Abstract
Methionine enkephalin (MENK), the endogenous neuropeptide, is known to exert direct effects on the neuroendocrine and the immune systems and participates in regulation of various functions of cells related to both the innate and adaptive immune systems. Dendritic cells (DCs) play important role in initiating and regulating T cell responses. The aim of this work is to investigate the effects of MENK on differentiation, maturation, and function of DCs derived from murine bone marrow progenitors (BM-derived DCs). Our result showed that MENK could induce BM-derived DCs to polarize predominantly to mDC subtype, rather than pDC both in vivo and in vitro, and this was in favor of Th1 response. BM-derived DCs, after treatment with MENK, up-regulated the expressions of MHC class II and key costimulatory molecules. Result by RT-PCR showed MENK could increase expressions of delta and kappa receptors on BM-derived DCs. Also MENK promoted BM-derived DCs to secret higher levels of proinflammatory cytokines of IL-12p70, TNF-α. Furthermore, differentiated BM-derived DCs treated with MENK displayed higher activity to induce allogeneic T cell proliferation and MENK also inhibited tumor growth in vivo and induced apoptosis of tumor cells in vitro. Thus, it is concluded that MENK could be an effective inducer of BM-derived DCs and might be a new therapeutic agent for cancer, as well as other immune handicapped disease. Also we may consider MENK as a potential adjuvant in vaccine preparation.
Keywords: Methionine enkephalin, Dendritic cells, Differentiation, Induction, Tumor immunity
Introduction
MENK, an endogenous opioid peptide composed of five amino acids with the following sequence: Tyr–Gly–Gly–Phe–Met, is generated in adrenal gland and derived from proenkephalin [1]. Receptors of the mu, kappa, and delta types [2] have been detected on various immune cells in a number of studies [3]. In addition to their analgesic activity, the control of respiratory, cardiovascular, gastrointestinal functions, and antitumor effects via opioid receptors, MENK also exert stimulation on the cells in both innate and adaptive immune system at a moderate range of concentrations, such as T cell [4, 5], NK [6–9] LAK cells and TIL cells [10] macrophage [11, 12], and DCs [13–16].
Dendritic cells (DCs) function as highly efficient antigen presenting cells (APCs) with a crucial role in both innate immunity and adaptive immunity [17, 18]. Immature DCs reside in peripheral tissues, where they serve as sentinels for foreign antigens and microbial pathogens. Upon activation, immature DCs with the capacity to arrest antigens undergo maturation, becoming maturated DCs with capacity to accumulate and present peptides to T cell. Simultaneously, there is significant up-regulation of expression of major histocompatibility complex (MHC) class II molecules, costimulatory molecules such as CD40, CD80, and CD86 on the maturated DCs. Usually DCs are broadly classified into plasmacytoid dendritic cells (pDCs) and myeloid dendritic cells (mDCs) subtypes, which could be developed from bone marrow progenitors [19]. In addition, it has been widely considered that mDC, preferentially elicited Th1 type response, produced IFN-γ, TNF-α, TLR-9, and IL-12 [20, 21], whereas pDC promoted a Th2 response, secreted IL-4, IL-5, IL-13, and IL-10. Recent findings indicated that the different stimuli induced BM-derived DCs to differentiate into different DC subset, which serves different Th cell responses [22–24]. Thus, the type of DC stimulus may determine the DC-mediated polarization of Th cell responses. Several cytokines can influence the differentiation and the maturation status of DCs. GM-CSF, G-CSF, and Flt3-L were widely used for modulating DC function. GM-CSF has been routinely used in clinical post-chemo/radiotherapy for repopulating mDCs in cancer patients [25–30]. Although immunomodulating properties of MENK have been reported previously [31, 32], the effect of MENK on differentiation of BM-derived DC precursor into DC has not been studied so far and mechanisms remain unclear. We endeavored to conduct following exploration to try to reveal mechanisms, via which, MENK regulates cells of immune system, so as to support clinical application of MENK as a new drug fighting cancers in the future.
Materials and methods
Reagents
MENK was provided by Penta Biotech. Inc., USA (≥97% purity). RT-PCR Kits were purchased from Taka (Japan). Recombinant murine cytokines of IL-4 and GM-CSF were obtained from PeproTech Inc. LPS from Escherichia coli (serotype 055:B5) was a product of Sigma-Aldrich. The FCM antibodies were purchased from Biologend (CA, USA). Murine cytokines were measured using ELISA kits purchased from R&D System. RT-PCR primers were prepared by Nanking Kinsit Gene Tech. Co., China. Trizol was a product of Invitrogen (Carlsbad, CA). Other chemicals frequently used in our laboratory were all products from Sigma-Aldrich or BD Pharmingen.
Ethic statement
All animal experiments were conducted in strict accordance with the Guide for the Care and Use of Laboratory Animals of the China law of animal welfare. The protocol was approved by the Committee on the ethics of Animal Experiments of China National Institutes of Health (permit number GB 14923-2001).
Mice
Female 6- to 8-week-old C57BL/6 mice and BALB/C mice were obtained from Harlan Slac Laboratory Animals Co., Ltd. (Shanghai, China), and genotypes of all mice were tested and authenticated by multi locus DNA fingerprinting and multiplex-PCR DNA profiling analysis and were housed in the nonspecific pathogen-free (SPF) animal facility at China medical university. The mice were kept at 22°C with 14 h light: 10 h dark schedule and fed with standard food pellets and water ad libitum. Mice were sacrificed by isoflurane inhalation. All surgery was performed under Ketamine and Xylazine anesthesia, and all efforts were made to minimize suffering.
Cell lines
S180 and MCF-7 cell lines used for in vivo and in vitro experiments were purchased from Shanghai Institute of Cell Biology. Genotypes of all cell lines were tested and authenticated by multi locus DNA fingerprinting and multiplex-PCR DNA profiling analysis. And that they were passaged in our laboratory for 5 months after receipt.
BM-derived DCs differentiation in vitro
Bone marrow cells obtained from mice were seeded in 6-well culture plates at 107 cells/well in a final volume of 2 mL 1640 medium and cultured at 37°C in 5% humidified CO2 for 2 h. Nonadherent granulocytes cells were removed, and adherent cells were cultured to induce DCs differentiation. Various grade concentrations of MENK (10−8–10−14 mol/L) were added to the cultured bone marrow cells on 0 day, as well we set G+I (GM-CSF plus IL-4, 20 ng/mL GM-CSF and 10 ng/mL IL-4) and IL-3 (15 ng/mL) as control groups. pDC and mDC were tested on 7 days by Flow cytometry (FCM) according to surface markers.
Analysis of costimulatory molecules by FCM
The cultured bone marrow cells served as both a testing group, after treatment with 10−12 mol/L MENK, G+I separately for 7 days, and a RPMI 1640 control group. They were collected, stained with APC-anti-CD40, Percp-anti-CD80, PE-anti-MHC-II, FITC-anti-CD11c, APC-anti-CD11b, and PE-anti-CD45R/B220 antibodies for 30 min, then washed with 2% PBS twice, and subsequently collected using FCM Calibur (Becton–Dickinson, San Diego, CA). The data were then analyzed using Flow Jo software (Tree Star, Ashland, OR).
Cytokine assay
The production of cytokines in the BM-derived DC cultures was determined by ELISA. Supernatants of differentiated BM-derived DCs in each group were harvested and subjected to ELISA for IL-10, and IL-12p70 the absorbance at 450 nm (A450) was determined using a bichromatic-microplate reader.
