Abstract
New anticancer vaccines must overcome regulatory T cell (Treg)-mediated immunosuppression. We previously reported that oral ingestion of Lentinula edodes mycelia (L.E.M.) extract restores melanoma-reactive T cells in melanoma-bearing mice via a mitigation of Treg-mediated immunosuppression. In this study, we investigated the effect of oral ingestion of the extract on peptide vaccine-induced anti-tumor activity. The day after subcutaneous inoculation in the footpad with B16 melanoma, mice were freely fed the extract and were vaccinated with a tyrosinase-related protein 2180–188 peptide. The peptide vaccine was repeated thrice weekly. Melanoma growth was significantly suppressed in mice treated with both the peptide vaccine and L.E.M. extract compared with mice treated with vaccine or extract alone, and the effect was CD8+ T cell-dependent. The combination therapy increased H-2Kb-restricted and B16 melanoma-reactive T cells in the draining lymph nodes and spleen. Flow cytometric and immunohistological analyses revealed that the combination therapy significantly decreased the percentage of Tregs in the draining lymph nodes and spleen of melanoma-bearing mice compared to treatment with vaccine or extract alone. Kinetic analyses of peptide-specific T cells and Tregs revealed that induction of peptide-specific T cells by the peptide vaccine alone was transient, but when combined with L.E.M. extract, it efficiently prolonged the duration of peptide-specific T cell induction without increasing the percentage of Tregs. These results indicate that combination therapy enhances peptide vaccine-induced anti-tumor activity due to attenuation of the increase in the percentage of Tregs in tumor-bearing hosts.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-012-1275-8) contains supplementary material, which is available to authorized users.
Keywords: Regulatory T cells, Cytotoxic T-lymphocyte, Immunotherapy, Biological response modifier, Melanoma, Tumor peptide vaccine
Introduction
Tumor-reactive cytotoxic T lymphocytes recognize tumor-derived antigens on cancer cells and are the most potent cancer killing cells known [1–3]. Anticancer peptide vaccines are used clinically to induce proliferation of anticancer T cells [4–6], but thus far their therapeutic efficacy has proven unsatisfactory [7]. One obstacle to be overcome is the activity of immunosuppressive cells, including regulatory T cells (Tregs) and/or myeloid-derived suppressor cells (MDSCs), which increase in cancer patients and profoundly influence the clinical outcome of anticancer immunotherapies [8, 9]. In particular, an increase in the number of Tregs is thought to negatively correlate with cancer patient survival [10, 11].
Several treatment modalities have been proposed to alleviate Treg-mediated immunosuppression [12–17]. We recently reported that oral ingestion of Lentinula edodes mycelia (L.E.M.) extract mitigates Treg-mediated immunosuppression and restores melanoma-reactive CD8+ T cells in melanoma-bearing mice [18]. As a preliminary step toward extending the application of this treatment, we investigated the effect of oral ingestion of L.E.M. extract on the anti-tumor effects induced by vaccination with a peptide derived from tyrosinase-related protein 2 (TRP2), which is a melanocyte differentiation antigen. Combination therapy involving the peptide vaccine and ingestion of L.E.M. extract significantly suppressed the growth of B16 melanoma cells in a CD8+ T cell-dependent manner compared toc treatment with either therapy alone. The combination therapy increased H-2Kb-restricted and B16 melanoma-reactive T cells in the draining lymph nodes (LNs) and spleen of melanoma-bearing mice. Additionally, although induction of peptide-specific T cells was transient with peptide vaccination alone, combining the vaccine with L.E.M. extract prolonged the duration of T cell induction while reducing the proliferation of Tregs. Our results indicate that L.E.M. extract is a useful immunomodulating reagent and has the potential to enhance the therapeutic efficacy of various types of immunotherapies, including anticancer peptide vaccines.
Materials and methods
Mice
C57BL/6 (H-2b: 6 weeks old) female mice were purchased from Japan SLC, Inc. (Hamamatsu, Japan). Animals were maintained under specific pathogen-free conditions. Mice were used for experiments at 7 weeks of age. Experiments were performed according to the Ethical Guidelines for Animal Experiments of the Kobayashi Pharmaceutical Co., Ltd., and conformed to the provisions of the Declaration of Helsinki in 1995 (as revised in Tokyo 2004).
Tumor cell lines
B16BL6 (B16) is a melanoma cell line originating in C57BL/6 mice. B16L is an MHC class I loss variant of the B16 melanoma line, and B16L-Kb is a B16L subline expressing H-2Kb [19]. All cell lines were maintained in vitro in RPMI 1640 complete medium (Sigma-Aldrich Japan, Tokyo, Japan) supplemented with 10 % heat-inactivated FBS (Thermo Trace, Melbourne, Australia) and 1 % penicillin–streptomycin (Wako Japan, Osaka, Japan).
L.E.M. reagent
A dried powder extract of L.E.M. was prepared with hot water before germination and after culturing mycelia in a medium composed of bagasse and rice bran, as previously described [20].
