Abstract
Purpose
Adult patients with relapsed high-grade glioma are a very heterogenous group with, however, an invariably dismal prognosis. We stratified patients with relapsed high-grade glioma treated with re-operation and postoperative dendritic cell (DC) vaccination according to a simple recursive partitioning analysis (RPA) model to predict outcome.
Patients and methods
Based on age, pathology, Karnofsky performance score, and mental status, 117 adult patients with relapsed malignant glioma, undergoing re-operation, and postoperative adjuvant dendritic cell (DC) vaccination were stratified into 4 classes. Kaplan–Meier survival estimates were generated for each class of this HGG-IMMUNO RPA model. Extent of resection was documented but not included in the prognostic model.
Results
Kaplan–Meier overall survival estimates revealed significant (p < 0.0001) differences among the 4 HGG-IMMUNO RPA classes. Long-term survivors, surviving more than 24 months after the re-operation and vaccination, are seen in 54.5, 26.7, 11.5, and 0 % for the classes I, II, III, and IV respectively.
Conclusion
This HGG-IMMUNO RPA classification is able to predict overall survival in a large group of adult patients with a relapsed malignant glioma, treated with re-operation and postoperative adjuvant DC vaccination in the HGG-IMMUNO-2003 cohort comparison trial. The model appears useful for prognostic patient counseling for patients participating in DC vaccination trials. A substantial number of long-term survivors after relapse are seen in class I to III, but not in class IV patients.
Keywords: Malignant glioma, Cancer vaccine, Dendritic cell, RPA
Introduction
Recursive partitioning analysis (RPA) is known as a model to define prognostic classes based on treatment and pre-treatment prognostic variables [1, 2]. For newly diagnosed high-grade glioma (HGG), the radiation therapy and oncology group (RTOG) first described 6 prognostic classes in a recursive tree analysis in 1993 [3]. Thus far, the RTOG RPA model has been validated in groups of patients with newly diagnosed malignant gliomas treated in several ways. Validation has been performed for external beam radiation therapy [4], brachytherapy [5], chemotherapy [6], and radiosurgery [7]. More recently, the European Organisation for research and treatment of cancer (EORTC) adapted this RPA model to the current state of the art treatment strategy of radiotherapy and concomitant chemotherapy with temozolomide in patients with newly diagnosed glioblastoma (GBM), considering the importance of the dose of radiation, the concomitant, and the adjuvant temozolomide therapy [8]. However, a comparable validation in adult patients with relapsed high-grade glioma (HGG) treated in a uniform way, other than with chemotherapy, has not been done. Carson et al. [9] published a RPA model, built upon data of 10 large phase I and II chemotherapy trials in patients with relapsed GBM. As such, within this heterogeneous group of patients, the diverse treatment regimens pooled in this model of Carson et al. [9] potentially further confound the outcome data. Moreover, it is impossible to use the Carson classification in patients treated with dendritic cell (DC) vaccination, as administration of steroids to the patient (in up to almost 2/3 of patients in the pooled trials building the Carson classes) is an important third-line question in the recursive tree. For postoperative adjuvant DC-based vaccination, however, steroids are to be avoided because of their immunosuppressive properties.
Nevertheless, there is a high need for patient stratification according to validated prognostic parameters given the high number of phase I/II trials of experimental and purely innovative treatment strategies, of which the real or possible clinical value is often hard to estimate due to the usually very heterogeneous nature of such patients’ population. Especially, the importance of pre-treatment patient-related variables should be stressed in these models, given the wide-spread belief that in high-grade gliomas, pre-treatment prognostic factors have more impact on outcome than any (new) potentially active therapy or treatment strategy [10].
We advocate the use of a prognostic model based on simple, pre-treatment patient, and tumor-related parameters rather than surrogate immunological endpoints to estimate the clinical value and potentials of DC-based immunotherapy for many reasons. Indeed, since recently, a growing consensus is rising on the use of overall survival as the far most important and discriminatory endpoint in the design of clinical trials for cancer vaccines, as expressed in the consensus review report of the Association for Cancer Immunotherapy (CIMT) by Bilusic et al. [11]. We [12–15] and others [16–21] explored several immunological assays like delayed-type hypersensitivity tests, ELISPOT, MHC-tetramer analyses to detect tumor-specific T-cell clones, or different cytotoxicity assays, all trying to demonstrate tumor-specific antitumor T-cell cytotoxicity in glioma vaccination trials: apart from one group [22], all failed to show any correlation with clinical outcome. All these single immunological parameters can provide proof of the principle but are unlikely to accurately reflect a clinically relevant, complex in vivo immune response. Moreover, the lack of standardization in antitumor immune monitoring to date further confounds the field, preventing a comparison of different immune therapy approaches. On the other hand, it is increasingly being recognized that conventional response criteria may not adequately assess the full potential of immunotherapeutic strategies in general and DC vaccination in particular [23]. Therefore, immune-related response criteria have already been defined in melanoma patients undergoing immunotherapy [24]. However, even when immune response status trended toward significance in the E1696 melanoma trial, a Cox regression showed the clinical stage at time of diagnosis to be the strongest predictor of overall survival [25]. Therefore, we believe that to date, a RPA model based on simple, well-established, and generally accepted prognostic pre-treatment clinical patient parameters is much more likely to move the field forward than today’s unstandardized immune monitoring assays that of course are most valid to reveal modes of immunological action. These simple models indeed can easily be used to compare trials and to help decide which innovative strategies are worth to be tested in large-scale, expensive randomized controlled trials.