Confirmation of opioid receptors, intracellular TNF-α, and TLR-9 by RT-PCR
The gene expressions of mu, kappa, delta receptors, and intracellular TNF-α, TLR-9 were detected by RT-PCR. The RT-PCR reaction was carried out as shown in Table 1. Meanwhile, the total cultured BM-derived DCs cellular RNA was extracted and reversed transcripted into complementary DNA (cDNA). The mRNA level of each gene was indicated by the gel electrophoresis with same amounts of PCR products. The amount of DNA was calculated using standard curves, and the results were normalized to the housekeeping gene β-actin.
Table 1.
The primers of genes
| Gene | Sequence | GC | Tm | Products length |
|---|---|---|---|---|
| (%) | (°C) | |||
| β-actin | 5′-TGCTGTCCCTGTATGCCTCT-3′ | 55 | 56 | 333 bp |
| 5′-CAGGATTCCATACCCAAG-3′ | 50 | |||
| TNF-α | 5′-GGCGGTGCCTATGTCTC-3′ | 64 | 52 | 462 bp |
| 5′-GCAGCCTTGTCCCTTGA-3′ | 58 | |||
| TLR-9 | 5′-TGGACGGGAACTGCTACT-3′ | 55 | 59 | 768 bp |
| 5′-GCCCACTGATGCGATTGT-3′ | 55 | |||
| Mu | 5′-CCATTGGTCTGCCCGTAA-3′ | 55 | 58 | 218 bp |
| 5′-TTTGGAGCCCGACAGCAT-3′ | 55 | |||
| Kappa | 5′-TCCGACAGTAATGGCAGTG-3′ | 52 | 55 | 609 bp |
| 5′-TGGGATCACAAAGGCAAA-3′ | 44 | |||
| Delta | 5′-CCAGTCGCAGTCAATCTAA-3′ | 47 | 54 | 756 bp |
| 5′-TCTTCCCTCAATCCCTTC-3′ | 50 |
Mixed lymphocyte reaction (MLR)
The differentiated BM-derived DCs were induced with 10−12 mol/L MENK, G+I (20 ng/mL GM-CSF and 10 ng/mL IL-4), IL-3 (15 ng/mL), and LPS (1 ng/mL), respectively, for 7 days, and they were purified by immunomagnetic beads and analyzed using FCM Calibur for DC, NK, Macrophage, and CTL. After that, allogenic T cells purified by immunomagnetic beads from BALB/C mice (2–5 × 105/well) were incubated in 96-well cell-culture plates with graded numbers of differentiated BM-derived DCs for 5 days. Proliferation of T cells was monitored by MTT at 490 nm (A490) using a bichromatic-microplate reader. Results were expressed as the mean ± SD from triplicate wells.
BM-derived DC-mediated inhibition to tumor cell in vitro
The BM-derived DCs induced with 10−12 mol/L MENK, G+I, IL-3, and LPS, respectively, were purified by immunomagnetic beads and analyzed using FCM Calibur for DC, NK, Macrophage, and CTL. MCF-7 cells (105/mL) were seeded in a 96-well plate and cultured with graded doses of differentiated BM-derived DCs in per triplicate/well, respectively, for 96 h. The optical value of each well was quantified using 3H-TdR penetration method. Statistical analysis of the inhibition ratio mediated by BM-derived DCs were done as per formula:
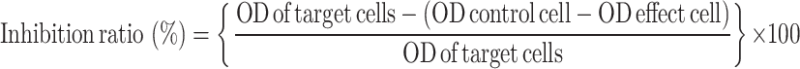 |
Confirmation of tumor apoptosis mediated by differentiated BM-derived DCs
Differentiated BM-derived DCs of each group were cocultured with (105/mL) MCF-7 tumor cells for 3 days. The harvested cells were for apoptotic detection using annexin V-FITC kit per manufacturer’s protocol by FCM, and this result was reconfirmed with inverted fluorescent light microscope.
Mice grouping and studying in vivo
C57BL/6 mice were randomly divided into three groups, with five mice in each group, and one group was for the establishment of S180 model (injected subcutaneously with 106 of Sarcoma 180 cells). The mice in S180 model group and in MENK group (normal C57BL/6 mice treated with MENK) were treated by s.c. with 20 mg/kg MENK, every other day for a period of 30 days. All the mice were sacrificed on 30 days. The bone marrow cells and splenocytes of the mice in each group were separated; subsequently, both the DC subtypes and expressions of costimulatory molecules were identified with FCM.
Antitumor activity of MENK in vivo
S180 cells (1.0 × 106/mL) were injected subcutaneously into C57BL/6 mice (0.2 mL/mouse). After establishment of tumor model for 24 h, mice were divided into two groups (eight mice/group). Mice in the MENK group were injected (i.p.) with MENK (20 mg/kg, Penta Biotech Inc., USA, ≥97 purity) once a day for consecutive 30 days. During the administration of MENK, the mice were observed daily. The tumor sizes and survival rate were measured. The tumors apoptosis was detected using annexin V-FITC kit per manufacturer’s protocol by FCM.
Inhibiting effect on tumor growth by infusing differentiated BM-derived DCs in vivo
C57BL/6 mice bearing S180 tumors were randomly divided into two groups with five mice in each group. The mice in testing groups received injection of 0.25 × 106 differentiated BM-derived DCs induced by 10−12 mol/L MENK. The mice were observed daily. The tumor sizes and survival rate were measured.
Statistical analysis
Mean, standard deviation, and statistical significance were calculated using the SPSS application software and Prism (Graph Pad Software). The results were expressed as mean ± SD of three or more independent experiments. Tukey’s test was used to compare testing group with control group. Statistical result used for post hoc analysis when p < 0.05 indicated significance. The results will be discussed later.