In vivo anti-tumor assay
A total of 7.5 × 105 B16 melanoma cells were inoculated s.c. into the footpad of C57BL/6 mice. Beginning the next day, mice were freely fed food containing 2 % L.E.M. extract. Tumor size was measured twice weekly thereafter. On day 21, all mice were euthanized and their feet were amputated. The tumor weights were calculated by subtracting the mean weight of the feet of naïve mice (0.126 g) from the weight of the feet of B16 melanoma-bearing mice.
Therapeutic immunization
The day after melanoma inoculation, mice (6 mice/group) received an s.c. injection of complete Freund’s adjuvant (CFA) containing TRP2180–188 peptide (200 μg of peptide/mouse). One and 2 weeks after the first immunization, mice received s.c. injections of incomplete Freund’s adjuvant (IFA) containing TRP2180–188 peptide (200 μg of peptide/mouse) for boosting.
In vivo depletion of CD8+ T cells
In some experiments, melanoma-bearing mice received i.p. injections of 200 μg of either anti-CD8 mAb (53–6.72: rat IgG2a) or an isotype-matched control antibody on days −1 and +7 relative to tumor inoculation. Both antibodies were purchased from eBioscience (Kobe, Japan).
In vivo transfer of Tregs
In some experiments, melanoma-bearing and L.E.M. extract-fed mice were injected i.v. with Tregs of 81–91 % purity on day 3 (3.2 × 105 cells/mouse) and day 7 (3.0 × 106 cells/mouse) after tumor inoculation. Tregs were prepared from naïve C57BL/6 mice using MACS beads (Miltenyi Biotec, Bergisch Gladbach, Germany).
Assay for peptide-specific or melanoma-specific T cells
To test T cell responses against melanoma cells, an H-2Kb-binding TRP2180–188 peptide (SVYDFFVWL) [21] was used. Ovalbumin (OVA)257–262 peptide (SIINFEKL) was used as an H-2Kb-binding control peptide. Both peptides were purchased from PH Japan (Hiroshima, Japan) and were of >90 % purity. Tumor-draining LNs and the spleen were harvested from each animal and stimulated in vitro with each of the indicated peptides in the presence of 20 U/ml interleukin (IL)-2 for 3 days. To test for anti-melanoma cell activity, tumor-draining LNs and spleen cells were stimulated in vitro with B16, B16L, or B16L-Kb in the presence of 20 U/ml IL-2 for 3 days. Tumor cells were inactivated by treatment with 100 μg/ml mitomycin C (Kyowa Hakko Kirin, Tokyo, Japan) for 90 min. The level of IFN-γ in the culture supernatants was determined using an ELISA kit (Invitrogen Japan, Tokyo, Japan).
Flow cytometry
Tumor-draining LNs and spleen cells were stained with FITC-conjugated anti-mouse CD4 mAb (eBioscience) and PE-Cy5-conjugated anti-mouse/rat Foxp3 mAb (eBioscience) and were analyzed using a Beckman Coulter (Tokyo, Japan) EPICS flow cytometer.
Immunohistochemistry
Immunohistochemical staining of draining LNs to identify Foxp3+ cells was performed at the Nara Pathological Institute, Nara, Japan. Draining LNs were harvested and fixed with formalin. Thereafter, samples were stained with anti-Foxp3 antibody (Bey Bioscience, Kobe, Japan), followed by a biotin-conjugated anti-rat antibody (DAKO, Tokyo, Japan). The development was carried out using DAB enhancer (Dako, Tokyo, Japan).
Kinetic analysis of spleen and draining LNs
A total of 7.5 × 105 B16 melanoma cells were inoculated s.c. into the footpad of C57BL/6 mice. Beginning the next day, mice were freely fed food containing 2 % L.E.M. extract and were vaccinated with the TRP2 peptide thrice weekly as described above. On days 0, 7, 14, and 21, the draining LNs and spleen were harvested from each animal and examined for reactivity to the TRP-2180–188 peptide and to determine the percentage of Foxp3+ cells among CD4+ T cells.
Results
Significant growth suppression of B16 melanoma in mice treated with both peptide vaccine and L.E.M. extract
First, we examined the effect of oral ingestion of L.E.M. extract on peptide vaccine-induced anti-tumor activity against B16 melanoma established by s.c. inoculation. As described above, beginning 1 day after melanoma inoculation, mice were freely fed food containing 2 % L.E.M. extract and received an s.c. injection of CFA containing the TRP2180–188 peptide. One and 2 weeks after the first immunization, mice received s.c. injections of IFA containing the TRP2180–188 peptide for boosting. As a control for the peptide vaccine, mice were injected s.c. with the same volume of CFA or IFA without the TRP2 peptide. On average, each mouse was fed approximately 4 g of the 2 % L.E.M. food, equivalent to 0.08 g of L.E.M. extract. No difference in tumor growth was noted between mice fed 2 or 8 % L.E.M. extract (data not shown); therefore, 2 % L.E.M. extract was used in all experiments.