We built a HGG-IMMUNO RPA model based on age, reference pathology grade according to WHO criteria, KPS, and mental status in 117 consecutive adult patients included in the HGG-IMMUNO-2003 cohort comparison trial [13]. The inherent heterogeneity in this population is further being explicited in the variable number of relapses that patients had before vaccination treatment varying from 1 to 4. The main objective is to define and compare survival categories within this heterogeneous patient population by obtaining simple, homogenous subsets of patients with comparable outcome.
Patients and methods
One hundred and seventeen adult patients (>18 years) with relapsed HGG included in the HGG-IMMUNO-2003 trial to undergo re-operation at time of recurrence, followed by vaccination with autologous DC loaded with autologous tumor lysate, were analyzed in an intent to treat analysis. In this cohort comparison trial, which has been approved by the local ethics committee, patients have been included in consecutive cohorts A, B, C, or D, all treated according to the general concept of postoperative adjuvant autologous DC vaccination according to the previously reported strategy [13, 14, 26]. Quality control of the cell product (monocyte-derived early mature dendritic cells loaded with autologous whole tumor cell lysate) being injected intradermally included viability (trypan blue exclusion), purity based on cell morphology (DC should display cytoplasmic veils) and flowcytometry (DC should express MHC class II and CD86 and should not express CD14), and sterility (bacterial and fungal cultures and mycoplasma testing in a validated clinical microbiology laboratory). Patients could only be included in the trial after filling out a written informed consent.
All these patients have previously been treated with surgery, external beam radiotherapy, and (sometimes multiple) chemotherapy regimens. As such, the main criterium for possible inclusion was the possibility of at least a partially operable lesion, allowing a stable weaning and stop of steroids and the harvesting of enough viable tumor tissue for vaccine production.
The extent of resection could be partial, subtotal, or total but is not included in the model. Extent of resection was assessed by the neurosurgical report and an early postoperative MRI with and without gadolinium within 72 h after the resection. Gross total resection was defined as the absence of any nodular postoperative contrast enhancement.
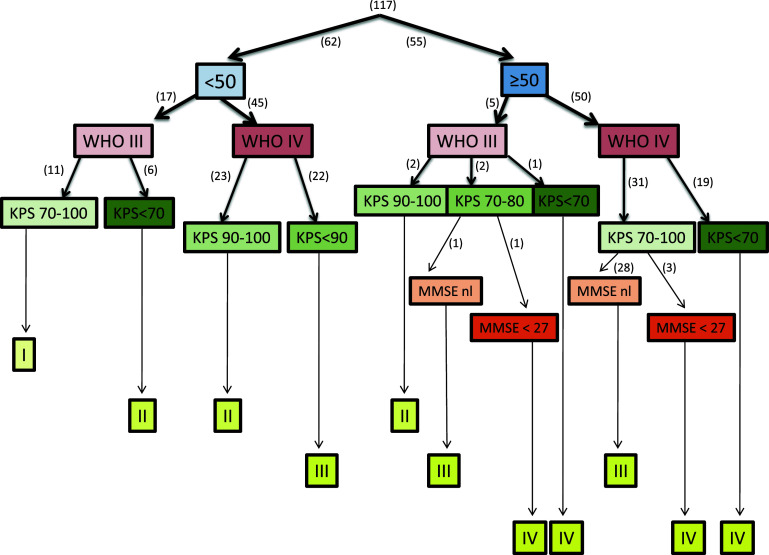
To build the model, we only used well-established and generally accepted pre-treatment variables; age with a cut-off at 50 years and reference pathology being classified according to the WHO classification [27]. Performance status was scored using Karnofsky Performance Score [28], ranging from 0 to 100 with cut-offs at 90 and 70 depending on the RPA class. Mental Status was assessed using the mini mental state examination (MMSE) [29], with a cut-off value of 27. These predefined variables, that is, age, performance status, reference pathology, mental status, and extent of resection, were tested in an univariate, log-rank survival analysis as possible prognostic parameters and hazard ratios (HR) with 95 % confidence intervals (CI) were calculated. Considering the resulting sample size of partitioning (by dichotomization or categorization) by the respective prognostic parameters and the magnitude of the respective statistically significant hazard ratios in univariate analysis, a recursive tree was built in a stepwise matter. At each partitioning step, univariate survival analysis according to the remaining parameters was repeated. As the investigated treatment consists of surgical resection followed by postoperative, autologous DC vaccination, extent of resection was not considered as a “pre-treatment” variable and as such, it was not considered to contribute to the partitioning tree. Given the limited sample size, the number of prognostic parameters to build the RPA tree was restricted to maximally four. The recursive tree built by these parameters and resulting in 4 prognostic classes is depicted in Fig. 1. All analyses were performed using SAS software, version 9.2 of the SAS System for Windows (Copyright © 2002 SAS Institute Inc.) SAS, and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA. Statistical survival analysis was done by log-rank test on Kaplan–Meier survival estimates.