Results
Effect of grade concentration of MENK on differentiation of BM-derived DC progenitor
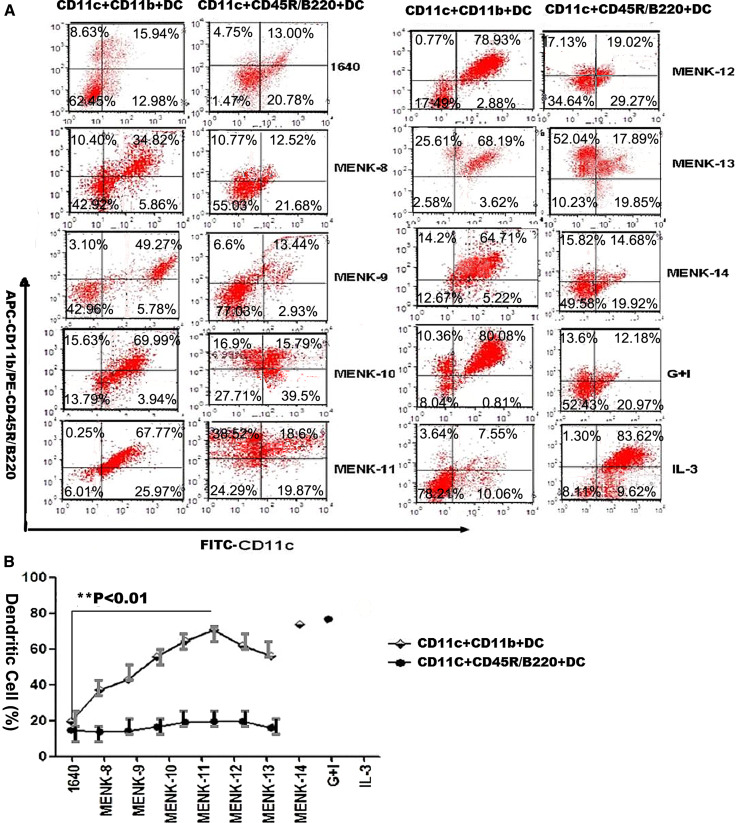
With increasing concentrations of MENK (10−8–10−14 mol/L) in testing group, the expression of CD11c+CD11b+ (mDC marker) on BM-derived DCs increased, while that of CD11c+CD11b−CD45R/B220+ (pDC marker) did not change significantly compared with expression in the control groups. The optimal concentration of MENK at used range was 10−12 mol/L. Under the influence of this concentration, CD11c+CD11b+ BM-derived DCs in the MENK-12 (10−12 mol/L MENK) group were higher than that in the other concentration MENK groups and much higher (p < 0.01) than that in the RPMI 1640 group, although it had no obvious difference (p > 0.05) compared with that in the G+I group, and CD11c+CD11b−CD45R/B220+ BM-derived DCs in the MENK-12 group had no difference (p > 0.05) versus that in the RPMI 1640 group and had a little higher (p < 0.05) than that in the G+I group, as shown in Fig. 1. These data demonstrated that MENK was a potentially effective factor that can reverse BM-derived DCs to mDC by immunophenotyping, and the optimal concentration was 10−12 mol/L MENK.
Fig. 1.
The effect of graded concentrations of MENK on the differentiation of BM-derived DC progenitors in vitro. BM-derived DC progenitors were cultured for 7 days with MENK at range of concentrations (10−8–10−14 mol/L) and phenotypes of BM-derived DCs were analyzed by FCM. a Immunophenotypes were characterized by FCM. Similar results were obtained from four separate experiments. b The results were summarized in the curve. **p < 0.01 versus in the RPIM 1640 group
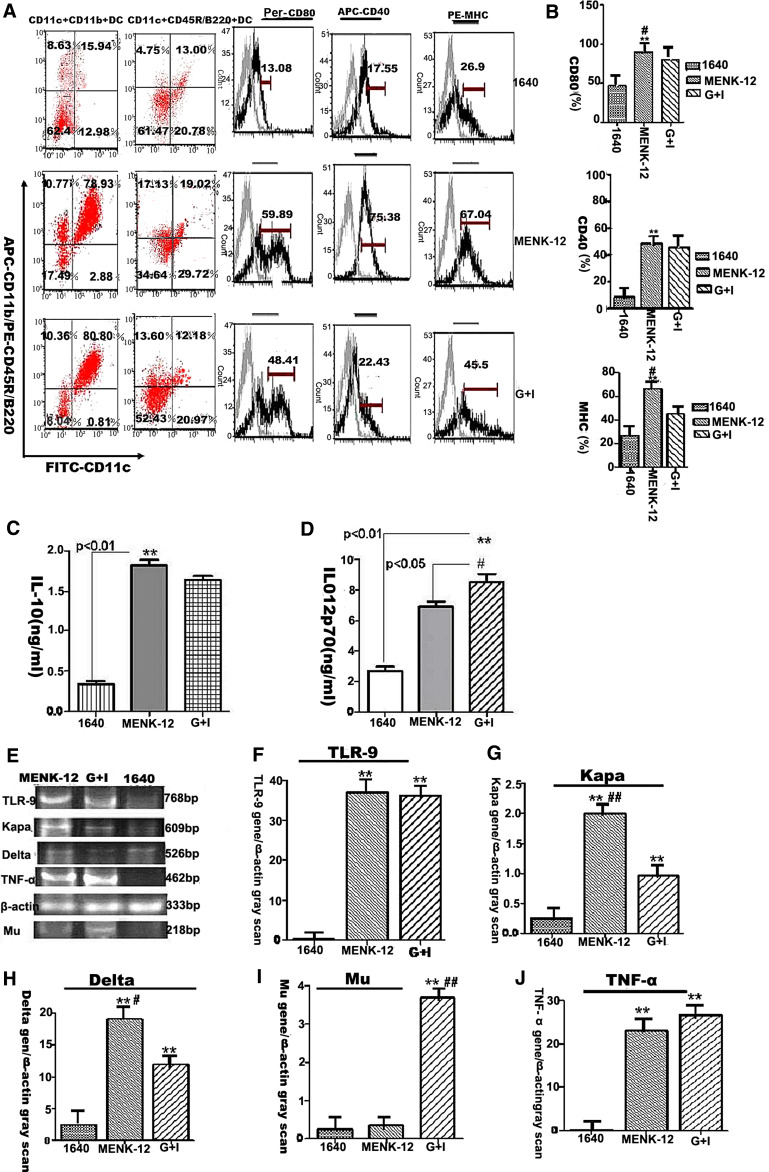
Expression of MHCII and costimulatory molecules on BM-derived DCs treated with 10−12 M MENK
The surface markers on the differentiated BM-derived DCs after treatment with MENK-12 were analyzed using four-color FCM. Concretely MHCII, CD40, and CD80 in the MENK-12 group expressed much higher level (p < 0.01) than that in the RPMI 1640 group, and then, there were also a significant increase in the number of CD80 and MHCII in the MENK-12 group (p < 0.05) than that in the G+I group, while the expression of CD40 had no obvious difference (p > 0.05) between the MENK-12 group and the G+I group, as shown in Fig. 2a, b. These data demonstrated that 10−12 mol/L MENK could effectively enhance the expressions of costimulatory molecules compared with the other control groups.
Fig. 2.
The characteristics of BM-derived DCs induced by MENK-12 (10−12 mol/L MENK). a, b The expressions of costimulatory molecules on the DCs derived from BM progenitors after treatment with 10−12 mol/L MENK (MENK-12). DCs expressed higher level of MHCII at the concentrations of 10−12 mol/L MENK as well as a significant increase in the number of CD80+ and CD40+ cells on the DC populations. a Dotplot analysis by FCM. b The statistical analysis of histogram by GraphPad prism5. **p < 0.01 versus in the RPIM 1640 group; # p < 0.05 versus in the G+I (GM-CSF plus IL-4) group. c, d IL-12p70 and IL-10 production by BM-derived DCs. Dendritic cells derived from bone marrow progenitors were cultured with GM-CSF plus IL-4 and 10−12 mol/L MENK, respectively, for 7 days. The supernatants were evaluated for the presence of IL-12p70 and IL-10 by ELISA. Results are shown as mean values (±SD) of five independent experiments. **p < 0.01 versus RPIM 1640 group, # p < 0.05 versus G+I group. e–j RT-PCR analysis at mRNA levels of delta, kappa, mu receptors, TNF-α and TLR-9 after treatment with 10–12 mol/L MENK. BM-derived DCs treated with MENK-12 was test group and G+I, RPIM 1640 were control groups. Delta yielded 576 bp. Kappa yielded 609 bp. Mu yielded 218 bp. TNF-α yielded 462 bp and TLR-9 yielded 768 bp. **p < 0.01 versus in the RPIM 1640 group; # p < 0.05 versus in the G+I group; ## p < 0.01 versus in the G+I group
Production of cytokine IL-12p70 and IL-10 by ELISA
Usually, the mDC produces high level of IL-12p70 while pDC produces high level of IL-10. The cytokine secretion of differentiated BM-derived DCs after treatment with MENK-12 and G+I, respectively, were analyzed by ELISA. Our results showed that the levels of IL-10 and IL-12p70 in the MENK-12 group increased significantly (p < 0.01) compared with those in the RPMI 1640 group. Correspondingly, the secretion of IL-10 in the MENK-12 group could be elevated compared with that in G+I group (p < 0.05), the levels of IL-12p70 in the MENK-12 group had no difference (p > 0.05) with that in G+I group. These data showed that 10−12 mol/L MENK could up-regulate the cytokine production and the productions of cytokine IL-12p70 were higher than that of IL-10 in each group as shown in Fig. 2c, d.