As shown in Fig. 1a, although both L.E.M. extract and the peptide vaccine exhibited some degree of anti-tumor activity individually, the combination therapy significantly suppressed tumor growth. A similar result was obtained when the anti-tumor activity was assessed according to tumor weight (Fig. 1b). Different results were observed with peptide vaccine treatment alone: tumor growth (as measured by size) was significantly suppressed, but there was no effect on tumor weight. Nevertheless, it is of note that both measures indicated that combination therapy significantly suppresses melanoma growth.
Fig. 1.
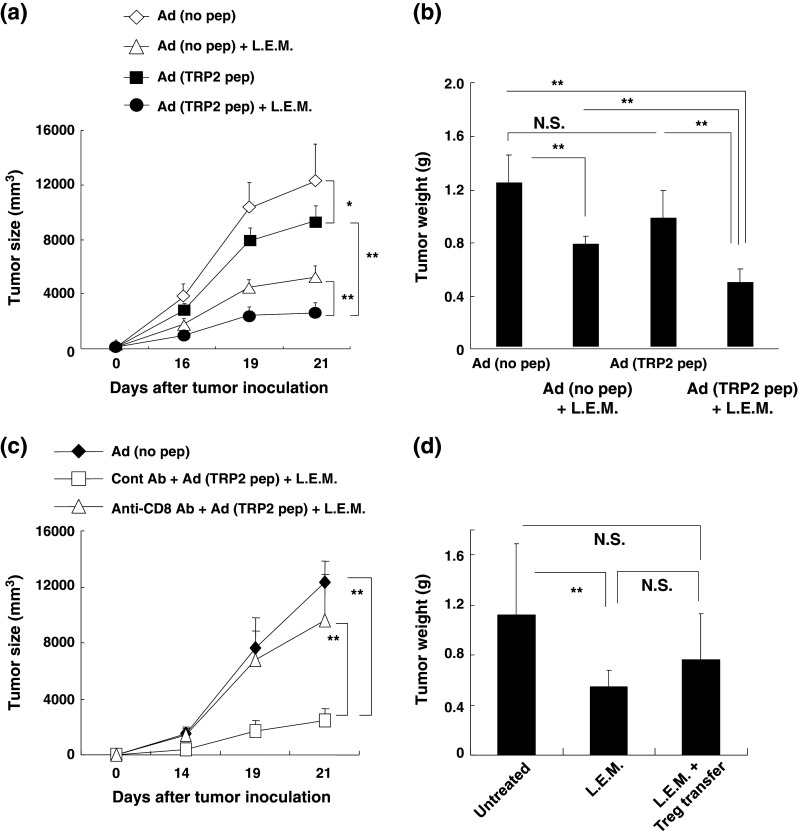
Anti-tumor activity induced by L.E.M. extract and/or TRP2 peptide. C57BL/6 mice were injected s.c. in the right foot pad with 7.5 × 105 B16 cells. Beginning the next day, mice were freely fed food containing 2 % L.E.M. extract and received an s.c. injection of CFA containing the TRP2180–188 peptide (200 μg of peptide/mouse). One and 2 weeks after the first immunization, mice received s.c. injections of IFA containing the TRP2180–188 peptide (200 μg of peptide/mouse) for boosting. a Measurement of tumor size. *P < 0.05; **P < 0.01. Ad adjuvant. b On day 21, all mice were euthanized and their feet were amputated. The tumor weights were calculated by subtracting the mean weight of the feet of naïve mice (0.126 g) from the weight of the feet of B16 melanoma-bearing mice. Similar results were obtained in three independent experiments. *P < 0.05; **P < 0.01. NS not significant. c B16 melanoma-bearing mice, which were injected i.p. with either anti-CD8 mAb (200 μg/mouse) or isotype-matched control antibody on days −1 and +7, were treated with or without the combination therapy and tumor size was measured. **P < 0.01. d B16 melanoma-bearing mice were treated with or without L.E.M. extract and Tregs were injected i.v. on days 3 and 7 after tumor inoculation. On day 21, all mice were euthanized, their feet were amputated, and tumor weights were determined. The mean ± SD for each group of 5 or 6 mice is shown. **P < 0.01; NS not significant
We next examined the effect of CD8+ T cell depletion on the anti-tumor effect induced by the combination therapy. On the day following the second administration of anti-CD8 mAb, the percentage of CD8+ T cells in the draining LNs decreased from 27.2 to 6.2 %. As shown in Fig. 1c, depletion of CD8+ T cells abolished the combination therapy-induced anti-tumor effect, suggesting that CD8+ T cells play an essential role in the anti-tumor effect induced by the combination therapy. Although we previously reported that oral ingestion of L.E.M. extract can significantly decrease the number of Tregs in melanoma-bearing mice [18], in this study, we directly addressed the question whether transfer of Tregs could reverse the anti-tumor effect induced by L.E.M. extract (Fig. 1d). Tregs that were prepared from spleen cells of naïve mice were transferred i.v. on days 3 and 7 after tumor inoculation. As a result, although no significant difference in tumor size was observed (data not shown), the adoptive transfer of Tregs reversed the decrease in tumor weight in mice that were fed L.E.M. extract. These findings indicate that in vivo growth suppression of melanoma after the combination therapy primarily depends on CD8+ T cells and support the hypothesis that the anti-tumor effect associated with oral ingestion of L.E.M. extract derives at least partially from mitigation of Treg-mediated immunosuppression.