Fig. 1.
HGG-IMMUNO recursive partitioning analysis (RPA) tree in relapsed high-grade glioma (rHGG) patients. <50 = age less than 50 years; ≥50 = age 50 years or older; WHO III = grade III pathology according to the World Health Organization criteria; WHO IV = grade IV pathology according to the World Health Organization criteria; KPS karnofsky performance score, MMSE mini mental state examination, nl normal, that is, MMSE ≥ 27; I = HGG-IMMUNO RPA class I; II = HGG-IMMUNO RPA class II; III = HGG-IMMUNO RPA class III; IV = HGG-IMMUNO RPA class IV. Numbers between () refer to the number of patients allocated to the respective arm
Results
Distribution of variables
In Table 1, the relative distribution of the prognostic variables is depicted for the 117 patients included in the 4 classes. Remarkably, 70.1 % of patients belong to class II and III, reflecting the classical majority of patients in this setting of (multi-)relapsed HGG. 20.5 % of patients belong to class IV, reflecting the patients above 50 with a poor mental status, a poor performance status or both. For obvious reasons, patients in class IV are only rarely eligible for inclusion in trials. The vast majority of patients in class III and IV have a glioblastoma (WHO grade IV). Glioblastoma patients are, as by definition, absent in class I: this class only harbors younger patients with recurrent WHO grade III lesions. From classes I to IV, median age of patients seems to increase. Median KPS, expressed as the preoperative performance state before vaccination, declines from class II to IV. Patients with an abnormal mental status, defined as a mini mental state examination score of less than 27, are only encountered in class IV. Although not mandatory for this RPA classification, extent of resection was routinely assessed using an early postoperative MRI. The percentage of patients with a gross total resection before vaccination seems to be fairly equal over the different classes, with a gradual and slight decrease over class II to class IV, and class IV being equal to class I. Considering the presumed beneficial effect of a gross total resection before vaccination [13, 14, 30], one might assume that the actual percentages of gross total resections in the different classes of this series further strengthen the impact of this RPA classification on overall survival at least for the differences between class II, III, and IV. The total numbers of patients belonging to cohort A, B, C, and D were 15, 19, 32, and 51 patients respectively, all proportionally distributed over the final 4 prognostic RPA classes. A non-significant trend toward better OS was noted in each cohort as compared to the previous cohort, but cohort membership did not contribute to the RPA model.
Table 1.
Distribution of variable predictors in the RPA classes
| RPA class | Number | Median age (range) | Pathology (% GBM) | Median karnofsky (range) | Mental status (% abnormal) | Surgery (% total) |
|---|---|---|---|---|---|---|
| I | 11 | 33.5 (19.4–49.9) | 0 | 90 (70–100) | 0 | 36.4 |
| II | 31 | 39.4 (18.6–55.4) | 74.2 | 90 (40–100) | 0 | 58 |
| III | 51 | 50.1 (19.9–68) | 98 | 70 (40–100) | 0 | 43.1 |
| IV | 24 | 61.3 (50–77.8) | 92 | 60 (40–80) | 25 | 37.5 |
RPA recursive partitioning analysis; median age in years; % GBM = percentage of patients in the corresponding class harboring WHO grade IV tumors at reference pathology; Karnofsky = Karnofsky performance score; % abnormal = the percentage of patients in the corresponding class having a mini mental state examination of less than 27; % total = the percentage of patients in the corresponding class having a gross total resection before start of vaccination therapy
Prognostic parameters
Age, dichotomized at less than 50 years versus 50 years or older, resulted in a HR of 0.4894 (95 % CI; 0.3420–0.8248 and p = 0.0048). Reference pathology at time of re-operation, dichotomized and recoded as non-GBM HGG (WHO grade III) versus GBM (WHO grade IV) resulted in a HR of 0.5923 (95 % CI; 0.3609–0.8460 and p = 0.0063). Extent of resection dichotomized and recoded as total versus non-total resulted in a HR of 0.5512 (95 % CI; 0.3587–0.8022 and p = 0.0024). Mental status dichotomized as normal (MMSE ≥27) versus abnormal (MMSE <27) resulted in a HR of 0.2546 (95 % CI; 0.0297–0.1638 and p < 0.0001). Only 20 patients, however, were found to have an abnormal mental status. Finally, performance status, categorized as KPS 90/100 versus 70/80 versus 60 or less, resulted in statistically significantly different (p < 0.0001) median OS of 14.1, 9.9 and 6 months respectively with HR’s of 0.5793 (95 % CI; 0.3218–0.9217 and p = 0.024) and 0.2761 (95 % CI; 0.0802–0.2710 and p < 0.0001) respectively.