Expression of opioid receptors, intracellular TNF-α, and TLR-9 at the mRNA level in BM-derived DCs by RT-PCR
The intracellular relative mRNA levels of TLR-9 were significantly higher in the MENK-12 group and G+I group, while there was no significance (p > 0.05) between them as shown in Fig. 2e, f. Opioid receptors of kappa and delta both increased in the MENK-12 group compared with that in RPIM 1640 group (p < 0.01) and G+I group (p < 0.01) as shown in Fig. 2e, g, h. However, the relative mRNA level of mu receptor was significantly lower in the MENK-12 group (p < 0.01) than those in other groups as shown in Fig. 2e and i. The relative mRNA level of intracellular TNF-α was significantly higher in the MENK-12 group (p < 0.01) than that in the RPIM1640 group, and there was no significance (p > 0.05) with that in G+I group as shown in Fig. 2e and j. These data demonstrated that 10−12 mol/L MENK could up-regulate the mRNA levels of kappa and delta and intracellular mRNA expressions of TNF-α and TLR-9 effectively, while the expression of mu mRNA was down-regulated simultaneously.
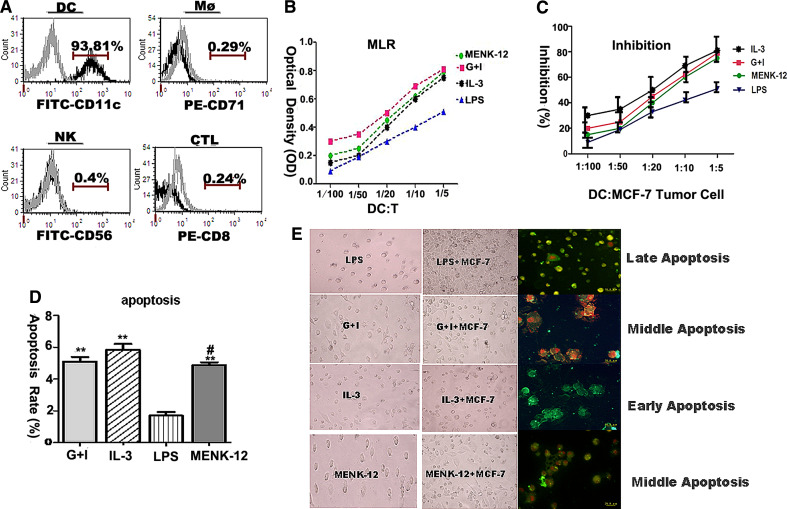
Stimulatory activity of BM-derived DCs in MLR
Allogeneic T cells from BALB/C mice displayed a significantly higher proliferative response to BM-derived DCs treated with 10−12 mol/L MENK. The OD value in the MENK-12 group was markedly higher (p < 0.01) than that in the LPS group, a little higher than that in G+I group (p > 0.05) and that in IL-3 group (p > 0.05). This means the activity to stimulate proliferation of allogeneic CD4+T cells by MENK-12 was great at the ratio of DC:T 1:5, as shown in Fig. 3a, b. These data demonstrated that 10−12 mol/L MENK could promoted proliferation activity of T cells in MLR.
Fig. 3.
Functional activity of differentiated BM-derived DCs with MENK-12. a, b MLR for differentiated BM-derived DCs. BM-derived DCs were cocultured for 5 days with 2–5 × 105 purified allogeneic T lymphocytes in the presence of 10−12 mol/L MENK, G+I, IL-3, and LPS, respectively. Proliferation of T cells was assayed by MTT. a The purity analysis of differentiated BM-derived DCs by FCM for DC, NK, Mφ, and CTL. b The result of MLR reaction. **p < 0.01 versus in the RPIM 1640 group, *p < 0.05 versus in the RPIM 1640 group, ## p < 0.01 versus in the G+I group. # p < 0.05 versus in the G+I group. c–e Inhibitory effects on MCF-7 tumor cells by differentiated BM-derived DCs in vitro. BM-derived DCs induced with IL-3, G+I, LPS, and MENK-12 for 7 days separately were cocultured with 105 MCF-7 tumor cells for 3 days. The MCF-7 tumor apoptosis was confirmed by FCM and reconfirmed by staining with both annexin V and propidium iodide (PI) for late apoptosis, with annexin V only for early apoptosis and with more annexin V, little PI for middle apoptosis under inverted fluorescent light microscopy. c The inhibition rate by 3H-TdR penetration method. d The apoptosis analysis of histogram by FCM. e Morphology of MCF-7 tumor cells under inverted fluorescent light microscopy. Representative data of three separate experiments. **p < 0.01 versus in the LPS group, # p < 0.05 versus in the IL-3 group, p > 0.05 versus in the G+I group
Inhibitory effects on MCF-7 tumor cells by differentiated BM-derived DCs
The inhibition on the MCF-7 tumor cells with differentiated BM-DCs was evaluated by 3H-TdR penetration method. Our data showed the inhibition rate in the MENK-12 group was higher (p < 0.01) compared with that in the LPS group, while the difference was not obvious with that in the G+I group (p > 0.05) and that in the IL-3 group (p > 0.05) as shown in Fig. 3a, c. Annexin V/PI staining was performed as described earlier, the apoptotic ratio of the MCF-7 tumor cells mediated by differentiated BM-derived DCs using FCM in the MENK-12 group was markedly higher (p < 0.01) than that in the LPS group and had significant differences with that in IL-3 group (p < 0.05), while had no significant differences with that in G+I group (p > 0.05), as shown in Fig. 3a, d. The morphologies of apoptotic MCF-7 tumor cells induced by differentiated BM-derived DCs under inverted fluorescent light microscope revealed a middle stage of apoptosis with nuclear fragmentation of the MCF-7 cells in the MENK-12 group as well as that in the G+I group, as shown in Fig. 3e. These data demonstrated that the differentiated BM-derived DCs with 10−12 mol/L MENK could effectively induce the apoptosis of tumor cells.
