Abstract
Cells with “stemness” and tumor-initiating properties have been isolated from both hematological and solid tumors. These cells denominated as cancer stem cells (CSCs), representing rare populations within tumors, have the ability to metastasize and are resistant to standard therapies and immunotherapy. Heterogeneity and plasticity in the phenotype of CSCs have been described in relation to their tissue origin. Few definitive markers have been isolated for CSCs from human solid tumors, limiting their usage for in vivo identification of these cells. Nevertheless, progress in the emerging CSCs concept has been achieved gaining, at least for some type of tumors, their biological and immunological characterization. The recent identification of molecules and signaling pathways that are up-regulated or aberrantly induced in CSCs allowed the development of small agents for specifically targeting of CSCs. A general low immunogenic profile has been reported for CSCs with, in some cases, the identification of the mechanisms responsible of the impairment of cell-mediated immune responses. These concepts are discussed in the context of this review. Although CSCs still need to be fully characterized, potential candidate markers and/or signaling pathways, to be exploited for the design of novel CSC-targeting therapeutic strategies, are described in this review.
Keywords: Cancer stem cells, Cancer stem cells-associated signaling pathways, Immunological profile, Immune modulation, CSC-targeted therapies, NIBIT 2013
Introduction
Tumor tissues comprise heterogeneous populations of cells with a minority of them displaying stemness/tumor-initiating properties, defined as cancer stem cells (CSCs) or cancer-initiating cells (CICs). These cells have been characterized by self-renewal, multipotency, and tumorigenic properties [1–4]. Tumor cells with stemness/tumor-initiating properties have been isolated from both hematological and solid tumors [2–4].
A variety of markers have been used for ex vivo isolation of CSCs [5], though recent evidences have revealed that most of them are limited to the enrichment in these cell populations and are widely expressed in many organs as well. These markers have been chosen on the evidences that they can discriminate cell subpopulations enriched or negative for their expression. However, the heterogeneity of tumors, the interaction with the microenvironment, the origin of CSCs, and the genetic background can influence the phenotype, the biological properties, and the plasticity of these rare cells [4–6]. Few definitive CSC markers have been identified based on the biological characteristics and tissue origin of these cells, such as Lgr5 and CD44v6 in colorectal cancer (CRC), which are both target molecules of Wnt pathway, and are required for clonogenic and metastatic properties of CSCs [7, 8]. The identification of functional markers that are related to the “stemness properties” represents more precise tools for the ex vivo isolation of CSCs. The increasing efforts in understanding the signaling pathways up-regulated or aberrantly activated in CSCs allowed better biological and functional characterization of these cells [7, 8].
CSCs are resistant to chemotherapy, radiotherapy and possibly to immunotherapy [9–11] although studies in the sensitivity of CSCs to immunotherapeutic approaches are somewhat limited. CSC-targeted therapeutic interventions are desirable to achieve complete tumor eradication. A comprehensive identification of biological and immunological properties of CSCs in relation to their origin and tumor microenvironment is needed to develop novel and more effective therapeutic interventions for cancer patients. A number of small molecules targeting signaling pathways associated with CSCs or monoclonal antibodies (mAb) specifically directed to these cells have been pre-clinically developed, and it can be envisaged that they will be the object of future clinical studies. Notably, the introduction in clinical practice of agents that target the blockade of immune checkpoints has improved the survival of patients with different solid tumors [12, 13]. The combination of these therapies with other interventions, such as chemotherapy, radiotherapy, targeted therapies, and vaccination with tumor antigens, opens a window of opportunity for the cure of solid tumors. Thus, next generation therapies based on increased knowledge of CSC characteristics and possibly, on the combination of therapeutic interventions, such as immunotherapy and CSC-specific-targeted therapies, need to be developed to achieve complete eradications of tumors.
Cancer stem cells: phenotype and signaling pathway
Initial phenotypic characterization of CSCs has been achieved through the prospective identification of tumorigenic or clonogenic cells isolated from the bulk tumor mass by flow cytometry or immunomagnetic selection. Using these assays, the presence or the absence of some surface molecules have been proposed as stem cell markers, such as CD34+/CD38− [3], CD44+/CD24−/low [14], CD133 [15], CD44 alone or in combinations with other antigens [16], and ephrin receptors [17, 18]. In other instances, the use of fluorescent dyes has allowed a similar enrichment of CSCs. While the property of excluding a Hoechst dye originally developed for normal stem cells has been attempted with limited success, the high enzymatic activity of ALDH has been more convincingly demonstrated to characterize the tumor-initiating cells in breast and thyroid cancer [19, 20]. More recently, the efforts have been concentrated on detection of functional markers that promote survival and migration of CSCs, such as integrin alpha 6 in glioblastoma [21] or CD44v6 in colorectal cancer [8]. The search for phenotypic similarities between normal and cancer stem cells has been primarily investigated based on key genes involved in embryonic stem cell maintenance and cell reprogramming. Sox2, Nanog, and Oct4 are expressed in CSCs from different tumors and have been extensively proposed as prognostic biomarkers [22].