Combination therapy with peptide vaccine and L.E.M. extract increases H-2Kb-restricted and melanoma-reactive T cells in melanoma-bearing mice
Next, we examined the effect of oral L.E.M. ingestion on induction of tumor peptide-reactive and/or melanoma-reactive T cells in the draining LNs and spleen of B16 melanoma-bearing mice. We used the TRP2180–188 peptide as a melanoma antigen-derived and H-2Kb binding peptide, as previously reported [18]. Although both the L.E.M. extract and the peptide vaccine significantly increased TRP2180–188 peptide-specific and IFN-γ-producing T cells in the draining LNs and spleen of melanoma-bearing mice when administered individually, the combination therapy significantly enhanced the levels of these T cells (Fig. 2).
Fig. 2.
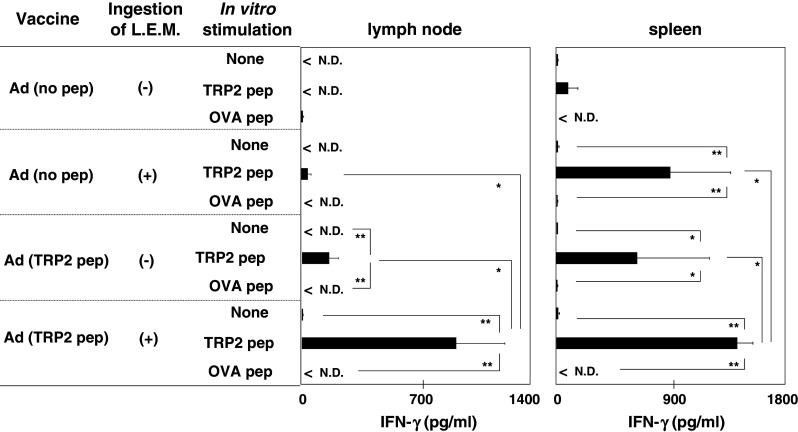
Reactivity of draining LNs and spleen to the TRP-2 peptide. B16 melanoma-bearing mice were treated as described in Fig. 1. On day 21, the draining LNs and spleen were harvested and stimulated in vitro with the indicated peptide in the presence of 20 U/ml IL-2, and the level of IFN-γ in the supernatant was determined using ELISA. Each data point represents three wells. Similar results were obtained in three independent experiments. *P < 0.05; **P < 0.01. Ad adjuvant, ND not detected
We further tested the reactivity of H-2Kb-restricted T cells to melanoma cells. Draining LNs and spleen cells were stimulated in vitro with either B16, B16L (an MHC class I loss variant of B16) or B16L-Kb (H-2Kb-expressing B16L), and the level of IFN-γ in the supernatants was determined (Fig. 3). Both the L.E.M. extract and the peptide vaccine significantly increased B16 melanoma-reactive T cells in the draining LNs and spleen of melanoma-bearing mice. Similar to tests of reactivity to the TRP2 peptide, the combination therapy significantly enhanced the level of IFN-γ in draining LNs, but not in the spleen. The combination therapy-induced enhancement of the activity of melanoma-reactive T cells was more distinguishable in the draining LNs than in the spleen. These results indicate that combining the L.E.M. extract and peptide vaccine therapies effectively increases MHC class I-restricted and melanoma antigen-reactive T cells in melanoma-bearing mice, especially in the draining LNs.
Fig. 3.
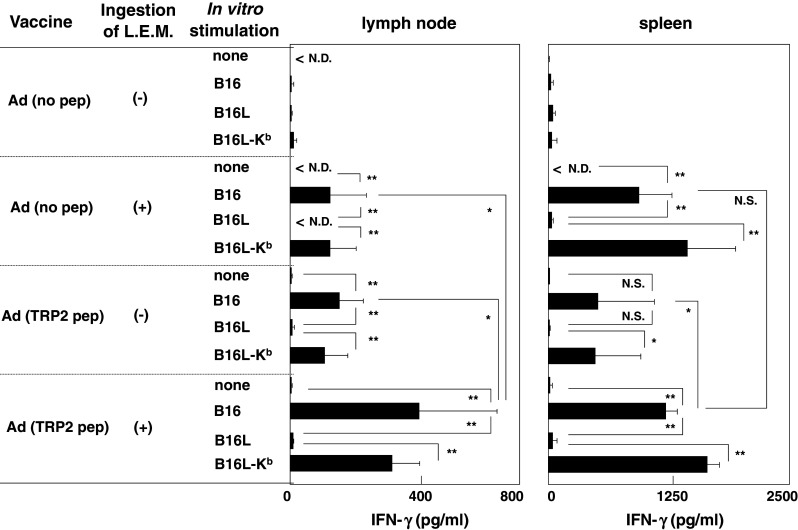
Reactivity of draining LNs and spleen to B16 melanoma and its sublines. B16 melanoma-bearing mice were treated as described in Fig. 1. On day 21, the draining LNs and spleen were harvested and stimulated in vitro with each of the indicated cell lines in the presence of 20 U/ml IL-2, and the level of IFN-γ in the supernatant was determined using ELISA. Each data point represents three wells. Similar results were obtained in three independent experiments. *P < 0.05; **P < 0.01. Ad adjuvant, ND not detected, NS not significant
Combination therapy can relieve the increase of Tregs in melanoma-bearing mice
We then examined the frequency of Tregs in the draining LNs and spleen of melanoma-bearing mice treated with L.E.M. extract and/or the peptide vaccine. Neither treatment alone had an impact on the melanoma-induced increase in the percentage of Tregs in the draining LNs and spleen. However, the combination therapy significantly decreased the percentage of Tregs in both the draining LNs and the spleen (Fig. 4a). Representative results are shown in Fig. 4b. Although we previously reported that ingestion of L.E.M. extract alone decreases the frequency of Tregs in melanoma-bearing mice [18], no significant decrease was observed in this experiment. This discrepancy might relate to the s.c. injection of CFA without peptide, since injection of adjuvant alone tended to increase the frequency of Tregs in naïve mice (data not shown).