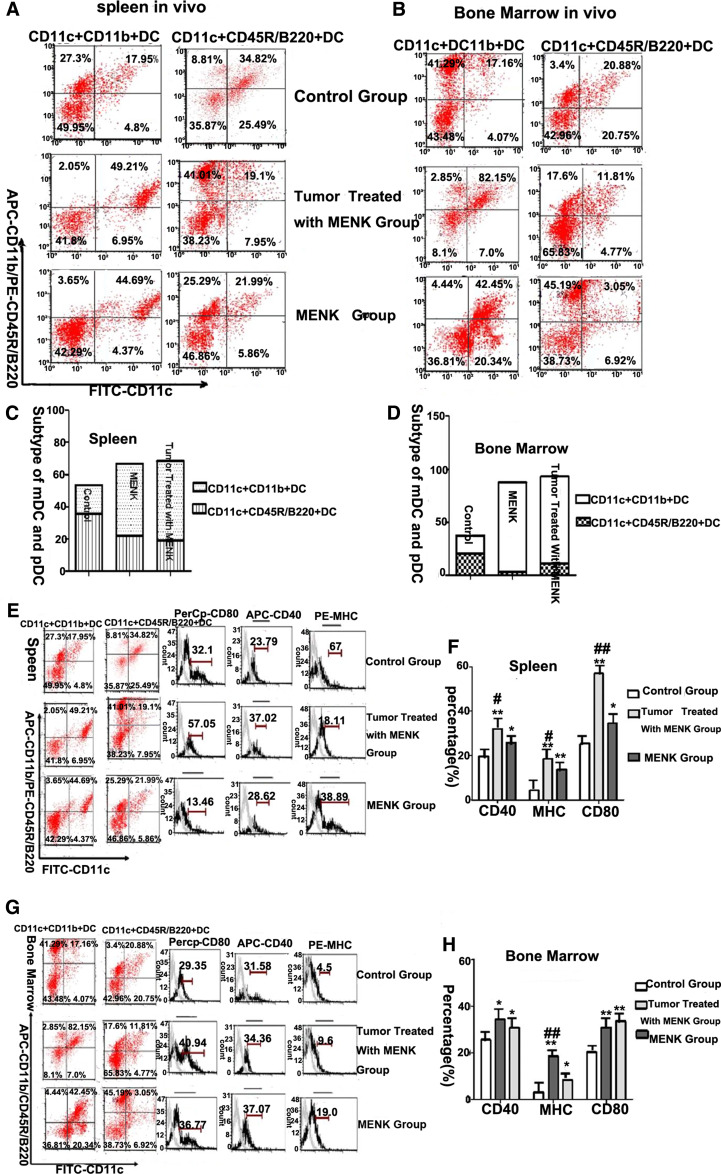
Differentiation of DC induced by MENK in vivo
Normally, expression of CD11c+CD11b+ acted as mDC markers and CD11c+CD11b−CD45R/B220+ acted as pDC markers on the DCs in both bone marrow and spleen of mice. Under the influence of MENK, there were much higher levels of CD11c+CD11b+ DCs in both the tumor group treated with MENK and the MENK group. Concretely, in spleen, CD11c+CD11b+ DCs yielded 44.69 ± 8.74% in the MENK group (p < 0.01) versus 17.95 ± 9.15% in the C57BL/6 normal control group, and (p < 0.05) versus 49.21 ± 2.41% in the tumor group treated with MENK group. CD11c+CD11b−CD45R/B220+ DCs yielded 24.99 ± 1.42% in the MENK group (p < 0.05) versus 34.82 ± 4.5% in the C57BL/6 normal control group, and (p > 0.05) versus 21.76 ± 2.4% in the tumor group treated with MENK group as shown in Fig. 4a, c. In bone marrow, CD11c+CD11b+ DCs yielded 42.45 ± 6.78% in the MENK group (p < 0.01) versus 17.16 ± 7.32% in the C57BL/6 normal control group, and (p < 0.01) versus 82.15 ± 9.32% in the tumor group treated with MENK group. CD11b−CD45R/B220+ DCs yielded 3.05 ± 0.61% in the MENK group (p < 0.01) versus 20.88 ± 4.71% in the C57BL/6 normal control group, and (p < 0.05) versus 11.84 ± 3.61% in the tumor group treated with MENK group as shown in Fig. 4b, d. These data demonstrated that MENK was a potentially effective factor that can polarize DCs in bone marrow and spleen to mDC in vivo.
Fig. 4.
The induction effect of MENK for the differentiation of DCs in vivo. a–d The subtypes distribution of myeloid DC (mDC, CD11c+CD11b+ DC) and plasmacytoid DC (pDC, CD11c+CD45R/B220+ DC) in tumor-bearing mice and normal mice by FCM. a The results of immunophenotypes of DCs in spleen. Similar results were obtained from four separate experiments. b The results of immunophenotypes of DCs in bone marrow. Similar results were obtained from four separate experiments. c, d The analysis of histogram with the subtypes of mDC and pDC in spleen and bone marrow. e, f The expressions of costimulatory molecules on the DCs in spleen in the C57BL/6 normal control group, tumor treated with MENK group and MENK group. e Dotplot analysis by FCM. f The statistical analysis of histogram by GraphPad prism5. **p < 0.01 versus in the C57BL/6 normal control group, *p < 0.05 versus in the C57BL/6 normal control group, ## p < 0.01 versus in the tumor treated with MENK group, # p < 0.05 versus in the tumor treated with MENK group. g, h The expressions of costimulatory molecules on the DCs in bone marrow in the C57BL/6 normal control group, tumor treated with MENK group and MENK group. g Dotplot analysis by flow cytometry. h The statistical analysis of histogram by GraphPad prism5. *p < 0.05 versus in the C57BL/6 normal control group; # p < 0.05 versus in the tumor treated with MENK group. **p < 0.01 versus in the C57BL/6 normal control group; ## p < 0.01 versus in the tumor treated with MENK group
The expression of costimulatory molecules induced by MENK in vivo
When treated with MENK, the DCs in mice gradually maturated with the up-regulated expression of key surface molecules. The results of the test were that CD80 in spleen DCs yielded 34.62 ± 3.75% in the MENK group, (p < 0.05) versus 25.43 ± 2.86% in the C57BL/6 normal control group, and (p < 0.01) versus 57.05 ± 6.04% in the tumor group treated with MENK group as shown in Fig. 4e, f and that CD80 in bone marrow DCs yielded 30.84 ± 3.41% in the MENK group, (p < 0.05) versus 20.24 ± 2.67% in the C57BL/6 normal control group, as shown in Fig. 4g, h. Similarly, CD40 in spleen DCs yielded 25.78 ± 1.67% in the MENK group, (p < 0.05) versus 19.73 ± 2.35% in the C57BL/6 normal control group, and (p < 0.05) versus 32.04 ± 3.06% in the tumor group treated with MENK group, and CD40 in bone marrow DCs yielded 36.19 ± 1.89% in the MENK group, (p < 0.05) versus 28.16 ± 2.23% in the C57BL/6 normal control group, as shown in Fig. 4g, h. Furthermore, MHCII in spleen DCs yielded 16.18 ± 0.22% in the MENK group, (p < 0.01) versus 3.9 ± 0.26% in the C57BL/6 normal control group, and MHCII in bone marrow DCs yielded 19.61 ± 1.69% in the MENK group, (p < 0.01) versus 2.8 ± 0.42% in the C57BL/6 normal control group, and (p < 0.01) versus 8.49 ± 0.39% in the tumor group treated with MENK group as shown in Fig. 4g, h. These data demonstrated that MENK was a potentially effective factor that could up-regulate the expressions of costimulatory molecules of DCs in vivo.