The peculiar signaling pathways active in CSCs results from the combination of genomic aberrations relative to the oncogenic transformation and the epigenetic status of immature stem-like cells. Some of the so-called stem cell genes have a direct impact on the signal transduction. For instance, Nanog can complex with Stat-3 and promote the activation of genes that contribute to increase survival and therapy resistance in CSCs, such as MDR1/ABCB1 [23]. The PI3K/AKT seems one of the most critical pathways in CSCs [8]. PTEN is epigenetically silenced in colorectal CSCs. Its expression is absent in the more immature compartment and gradually acquired during differentiation [24, 25]. Although it is still unclear how PTEN silencing occurs, recent evidence clarified the pivotal role played of PI3K in maintaining the tumorigenic activity of colorectal CSCs and the ability of PI3K activation to reprogram non-tumorigenic progenitors into tumorigenic CSCs [8]. Proteins of the extracellular matrix deliver other critical signals in CSCs. The interaction between hyaluronic acid and CD44 as well as between integrins and laminin or fibronectin triggers a series of biochemical events leading to increase survival and invasion, which are two key properties of CSCs, essential for tumor growth and progression [8, 9]. Although the ability of CSCs to generate metastasis has been postulated for years, only recently the mechanisms responsible for increase in invasiveness and metastatic activity of CSCs are beginning to become clear [26, 27]. In carcinomas, CSCs show traits of EMT that contribute to enhance the metastatic potential conferred by cMET and CD44 isoforms [8, 28]. The activity of such receptors is not confined to CSCs from carcinomas, but it is shared by tumorigenic cells in glioblastoma and melanoma [28]. Thus, intense efforts are directed on the targeting of relevant receptor–ligand interaction or their signaling pathways.
Therapeutic targeting of cancer stem cells
Targeting of CSCs carries the hope of curing cancer by hitting the source that feeds the tumor cell mass. However, this task appears extremely challenging. CSCs have been shown to be resistant to chemotherapy and radiotherapy [10, 11, 29, 30]. In vitro drug screenings have shown that only a limited number of compounds are able to kill CSCs at nanomolar concentrations [31, 32]. Likewise, targeting of death receptors may not be an easy task, at least in the systems explored so far, such as glioblastoma multiforme (GBM) and breast cancer. Neural stem cells do not express caspase-8, which is required for generation of death receptor signals [23]. Caspase-8 promoter methylation occurs in GBM stem cells and their progeny [33]. Thus, the possible use of death receptor agonists needs to be combined with drugs or molecules that are able to up-regulate caspase-8. As for breast cancer, although some CSCs from established cell lines have been proposed to be sensitive to TRAIL receptor agonists [34, 35], suppression of the caspase-8 inhibitory partner c-Flip may be required to enhance the efficacy of TRAIL receptor targeting [36].
CSC resistance to radiotherapy may be overcome by the use of small molecules that sensitize resistant cells to apoptosis. This may be the case of XIAP inhibition in GBM stem cells [37]. The radioresistance of glioma stem cell seems mostly to be mediated by the activity of checkpoints that allow the irradiated stem cell to undergo cell cycle arrest and repair the DNA breaks without undergoing mitotic catastrophe [29]. This mechanism has been proposed for chemotherapy resistance in CSCs of NSCLC and colorectal cancer [38], raising the possibility to develop effective combined therapies using chemotherapy and cell cycle checkpoint inhibitors [30].
