Abstract
Pancreatic ductal adenocarcinoma (PDAC) represents the fourth leading cause of cancer-related death in western countries. The patients are often diagnosed in advanced metastatic stages, and the prognosis remains extremely poor with an overall 5-year survival rate less than 5 %. Currently, novel therapeutic strategies are being pursued to combat PDAC, including oncolytic viruses, either in their natural forms or armed with immunostimulatory molecules. Natural killer cells are critical players against tumours and infected cells. Recently, we showed that IL-2-activated human NK cells displayed killing activity against PDAC cells, which could further be enhanced through the infection of PDAC cells with the rodent parvovirus H-1PV. In this study, the therapeutic efficacy of parvovirus-mediated delivery of three distinct cyto/chemokines (Il-2, MCP-3/CCL7 and IP-10/CXCL10) was evaluated in xenograft models of human PDAC. We show here that activated NK and monocytic cells were found to be recruited by PDAC tumours upon infection with parvoviruses armed with IL-2 or the chemokine MCP-3/CCL7, resulting in a strong anti-tumour response.
Keywords: IL-2, Chemokine, Parvovirus, Pancreatic carcinoma
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a devastating disease, affecting more than 43.000 people in the US every year. Due to the lack of effective therapeutic options, PDAC is associated with an extremely poor prognosis [1]. Therefore, new approaches for more effective treatment, for example, application of oncolytic viruses, are urgently needed. Only few virus-based approaches have already been therapeutically tested in advanced clinical trials, including the adenovirus ONYX-015 [2], a combination of recombinant vaccinia and fowlpox viruses [3] and a PDAC-retargeted retroviral vector [4, 5]. Further virotherapeutical approaches are evaluated in pre-clinical studies based on adeno-, herpes- and retro-viruses [6–8].
The autonomous rodent parvoviruses (PV) H-1PV and MVMp have been associated with oncotropic and oncolytic properties in vitro [[9], and references therein]. Indeed, PV have been shown to infect and—by virtue of their lytic life cycle—to kill a large number of human tumour cells, whereas cultures of their corresponding normal cells were not or scantily susceptible to virus-mediated cytotoxicity [9, 10]. Preferential parvoviral replication in neoplastic cells can be explained in part by their life cycle’s dependence on host cellular factors whose expression is restricted to proliferating cells [9]. The cytotoxicity elicited by H-1PV and MVMp is largely dependent on the viral non-structural protein NS1 whose expression is controlled by the early viral promoter P4 that is activated through a TATA-box, an SP1-binding GC-, E- and Y-boxes, cyclic AMP response elements and Smad-binding sites [11]. The tumour-suppressive activities of wild-type H-1PV have long been recognized [12] and recently confirmed in orthotopic tumour models of PDAC and malignant glioblastoma [13, 14].
Although quite efficient as anti-tumour agents in many animal tumour models [[9], and references therein], rodent PVs are, in their natural forms, not always potent enough to eradicate established tumours [15], in keeping with their frequent isolation from tumour specimens [12, 16]. Hence, in order to improve their anti-neoplastic properties, recombinant vectors based on H-1PV and MVMp have been designed [17] to deliver toxic [18] or immunostimulatory transgenes [19–22]. These immunomodulators, such as cytokines, are expected to be secreted from infected cells, to subsequently induce the infiltration and/or activation of specific leucocytes, and eventually to lead to tumour regression by exerting bystander effects on the neoplasm and its surrounding microenvironment.
The present study aims to evaluate the therapeutic efficacy of PV-mediated delivery of three distinct cyto/chemokines in a xenograft model of human PDAC.