Fig. 4.
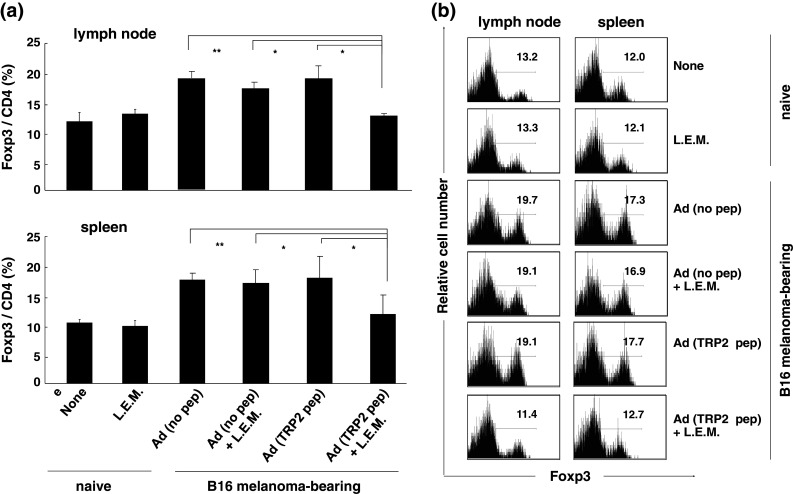
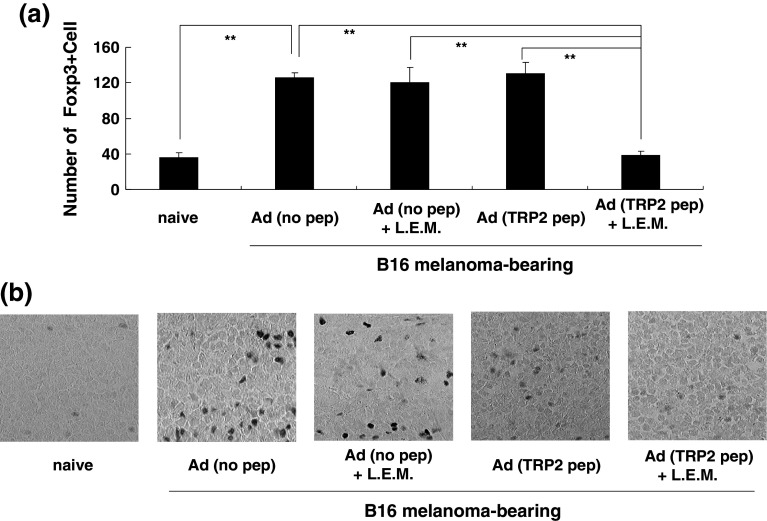
Significant decrease in the percentage of Tregs in melanoma-bearing mice treated with the combination therapy. B16 melanoma-bearing mice were treated as described in Fig. 1. On day 21, the tumor-draining LNs and spleen were harvested and flow cytometric analysis was performed. a The mean ± SD for each group of 6 mice is shown. Similar results were obtained in three independent experiments. *P < 0.05; **P < 0.01. b Representative results gated on CD4+ T cells. Numbers represent the percentage of Foxp3+ cells among CD4+ T cells. Ad adjuvant
To directly explore the effect of combination therapy on Tregs in vivo, we examined Tregs in the draining LNs using immunohistochemical staining. While administration of either L.E.M. extract or peptide vaccine alone had no impact on the melanoma-induced increase in the number of Treg cells in the draining LNs, the combination therapy successfully diminished the number of these cells in the draining LNs (Fig. 5a). Representative photos are shown in Fig. 5b.
Fig. 5.