Upon terminal differentiation, CSCs lose the clonogenic and tumorigenic activity [16, 39, 40]. Thus, another possible therapeutic strategy for effective CSC targeting may involve differentiation therapy. The use of retinoic acid in combination with either chemotherapy or arsenic trioxide in promyelocytic leukemia has completely turned deadly disease in one of the most curable malignancies, thus creating a paradigm for the beneficial therapeutic effect of enhancing cancer stem cell differentiation [41]. Although similar attempts in solid tumors have had limited success, the technologies to purify and expand CSC from solid tumors allow for the discovery of new molecules and mechanisms that promote the differentiation therapy in preclinical models of solid tumors. The first evidence has come in GBM, where BMP4 has been shown to promote terminal differentiation of CSCs. Moreover, in vivo delivery of bead-conjugated BMP4 showed a therapeutic effect in CSC-based orthotopic tumor xenografts [39]. Again in GBM, similar data were obtained with a modified version of BMP7 that does not require delivery attached to beads [42]. As for other tumors, BMP4 has proven to induce CSC differentiation and sensitization to chemotherapy in colorectal cancer [25], suggesting that such potential therapeutic effect is not confined into glioblastoma, but may be relevant in some carcinomas.
Another promising field involves the development of Abs targeting CSC-related pathways, such as Notch, whose prototype appears to be an anti-DLL4 [43] that is currently in clinical trials. Other CSC-related pathways that are targeted successfully in preclinical studies are Indian and Sonic Hedgehog, for which there is a human-specific mAb targeting the interaction between CSCs and stroma [44]. Finally, there are mAbs targeting CSC surface markers that have been shown effective in preclinical studies. These include anti-CD44 [45], anti-CD133 [46], and integrin alpha 6 [21]. Although these are not clinically approved therapeutic agents specifically targeting CSCs as yet, there is considerable expectation among the scientific community that a number of new preclinical molecules targeting CSCs will enter clinical trials in the near future.
Immunological profile of CSCs
The improvements of methods to isolate and to expand in vitro CSCs have allowed a few groups to analyze the immunological properties of these cells. A comprehensive immune profile was gained for CSCs isolated from GBM. A comparison of the expression MHC and the antigen processing machinery molecules between GBM and CSCs and their autologous non-CSC counterparts have been carried out, highlighting a defective expression of these molecules by CSCs and low sensibility to the modulation by IFN (both α and γ) as well as de-methylating (5-Aza CdR) treatments [47]. Thus, a general down-modulation of antigen processing and presentation may affect the ability of CSCs to elicit a T cell-mediated immune responses. Along these lines, ligands of NKG2D (MICA/B and ULBPs) that engage directly or by co-stimulation the NKG2D receptor on NK or T lymphocytes, respectively [48], were also found to be down-modulated on GBM-CSCs, leading to impairment of cell-mediated immune responses. Differential gene expression, which was confirmed at the protein levels for some immunological-related molecules, was detected in GBM-CSCs as compared to differentiated autologous non-CSCs, corroborating the hypothesis that CSCs from GBM display peculiar biological and immunological profiles that differ from that of cells lacking “stemness” functions within the same tumor [47, 49]. The evidence that GBM-CSCs can play negative regulatory functions on lymphocytes was provided by the observation that impairment of the proliferation of T cells and that a preferential differentiation toward a TH2 type subset occurred by the co-culture in vitro of CSCs with autologous or allogeneic lymphocytes [47]. The immunosuppressive activity of CSCs has been described both in GBM and melanoma, showing that these cells can release soluble suppressive factors, such as Galectin-3, TGF-β2, IL-10, IL-13, PGE2, PD-1, B7-H1, B7-H3, and GDF-15 and can then induce the differentiation of T cell regulatory functions [50–53]. The variability in the quality and quantity of immune-suppressive factors associated with CSCs can be detected in relation to their tissue of origin; nonetheless, common immunosuppressive features are found despite the levels of heterogeneity of these cells.
In line with these observations, scant immunogenic properties were found also in CRC-derived CSCs, indicating that this feature is not merely affected by the histological origin of these cells, but is determined by the “stemness” functions of these cells [54]. CRC–CSCs have been found to express IL-4 and IL-4R, at elevated levels compared with the autologous non-CSCs [54, 55].