(1) A first molecule of interest is the monocyte chemoattractant protein (MCP)-3/CCL7. MCP-3 is known to attract a variety of innate immune cells because of its ability to interact with at least three chemokine receptors (CCR1, −2 and −3), which are expressed by a large subset of leucocytes [23]. (2) Pro-angiogenic factors, like vascular endothelial growth factor and interleukin (IL)-8/CXCL8, are known to be over-expressed in PDAC [24–26]. Hence, the angiostatic CXC chemokine interferon-γ inducible protein (IP)-10/CXCL10 was also examined in this model, since it has been reported to suppress endothelial cell differentiation, migration and proliferation [27]. Furthermore, through its binding to CXCR3 [28], IP-10 is able to chemoattract activated NK and cytotoxic T cells, monocytes and a subset of dendritic cells [29, 30] and has been shown to be endowed with anti-tumoral efficacy in various mouse tumour models [22, 31]. Moreover, it is well established that cancer prognosis is correlated with the innate immune response mounted by natural killer (NK) cells [32]. (3) Recently, we showed that, upon stimulation with recombinant IL-2, NK cells displayed killing activity against PDAC cells in vitro, which could further be enhanced through the infection of PDAC-target cells with oncolytic H-1PV [33]. Therefore, the therapeutic efficacy of a PV-mediated delivery of this cytokine was also tested.
We show here that the intrinsic oncosuppressive capacity of H-1PV could strongly be enhanced by arming this parvovirus with IL-2 or MCP-3. Activated NK and monocytic cells were found to be attracted by PDAC tumours of human origin upon infection with recombinant H-1PVs armed with these cytokines, resulting in a strong anti-tumour response and a significant increase in the survival of the animals.
Materials and methods
Cell culture
Human PDAC cell lines Panc-1 and MiaPaCa-2 (kindly provided by A. Vecchi), human embryonic 293T and newborn human NB-324K kidney cells were cultured as described elsewhere [11]. Human NK cells were isolated by negative depletion from PBMCs, resulting in a cell population that consisted of 90–99 % of CD3− CD56+ cells, or obtained by co-culturing PBMCs and irradiated RPMI 8866 cells [33].
Plasmids
The existing parvovirus infectious molecular clone (pH1) [17] was modified as following: After linearization and mung bean nuclease-mediated removal of the SalI restriction site, the pH1 vector was ligated with the annealed oligonucleotides, 5′P-atccaaactccctgaaccgcttatcatttttagaa-3′ and 5′P-ttctaaaaatgataagcggttcagggagtttggat-3′, yielding pdBH1. The plasmid pChi-H1/∆800 was generated by inserting the 2.5kbp EcoRI/BglI DNA fragment of pdBH1 into pChi-hH1/∆800 [34]. Accordingly, the recombinant vectors pChi-H1/MCP-3 and pChi-H1/IP-10 were modified from their respective Chi-hH1/-based parental vectors. The IL-2 and Gaussia luciferase (GLuc) expressing vectors were produced by inserting the MluI/HpaI DNA fragment (699 bp) of phH1/IL-2 [20] or the SacI/NotI DNA fragment (574 bp) of the pGLuc-Basic vector (New England Biolabs) into pChi-H1/∆800, respectively.
Virus infection and production
Virus infections were performed at 37 °C for 1 h with a virus inoculum of purified (recombinant) H-1PV and occasional rocking of the plate. Stocks of recombinant H-1PV were produced by transfection of 293T cells and wild-type H-1PV by infection of NB-324K cells, purified by iodixanol gradient centrifugation, titered by infected cell hybridization assay on NB-324K indicator cells [20] and expressed as replication units (RU) per millilitre of virus suspension.
Quantification of transgene secretion
Levels of secreted cytokines were measured in the cell culture supernatants of recombinant H-1PV-infected PDAC cells at various time-points post-infection (p.i.) using specific ELISAs for MCP-1/CCL2 (R&D Systems), MCP-3 [21], IP-10 [19] or IL-2 (Biosource). The activity of the GLuc was assessed in vivo after intraperitoneally injection of 100 μg of coelenterazine in 150 μL of a water/CH3OH formulation. Following injection, mice were anesthetized by isoflurane gas (1L/min), luciferase activity was measured after 7 min for 5 min using a CCD camera (IVIS 100, Caliper Life Sciences, Mainz, Germany) and photon emission quantified using the Living Image software package (Caliper Life Sciences).
