (CTLA-4, PD-1, and PD-L1) cured nearly 70 % of the mice [17]. Arlene Sharp has described an additional T cell inhibitory interaction within the PD-1 circuit consisting of engagement of PD-L1 expressed on T cells by B7-1 expressed by myeloid cells [18]. We hypothesize that the additional efficacy of αPD-L1 in combination with αPD-1 results from blockade of this B7-1/PD-L1 checkpoint. Within the tumor microenvironment, triple co-inhibitory blockade dramatically increased the ratios of effector CD8 and CD4 relative to suppressive CD4+FoxP3+ regulatory T cells (Treg) relative to untreated tumors and to treatment with each monotherapy. These data provided a compelling rationale for clinical translation of this combination immunotherapy.
Opposing the argument for clinical combination blockade of CTLA-4 and PD-1, however, was the reality of IRAE associated with these therapies [19]. CTLA-4 blockade causes a spectrum of sometimes high grade autoimmune-like side effects including colitis, hypophysitis, hepatitis, a variety of rashes, and others. PD-1 blockade also causes a partially overlapping spectrum of IRAE, the most severe of which is pneumonitis. Thus, while CTLA-4 and PD-1 elicited IRAE can be managed with steroids and tumor necrosis factor alpha (TNF-α)-blocking antibodies, the concern was that the combination of the two might elicit IRAE with an acute severity which defied management by established interventions.
Aided by their own preclinical CTLA-4/PD-1 blockade studies performed by Alan Korman and colleagues, Bristol-Myers Squibb decided that the potential benefits of this combination checkpoint blockade outweighed the associated risks. In this Phase I trial, concurrent administration of the CTLA-4-blocking antibody ipilimumab (3 mg/kg) and the PD-1-blocking antibody nivolumab (1 mg/kg) achieved Response Evaluation Criteria In Solid Tumors (RECIST) responses (>30 % tumor volume rejection) in 53 % of patients suffering from metastatic melanoma [20]. As compelling as these responses were, the speed with which they occurred (41 % of patients achieved >80 % reduction in tumor volume) was unprecedented in comparison to CTLA-4 or PD-1 monotherapy in which most clinical responses develop slowly over many months. According to the 2008 AJCC Melanoma Staging Database, the 2-year survival rate for Stage IV melanoma was under 25 % with a <1 % cure rate. In contrast, 88 % of patients receiving combination checkpoint blockade in this trial are alive at 2 years and a substantial percentage are likely permanently cured [21]. In contrast to prior studies, this trial yielded large cohorts of near-complete responders, partial responders, and non-responders, making it an ideal patient population in which to search for biomarkers associated with clinical responsiveness.
The detailed mechanistic studies of CTLA-4 and PD-1 combination blockade previously performed in mice suggested biomarkers with which to begin assessing responses in patients. In mice, increases in CTLA-4/PD-1 double-positive effector T cells within the tumor correlated with increased therapeutic efficacy and cure rates [17]. In an untreated tumor, few effector T cells can be identified which express both co-inhibitory molecules as these are rapidly inactivated and lost. In contrast, when CTLA-4-, PD-1-, and PD-L1-mediated negative signals are all shut down, up to 80 % of CD8 T cells in the tumor are CTLA-4/PD-1 positive. One week after combination ipilimumab/nivolumab therapy, an increased percentage of CTLA-4/PD-1-positive effector T cells becomes apparent in patient peripheral blood mononuclear cells (PBMC) and can be observed to persist throughout the 12-week monitoring period in some patients who experience complete responses, while it is lost in many who do not. Further studies will be required to determine any predictive significance of these changes.