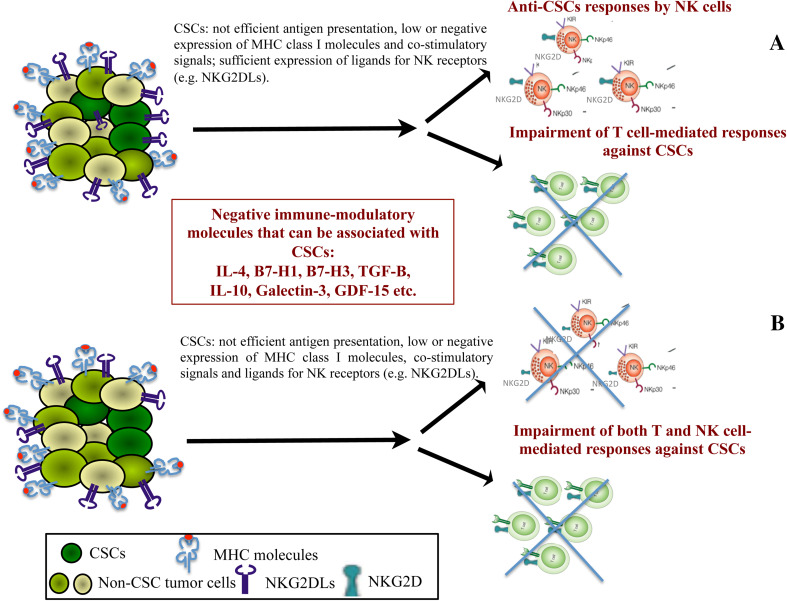
The IL-4 signaling in epithelial tumor is linked to unresponsiveness to standard therapies such as chemotherapy by inducing resistance to apoptosis [55, 56]. Recently, an immune-suppressive role of CSC-associated IL-4 was documented [54]. Notably, this phenomenon was dependent on in vitro cell-to-cell contact between CSCs and T cells and was not mediated by the autocrine engagement of IL-4. The evidence that the negative cross talk between CSCs and T lymphocytes needs a joint cellular interaction was demonstrated by the fact that soluble IL-4 released by CRC–CSCs, only partially affected T cell functions. The neutralization of IL-4, e.g., by specific monoclonal antibodies, can restore the immunogenicity of CRC–CSCs, leading to the efficient T cell proliferation and induction in vitro of anti-CSC TH1 type responses. Interestingly, following the neutralization of IL-4 signaling an enrichment of CD8+ T effector memory cells was also observed, indicating that the blocking of IL-4 can prevent at least one of the immunosuppressive mechanisms associated with CRC–CSCs and can modify the tumor–lymphocyte interaction, supporting activation and proliferation of T rather than NK cells. Indeed, CRC–CSCs have been described to be highly susceptible to NK cell recognition due to the efficient expression by these cells of NK-receptor ligands and the lack or low expression of MHC class I molecules [57]. The expression of NK-receptor ligands by CRC–CSCs was in disagreement with previous data by our and other groups in the context of GBM model, where an immune-suppressive phenotype prevented anti-tumor activity by NK cells [47, 58]. However, the activation of NK cell functions was shown following their culture in vitro with primary oral squamous carcinoma or ovarian CSCs [59, 60], representing further evidences of the high heterogeneity of CSCs deriving from different tumors. Multiple mechanisms of immunoregulatory activity are exploited by CSCs to escape from cell-mediated immune surveillance, indicating the complexity of the immunological profile of these cells (Fig. 1). Moreover, none of this negative immunoregulatory signaling is CSC-specific, being shared with normal stem cells [61, 62] and thus associated with “stemness” functions.
Fig. 1.
Escape from cell-mediated immune surveillance by CSCs. CSCs have impairment of antigen processing and presentation machinery. In the presence of efficient expression of ligands of NK receptors, they can be susceptible to NK but not to T cell recognition (a). Cell-mediated immune reactivity is totally impaired whether both antigen processing and presentation machinery and ligands of NK receptors are down-modulated on CSCs (b). In addition, CSCs can either express on the membrane or secrete a variety of negative immune-modulatory molecules that can affect cell-mediated immune surveillance
Further efforts are needed to dissect the relationship between CSCs and anti-tumor immunity; however, thus far, the available data provide clues for the identification of strategies that can revert CSC-based immunosuppression, for the design of new immunotherapeutic approaches for patients with solid tumors (Fig. 1).
What type of tumor-associated antigens is expressed by CSCs?
An exhaustive antigenic profile of CSCs still needs to be assessed. Several groups have reported the expression of human tumor antigens shared with the non-CSC counterparts of the tumors and the isolation of T cell responses targeting these molecules [5]. Nevertheless, besides scant antigen processing and presentation (as discussed below), low levels of expression of these molecules were found to be associated with CSCs [47], preventing from an efficient induction of tumor-specific T cell responses. Of interest, T cell responses elicited by CSCs and directed to the COA-1 antigen have been detected in CRC patients. These results highlighted that COA-1 may represent a relevant target molecule for T cell responses against CRC–CSCs. Moreover, highly efficient antigen-specific anti-tumor immune responses could be achieved when CSCs with neutralized IL-4 signaling where used as stimulators [54]. In future studies, it would be worthy to investigate the mechanisms that can lead to differential antigen processing and presentation between CSCs and the non-CSC tumor cells. The determination of comprehensive genomic and immunological profiles of CSCs is desirable to identify new CSC-specific antigens that can represent novel target molecules for immunotherapy.