Immunohistochemical staining of Foxp3+ cells in draining LNs. a B16 melanoma-bearing mice were treated as described in Fig. 1. On day 21, draining LNs were harvested and stained with methyl green. Foxp3+ cells in five different fields were counted and the means ± SD are shown. **P < 0.01. b Representative photos. Foxp3+ cells are shown as black dots. Ad adjuvant
L.E.M. extract prolongs the peptide vaccine-induced increase of specific T cells while attenuating the increase in Tregs in combined therapy
To elucidate the mechanism behind the efficacy of the combination therapy, we examined the kinetics of TRP2 peptide-specific T cells and Tregs in the draining LNs and spleen of melanoma-bearing mice. The peptide vaccine increased peptide-specific T cells in the draining LNs on day 7, but this increase was transient and did not last until day 14 (Fig. 6a). Ingestion of L.E.M. extract increased peptide-specific T cells in the draining LNs for up to 2 weeks, but the increase was relatively small. In contrast, the combination therapy significantly increased TRP2 peptide-reactive and IFN-γ-producing T cells in draining LN cells and maintained them at a high level even 21 days after melanoma inoculation. Although a similar trend was observed in the spleen, the increase was not as prominent as in the draining LNs. With respect to the frequency of Tregs, the combination therapy significantly attenuated the Treg increase in both the draining LNs and spleen (Fig. 6b).
Fig. 6.
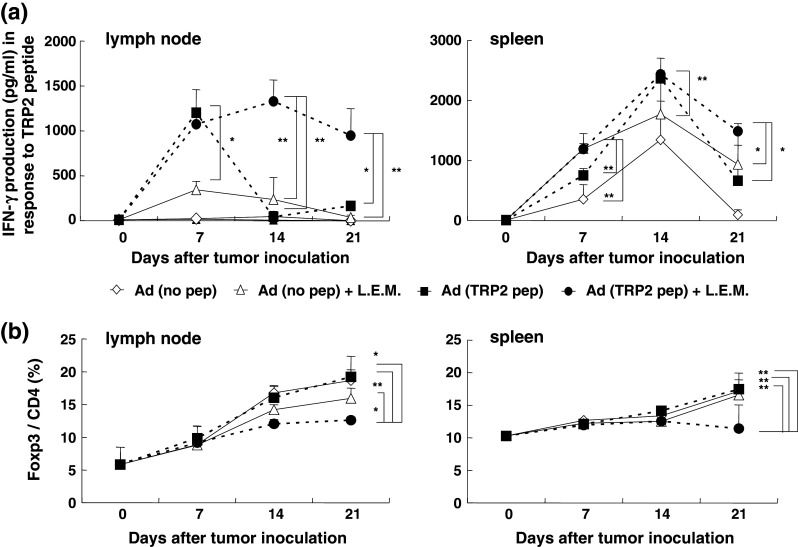
Kinetic analyses of draining LNs and spleen cells of melanoma-bearing mice. B16 melanoma-bearing mice were treated as described in Fig. 1. a On days 0, 7, 14, and 21, the draining LNs and spleen were harvested and stimulated in vitro with each of the indicated peptides in the presence of 20 U/ml IL-2 and the level of IFN-γ in the supernatant was determined using ELISA. The mean ± SD for each group is shown. Similar results were obtained in three independent experiments. *P < 0.05; **P < 0.01. b Flow cytometric analysis for determination of the percentage of Tregs. The mean ± SD for each group of 6 mice is shown. Similar results were obtained in three independent experiments. *P < 0.05; **P < 0.01. Ad adjuvant
Discussion
Treg-mediated immunosuppression significantly diminishes the efficacy of vaccine-induced anticancer therapies in cancer-bearing hosts [10, 11]. We recently reported that oral ingestion of L.E.M. extract mitigates Treg-mediated immunosuppression in B16 melanoma-bearing mice, and this effect is accompanied by efficient induction of melanoma-reactive T cells [18]. In this study, we determined whether oral ingestion of L.E.M. extract can enhance anti-tumor activity in B16 melanoma-bearing mice vaccinated with a TRP2 peptide that induces H-2Kb-restricted and melanoma-reactive CTLs.
We attempted to elucidate the mechanism underlying the significantly greater suppression of tumor growth by the combination therapy relative to the use of L.E.M. extract and the peptide vaccine alone. First, we focused on the reactivity of the spleen and draining LNs against a TRP2 peptide and examined the level of IFN-γ 21 days after melanoma inoculation. The draining LNs and spleen of melanoma-bearing mice treated with the combination therapy had higher levels of IFN-γ than mice treated with either the peptide vaccine or L.E.M. extract alone (Fig. 2). Similar results were obtained when reactivity to B16 melanoma and its sublines was examined (Fig. 3). In addition, we showed that depletion of CD8+ T cells abolishes the combination therapy-induced anti-tumor effect, indicating an essential role for CD8+ T cells in this experimental system. Together, these results indicate that the combination therapy effectively increases the amplitude of the reactivity of CTLs toward melanoma cells.