Internal validation
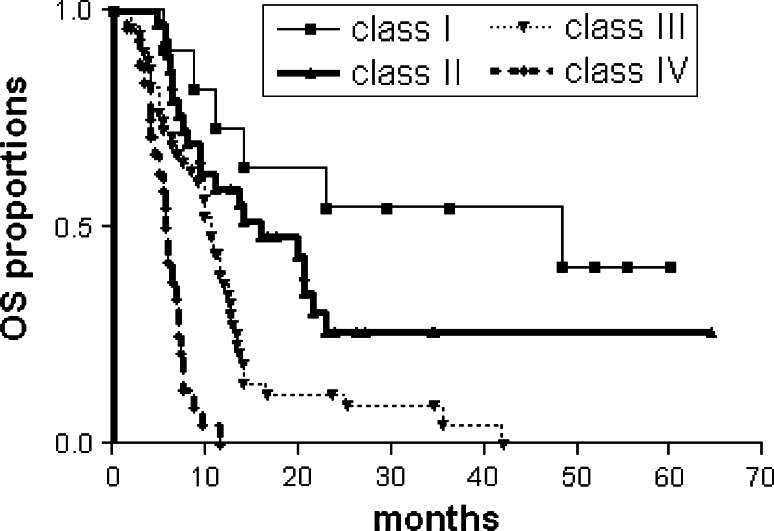
Log-rank analysis of the Kaplan–Meier survival estimates for overall survival (OS) is depicted in Fig. 2 for each HGG-IMMUNO RPA class. The global difference in OS between the classes, reflecting different survival categories, is highly statistically significant (p < 0.0001). In Table 2, median OS with 95 % CI, ranges of OS, 2-year survival rates with 95 % CI calculated from the moment of the re-operation and the numbers of patients still alive at latest follow-up (FU) are depicted. The decreasing median OS from class I to IV, but especially the differences in long-term survivors (>24 months after pre-vaccine re-operation), reflects clinically relevant prognostic differences: in class I, median OS is 48.4 months with 54.5 % of patients surviving 24 months and much longer. For classes II and III, these figures are 16 months with 26.7 % and 10.6 months with 11.5 % long-term survivors respectively. With a median FU of 25.5 months (range, 5.2–64.6 months), 24 of the 117 patients with relapsed HGG are still alive after re-operation and vaccination for recurrent HGG. No long-term survivors, however, are found in class IV.
Fig. 2.
Kaplan Meier Overall survival estimates according to the 4 HGG-IMMUNO RPA classes (class I–IV) in 117 adult patients with a (multi-)relapsed malignant glioma, treated with re-operation and adjuvant postoperative dendritic cell vaccination. Survival data are calculated from the time of the pre-vaccine re-operation. Log-rank analysis, p < 0.0001
Table 2.
Survival data according to RPA classes
| RPA class | Number | Median OS in months (95%CI) | Range of OS (months) | Percentage 2-year survival (95%CI) | Number still alive (%) |
|---|---|---|---|---|---|
| I | 11 | 48.4 (23.1-ND) | 8.8 till >60 | 54.5 (22.9–78) | 5 (45.5 %) |
| II | 31 | 16 (8.1–21.5) | 4.7 till >64.6 | 26.7 (11.1–43.9) | 13 (42 %) |
| III | 51 | 10.6 (7.6–12.5) | 2.9 till 42 | 11.5 (4.3–22.7) | 6 (11.7 %) |
| IV | 24 | 6 (4.2–7.0) | 1.5 till 11.6 | 0 (0–17.6) | 0 |
OS overall survival, CI confidence interval; percentage 2-year survival = percentage of patients alive 24 months or more after the pre-vaccine re-operation; number still alive = the number and percentage () of patients still alive at latest follow-up: patients are being controlled each 3 months clinically
Although all survival data were based on an intent to treat analysis, the proportion of patients from the different RPA classes who were treated per protocol (i.e. who received at least three DC vaccines postoperatively) was different: in class I and II, 100 % of patients were treated per protocol, as compared to only 46 out of 51 (90.2 %) and 18 out of 24 (75 %) patients from class III and IV respectively.