Conclusions and perspectives
The biological and immunological features of CSCs isolated from solid tumors have been summarized as discussed in the context of the XIth NIBIT meeting.
Progress in the development and in understanding the CSCs concept has allowed for a better biological characterization of these cells. Indeed, aberrantly up-regulated and/or activated CSC-associated signaling pathways have been identified that are related with “stemness” and the ability to metastasize. Moreover, this information has allowed for the isolation of CSC-associated markers that are relevant tools for ex vivo characterization of cells with tumor initiation and propagation properties. Thus, small molecules and mAbs that target these structures have been developed and will enter soon clinical studies.
Despite some possible differences depending on the protocols for the isolation in vitro of CSCs and on their genomic background and/or histological origin, these cells can display immune-modulatory activities. The information provided by the analysis of the immunological profile of CSCs from GBM and CRC are important in enabling appropriate targeting of the negative immune-modulatory molecules expressed by these cells (Fig. 1). Further efforts are needed to achieve a comprehensive biological and antigenic characterization of CSCs. This information will have relevant implications for the design of novel CSC-targeting agents including immunotherapy and/or of combinatorial therapies based on targeted therapies and immune-modulatory agents.
Conflict of interest
The authors have no conflict of interest.
Abbreviations
- ALDH
Aldehyde dehydrogenase
- BMP4
Bone morphogenetic protein 4
- COA-1
Colon antigen-1
- CRC
Colorectal cancer
- CSCs
Cancer stem cells
- DLL4
Delta-like ligand 4
- GDF-15
Growth differentiation factor-15
- GBM
Glioblastoma multiforme
- IFN
Interferon
- mAb
Monoclonal antibody
- MDR1
Multi-drug resistance 1 gene
- PD-1
Programmed death 1
- PGE2
Prostaglandin E2
- PI3K
Phosphatidylinositide 3-kinase
- PTEN
Phosphatase and tensin homolog
- TGFB-1
Transforming growth factor beta 1
- XIAP
X-linked inhibitor of apoptosis
References
- 1.Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Clevers H. The cancer stem cells: premises, promises and challenges. Nat Med. 2011;17:313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 4.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 5.Maccalli C, Volontè A, Cimminiello C, Parmiani G. Immunology of cancer stem cells in solid tumor. Eur J Cancer. 2013;50:649–655. doi: 10.1016/j.ejca.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Hjelmeland AB, Rich JN. The quest for self-identity: not all cancer stem cells are the same. Clin Cancer Res. 2012;18:3495–3498. doi: 10.1158/1078-0432.CCR-12-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kemper K, Presetyanti PR, de Lau W, Rodermond H, Clevers H, Medema JP. Monoclonal antibodies against Lgr5 identify human colorectal cancer stem cells. Stem Cells. 2012;30:2378–2386. doi: 10.1002/stem.1233. [DOI] [PubMed] [Google Scholar]
- 8.Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M, Apuzzo T, Sperduti I, Volpe S, Cocorullo G, Gulotta G, Dieli F, De Maria R, Stassi G. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014;14:342–356. doi: 10.1016/j.stem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Maugeri-Saccà M, Vigneri P, De Maria R. Cancer stem cells and chemosensitivity. Clin Cancer Res. 2011;17:4942–4947. doi: 10.1158/1078-0432.CCR-10-2538. [DOI] [PubMed] [Google Scholar]
- 10.Diehn M, Clarke MF. Cancer stem cells and radiotherapy: new insights into tumor radioresistance. J Natl Cancer Inst. 2006;98:1755–1757. doi: 10.1093/jnci/djj505. [DOI] [PubMed] [Google Scholar]