We then examined the kinetics of the reactivity of the draining LNs and spleen to the TRP2 peptide and the percentage of Tregs in these tissues (Fig. 6). Notably, combination therapy significantly prolonged the duration of the anti-tumor responses of the draining LNs and spleen in melanoma-bearing mice. The prolongation was more prominent in the draining LNs than in the spleen. Intriguingly, the lasting increase in the reactivity of T cells in the draining LNs and spleen contrasted with the lowered percentage of Tregs in melanoma-bearing mice treated with the combination therapy. These findings led us to conclude that combining the peptide vaccine with oral ingestion of L.E.M. extract elicits anti-tumor activity in melanoma-bearing mice through an enhancement of anti-melanoma T cell responses at the levels of ‘amplitude’ and ‘duration’, while attenuating the increase in the percentage of Tregs.
It remains unknown how oral ingestion of L.E.M. extract enhances induction of melanoma-reactive T cells in tumor-bearing mice. We previously reported that oral ingestion of L.E.M. extract can decrease the number of Tregs in B16 melanoma-bearing mice [18]. In the present study, we showed that adoptive transfer of Tregs can reverse the L.E.M. extract-induced anti-tumor effect as demonstrated by evaluation of tumor weight (Fig. 1d). These lines of evidence indicate that oral ingestion of L.E.M. extract inhibits B16 melanoma growth via mitigation of Treg-mediated immunosuppression. In this regard, we tested the possibility that L.E.M. extract can directly inhibit the induction of Tregs in an in vitro culture system, whereas the addition of a high dose of L.E.M. extract (e.g., 200 μg/ml) failed to inhibit Treg induction (Suppl. Fig. 1). On the other hand, it has been reported that L.E.M. extract contains β(1,3)(1,6)d-glucan [20], which potentially provides signaling through a Dectin-1 receptor [22]. Signaling via this receptor can elicit a Th1-type response without inducing Tregs [23]. This information suggests that Dectin-1-mediated signaling was involved in enhancing induction of melanoma-reactive T cells in melanoma-bearing mice treated with the combination therapy in this study. In addition, a recent report revealed that vaccination with an MHC class I-binding tumor-antigen-derived peptide along with IFA results in enhanced tumor growth in association with apoptosis of CD8+ T cells, and that the additional use of toll-like receptor (TLR) 9 ligand rescues these T cells from apoptosis [24]. Furthermore, lentinan and the protein-bound polysaccharide PSK reportedly contain ligands for TLR2 or TLR4 [25, 26]. Signaling though TLR2 can induce production of cytokines and chemokines synergistically and in a MyD88-dependent manner when combined with stimulation of TLR4 and TLR9 [27]. Although we have not determined whether L.E.M. extract contains candidate TLR ligands, we observed that TNF-α production by human monocytes in the presence of L.E.M. extract is inhibited by the addition of anti-TLR4 antibody (unpublished observation). These lines of evidence suggest that L.E.M. extract can provide TLR-mediated signaling that results in optimal activation of professional antigen-presenting cells and avoids apoptosis of T cells induced by MHC class I-binding peptide vaccines. We are currently undertaking experiments to test these possibilities.
We also examined the percentage of Tregs in the draining LNs and spleen of melanoma-bearing mice treated with the peptide vaccine and/or L.E.M. extract and found that the percentage of Foxp3+ cells among CD4+ T cells decreased to the same level as in naïve mice (Fig. 4). This observation was also confirmed by immunohistochemical staining experiments (Fig. 5). On the other hand, no decrease in the percentage of Tregs was observed in melanoma-bearing mice that were fed L.E.M. extract and injected with CFA/IFA without a TRP2 peptide. Although this result seemed to contradict our previous report that oral ingestion of L.E.M. extract decreases the percentage of Tregs in melanoma-bearing mice [18], we suppose that the discrepancy might have been due to the use of CFA, which contains mycobacterial antigens that strongly induce inflammation in vivo. The observation that injection of adjuvant alone tended to increase the frequency of Tregs in naïve mice supports this explanation. Concurrent injection of CFA might prevent L.E.M. extract from decreasing the percentage of Tregs in melanoma-bearing mice. Alternatively, it is significant that no decrease in the percentage of Tregs was observed when naïve mice were fed L.E.M. extract, excluding the possibility of L.E.M. extract-induced autoimmune disease.