Antitumor activity of MENK in vivo
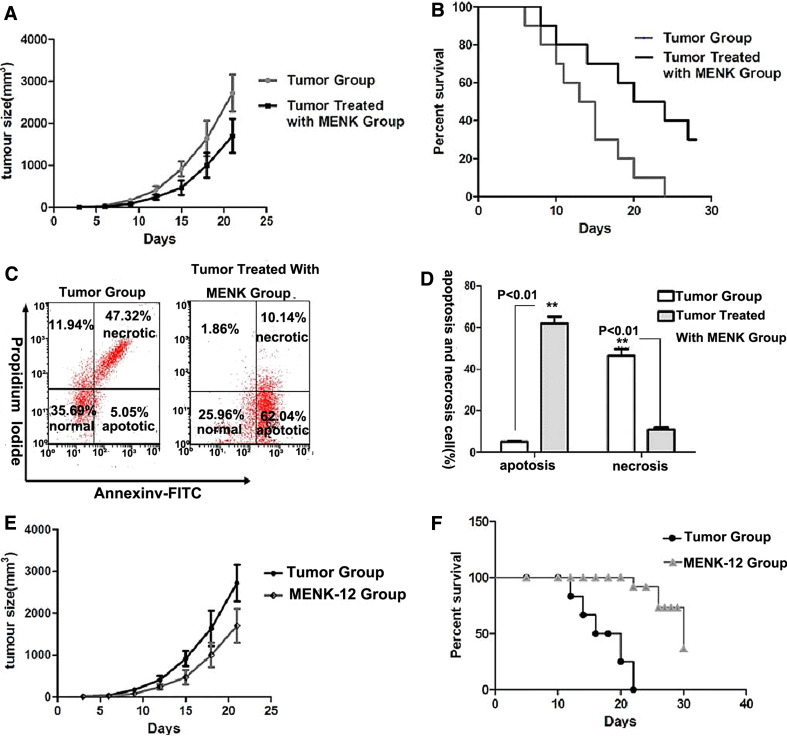
Upon 30 days period of treatment with MENK, the efficacy was assessed by survival rate, tumor size, and apoptosis. Our results showed that mean tumor sizes yielded 504 ± 49.2 mm3 in the MENK group, (p < 0.05) versus 849.2 ± 51.4 mm3 in the tumor group as shown in Fig. 5a. Survival rate in the MENK group was significantly higher compared with those in the tumor group. As measured, we had 38 ± 1.2% in the MENK group, (p < 0.05) versus 0% in the tumor group as shown in Fig. 5b. The analysis of tumor tissue apoptosis by FCM demonstrated that apoptotic rate yielded 62.04 ± 3.32% in the MENK group, (p < 0.01) versus 5.05 ± 0.61% in the tumor group. In parallel, necrotic rate yielded 47.32 ± 2.1% in tumor group, (p < 0.01) versus 10.14 ± 1.9% in the MENK group as shown in Fig. 5c, d. The results showed that MENK could delay tumor growth and significantly enhance survival of mice bearing S180 tumors.
Fig. 5.
Antitumor effect by MENK in vivo. a–d The tumor models were established in C57BL/6 mice and then one group was treated with MENK (20 mg/kg) every other day for successive 30 days. Tumor sizes of each mouse (5/group) were measured every 3 days, and survival condition in each group (5/group) was monitored daily. The effect of apoptosis on tumor tissue of tumor group and MENK-treated group were analyzed. a Tumor sizes in the MENK-treated group showed significance. b Significant increase in survival rate and prolonged survival times were observed in MENK-treated group. c, d The apoptosis analysis of tumor tissue stained with both annexin V and propidium iodide (PI) by FCM. *p < 0.05 versus tumor group, **p < 0.01 versus tumor group. e, f Inhibiting effect on tumor growth by infusing differentiated BM-derived DCs into mouse. With use of 10−12 mol/L MENK, we got differentiated BM-derived DCs. Subsequently, we injected these differentiated BM-derived DCs into tumors bearing mouse. Tumor sizes and survival rate were measured separately. e Significant increase in survival rate in MENK-12 group compared with tumor group. f Tumor sizes in MENK-12 group showed significance compared with those in the tumor group. *p < 0.05 versus tumor group, **p < 0.01 versus tumor group
Inhibiting effect on tumor growth by reinfusing differentiated BM-derived DCs into mouse
Our data directly showed that the reinfusing differentiated BM-derived DCs treated with MENK-12 in vitro into the tumor bearing mice could significantly increase survival rate, compared with that of the tumor groups. Tumor sizes yielded 503.3 ± 22.2 mm3 in the MENK-12 group, (p < 0.01) versus 842.9 ± 26.61 mm3 in the tumor group as shown in Fig. 5e. Survival rate yielded 51 ± 6.2% in the MENK-12 group, (p < 0.01) versus 0% in the tumor group as shown in Fig. 5f.
Discussion
As we know, the immune system is a very complicated and diverse entity, controlled by endocrine system via signaling molecules that act as activating agents, and thus formulating a coordinated interaction between the immune and endocrine system [33, 34]. Immunomodulation property of MENK has been reported previously [35]. DCs can be derived from bone marrow and play critical roles in initiating T cell responses.
In our study, we found that MENK, at used concentration, could induce DC phenotypic and functional maturation toward mDC predominately in vitro. As evidenced, under the influence of MENK, the DCs developed from bone marrow progenitors differentiated into mDC characterized by high expression of CD11c+CD11b+ and low expression of CD11c+CD11b−CD45R/B220+ phenotype (Fig. 1). Simultaneously, 10−12 mol/L MENK stimulated higher expression of CD40, CD80, and MHCII compared with that in G+I, RPMI1640 groups, and the optimal concentration of 10−12 mol/L MENK was identified (Fig. 2a, b). Furthermore, the higher level of IL-12p70 and lower level of IL-10 secreted in the 10−12 mol/L MENK group reconfirmed induction of BM-derived DCs toward mDC (Fig. 2c, d). Additionally, the expressions of receptors of TNF-α and TLR-9 on DCs showed consistence to BM-derived DCs differentiation induced by MENK-12 (Fig. 2e, f, j). Moreover, we quantified expressions of mu, kappa, and delta receptors using RT-PCR and proved that 10−12 mol/L MENK could up-regulate expressions of delta and kappa except mu receptor (Fig. 2e, g–i). Also found from our data were BM-derived DCs treated with 10−12 mol/L MENK displayed a stronger capacity to induce allogeneic T cell proliferation (Fig. 3a, b) and exerted inhibitory effect on MCF-7 tumor cells accompanied with middle apoptosis (Fig. 3a–d). Meanwhile, in vivo experiment, we administrated both C57BL/6 mice bearing tumors and C57BL/6 normal mice with MENK (20 mg/kg) by s.c. to see the ways of differentiation and demonstrated that, under the influence of MENK, the DC progenitors in bone marrow differentiated into mDC characterized by high expression of CD11c+CD11b+ and low expression of CD11c+CD11b−CD45R/B220+ phenotype (Fig. 4a–d). As evidenced, MENK up-regulated the expressions of CD40, CD80, and MHC-II significantly compared with other control groups regardless of whether in spleen or in bone marrow (Fig. 4e–h). Correspondingly, the group treated with the MENK had higher survival rate with marked tumor inhibition than those in the tumor group, also accompanied with apoptosis (Fig. 5a–d). Furthermore, we saw the increased survival rate by reinfusing differentiated BM-derived DCs treated with MENK-12 in vitro into the tumor bearing mice, compared with those in the tumor control groups (Fig. 5e, f). We hereby summarize our data as following points:
(1) MENK could induce more than 85% BM-derived DCs to polarize predominantly to mDC subtype, which is also supported by both the expressions of costimulatory molecules, like CD40, CD80, and MHC-II, and secretion of cytokines, like IL-12. (2) MENK could up-regulate expressions of kappa and delta receptors, except mu receptor on the DCs, which will ligate MENK and trigger a chain of T cell responses, which provided direct evidence that kappa and delta are involved in immune responses. (3) Differentiated BM-derived DCs treated with MENK could directly induce allogeneic T cell proliferation and mount activity causing the apoptosis of tumor cell in vitro and in vivo. Our data demonstrated that MENK delayed tumor growth and significantly prolong survival rate of mice, accompanied with middle apoptosis.