- 11.Irvin DK, Jouanneau E, Duvall G, Zhang XXY, Zhai Y, Sarayba D, Seksenyan A, Panwar A, Black KL, Wheeler CJ. T cells enhance stem-like properties and conditional malignancy in gliomas. PLoS One. 2010;5:e10974. doi: 10.1371/journal.pone.0010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Postow MA, Harding J, Wolchok JD. Targeting immune checkpoints: releasing the restraints on anti-tumor immunity for patients with melanoma. Cancer J. 2012;18:153–159. doi: 10.1097/PPO.0b013e31824b2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 16.Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 17.Batlle E, Bacani J, Begthel H, Jonkheer S, Gregorieff A, van de Born M, Malats N, Sancho E, Boon E, Pawson T, Gallinger S, Pals S, Clevers H. EphB receptor activity suppresses colorectal cancer progression. Nature. 2005;435:1126–1130. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]
- 18.Binda E, Visioli A, Giani F, Lamorte G, Copetti M, Pitter KL, Huse JT, Cajola L, Zanetti N, DiMeco F, De Filippis L, Mangiola A, Maira G, Anile C, De Bonis P, Reynolds BA, Pasquale EB, Vescovi AL. The EphA2 receptor drives self-renewal and tumorigenicity in stem-like tumor-propagating cells from human glioblastomas. Cancer Cell. 2012;22:765–780. doi: 10.1016/j.ccr.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquermier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todaro M, Iovino F, Eterno V, Cammareri P, Gambara G, Espina V, Gulotta G, Dieli F, Giordano S, De Maria R, Stassi G. Tumorigenic and metastatic activity of human thyroid cancer stem cells. Cancer Res. 2010;70:8874–8885. doi: 10.1158/0008-5472.CAN-10-1994. [DOI] [PubMed] [Google Scholar]
- 21.Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB, Rich JN. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6:421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang ML, Chiou SH, Wu CW. Targeting cancer stem cells: emerging role of Nanog transcription factor. Onco Targets Ther. 2013;6:1207–1220. doi: 10.2147/OTT.S38114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Signore M, Ricci-Vitiani L, De Maria R. Targeting apoptosis pathways in cancer stem cells. Cancer Lett. 2013;332:374–382. doi: 10.1016/j.canlet.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Ricci-Vitiani L, Mollinari C, di Martino S, Biffoni M, Pilozzi E, Pagliuca A, de Stefano MC, Circo R, Merlo D, De Maria R, Garaci E. Thymosin beta4 targeting impairs tumorigenic activity of colon cancer stem cells. FASEB J. 2010;24:4291–4301. doi: 10.1096/fj.10-159970. [DOI] [PubMed] [Google Scholar]
- 25.Lombardo Y, Scopelliti A, Cammareri P, Todaro M, Iovino F, Ricci-Vitiani L, Gulotta G, Dieli F, de Maria R, Stassi G. Bone morphogenetic protein 4 induces differentiation of colorectal cancer stem cells and increases their response to chemotherapy in mice. Gastroenterology. 2011;140:297–309. doi: 10.1053/j.gastro.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2011;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 28.Peters S, Adjei AA. MET: a promising anticancer therapeutic target. Nat Rev Clin Oncol. 2012;9:314–326. doi: 10.1038/nrclinonc.2012.71. [DOI] [PubMed] [Google Scholar]
- 29.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 30.Bartucci M, Svensson S, Romania P, Dattilo R, Patrizii M, Signore M, Navarra S, Lotti F, Biffoni M, Pilozzi E, Duranti E, Martinelli S, Rinaldo C, Zeuner A, Maugeri-Saccà M, Eramo A, De Maria R. Therapeutic targeting of Chk1 in NSCLC stem cells during chemotherapy. Cell Death Differ. 2012;19:768–778. doi: 10.1038/cdd.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francescangeli F, Patrizii M, Signore M, Federici G, Di Franco S, Pagliuca A, Baiocchi M, Biffoni M, Ricci Vitiani L, Todaro M, De Maria R, Zeuner A. Proliferation state and polo-like kinase 1 dependence of tumorigenic colon cancer cells. Stem Cells. 2012;30:1819–1830. doi: 10.1002/stem.1163. [DOI] [PubMed] [Google Scholar]
- 33.Eramo A, Pallini R, Lotti F, Sette G, Patti M, Bartucci M, Ricci-Vitiani L, Signore M, Stassi G, Larocca LM, Crinò L, Peschle C, De Maria R. Inhibition of DNA methylation sensitizes glioblastoma for tumor necrosis factor-related apoptosis-inducing ligand-mediated destruction. Cancer Res. 2005;65:11469–11477. doi: 10.1158/0008-5472.CAN-05-1724. [DOI] [PubMed] [Google Scholar]