Also, combination CTLA-4 and PD-1 blockade was shown in multiple murine models to increase proliferation of tumor-infiltrating T cells [17, 22]. Callahan et al. [23] from Memorial Sloan-Kettering Cancer Center discovered unprecedented increases in the proliferation of CD4 and CD8 effector T cells, measured as Ki67 upregulation, in the peripheral blood of patients treated with the ipilimumab/nivolumab combination as early as 7 days following the first combined infusion of antibody. While the capacity of ipilimumab to induce proliferation of effector T cells in patient PBMC had been previously observed [24], concurrent combination-treated patients exhibited frequencies of proliferating cells which were as much as tenfold greater than those reported for ipilimumab alone (i.e., 1.5–3.5 % Ki67+ vs. 15–35 % Ki67+). It remains to be determined whether the degrees of proliferation seen in these patients predict later outcomes.
Studies from the Sharma laboratory at MD Anderson Cancer Center identified sustained expression of ICOS on CD4 effector T cells as a positive biomarker of response to ipilimumab [25, 26]. Follow-up studies in mice showed that ICOS expression is actually functionally correlated with the efficacy of CTLA-4 blockade and that overexpression of ICOS ligand (B7h) could augment tumor immunity in the context of checkpoint blockade [27, 28]. In patients receiving combination blockade of CTLA-4 and PD-1, ICOS levels rose dramatically both in peripheral CD4 effector T cells and, in contrast to most patients treated with monotherapy, in CD8 T cells. ICOS is unusually compelling as a potential biomarker in these studies because of its mechanistic association with therapeutic efficacy. In addition, multiple therapeutic ICOS agonist antibodies are in clinical development suggesting a possible avenue for future combination studies.
Guided by a strong rationale rooted in basic tumor immunology data and mechanistic preclinical studies, blockade of CTLA-4 and PD-1 in combination has emerged as a potentially transformative immunotherapy for melanoma patients. Studies are ongoing in multiple tumor types including renal cell carcinoma, both non-small cell and small cell lung cancer, gastric cancer, pancreatic adenocarcinoma, colon cancer, triple-negative breast cancer, and a variety of hematologic malignancies to assess the therapeutic breadth of this potent immunotherapy. While the individual IRAE observed in combination-treated melanoma patients was no more severe than in patients receiving ipilimumab or nivolumab alone, the rate of Grade III/IV adverse events was very high (53 %). Thus, CTLA-4/PD-1 combination therapy will likely provide an important base for developing broadly curative immunotherapy regimens for the future; however, one goal in the field of immunotherapy is to identify combination therapies which offer similar efficacy but reduced toxicity.
4-1BB activation and CTLA-4 blockade: a perfect partnership
Activation of the TNF receptor superfamily member 4-1BB (CD137) on T cells augments tumor-specific T cell responses, particularly cytotoxic CD8s, against tumors of a variety of lineages [29]. In response to 4-1BB activation, T cells significantly elevate interferon gamma (IFN-γ) and TNF-α production, proliferate robustly, and demonstrate extended survival and memory potential [30]. Early observations also indicated a capacity of 4-1BB activation to elicit higher levels of tumor-specific cytotoxicity; however, no detailed mechanism to explain this effect was described [31]. In subsequent studies of 4-1BB agonist antibody, we found that α4-1BB induced a novel, highly cytotoxic phenotype of both CD8 and CD4 T cells driven by the T-box transcription factor eomesodermin which we termed ThEO/TcEO. Compared to Th1-/Tc1-polarized tumor-infiltrating T cells, these ThEO/TcEO cells expressed highly elevated levels of multiple granzymes, perforin, and Fas ligand and killed tumor cells with significantly enhanced potency [32]. This capacity of 4-1BB activation to enhance T cell expansion and effector function was counterbalanced, however, by the reality that these T cells remained subject to attenuation due to their expression of the co-inhibitory receptors CTLA-4 and PD-1.