Discussion
We built a HGG-IMMUNO RPA classification for patients with malignant glioma and applied it to a large group of adult patients with relapsed HGG who were re-operated upon and vaccinated postoperatively as previously described [13, 14, 30]. In this inherently heterogeneous group of patients with relapsed malignant glioma, we defined 4 simple, clinically relevant prognostic classes to construct this model. The prognostic variables considered and analyzed in this model are all commonly accepted, well-established prognostic pre-treatment patient variables, easy to assess, and deemed relevant for this particular group of patients with (multi-)relapsed high-grade glioma: age (cut-off 50 years), WHO grading of malignancy (III or IV), Karnofsky Performance score (cut-off at 90 and 70), and mental status (MMSE score cut-off of 27), all measured at re-operation before inclusion. Other pre-treatment variables like the number of prior episodes of progressive disease (ranging from 0 to 3) or the number of prior therapies (ranging from 3 to 6) did not have a discriminatory impact on the model. The extent of resection, ranging from partial to gross total, has shown to be a prognostic variable in our previous publications [13, 14] and has been registered and found to be a predictor of survival in an univariate survival analysis but not included in the model because of three reasons: it did not add to the discriminatory power of the classes, it further divided the group into a larger number of classes with smaller numbers of patients per class and most importantly, it is a parameter that is not available at the moment of pre-treatment counseling of the patient, who is possibly eligible for the HGG-IMMUNO trial. Pre-treatment counseling of patients is indeed a major reason to use this RPA classification in the future.
Theoretically, parameters of the patients’ immune status, preferentially measured at the glioma microenvironment itself, could be considered as a relevant prognostic parameter for vaccination therapies [31]. However, several practical and theoretical considerations withheld us from its use in this model. First, each additional possible predictor that is being considered to contribute to the model will inevitably reduce the number of patients in the final prognostic classes. Secondly, we often lack large tumor samples for pathological and molecular analysis as we do need a critical volume of at least 3 cm3 to prepare the whole tumor cell lysate, used to prepare the vaccine. The remaining tumor volumes are used for reference pathological examination: determining tumor-infiltrating immune cells on these small samples would definitely be subject to a large variability within one tumor specimen due to sampling variations leading to non-representative results. Finally, we pointed out that there is no correlation at all between subtypes of immune cells in the blood as compared to the same subtypes in the glioma micro-environment (unpublished results): as such, we were reluctant to introduce results of the patient’s immune status in the blood as a relevant discriminatory parameter in this clinical model.
This HGG-IMMUNO RPA classification resulted in 4 classes (I to IV) with a significant difference in OS in log-rank analysis of Kaplan–Meier survival estimates. Apart from the differences in median OS and ranges of OS, the differences in percentage long-term survivors, defined as adult patients with relapsed malignant glioma surviving 24 months or more after the pre-vaccine re-operation, were strikingly distinct and especially encouraging for patients in classes I to III; only in class IV patients, being patients of 50 years or older with a poor global or mental performance status at time of inclusion, no long-term survivors have been found. For obvious reasons, however, many of these class IV patients are not eligible for inclusion in most classical trials of relapsed HGG patients. The long-term survivors—even up to more than 60 months after relapse—in class I to III are a remarkable and most relevant clinical finding, comparing favorably to almost all other trials in this population of (multi-) relapsed patients with HGG. Unfortunately, no real comparisons can be made as the classification in comparable prognostic subgroups is missing in all studies in larger series of relapsed malignant glioma patients. Compared to studies using the Carson classification [9, 32], the overall long-term survival data appear promising. Re-operation at time of recurrence, alone or in combination with several chemotherapy regimens, has been extensively studied in the past, but invariably showed to be disappointing [33]: the additional survival benefit was barely half the interval between the first diagnosis and the relapse. Temozolomide in recurrent glioblastoma did not result in any long-term survivors [34], although in anaplastic astrocytoma, it seemed to result in some [35]. Recently, bevacizumab (with chemotherapy) in recurrent malignant glioma in a large single-institution trial showed comparable results to TMZ [36]. The combination of bevacizumab and irinotecan, however, showed promising numbers of responders with maybe some long-term survivors in anaplastic oligodendroglioma [37]. No information, however, was given on prognostic classes in this trial. It should be stated that in this series, no one received bevacizumab at any given time.
To our believe, this distinction in prognostically homogenous subgroups creates the opportunity to better assess the possible value of innovative strategies as such and in comparison with other trials. This type of refined stratification to obtain a more homogenous study population might result in the identification of clinically meaningful modifications in therapy that might map the road for further innovation within a certain field of therapeutic research. The efforts and financial investments for randomized controlled trials (RCT’s) could in this way be directed toward comparisons of therapeutic regimens that have already shown some benefit after stratified analysis according to validated RPA classifications.
It has to be stressed, however, that the HGG-IMMUNO trial is only applicable to a subset of patients with recurrent HGG who can have second surgery. This subgroup is still a minority, even if the notion of operability is changing over time. In a national, French GBM management report [38], Bauchet et al. [39] included 952 consecutive patients with newly diagnosed GBM of whom only 9.6 % underwent reoperation at time of progression. At the other end of the spectrum, Filippini et al., in national Italian study, reported on 676 patients with newly diagnosed GBM and mentioned a reoperation in 26 % of patients. Interestingly, a multivariate analysis in this series showed no survival benefit for reoperation in these patients, regardless of the timing of the reoperation before or later than 9 months after the first diagnosis. Older studies, like those from Harsh et al. [40] and Sipos et al. [41], already showed that reoperation could be beneficial for patients with recurrent anaplastic astrocytoma, but not for those with recurrent GBM.