- 34.Londoño-Joshi AI, Oliver PG, Li Y, Lee CH, Forero-Torres A, LoBuglio AF, Buchsbaum DJ. Basal-like breast cancer stem cells are sensitive to anti-DR5 mediated cytotoxicity. Breast Cancer Res Treat. 2012;133:437–445. doi: 10.1007/s10549-011-1763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li M, Knight DA, Smyth MJ, Stewart TJ. Sensitivity of a novel model of mammary cancer stem cell-like cells to TNF-related death pathways. Cancer Immunol Immunother. 2012;61:1255–1268. doi: 10.1007/s00262-012-1200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piggott L, Omidvar N, Pérez SM, Eberl M, Clarkson RW. Suppression of apoptosis inhibitor c-FLIP selectively eliminates breast cancer stem cell activity in response to the anti-cancer agent, TRAIL. Breast Cancer Res. 2011;13:R88. doi: 10.1186/bcr2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vellanki SH, Grabrucker A, Liebau S, Proepper C, Eramo A, Braun V, Boeckers T, Debatin KM, Fulda S. Small-molecule XIAP inhibitors enhance gamma-irradiation-induced apoptosis in glioblastoma. Neoplasia. 2009;11:743–752. doi: 10.1593/neo.09436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maugeri-Saccà M, Bartucci M, De Maria R. Checkpoint kinase 1 inhibitors for potentiating systemic anticancer therapy. Cancer Treat Rev. 2013;39:525–533. doi: 10.1016/j.ctrv.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Piccirillo SGM, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 40.Ricci-Vitiani L, Pallini R, Larocca LM, Lombardi DG, Signore M, Pierconti F, Petrucci G, Montano N, Maira G, De Maria R. Mesenchymal differentiation of glioblastoma stem cells. Cell Death Differ. 2008;15:1491–1498. doi: 10.1038/cdd.2008.72. [DOI] [PubMed] [Google Scholar]
- 41.Lo Coco F, Diverio D, Avvisati G, Petti MC, Meloni G, Pogliani EM, Biondi A, Rossi G, Carlo-Stella C, Selleri C, Martino B, Specchia G, Mandelli F. Therapy of molecular relapse in acute promyelocytic leukemia. Blood. 1999;94:2225–2229. [PubMed] [Google Scholar]
- 42.Tate CM, Pallini R, Ricci-Vitiani L, Dowless M, Shiyanova T, D’Alessandris GQ, Morgante L, Giannetti S, Larocca LM, di Martino S, Rowlinson SW, De Maria R, Stancato L. A BMP7 variant inhibits the tumorigenic potential of glioblastoma stem-like cells. Cell Death Differ. 2012;19:1644–1654. doi: 10.1038/cdd.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoey T, Yen WC, Axelrod F, Basi J, Donigian L, Dylla S, Fitch-Bruhns M, Lazetic S, Park IK, Sato A, Satyal S, Wang X, Clarke MF, Lewicki J, Gurney A. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5:168–177. doi: 10.1016/j.stem.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 44.Michaud NR, Wang Y, McEachern KA, Jordan JJ, Mazzola AM, Hernandez A, Jalla S, Chesebrough JW, Hynes MJ, Belmonte MA, Wang L, Kang JS, Jovanovic J, Laing N, Jenkins DW, Hurt E, Liang M, Frantz C, Hollingsworth RE, Simeone DM, Blakey DC, Bedian V. Novel neutralizing hedgehog antibody MEDI-5304 exhibits antitumor activity by inhibiting paracrine hedgehog signaling. Mol Cancer Ther. 2014;13:386–398. doi: 10.1158/1535-7163.MCT-13-0420. [DOI] [PubMed] [Google Scholar]
- 45.Du YR, Chen Y, Gao Y, Niu XL, Li YJ, Deng WM. Effects and mechanisms of anti-CD44 monoclonal antibody A3D8 on proliferation and apoptosis of sphere-forming cells with stemness from human ovarian cancer. Int J Gynecol Cancer. 2013;23:1367–1375. doi: 10.1097/IGC.0b013e3182a1d023. [DOI] [PubMed] [Google Scholar]