In conclusion, we demonstrated that anti-tumor activity induced by peptide vaccines can be effectively enhanced by combining the vaccine with oral ingestion of L.E.M. extract. This enhancement presumably occurs through an increase in the amplitude and duration of the heightened response of melanoma-reactive T cells in tumor-bearing hosts. An advantage of L.E.M. extract is that it can be administered orally on an outpatient basis [28, 29]. We hope that this type of immunomodulating drug will be reevaluated and eventually used together with current anticancer immunotherapies, including peptide-based anticancer vaccines.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide-or tumor lysatepulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 3.Jager E, Gnjatic S, Nagata Y, et al. Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers. Proc Natl Acad Sci USA. 2000;97:12198–12203. doi: 10.1073/pnas.220413497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oka Y, Tsuboi A, Fujiki F, et al. WT1 peptide vaccine as a paradigm for “cancer antigen-derived peptide”-based immunotherapy for malignancies: successful induction of anti-cancer effect by vaccination with a single kind of WT1 peptide. Anticancer Aqents Med Chem. 2009;9:787–797. doi: 10.2174/187152009789056958. [DOI] [PubMed] [Google Scholar]
- 5.Mine T, Sato Y, Noguchi M, et al. Humoral responses to peptides correlate with overall survival in advanced cancer patients vaccinated with peptides based on pre-existing, peptide-specific cellular responses. Clin Cancer Res. 2004;10:929–937. doi: 10.1158/1078-0432.CCR-1117-3. [DOI] [PubMed] [Google Scholar]
- 6.Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol. 2006;16:115–123. doi: 10.1016/j.semcancer.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 11.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loeffler M, Kruger JA, Reisfeld RA. Immunostimulatory effects of low-dose cyclophosphamide are controlled by inducible nitric oxide synthase. Cancer Res. 2005;65:5027–5030. doi: 10.1158/0008-5472.CAN-05-0646. [DOI] [PubMed] [Google Scholar]
- 13.Wada S, Yoshimura K, Hipkiss EL, et al. Cyclophosphamide augments antitumor immunity: studies in an autochthonous prostate cancer model. Cancer Res. 2009;69:4309–4318. doi: 10.1158/0008-5472.CAN-08-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+ CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 15.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 16.Tawara I, Take Y, Uenaka A, Noguchi Y, Nakayama E. Sequential involvement of two distinct CD4+regulatory T cells during the course of transplantable tumor growth and protection from 3-methylcholanthrene-induced tumorigenesis by CD25-depletion. Jpn J Cancer Res. 2002;93:911–916. doi: 10.1111/j.1349-7006.2002.tb01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi T, Hirota K, Nagahama K, et al. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity. 2007;27:145–159. doi: 10.1016/j.immuni.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka K, Ishikawa S, Matsui Y, Tamesada M, Harashima N, Mamoru H. Oral ingestion of Lentinula edodes mycelia extract inhibits B16 melanoma growth via mitigation of regulatory T cell-mediated immunosuppression. Cancer Sci. 2011;102:516–521. doi: 10.1111/j.1349-7006.2010.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harada M, Tamada K, Abe K, et al. Characterization of B16 melanoma-specific cytotoxic T lymphocytes. Cancer Immunol Immunother. 1998;47:198–204. doi: 10.1007/s002620050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kojima H, Akaki J, Nakajima S, Kamei K, Tamesada M. Structural analysis of glycogen-like polysaccharides having macrophage-activating activity in extracts of Lentinula edodes mycelia. J Nat Med. 2010;64:16–23. doi: 10.1007/s11418-009-0357-1. [DOI] [PubMed] [Google Scholar]
- 21.Zeh HJ, III, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol. 1999;162:989–994. [PubMed] [Google Scholar]
- 22.Kato Y, Adachi Y, Ohno N. Characterization of rat beta-glucan receptor dectin-1. Microbiol Immunol. 2008;52:418–428. doi: 10.1111/j.1348-0421.2008.00055.x. [DOI] [PubMed] [Google Scholar]
- 23.Manicassamy S, Ravindran R, Deng J, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muraoka D, Kato T, Wang L, et al. Peptide vaccine induces enhanced tumor growth associated with apoptosis induction in CD8+ T cells. J Immunol. 2010;185:3768–3776. doi: 10.4049/jimmunol.0903649. [DOI] [PubMed] [Google Scholar]
- 25.Zhou LD, Zhang QH, Zhang Y, Liu J, Cao YM. The shiitake mushroom-derived immuno-stimulant lentinan protects against murine malaria blood-stage infection by evoking adaptive immune-responses. Int Immunopharmacol. 2009;9:455–462. doi: 10.1016/j.intimp.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Lu H, Yang Y, Gad E, et al. Polysaccharide krestin is a novel TLR2 agonist that mediates inhibition of tumor growth via stimulation of CD8T cells and NK cells. Clin Cancer Res. 2011;17:67–76. doi: 10.1158/1078-0432.CCR-10-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KS, Scanga CA, Bachelder EM, Chen Q, Snapper CM. TLR2 synergizes with both TLR4 and TLR9 for induction of the MyD88-dependent splenic cytokine and chemokine response to Streptococcus pneumoniae . Cell Immunol. 2007;245:103–110. doi: 10.1016/j.cellimm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshioka Y, Tamesada M, Nagayama A. The safety of excessive intake of the food containing extract of cultured Lentinula edodes mycelia (LEM) in healthy adult volunteers. JCAM. 2009;6:9–15. [Google Scholar]
- 29.Yoshioka Y, Matsui Y, Kobayashi M, et al. Safety evaluation of extract from cultured Lentinula edodes mycelia; study of acute toxicity, genotoxicity and inhibiting effect of drug-metabolizing enzyme, cytochrome P-450 3A4. JCAM. 2010;7:51–57. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.