Finally, we deliberately built this classification based on patients’ data from the HGG-IMMUNO trials to increase the uniformity of the treatment applied. Several other related and unrelated types of cancer vaccines are under investigation for glioma patients, but given the large heterogeneity in the different approaches in terms of technology, patient selection and eligibility, and the relatively small numbers of patients in most trials, a meta-analysis approach after pooling of these data (if available) would largely confound the results. Therefore, rather than being used for overall counseling of patients for inclusion in any type of immunotherapy trials, this classification could be used to compare outcomes in comparable classes of different trials for innovative treatments.
Conclusions
This HGG-IMMUNO RPA classification could be validated in a large group of adult patients with relapsed malignant glioma, treated with re-operation and postoperative adjuvant DC vaccination in the HGG-IMMUNO-2003 cohort comparison trial. This model is an important tool for stratification of patients with relapsed malignant glioma, useful for patient counseling and, in our opinion, mandatory for comparing outcomes in more homogenous subgroups of patients with relapsed malignant glioma undergoing different, especially non-chemotherapy-based treatment strategies as a useful indicator of their possible clinical value. This RPA classification could be used to move the field forward, as there is no standard treatment for patients with relapsed high-grade glioma at the moment. Based upon the important numbers of long-term survivors, adjuvant postoperative DC-based vaccination can be considered in re-operable patients with relapsed HGG.
Acknowledgments
We thank Katja Vandenbrande, Goedele Stegen, Vallentina Schaiko, Elke Nackers, and Anaïs Van Hoylandt for their excellent technical assistance. We thank the department of hematology for the care provided at time of the leukapheresis. We thank the clinic for radiotherapy for irradiating the tumor lysate. We thank Steffen Fieuws for statistical support. This project is supported by the Olivia Hendrickx Research Fund (www.olivia.be), the Herman Memorial Research Fund (www.hmrf.be), the James E. Kearney Foundation (www.jekfoundation.org), the TBM program of the IWT – Flanders (www.iwt.be), the Belgian Foundation against Cancer (www.cancer.be), and private initiatives. Human Serum Albumin has been provided by the Belgian Red Cross and Baxter. Steven De Vleeschouwer was supported by the “Klinisch Onderzoeksfonds” from the University Hospitals Leuven, and Stefaan Van Gool is Senior Clinical Investigator of the Fund for Scientific Research—Flanders (Belgium) (F.W.O.—Vlaanderen).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Friedman JH. A recursive partitioning decision rule for nonparametric classification. IEEE Trans Comput. 1977;C26:404–408. doi: 10.1109/TC.1977.1674849. [DOI] [Google Scholar]
- 2.Ruberg SJ, Chen L, Wang Y. The mean does not mean as much anymore: finding sub-groups for tailored therapeutics. Clin Trials. 2010;7:574–583. doi: 10.1177/1740774510369350. [DOI] [PubMed] [Google Scholar]
- 3.Curran WJ, Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO, Krisch RE. Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 4.Scott CB, Scarantino C, Urtasun R, Movsas B, Jones CU, Simpson JR, Fischbach AJ, Curran WJ., Jr Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients: a report using RTOG 90-06. Int J Radiat Oncol Biol Phys. 1998;40:51–55. doi: 10.1016/S0360-3016(97)00485-9. [DOI] [PubMed] [Google Scholar]
- 5.Videtic GM, Gaspar LE, Zamorano L, Fontanesi J, Levin KJ, Kupsky WJ, Tekyi-Mensah S. Use of the RTOG recursive partitioning analysis to validate the benefit of iodine-125 implants in the primary treatment of malignant gliomas. Int J Radiat Oncol Biol Phys. 1999;45:687–692. doi: 10.1016/S0360-3016(99)00244-8. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R, Dietrich PY, Ostermann Kraljevic S, Pica A, Maillard I, Maeder P, Meuli R, Janzer R, Pizzolato G, Miralbell R, Porchet F, Regli L, de Tribolet N, Mirimanoff RO, Leyvraz S. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002;20:1375–1382. doi: 10.1200/JCO.20.5.1375. [DOI] [PubMed] [Google Scholar]
- 7.Sarkaria JN, Mehta MP, Loeffler JS, Buatti JM, Chappell RJ, Levin AB, Alexander E, III, Friedman WA, Kinsella TJ. Radiosurgery in the initial management of malignant gliomas: survival comparison with the RTOG recursive partitioning analysis. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1995;32:931–941. doi: 10.1016/0360-3016(94)00621-Q. [DOI] [PubMed] [Google Scholar]