NK assays
The chemotactic potential of unstimulated, freshly isolated NK cells was analysed using 96-well chemotaxis plates (Europrobe). Briefly, 50 μL of the conditioned media, harvested from transduced Panc-1 cells, were placed in the lower chamber with a 3-μm-pore-sized filter on top. 5 × 103 NK cells, suspended in 80 μL of RPMI, containing 10 % of FCS, were applied onto the porous membrane, incubated for 1–3 h at 37 °C, and transmigrated NK cells were collected and microscopically counted (four fields per well), as described elsewhere [35]. The assays were performed in triplicate. To determine NK cell-mediated cytotoxicity, 51Cr release assays were performed as described previously [33] using 5 × 105 target 51Cr-labelled K562 and effector NK cells that have been stimulated for 5 days with the conditioned media of transduced Panc-1 cells or 100 IU/mL of recombinant IL-2.
PDAC tumour model
Female nude Balb/c mice (Charles River), 5–7 weeks old, were maintained in a flexible film isolator under pathogen-free conditions within the central animal facility. PDAC tumour models were established in groups of 3–10 animals by subcutaneous (s.c.) injection at the right posterior flank of buffer-treated or in vitro (recombinant) H-1PV-infected 5 × 106 Panc-1 or 2.5 × 106 MiaPaCa-2 cells, suspended in 200 or 100 μL of calcium-, magnesium- and glucose-containing Dulbecco’s PBS (Invitrogen), respectively. Tumour growth was monitored every 3–4 days using an electronic digital calliper (Farnell), and mice were killed when the tumour volume exceeded 1,500 mm3, calculated using the following formula: volume = ½ x length × width2. The animal experimental procedures were approved by the responsible Animal Protection Officer at the German Cancer Research Centre and by the Regional Council according to the German Protection Law.
RT-PCR analysis of PDAC tumours
The recruitment of (activated) leucocytes was determined in PDAC tumours 5–11 days post-implantation (p.imp.). Briefly, mice were killed by cervical dislocation, and tumours were transferred into 2-mL ceramic beads-filled tubes (Precellys) and treated with RNAlater (Qiagen). Three micrograms of total RNA, isolated using the RNeasy Kit (Qiagen), was reverse-transcribed using 200U of the RNA-dependent DNA polymerase M-MLV RT (Promega) in the presence of 500 μM of dNTPs (Invitrogen), 20U of RNasin (Promega) and 500 ng of random primers (Promega) in a reaction volume of 25 μL for 1 h at 38 °C. After heat-inactivation of the reverse-transcriptase, the cDNA was diluted 1:8 in RNAse/DNAse-free water (Invitrogen) and the specific amplification of leucocyte markers achieved in reaction volumes of 25 μL, using 5 μL of the cDNA-pool, 200 μM of dNTPs (Invitrogen), 1.5 mM of MgCl2 (Invitrogen), 500nM of each specific forward and reverse primer (Eurofins MWG Operon) and 2U of Taq DNA polymerase (Invitrogen). Species-specific primers, designed using the NCBI-software package, respective annealing temperatures and number of amplification cycles are listed in Table 1. PCR cycles were chosen as follows: 94 °C for 3 min (94 °C for 30 s, annealing 53–62 °C for 30 s, extension at 72 °C for 30 s)25–35x and 72 °C for 10 min. The amplified PCR products were analysed by 1 % agarose gel electrophoresis.
Table 1.