Multiple groups, including ours, have demonstrated cooperative and even synergistic therapeutic benefit to combining CTLA-4 blockade with 4-1BB agonist antibody for tumor immunotherapy [33–35]. Freed from the confines of CTLA-4-mediated attenuation, 4-1BB can promote higher levels of proliferation and effector cytokine production in tumor-specific T cells which are likely to enjoy greatly extended persistence in the tumor microenvironment. Whereas CTLA-4 blockade alone can protect T cells from being inactivated, it does little to change their polarity and only modestly increases effector function. In combination, then, CTLA-4 allows unfettered expansion and survival in the tumor while 4-1BB drives enhanced effector function and promotes a highly tumoricidal polarity in both CD4 and CD8 effector T cells. The unique advantage of the αCTLA-4/α4-1BB combination, however, extends beyond this therapeutic synergy.
In contrast to checkpoint blockade which can worsen autoimmunity and engender IRAE, 4-1BB agonist antibodies actually ameliorate autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE) [36]. The first insight into the mechanism underlying this paradoxical capacity to promote tumor immunity but suppress autoimmunity was the discovery that 4-1BB agonist antibodies suppressed Th17 responses [37]. We later confirmed this Th17 suppression and showed that Eomesodermin upregulation in T cells and IL-27 induction in myeloid cells were the likely downstream mechanisms mediating this effect [32]. Kocak et al. [35] elegantly demonstrated that autoimmune suppression by 4-1BB antibodies also extended to suppression of autoimmunity elicited by persistent CTLA-4 blockade. Knowing the mechanism of α4-1BB-induced IRAE suppression (i.e., reduction in Th17) in mice strongly suggests that, in the clinic, 4-1BB agonist antibodies will also be able to suppress αCTLA-4 toxicity as colitis in ipilimumab-treated patients has been linked to elevation in serum IL-17 [38]. While inflammatory liver IRAE caused by 4-1BB and CD40 agonist antibodies can be induced and studied in animal models with kinetics and pathology closely mimicking their clinical presentation, the autoimmune-like adverse events elicited in disparate tissues by checkpoint blockade cannot be faithfully replicated in pathogen-free mice. Measuring anti-dsDNA antibodies induced by persistent CTLA-4 blockade as demonstrated by Kocak et al. can be used effectively, however, to study whether various combinations either exacerbate or ameliorate CTLA-4-induced IRAE. More recent mouse models using antigen-exposed “pet store” mice or humanized mice may prove more capable of modeling the detailed pathologies of clinical IRAE [39].
4-1BB antibodies entered clinical trials before PD-1 and PD-L1 antibodies, yet have not advanced much beyond Phase I trials while the latter are already reaching FDA approval. Unfortunately, treatment with 4-1BB agonist antibody causes life-threatening hepatitis in a minority of patients which has stalled the progress of these promising drugs in trials [40]. Again, Kocak et al. [35] were among the first to demonstrate liver pathology in α4-1BB-treated mice; however, they also showed that co-administration of CTLA-4-blocking antibody substantially ameliorated this hepatitis. Remarkably then, α4-1BB blocks the autoimmunity elicited by CTLA-4 blockade, and αCTLA-4 dramatically reduces the hepatitis resulting from 4-1BB agonist antibody treatment.
Evidence continues to accumulate in preclinical models that 4-1BB agonist and CTLA-4-blocking antibodies, when given in combination, not only synergize therapeutically in promoting immune-mediated tumor rejection, but also reduce or eliminate one another’s adverse events. In patients, this combination has the potential to increase efficacy across a variety of malignancies relative to either antibody alone, allow these antibodies to be administered at higher doses than are currently possible, reduce or eliminate IRAE associated with therapy relative to each monotherapy, and expand the pool of patients eligible to receive and remain on immunotherapy for their disease.