- 8.Mirimanoff RO, Gorlia T, Mason W, van den Bent MJ, Kortmann RD, Fisher B, Reni M, Brandes AA, Curschmann J, Villa S, Cairncross G, Allgeier A, Lacombe D, Stupp R. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol. 2006;24:2563–2569. doi: 10.1200/JCO.2005.04.5963. [DOI] [PubMed] [Google Scholar]
- 9.Carson KA, Grossman SA, Fisher JD, Shaw EG. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol. 2007;25:2601–2606. doi: 10.1200/JCO.2006.08.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003;361:323–331. doi: 10.1016/S0140-6736(03)12328-8. [DOI] [PubMed] [Google Scholar]
- 11.Bilusic M, Gulley JL. Endpoints, patient selection, and biomarkers in the design of clinical trials for cancer vaccines. Cancer Immunol Immunother. 2012;61:109–117. doi: 10.1007/s00262-011-1141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Vleeschouwer S, Van Calenbergh F, Demaerel P, Flamen P, Rutkowski S, Kaempgen E, Wolff JEA, Plets C, Sciot R, Van Gool SW. Transient local response and persistent tumor control of recurrent malignant glioma treated with combination therapy including dendritic cell therapy. J Neurosurg (pediatrics) 2004;100:492–497. doi: 10.3171/ped.2004.100.5.0492. [DOI] [PubMed] [Google Scholar]
- 13.De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J, Sciot R, Wilms G, Demaerel P, Warmuth-Metz M, Soerensen N, Wolff JE, Wagner S, Kaempgen E, Van Gool SW. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14:3098–3104. doi: 10.1158/1078-0432.CCR-07-4875. [DOI] [PubMed] [Google Scholar]
- 14.Rutkowski S, De Vleeschouwer S, Kaempgen E, Wolff JEA, Kuhl J, Demaerel P, Warmuth-Metz M, Flamen P, Van Calenbergh F, Plets C, Sorensen N, Opitz A, Van Gool SW. Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br J Cancer. 2004;91:1656–1662. doi: 10.1038/sj.bjc.6602195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ardon H, Van Gool S, Lopes IS, Maes W, Sciot R, Wilms G, Demaerel P, Bijttebier P, Claes L, Goffin J, Van Calenbergh F, De Vleeschouwer S. Integration of autologous dendritic cell-based immunotherapy in the primary treatment for patients with newly diagnosed glioblastoma multiforme: a pilot study. J Neurooncol. 2010;99:261–272. doi: 10.1007/s11060-010-0131-y. [DOI] [PubMed] [Google Scholar]
- 16.Kikuchi T, Akasaki Y, Abe T, Fukuda T, Saotome H, Ryan JL, Kufe DW, Ohno T. Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. J Immunother. 2004;27:452–459. doi: 10.1097/00002371-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, Lin JW, Chute DJ, Mischel PS, Cloughesy TF, Roth MD. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 18.Okada H, Lieberman FS, Walter KA, Lunsford LD, Kondziolka DS, Bejjani GK, Hamilton RL, Torres-Trejo A, Kalinski P, Cai Q, Mabold JL, Edington HD, Butterfield LH, Whiteside TL, Potter DM, Schold SC, Jr, Pollack IF. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of patients with malignant gliomas. J Transl Med. 2007;5:67. doi: 10.1186/1479-5876-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Herndon JE, Lally-Goss D, McGehee-Norman S, Paolino A, Reardon DA, Friedman AH, Friedman HS, Bigner DD. An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther. 2009;8:2773–2779. doi: 10.1158/1535-7163.MCT-09-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker DG, Laherty R, Tomlinson FH, Chuah T, Schmidt C. Results of a phase I dendritic cell vaccine trial for malignant astrocytoma: potential interaction with adjuvant chemotherapy. J Clin Neurosci. 2008;15:114–121. doi: 10.1016/j.jocn.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 22.Wheeler CJ, Black KL, Liu G, Mazer M, Zhang XX, Pepkowitz S, Goldfinger D, Ng H, Irvin D, Yu JS. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 2008;68:5955–5964. doi: 10.1158/0008-5472.CAN-07-5973. [DOI] [PubMed] [Google Scholar]