When MENK works in vivo, regardless of whether it works alone, or is combined with other agents, it can regulate DCs not only via a direct pathway, but also via an indirect route by triggering other immune cells such as macrophages and CD4+T cells, which can secrete high levels of IL-12, adding a synergistic effect to DCs. NK cells and CD4+T cells can also secrete IFN-γ to co-activate DCs. Thus, there is an immune net to coordinate the immune system. However, when MENK works in vitro, there is no such endocrine or immune interaction among the immune cells. This means that for MENK to have an effect in vitro, it regulates DCs only via a direct route to bind to the receptors on the DCs without other co-stimulating processes involved, regardless of whether it works alone or is combined with other agents. This helps us better understand the unparallel results of tumor apoptosis in vitro and in vivo, which is consistent with the report by Jankovic and Marie, who found that opioid peptides including MENK modulate both cellular and humoral immune function via an opiate receptor–mediated action [36], and data by Murgo demonstrated the antitumor effect of MENK in C57BL/6 mice inoculated with B16-BL6 melanoma cells [37–39].
It may be further noted that the up-regulation of IL-12 secretion not only augments the increase of CD4+T cells [40], but also increases the stability of the pathway between the DCs and CD4+T cells, which, in turn, will result in more secretion of cytokines like IL-12 and IFN-γ by the CD4+T cells.
Dendritic cells have emerged as the most potent and professional antigen presenting cells that possess the ability to stimulate naïve T cells and initiate T cell responses, acting as messengers between the innate and adaptive immunities. They can thus potentially be used in therapeutic vaccines in cancer immunotherapy and for other threatening diseases. This study can therefore contribute to a broader understanding of MENK’s positive modulating effects on the immune system. Furthermore, this study also provides a meaningful mechanism of action for MENK and highlights the clinical significance of a cancer immunotherapeutic vaccine preparation, which may play a critical role in combating cancer. Likewise, we may consider MENK as a possible adjuvant to be used in vaccine preparations against life-threatening diseases like AIDS.
Despite the fruitful results we obtained earlier, there are still quite a few detailed approaches to pursue in depth, such as the concrete signal pathways inside the DC cell, via which MENK modulate polarization and the pathway as well as how DC induces apoptosis of tumor cells.
Conclusion
We believe this is the first ever publication to provide evidence that MENK can induce BM-derived DCs to polarize predominantly to mDC and can markedly enhance BM-derived DCs maturation with functions, which mounted tumoricidal activity.
Acknowledgments
Thanks for all researchers who contributed to the work and we apologize to the researchers whose works could not be discussed here due to space limitations. This work was supported financially by China Liaoning provincial foundation for international collaboration, No. 2006305007 (to Fengping Shan).
Conflict of interest
The authors declare that we have no conflict of interest.
Abbreviations
- MENK
Methionine enkephalin
- DC
Dendritic cell
- BM-derived DCs
DCs derived from murine bone marrow progenitors
- GM-CSF
Granulocyte macrophage colony stimulating factor
- IL-4
Interleukin-4
- IL-3
Interleukin-3
- LPS
Lipopolysaccharide
- FCM
Flow cytometry
- IFN-γ
Interferon-γ
- OGFr
Opioid growth factor receptor
- RT-PCR
Reverse transcriptase polymerase chain reaction
- pDCs
Plasmacytoid dendritic cells
- mDCs
Myeloid dendritic cells
- MLR
Mixed lymphocyte reaction
- MCF-7
Human breast adenocarcinoma cell line
References
- 1.Marcotte I, Dufourc EJ, Ouellet M, Auger M. Interaction of the neuropeptide met-enkephalin with zwitterionic and negatively charged bicelles as viewed by 31P and 2H solid state NMR. Biophys J. 2003;85:328–339. doi: 10.1016/S0006-3495(03)74477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharp BM. Multiple opioid receptors on immune cells modulate intracellular signaling. Brain Behav Immun. 2006;20:9–14. doi: 10.1016/j.bbi.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Finley MJ, Happel CM, Kaminsky DE, Rogers TJ. Opioid and nociceptin receptors regulate cytokine and cytokine receptor expression. Cell Immunol. 2008;252:146–154. doi: 10.1016/j.cellimm.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohmori H, Fujii K, Sasahira T, Luo Y, Isobe M, et al. Methionine-enkephalin secreted by human colorectal cancer cells suppresses T lymphocytes. Cancer Sci. 2009;100:497–502. doi: 10.1111/j.1349-7006.2008.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shan F, Xia Y, Wang N, Meng J, Lu C, et al. Functional modulation of the pathway between dendritic cells (DCs) and CD4+T cells by the neuropeptide: methionine enkephalin (MENK) Peptides. 2011;32:929–937. doi: 10.1016/j.peptides.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 6.Hsueh CM, Chen SF, Huang HJ, Ghanta VK, Hiramoto RN. Activation of mu-opioid receptors are required for the conditioned enhancement of NK cell activity. Brain Res. 1996;737:263–268. doi: 10.1016/0006-8993(96)00740-8. [DOI] [PubMed] [Google Scholar]
- 7.Burger RA, Warren RP, Huffman JH, Sidwell RW. Effect of methionine enkephalin on natural killer cell and cytotoxic T lymphocyte activity in mice infected with influenza A virus. Immunopharmacol Immunotoxicol. 1995;17:323–334. doi: 10.3109/08923979509019754. [DOI] [PubMed] [Google Scholar]
- 8.Kowalski J, Belowski D, Wielgus J. Bidirectional modulation of mouse natural killer cell and macrophage cytotoxic activities by enkephalins. Pol J Pharmacol. 1995;47:327–331. [PubMed] [Google Scholar]
- 9.Mosnaim AD, Wolf ME, Maturana P, Mosnaim G, Puente J, et al. In vitro studies of natural killer cell activity in post traumatic stress disorder patients. Response to methionine-enkephalin challenge. Immunopharmacology. 1993;25:107–116. doi: 10.1016/0162-3109(93)90014-H. [DOI] [PubMed] [Google Scholar]
- 10.Plotnikoff NP, Faith RE, Murgo AJ, Herberman RB, Good RA. Methionine enkephalin: a new cytokine-human studies. Clin Immunol Immunopathol. 1997;82:93–101. doi: 10.1006/clin.1996.4287. [DOI] [PubMed] [Google Scholar]