Outside of the box: checkpoint blockade and other therapies
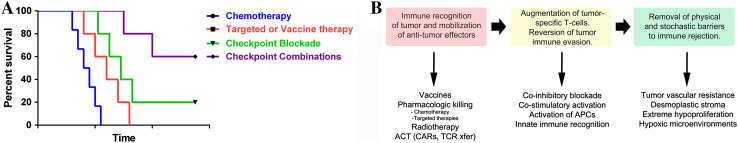
Antibodies that modulate the activity of tumor-specific T cells by blocking co-inhibitory or activating co-stimulatory receptors have ushered in a new era of cancer therapy. In contrast to chemotherapy or targeted therapies, immunotherapy has demonstrated the capacity to truly cure disseminated solid tumors and ward against their return, albeit in a minority of patients (Fig. 2a). Moving forward, the overarching goal is to find combination therapies which allow a greater percentage of patients suffering from a broader range of malignancies to experience durable, anti-tumor immunity. While this article focuses on the identification of optimal combinations of immune checkpoint antibodies, combinations of these with other types of interventions will be necessary to substantially extend the efficacy of immunotherapy (Fig. 2b).
Fig. 2.
Strategies for immune checkpoint combinations. a Shown is the current goal of the field of immunotherapy to increase the percentage of patients experiencing durable, complete responses through combination therapy approaches. b Different classes of therapeutic approaches which have synergistic potential for future combination immunotherapies are depicted
It is important to realize that checkpoint blockade does not create tumor-specific T cell responses out of nothing. In a patient who is completely immunologically ignorant of their tumor, CTLA-4 and/or PD-1 blockade will do very little to foster tumor rejection. In addition to its capacity to protect effector T cells from attenuation by CTLA-4 engagement, CTLA-4 blockade can also enhance T cell priming. While one study demonstrated the capacity of CTLA-4 blockade to elevate undetectable T cell responses against a variety of melanoma tissue-specific and cancer-testis antigens to measurable levels, the patients studied were a selected subset with likely preexisting immune recognition of their tumors [41]. The Gajewski laboratory at the University of Chicago has shown that only restoration of antigen presentation, not checkpoint blockade, can restore tumor-specific T cell responses to entirely unrecognized, non-infiltrated melanomas [42]. Also, it is becoming increasingly clear that the relevant T cell responses that are targeted by checkpoint blockade to mediate tumor rejection are specific to mutated proteins individual to each patient’s tumor [43, 44]. It has also been theorized that the relative fraction of a patient’s active T cells which recognize the tumor versus other antigens will reflect in the extent that patient experiences tumor regression versus IRAE when given checkpoint blockade. In this context, therapeutic interventions which increase the abundance of tumor-specific T cells are likely to synergize with checkpoint blockade and/or co-stimulatory agonist antibody therapy and may even lower the incidence of IRAE. Some traditional chemotherapies and some targeted therapies which cause tumor cells to die in an immunologic context could certainly play this role. Also, vaccines which augment the frequency of tumor-specific T cells could provide a fertile base of effectors for checkpoint blockade to expand and protect as they engage the tumor. Finally, adoptive transfer of large numbers of tumor-specific T cells, whether they be expanded TIL, chimeric antigen receptor (CAR)-transduced T cells, or expanded, tumor-specific PBMC, may prove the most potent partner for combination immunotherapy. In this latter incidence, however, caution must be exercised to avoid exacerbating existing toxicities associated with these approaches such as cytokine release syndrome.
Even when high-potency, tumor-specific T cell responses can be mobilized in the periphery, they provide little therapeutic benefit if they either are excluded from entry into the tumor or prove incapable of survival in the face of the hostile metabolic nature of the microenvironment. Here, drugs which modulate angiogenesis in order to facilitate T cell entry into the tumor stroma will likely prove to be synergistic partners for immunotherapy. Also, drugs which combat hypoxia and its associated tumor-promoting signaling would also be of potential benefit when combined with checkpoint blockade. The web of suppressive myeloid cells which dominate in most tumors also presents a formidable barrier to T cell-driven immunotherapy. Agents such as colony stimulating factor 1 receptor (CSF1R) inhibitors and antibodies which diminish this burden will likely demonstrate synergistic benefit in combination with checkpoint blockade.