- 23.Butterfield LH, Palucka AK, Britten CM, Dhodapkar MV, Hakansson L, Janetzki S, Kawakami Y, Kleen TO, Lee PP, Maccalli C, Maecker HT, Maino VC, Maio M, Malyguine A, Masucci G, Pawelec G, Potter DM, Rivoltini L, Salazar LG, Schendel DJ, Slingluff CL, Jr, Song W, Stroncek DF, Tahara H, Thurin M, Trinchieri G, Der Burg SH, Whiteside TL, Wigginton JM, Marincola F, Khleif S, Fox BA, Disis ML. Recommendations from the iSBTc-SITC/FDA/NCI Workshop on Immunotherapy Biomarkers. Clin Cancer Res. 2011;17:3064–3076. doi: 10.1158/1078-0432.CCR-10-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 25.Kirkwood JM, Lee S, Moschos SJ, Albertini MR, Michalak JC, Sander C, Whiteside T, Butterfield LH, Weiner L. Immunogenicity and antitumor effects of vaccination with peptide vaccine±granulocyte-monocyte colony-stimulating factor and/or IFN-alpha2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin Cancer Res. 2009;15:1443–1451. doi: 10.1158/1078-0432.CCR-08-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Gool SW, Maes W, Ardon H, Verschuere T, Van Cauter S, De Vleeschouwer S. Dendritic cell therapy of high grade gliomas. Brain Pathol. 2009;19:694–712. doi: 10.1111/j.1750-3639.2009.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- 28.DA Karnofsky BJ. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. evaluation of chemotherapeutic agents. New York: Columbia University Press; 1949. pp. 191–205. [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.De Vleeschouwer S, Rapp M, Sorg RV, Steiger HJ, Stummer W, Van Gool S, Sabel M. Dendritic cell vaccination in patients with malignant gliomas: current status and future directions. Neurosurgery. 2006;59:988–999. doi: 10.1227/01.NEU.0000245595.38957.3E. [DOI] [PubMed] [Google Scholar]
- 31.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, Nelson SF, Liau LM. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603–1615. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyatake S, Kawabata S, Yokoyama K, Kuroiwa T, Michiue H, Sakurai Y, Kumada H, Suzuki M, Maruhashi A, Kirihata M, Ono K. Survival benefit of Boron neutron capture therapy for recurrent malignant gliomas. J Neurooncol. 2009;91:199–206. doi: 10.1007/s11060-008-9699-x. [DOI] [PubMed] [Google Scholar]
- 33.Nieder C, Grosu AL, Molls M. A comparison of treatment results for recurrent malignant gliomas. Cancer Treat Rev. 2000;26:397–409. doi: 10.1053/ctrv.2000.0191. [DOI] [PubMed] [Google Scholar]
- 34.Brada M, Hoang-Xuan K, Rampling R, Dietrich PY, Dirix LY, Macdonald D, Heimans JJ, Zonnenberg BA, Bravo-Marques JM, Henriksson R, Stupp R, Yue N, Bruner J, Dugan M, Rao S, Zaknoen S. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol. 2001;12:259–266. doi: 10.1023/A:1008382516636. [DOI] [PubMed] [Google Scholar]
- 35.Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, Albright R, Olson J, Chang SM, O’Neill AM, Friedman AH, Bruner J, Yue N, Dugan M, Zaknoen S, Levin VA. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17:2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 36.Nghiemphu PL, Liu W, Lee Y, Than T, Graham C, Lai A, Green RM, Pope WB, Liau LM, Mischel PS, Nelson SF, Elashoff R, Cloughesy TF. Bevacizumab and chemotherapy for recurrent glioblastoma: a single-institution experience. Neurology. 2009;72:1217–1222. doi: 10.1212/01.wnl.0000345668.03039.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taillibert S, Vincent LA, Granger B, Marie Y, Carpentier C, Guillevin R, Bellanger A, Mokhtari K, Rousseau A, Psimaras D, Dehais C, Sierra dR, Meng Y, Laigle-Donadey F, Hoang-Xuan K, Sanson M, Delattre JY. Bevacizumab and irinotecan for recurrent oligodendroglial tumors. Neurology. 2009;72:1601–1606. doi: 10.1212/WNL.0b013e3181a413be. [DOI] [PubMed] [Google Scholar]
- 38.Bauchet L, Mathieu-Daude H, Fabbro-Peray P, Rigau V, Fabbro M, Chinot O, Pallusseau L, Carnin C, Laine K, Schlama A, Thiebaut A, Patru MC, Bauchet F, Lionnet M, Wager M, Faillot T, Taillandier L, Figarella-Branger D, Capelle L, Loiseau H, Frappaz D, Campello C, Kerr C, Duffau H, Reme-Saumon M, Tretarre B, Daures JP, Henin D, Labrousse F, Menei P, Honnorat J. Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro Oncol. 2010;12:725–735. doi: 10.1093/neuonc/noq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Filippini G, Falcone C, Boiardi A, Broggi G, Bruzzone MG, Caldiroli D, Farina R, Farinotti M, Fariselli L, Finocchiaro G, Giombini S, Pollo B, Savoiardo M, Solero CL, Valsecchi MG. Prognostic factors for survival in 676 consecutive patients with newly diagnosed primary glioblastoma. Neuro Oncol. 2008;10:79–87. doi: 10.1215/15228517-2007-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harsh GR, Levin VA, Gutin PH, Seager M, Silver P, Wilson CB. Reoperation for recurrent glioblastoma and anaplastic astrocytoma. Neurosurgery. 1987;21:615–621. doi: 10.1227/00006123-198711000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Sipos L, Afra D. Re-operations of supratentorial anaplastic astrocytomas. Acta Neurochir (Wien) 1997;139:99–104. doi: 10.1007/BF02747187. [DOI] [PubMed] [Google Scholar]