- 11.Stanojević S, Mitić K, Vujić V, Kovacević-Jovanović V, Dimitrijević M. The influence of stress and methionine-enkephalin on macrophage functions in two inbred rat strains. Life Sci. 2007;80:901–909. doi: 10.1016/j.lfs.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Vujić V, Stanojević S, Dimitrijević M. Methionine-enkephalin stimulates hydrogen peroxide and nitric oxide production in rat peritoneal macrophages: interaction of mu, delta and kappa opioid receptors. Neuroimmunomodulation. 2004;11:392–403. doi: 10.1159/000080150. [DOI] [PubMed] [Google Scholar]
- 13.Esche C, Makarenkova VP, Kost NV, Lotze MT, Zozulya AA, et al. Murine dendritic cells express functional delta-type opioid receptors. Ann NY Acad Sci. 1999;885:387–390. doi: 10.1111/j.1749-6632.1999.tb08695.x. [DOI] [PubMed] [Google Scholar]
- 14.Makarenkova VP, Esche C, Kost NV, Shurin GV, Rabin BS, et al. Identification of delta and mu type opioid receptors on human and murine dendritic cells. J Neuroimmunol. 2001;117:68–77. doi: 10.1016/S0165-5728(01)00313-7. [DOI] [PubMed] [Google Scholar]
- 15.Bénard A, Boué J, Chapey E, Jaume M, Gomes B, et al. Delta opioid receptors mediate chemotaxis in bone marrow-derived dendritic cells. J Neuroimmunol. 2008;197:21–28. doi: 10.1016/j.jneuroim.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Shen Z, Reznikoff G, Dranoff G. Cloned dendritic cells can present exogenous antigens on both MHC class I and II molecules. J Immunol. 1997;58:2723–2730. [PubMed] [Google Scholar]
- 17.Bluml S, Zupkovitz G, Kirchberger S, Seyerl M, Valery N, et al. Epigenetic regulation of dendritic cell differentiation and function by oxidized phospholipids. Blood. 2009;114:5481. doi: 10.1182/blood-2008-11-191429. [DOI] [PubMed] [Google Scholar]
- 18.Khayrullina T, Yen J-H, Jing H, Doina G. Adenosine receptors in regulation of dendritic cell differentiation and function. J Immunol. 2008;181:721–735. doi: 10.4049/jimmunol.181.1.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephens TA, Nikoopour E, Rider BJ, Leon-Ponte M, Chau TA, et al. Dendritic cell differentiation induced by a self-peptide derived from apolipoprotein E1. J Immunol. 2008;181:6859–6871. doi: 10.4049/jimmunol.181.10.6859. [DOI] [PubMed] [Google Scholar]
- 20.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ardavín C, Martínez del Hoyo G, Martín P, Anjuère F, Arias CF, et al. Origin and differentiation of dendritic cells. Trends Immunol. 2001;22:691–700. doi: 10.1016/S1471-4906(01)02059-2. [DOI] [PubMed] [Google Scholar]
- 22.De Jong EC, Vieira PL, Kalinski P, Schuitemaker JH, Tanaka Y, et al. Microbial compounds selectively induce Th1 cell promoting or Th2 cell promoting dendritic cells in vitro with diverse Th cell polarizing signals. J Immunol. 2002;168:1704–1709. doi: 10.4049/jimmunol.168.4.1704. [DOI] [PubMed] [Google Scholar]
- 23.Manickasingham SP, Edwards AD, Schulz O, Reise Sousa C. The ability of murine dendritic cell subsets to direct T helper cell differentiation is dependent on microbial signals. Eur J Immunol. 2003;33:101–107. doi: 10.1002/immu.200390001. [DOI] [PubMed] [Google Scholar]
- 24.Wiethe C, Debus A, Mohrs M, Steinkasserer A, Lutz M, et al. Dendritic cell differentiation state and their interaction with NKT cells determine Th1/Th2 differentiation in the murine model of leishmania major infection. J Immunol. 2008;180:4371–4381. doi: 10.4049/jimmunol.180.7.4371. [DOI] [PubMed] [Google Scholar]
- 25.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 26.Maldonado-López R, De Smedt T, Michel P, Godfroid J, Pajak B, et al. CD8alpha+ and CD8alpha− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Shea JJ, Paul WE. Regulation of T(H)1 differentiation controlling the controllers. Nat Immunol. 2002;3:506–508. doi: 10.1038/ni0602-506. [DOI] [PubMed] [Google Scholar]
- 28.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, et al. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol. 2001;166:5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 29.Chtanova T, Mackay CR. T cell effector subsets: extending the Th1/Th2 paradigm. Adv Immunol. 2001;78:233–266. doi: 10.1016/S0065-2776(01)78005-4. [DOI] [PubMed] [Google Scholar]
- 30.Ganesh BB, Cheatem DM, Sheng JR, Vasu C, et al. GM-CSF induced CD11c CD8a-dendritic cells facilitate Foxp31 and IL-10 regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int Immunol. 2009;21:269–282. doi: 10.1093/intimm/dxn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jankovic BD, Marie D. Enkephalins and immunity. I: In vivo suppression and potentiation of humoral immune response. Ann NY Acad Sci. 1987;496:115–125. doi: 10.1111/j.1749-6632.1987.tb35754.x. [DOI] [PubMed] [Google Scholar]
- 32.Marie D, Jankovic BD. Enkephalins and immunity. II: In vivo modulation of cell-mediated immunity. Ann NY Acad Sci. 1987;496:126–136. doi: 10.1111/j.1749-6632.1987.tb35755.x. [DOI] [PubMed] [Google Scholar]
- 33.Sulowska Z, Majewska E, Krawczyk K, Klink M, Tchórzewski H. Influence of opioid peptides on human neutrophil apoptosis and activation in vitro. Mediators Inflamm. 2002;11:245–250. doi: 10.1080/096293502900000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menzebach A, Hirsch J, Hempelmann G, Welters ID. Effects of endogenous and synthetic opioid peptides on neutrophil function in vitro. Br J Anaesth. 2003;17:546–550. doi: 10.1093/bja/aeg219. [DOI] [PubMed] [Google Scholar]
- 35.Marotti T, Gabrilovac J, Rabatic S, Smejkal-Jagar L, Rocic B, et al. Met-enkephalin modulates stress induced alterations of the immune response in mice. Pharmacol Biochem Behav. 1996;54:277–284. doi: 10.1016/0091-3057(95)02112-4. [DOI] [PubMed] [Google Scholar]
- 36.Kraus J. Regulation of mu-opioid receptors by cytokines. Front Biosci. 2009;1:164–170. doi: 10.2741/s16. [DOI] [PubMed] [Google Scholar]
- 37.Stanojević S, Mitić K, Vujić V. The influence of stress and methionine enkephalin on macrophage functions in two in bred rat strains. Life Sci. 2007;80:901–909. doi: 10.1016/j.lfs.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 38.Vujić V, Stanojević S, Dimitrijević M. Methionine enkephalin stimulates hydrogen peroxide and nitric oxide production in rat peritoneal macrophages: interaction of mu, delta and kappa opioid receptors. Neuroimmunomodulation. 2004;11:392–403. doi: 10.1159/000080150. [DOI] [PubMed] [Google Scholar]
- 39.Stanojević S, Vujić V, Mitić K, Kuštrimović N, Kovačević-Jovanović V, et al. Methionine-enkephalin modulation of hydrogen peroxide (H2O2) release by rat peritoneal macrophages involves different types of opioid receptors. Neuropeptides. 2008;42:147–158. doi: 10.1016/j.npep.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Zagon IS, Donahue RN, Rogosnitzky M. Imiquimod up-regulates the opioid growth fact or receptor to inhibit cell proliferation independent of immune function. Exp Biol Med. 2008;233:968–979. doi: 10.3181/0802-RM-58. [DOI] [PubMed] [Google Scholar]