The potent immunotherapeutic combinations discussed here provide illustrative examples of how basic tumor immunology and preclinical studies of individual agents in mice can reveal optimal immunotherapy combinations for translation into the clinic. By pursuing combinations within and without the field of T cell immunotherapy, there is hope that more and more patients will experience the therapeutic impact which these agents have had in the treatment of metastatic melanoma.
Acknowledgments
The work from our laboratory cited in this review was partially funded by a U.T. MD Anderson Moonshot Knowledge Gap award.
Conflict of interest
Dr. Curran has a research collaboration with Threshold Pharmaceuticals for which his laboratory has received funding, and an active collaboration with Astrazeneca in which compounds are provided free of charge. Dr. Curran also receives royalties from the patent “Methods and Compositions for Localized Secretion of anti-CTLA-4 Antibodies.” Dr. Ai has no conflicts to disclose.
Abbreviations
- CAR
Chimeric antigen receptor
- CSF1R
Colony stimulating factor 1 receptor
- CTLA-4
Cytotoxic T lymphocyte-associated protein 4
- EAE
Experimental autoimmune encephalomyelitis
- FVAX
B16-Flt3 ligand
- ICOS
Inducible T cell co-stimulator
- IFN-γ
Interferon gamma
- IRAE
Immune-related adverse events
- MHC
Major histocompatibility complex
- PBMC
Peripheral blood mononuclear cells
- PD-1
Programmed death 1
- RECIST
Response Evaluation Criteria In Solid Tumors
- TNF
Tumor necrosis factor
- Treg
Regulatory T cells (CD4+FoxP3+)
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Twelfth Annual Meeting of the Association for Cancer Immunotherapy (CIMT), held in Mainz, Germany, 6th-8th May, 2014. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Ehrlich P. Über den jetzigen Stand der Chemotherapie. Ber Dtsch Chem Ges. 1909;42:17–47. doi: 10.1002/cber.19090420105. [DOI] [Google Scholar]
- 2.Zinkernagel RM, Doherty PC. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 1974;251:547–548. doi: 10.1038/251547a0. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wick M, Dubey P, Koeppen H, Siegel CT, Fields PE, Chen L, Bluestone JA, Schreiber H. Antigenic cancer cells grow progressively in immune hosts without evidence for T cell exhaustion or systemic anergy. J Exp Med. 1997;186:229–238. doi: 10.1084/jem.186.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 7.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg AD, Rivera MM, Prell R, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 10.Moran AE, Kovacsovics-Bankowski M, Weinberg AD. The TNFRs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy. Curr Opin Immunol. 2013;25:230–237. doi: 10.1016/j.coi.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov. 2013;12:147–168. doi: 10.1038/nrd3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Podojil JR, Miller SD. Targeting the B7 family of co-stimulatory molecules: successes and challenges. BioDrugs. 2013;27:1–13. doi: 10.1007/s40259-012-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 14.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/S1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 17.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013;19:997–1008. doi: 10.1158/1078-0432.CCR-12-2214. [DOI] [PubMed] [Google Scholar]
- 20.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. New Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sznol M, Kluger HM, Callahan MK, et al. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL) J Clin Oncol. 2014;32(5s Suppl):LBA9003. [Google Scholar]
- 22.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callahan MK, Horak CE, Curran MA, et al. Peripheral and tumor immune correlates in patients with advanced melanoma treated with combination nivolumab (anti-PD-1, BMS-936558, ONO-4538) and ipilimumab. J Clin Oncol. 2013;31(Suppl):3003. [Google Scholar]