Primers used for cDNA amplification
| Target genea | Primer sequence (5′ → 3′) | TAn. (°C) | Cycles | Amplicon size (bp) |
|---|---|---|---|---|
| m β-actin | catgtttgagaccttcaacacc1 | 58 | 25 | 497 |
| gaaagggtgtaaaacgcagctcagta2 | ||||
| h GAPDH | gccttccgtgtccccactgc1 | 62 | 30 | 334 |
| ggctggtggtccaggggtct2 | ||||
| PV NS1 | ctaaatggaaaggacatcggttggaatag1 | 62 | 30 | 570 |
| gcctccgtcccttggtgg2 | ||||
| PV MCS | tcttgctgcacagcagaggact1 | 63 | 30 | ≥227 |
| gcttcactcacccagttaaccccc2 | ||||
| m NKG2D | tgtggcttgccattttcaaagagacg1 | 58 | 25 | 446 |
| ttacaccgcccttttcatgcagatg2 | ||||
| m Perforin | cagaatgcaagcagaagcacaag1 | 61 | 30 | 486 |
| ggtggagtggaggtttttgtacc2 | ||||
| m Granzyme B | actcaaacacgctacaaga1 | 53 | 30 | 253 |
| atccaggataagaaactcg2 | ||||
| m IFN-γ | tggaggaactggcaaaaggatgg1 | 63 | 30 | 320 |
| cccaccccgaatcagcagcg2 | ||||
| m F4/80 | acaccctcgggctgtgagattgt1 | 58 | 25 | 561 |
| ccccgtctctgtattcaacc2 | ||||
| m iNOS | atggcttgcccctggaagttt1 | 61 | 30 | 827 |
| gcagcatcccctctgatggtg2 | ||||
| m CD11c | ccgtctgagtacccgggcca1 | 61 | 25 | 495 |
| cgctgccactgctggtcctc2 | ||||
| m CD83 | atgtcgcaaggcctccagctc1 | 61 | 25 | 634 |
| tctgatgtgcccttggctttgtaa2 |
T An annealing temperature, bp base pairs, PV parvovirus, GAPDH glyceraldehyde-3-phosphate dehydrogenase, MCS multiple cloning site, iNOS inducible nitric oxide synthase, CD cluster of differentiation
aTarget cDNAs of murine (m) and human (h) origin were amplified with the indicated pair of forward1 and reverse2 primers
Results
Efficient transduction of Panc-1 cells by means of H-1PV vectors
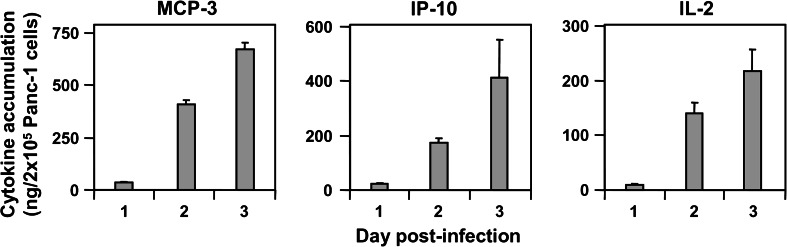
To determine the transduction efficiency of human PDAC cells by means of PV vectors, Panc-1 cells were infected at a multiplicity of infection (MOI) of 3 RU per cell (RU/c) with the vectors encoding MCP-3/CCL7 or IP-10/CXCL10. The chemokine accumulation was determined in the cell culture media at various time-points p.i. using specific ELISAs. The levels of both chemokines were below the limit of detection in the supernatants of Panc-1 cells infected with a control vector, devoid of transgene, Chi-H1/Δ800 (data not shown), whereas 3 days p.i. up to 730 ng/2 × 105 cells (MCP-3) and 410 ng/2 × 105 cells (IP-10) of the transduced chemokines accumulated in Panc-1 cell supernatants (Fig. 1). Similarly, high levels of IL-2 (220 ng/2 × 105 cells at day 3 p.i.) were measured after infection (MOI = 3 RU/c) with Chi-H1/IL-2, showing that Panc-1 cells can be efficiently transduced by means of H-1PV vectors.
Fig. 1.