- 46.Wang CH, Chiou SH, Chou CP, Chen YC, Huang YJ, Peng CA. Photothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with CD133 monoclonal antibody. Nanomedicine. 2011;7:69–79. doi: 10.1016/j.nano.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Di Tomaso T, Mazzoleni S, Wang E, Sovena G, Clavenna D, Franzin A, Mortini P, Ferrone S, Doglioni C, Marincola FM, Galli R, Parmiani G, Maccalli C. Immuno-biological characterization of cancer stem cells isolated from glioblastoma patients. Clin Cancer Res. 2010;16:800–813. doi: 10.1158/1078-0432.CCR-09-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maccalli C, Scaramuzza S, Parmiani G. TNK cells (NKG2D+ CD8+ or CD4+ T lymphocytes) in the control of human tumors. Cancer Immunol Immunother. 2009;58:801–808. doi: 10.1007/s00262-008-0635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 50.Wei J, Barr J, Kong L-Y, Wang Y, Wu A, Sharma AK, Gumin J, Henry V, Colman H, Sawaya R, Lang FF, Heimberger AB. Glioma-associated cancer-initiating cells induce immunosuppression. Clin Cancer Res. 2010;16:461–473. doi: 10.1158/1078-0432.CCR-09-1983. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Schatton T, Schütte U, Frank NY, Zhan Q, Hoerning A, Robles SC, Zhou J, Hodi FS, Spagnoli GC, Murphy GF, Frank MH. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010;70:697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schatton T, Frank MH. Antitumor immunity and cancer stem cells. Ann NY Acad Sci. 2009;1176:154–169. doi: 10.1111/j.1749-6632.2009.04568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roth P, Junker M, Tritschler I, Mittelbronn M, Dombrowski Y, Breit SN. GDF-15 contributes to proliferation and immune escape of malignant gliomas. Clin Cancer Res. 2010;16:3851–3859. doi: 10.1158/1078-0432.CCR-10-0705. [DOI] [PubMed] [Google Scholar]
- 54.Volonté A, Di Tomaso T, Spinelli M, Todaro M, Sanvito F, Albarello L, Bissolati M, Ghirardelli L, Orsenigo E, Ferrone S, Doglioni C, Stassi G, Dellabona P, Staudacher C, Parmiani G, Maccalli C. Cancer-initiating cells from colorectal cancer patients escape from T cell-mediated immunosurveillance in vitro through membrane-bound IL-4. J Immunol. 2014;192:523–532. doi: 10.4049/jimmunol.1301342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Francipane MG, Alea MP, Lombardo Y, Todaro M, Medema JP, Stassi G. Crucial role of interleukin-4 in the survival of colon cancer stem cells. Cancer Res. 2008;68:4022–4025. doi: 10.1158/0008-5472.CAN-07-6874. [DOI] [PubMed] [Google Scholar]
- 56.Hallett MA, Venmar T, Fingleton B. Cytokine stimulation of epithelial cancer cells: the similar and divergent functions of IL-4 and IL-13. Cancer Res. 2012;72:6338–6343. doi: 10.1158/0008-5472.CAN-12-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tallerico R, Todaro M, Di Franco S, Maccalli C, Garofalo C, Sottile R, Palmieri C, Tirinato L, Pangigadde PN, La Rocca R, Mandelboim O, Stassi G, Di Fabrizio E, Parmiani G, Moretta A, Dieli F, Kärre K, Carbone E. Human NK cells selective targeting of colon cancer-initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J Immunol. 2013;190:2381–2390. doi: 10.4049/jimmunol.1201542. [DOI] [PubMed] [Google Scholar]
- 58.Wolpert F, Roth P, Lamszus K, Tabatabai G, Weller M, Eisele G. HLA-E contributes to an immune-inhibitory phenotype of glioblastoma stem-like cells. J Neuroimmunol. 2012;250:27–34. doi: 10.1016/j.jneuroim.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 59.Jewett A, Tseng HC, Arasteh A, Saadat S, Christensen RE, Cacalano NA. Natural killer cells preferentially target cancer stem cells; role of monocytes in protection against NK cell mediated lysis of cancer stem cells. Curr Drug Deliv. 2012;9:5–16. doi: 10.2174/156720112798375989. [DOI] [PubMed] [Google Scholar]
- 60.Koh J, Lee SB, Park H, Lee HJ, Cho NH, Kim J. Susceptibility of CD24(+) ovarian cancer cells to anti-cancer drugs and natural killer cells. Biochem Biophys Res Commun. 2012;427:373–378. doi: 10.1016/j.bbrc.2012.09.067. [DOI] [PubMed] [Google Scholar]
- 61.Kim S-Y, Cho H-S, Yang S-H, Shin J-Y, Kim J-S, Lee S-T, et al. Soluble mediators from human neural stem cells play a critical role in suppression of T-cell activation and proliferation. J Neurosci Res. 2009;87:2264–2272. doi: 10.1002/jnr.22050. [DOI] [PubMed] [Google Scholar]
- 62.Ljujic B, Milovanovic M, Volarevic V, Murray B, Bugarski D, Przyborski S, Arsenijevic N, Lukic ML, Stojkovic M. Human mesenchymal stem cells creating an immunosuppressive environment and promote breast cancer in mice. Sci Rep. 2013;3:2298–2306. doi: 10.1038/srep02298. [DOI] [PMC free article] [PubMed] [Google Scholar]