- 24.Wang W, Yu D, Sarnaik AA, Yu B, Hall M, Morelli D, Zhang Y, Zhao X, Weber JS. Biomarkers on melanoma patient T cells associated with ipilimumab treatment. J Transl Med. 2012;10:146. doi: 10.1186/1479-5876-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng Tang D, Shen Y, Sun J, Wen S, Wolchok JD, Yuan J, Allison JP, Sharma P. Increased frequency of ICOS+ CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer Immunol Res. 2013;1:229–234. doi: 10.1158/2326-6066.CIR-13-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carthon BC, Wolchok JD, Yuan J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16:2861–2871. doi: 10.1158/1078-0432.CCR-10-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu T, He Q, Sharma P. The ICOS/ICOSL pathway is required for optimal antitumor responses mediated by anti-CTLA-4 therapy. Cancer Res. 2011;71:5445–5454. doi: 10.1158/0008-5472.CAN-11-1138. [DOI] [PubMed] [Google Scholar]
- 28.Fan X, Quezada SA, Sepulveda MA, Sharma P, Allison JP. Engagement of the ICOS pathway markedly enhances efficacy of CTLA-4 blockade in cancer immunotherapy. J Exp Med. 2014;211:715–725. doi: 10.1084/jem.20130590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SW, Croft M. 4-1BB as a therapeutic target for human disease. Adv Exp Med Biol. 2009;647:120–129. doi: 10.1007/978-0-387-89520-8_8. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev. 2009;229:192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 31.Li B, Lin J, Vanroey M, Jure-Kunkel M, Jooss K. Established B16 tumors are rejected following treatment with GM-CSF-secreting tumor cell immunotherapy in combination with anti-4-1BB mAb. Clin Immunol. 2007;125:76–87. doi: 10.1016/j.clim.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Curran MA, Geiger TL, Montalvo W, Kim M, Reiner SL, Al-Shamkhani A, Sun JC, Allison JP. Systemic 4-1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. J Exp Med. 2013;210:743–755. doi: 10.1084/jem.20121190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belcaid Z, Phallen JA, Zeng J, et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS One. 2014;9:e101764. doi: 10.1371/journal.pone.0101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One. 2011;6:e19499. doi: 10.1371/journal.pone.0019499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kocak E, Lute K, Chang X, et al. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 2006;66:7276–7284. doi: 10.1158/0008-5472.CAN-05-2128. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y, Lin X, Chen HM, Wu Q, Subudhi SK, Chen L, Fu YX. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J Immunol. 2002;168:1457–1465. doi: 10.4049/jimmunol.168.3.1457. [DOI] [PubMed] [Google Scholar]
- 37.Kim YH, Choi BK, Shin SM, et al. 4-1BB triggering ameliorates experimental autoimmune encephalomyelitis by modulating the balance between Th17 and regulatory T cells. J Immunol. 2011;187:1120–1128. doi: 10.4049/jimmunol.1002681. [DOI] [PubMed] [Google Scholar]
- 38.Callahan MK, Yang A, Tandon S, et al. Evaluation of serum IL-17 levels during ipilimumab therapy: correlation with colitis. J Clin Oncol. 2011;29(Suppl):2505. [Google Scholar]
- 39.Vudattu NK, Waldron-Lynch F, Truman LA, Deng S, Preston-Hurlburt P, Torres R, Raycroft MT, Mamula MJ, Herold KC. Humanized mice as a model for aberrant responses in human T cell immunotherapy. J Immunol. 2014;193:587–596. doi: 10.4049/jimmunol.1302455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol. 2010;37:508–516. doi: 10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Kvistborg P, Philips D, Kelderman S, et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med. 2014;6:254ra128. doi: 10.1126/scitranslmed.3008918. [DOI] [PubMed] [Google Scholar]
- 42.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signaling prevents T cell infiltration and anti-tumor immunity. J ImmunoTherapy Cancer. 2014;2(Suppl 3):O15. doi: 10.1186/2051-1426-2-S3-O15. [DOI] [Google Scholar]
- 43.Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Rooij N, van Buuren MM, Philips D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 2013;31:e439–e442. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]