Transgene production from recombinant H-1PV-transduced Panc-1 cells. 2 × 105 Panc-1 cells were infected with MCP-3-, IP-10- or IL-2-transducing Chi-H1/-based vectors (MOI = 3 RU/c). Cell-free conditioned media were analysed at the indicated time-points p.i. for cytokine accumulation using specific ELISAs and expressed in ng/2 × 105 cells. Data represent means ± SD of three measurements of a representative infection
IL-2 delivered by recombinant H-1PV in Panc-1 cells induces NK cell migration and increases their killing potential
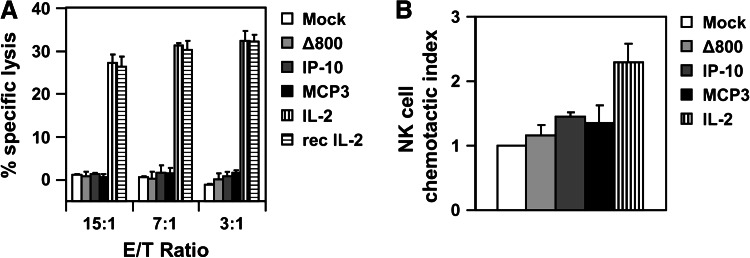
We next investigated the biological activity of the PV-delivered cytokines towards human NK cells. For this, NK cells were isolated from buffy coats of healthy blood donors, as described previously [33], and incubated for 5 days in the cell-free conditioned media harvested at day 2 p.i. from buffer-treated (Mock) or Panc-1 cells infected (MOI = 3 RU/c) with the control empty vector (∆800) or the vectors encoding IP-10, MCP-3 and IL-2. These NK cells were then tested for their ability to kill target K562 cells using the 51Cr release cytotoxicity assay. NK cells stimulated for 5 days with 100 IU/mL of recombinant IL-2 were included as a control. Compared to the supernatants from buffer-treated or control vector-infected (∆800) Panc-1 cells, the MCP-3 or IP-10-containing supernatants did not stimulate the killing efficacy of NK cells towards co-cultured K562 cells (Fig. 2a). In contrast, the supernatants of Chi-H1/IL-2-infected Panc-1 cells dramatically increased NK cell-mediated cytotoxicity (up to 30 % of specific killing) similarly to the stimulation obtained with 100 IU/mL of the recombinant IL-2.
Fig. 2.
IL-2-transduced Panc-1 cells induce human NK cell activation and chemotaxis. a 1 × 106 NK cells were stimulated for 5 days with recombinant (rec) IL-2 at 100 IU/mL or conditioned media harvested at day 2 p.i. from 1 × 106 Panc-1 cells buffer-treated (Mock) or infected (MOI = 3 RU/c) with control (∆800), MCP-3 or IL-2-encoding recombinant H-1PV vectors. Stimulated effector NK cells were then co-cultured with NK cell-susceptible Cr51-labelled target K562 cells for 4 h at the indicated effector to target (E/T) cell ratios, and the percentage of specific lysis was calculated as described in the M&M section. Data represent means ± SD of a representative experiment. b 5 × 103 NK cells were placed onto the porous membrane of a 96-well chemotaxis plate and allowed to transmigrate for 3 h into the lower chamber, filled with the conditioned media of (infected) Panc-1 cells, as described in a. NK cells present in the lower chamber were microscopically counted. Data represent the means ± SD of the chemotactic index above buffer-treated cells obtained from three independent experiments
We next evaluated whether the cytokines produced from recombinant H-1PV-infected Panc-1 cells could affect the chemotactic potential of human NK cells. For this, freshly purified NK cells were placed onto a porous filter membrane and allowed to migrate towards 30 μL of the cell-free conditioned media harvested at day 2 p.i. from 1 × 106 buffer-treated (Mock), Chi-H1/IL-2-, Chi-H1/MCP-3-, Chi-H1/IP-10- or control (∆800)-infected Panc-1 cells (MOI = 3RU/c). Chemotaxis was monitored after 3 h by microscopically counting NK cells present in the lower chamber. Compared to Mock- or Chi-H1/∆800-infected Panc-1 cells, the incubation of NK cells with the supernatants of Panc-1 cells infected with the H-1PV expressing IL-2 displayed increased migratory ability as shown in Fig. 2b. This may be due to the reported property of IL-2 to up-regulate the expression of the chemokine receptor CCR2 on NK cells [36], thereby allowing these cells to migrate to the ligand-producing cells. Indeed, this receptor binds to MCP-1/CCL2, reported to be released from cultured Panc-1 cells [25]. The constitutive accumulation of this chemokine (25 ng/mL) was confirmed in the conditioned media used in this study, as well as basal expression of CCR2 on NK cells. Yet, no further up-regulation of CCR2 could be observed on NK cells by IL-2 (data not shown). Whether IL-2 expressed by virus-infected Panc-1 cells may chemoattract NK cells [37] is still unclear. The supernatants harvested from virus-infected Panc-1 cells transducing MCP-3 or IP-10 could not significantly increase the number of transmigrated naïve NK cells in this assay (Fig. 2b). This is, however, not surprising as only activated NK cells are known to migrate towards MCP-3 [38] or IP-10 [39], indicating that Panc-1 cells per se do not secrete factors that activate the migratory capacity of NK cells. Taking together, IL-2, but not MCP-3 or IP-10 expressed from Panc-1-infected cells induces NK cell migration and increases their killing potential in vitro.
In vivo time-course expression of H-1PV-delivered transgene
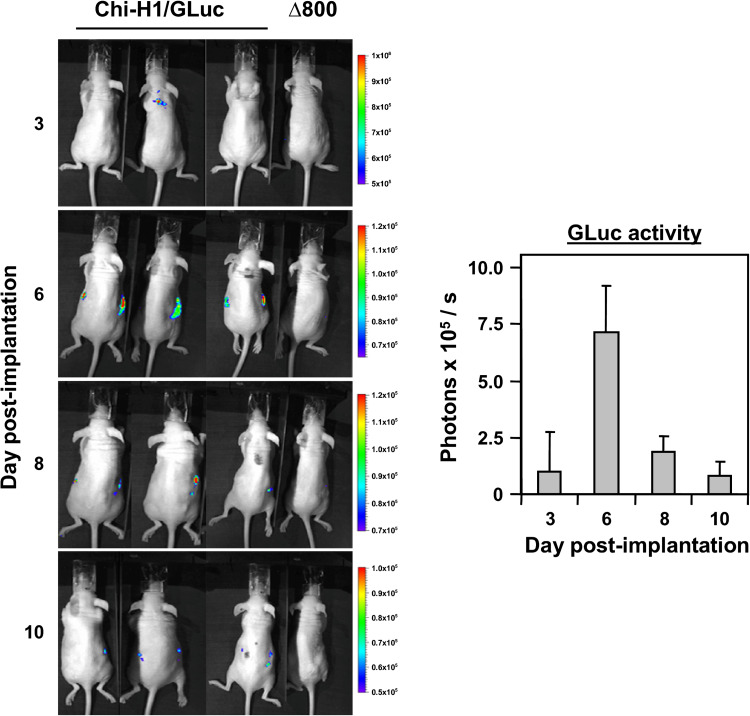
We previously showed that the expression of various transgenes by parvoviral vectors was transient in vitro [20]; hence, we next wanted to monitor the expression of a transgene in vivo. For this, a recombinant H-1PV vector encoding the GLuc reporter gene was designed and used to infect 5 × 106 Panc-1 cells (MOI = 3 RU/c) that were subcutaneously (s.c.) implanted into nude Balb/c recipient mice (n = 3). Panc-1 cells infected with Chi-H1/Δ800 for background luminescence were included as a control. GLuc activity was determined at various time-points post-implantation (p.imp.) after intraperitoneal injection of 100 μg of coelenterazine. As shown in Fig. 3, luciferase activity, measured by GLuc-dependent photon emission, was only detected in the mice challenged with Chi-H1/GLuc-infected Panc-1 cells, and peaked at day 6 p.imp. Most of the GLuc activity was detected around the site of the developing tumour (Fig. 3, left panel) but was also observed in the kidney region. This is, however, not surprising, since GLuc is a naturally secreted luciferase [40], which is renally cleared upon xenotropic expression. GLuc activity decreased rapidly after day 6 p.imp., indicating that the expression of the transgene by virus-infected Panc-1 cells only occurred transiently, in agreement with our previous in vitro observations with recombinant H-1PV vectors [20].
Fig. 3.
Secretion of Gaussia luciferase in Panc-1 xenografts infected with Chi-H1/GLuc. 5 × 106 Panc-1 cells were infected with Chi-H1/GLuc or control Chi-H1/∆800 (MOI = 3 RU/c), s.c. implanted into nude Balb/c recipient mice and 100 μg of coelenterazine was intraperitoneally injected at the indicated time-points p.imp. GLuc activity was monitored 7 min thereafter for 5 min using a CCD camera (left panel), photon emission quantified and expressed as photons × 105 released per second ± SD (right panel)
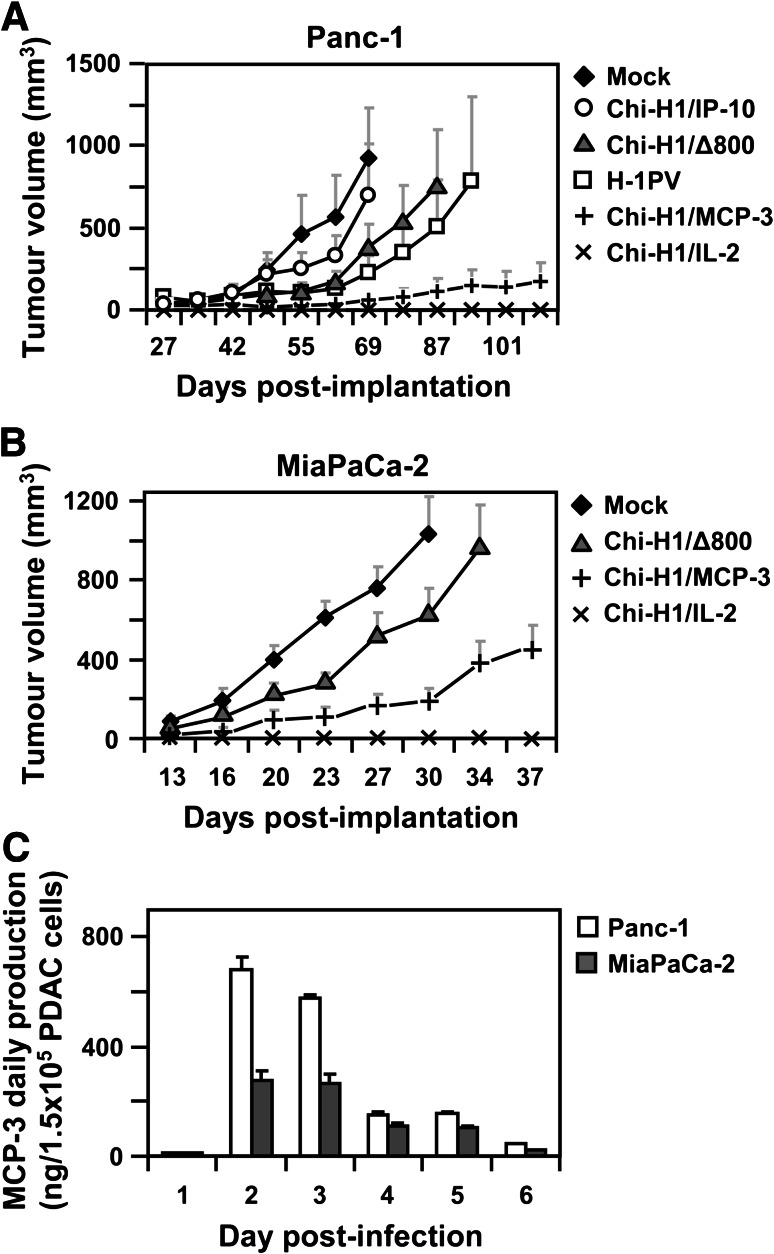
Chi-H1/IL-2 and Chi-H1/MCP-3 viruses suppress the growth of human pancreatic tumours
Since recombinant H-1PV-infected Panc-1 cells were shown to deliver and express a reporter gene in vivo, the anti-tumour potential of the virus-expressed cytokines IP-10, MCP-3 and IL-2 was investigated in this tumour model. 5 × 106 Panc-1 cells were buffer-treated (Mock) or infected (MOI = 1.5 RU/cell) with the corresponding chemokine/cytokine-transducing Chi-H1-based recombinant vector. At a MOI of 1.5 RU/cell, statistically only a fraction (77 %) of the cell population is efficiently infected by the virus. In order to evaluate the respective contributions of the viral vector and the transgene to their anti-tumour activity against PV-infected Panc-1 cells, the effects of the wild-type, fully replicative, H-1PV, and the control empty vector (Chi-H1/Δ800) were included. Following infection, the cells were s.c. implanted into nude Balb/c recipient mice and the tumour volumes were measured twice a week. As shown in Fig. 4a, compared to mice challenged with buffer-treated cells (Fig. 4, Mock, ♦), groups receiving Panc-1 cells infected with either wild-type H-1PV (Fig. 4, □) or the control vector (Fig. 4, Chi-H1/Δ800, ▲) displayed slower tumour growth rates. This can likely be assigned to the cytotoxic activity of the viral NS1 protein, as it was previously observed in other tumour models [9, 19–21]. Alternatively, the decrease in the presentation of MHC class I on the surface of H-1PV-infected Panc-1 cells contributes to render these cells more susceptible to NK cell-mediated killing [33]. It is worth mentioning that wild-type H-1PV albeit fully replicating in permissive Panc-1 cells in vitro [11] did not display a stronger anti-tumour activity than the replication-deficient vector Chi-H1/∆800. This may be explained by the fact that tumour-produced H-1PV progenies can be taken up by surrounding (non-permissive) mouse cells [34], hence, preventing efficient virus spread in the tumour microenvironment. No significant differences on tumour growth rates were observed after infection of Panc-1 cells with Chi-H1/IP-10 (Fig. 4, ○), compared with the control vector (Chi-H1/Δ800). In the nude Balb/c model, the lack of therapeutic efficacy with this vector may tentatively be traced back to the absence of IP-10 target CXCR3+ T-lymphocytes, reported to be involved in the anti-tumoral capacity of this chemokine [31]. In support of this, in a previous study [19], a protective anti-tumour immunity was induced in immunocompetent mice cured of glioblastoma, as a result of treatment with parvoviruses delivering IP-10 and TNF-α. This was apparent from their resistance to a subsequent challenge with uninfected glioma cells, suggesting that a specific CTL response was induced. However, IP-10 is also known to be endowed with strong angiostatic properties [29, 30] and could thus be expected to hamper the development of VEGF-producing Panc-1 tumours also in nude mice. The observation showing that IP-10 had no effect on the tumour growth is, therefore, puzzling. In sharp contrast, the transient H-1PV-mediated expression of IL-2 (Fig. 4×) or MCP-3 (Fig. 4, +) showed a dramatic inhibitory effect on the development of Panc-1 tumours. All animals, challenged with IL-2-transduced cells, remained tumour-free until the end of the experiment and only four out of eight animals injected with Chi-H1/MCP-3-infected Panc-1 cells developed tumours that grew very slowly. Given the strong anti-tumour effects elicited by both recombinant parvoviruses, these vectors were assessed in a second PDAC model. For this, 2.5 × 106 MiaPaCa-2 cells were buffer-treated (Mock) or infected (MOI = 2 RU/c) with control Chi-H1/∆800, Chi-H1/MCP-3 or Chi-H1/IL-2 viruses and s.c. injected into nude Balb/c mice. As shown in Fig. 4b, in contrast to rapidly developing buffer-treated (Mock) or control-infected (Chi-H1/Δ800) MiaPaCa-2 tumours, the infection with Chi-H1/MCP-3 markedly decreased tumour growth rates, resulting in an increased survival of the animals (data not shown). This effect, however, was less prominent than observed in the Panc-1 tumour model, which can be assigned, at least in part, to the different abilities of both PDAC cell lines to secrete the virus-delivered MCP-3 (Fig. 4c). Moreover, a higher fraction of virus-delivered MCP-3 might be processed into inactive forms in MiaPaCa-2 cells compared with Panc-1 cells. In addition, the more aggressive growth of MiaPaCa-2 tumours may overwhelm the immune system and, hence, reduce the anti-tumour effect of MCP-3. As it was observed for Panc-1 tumours, mice treated with IL-2-transduced MiaPaCa-2 cells remained tumour-free, confirming the high anti-tumour activity of this cytokine on PDAC.
Fig. 4.
IL-2- and MCP-3-encoding H-1PV vectors suppress human PDAC tumour development. a 5 × 106 Panc-1 cells were buffer-treated (Mock), infected at an MOI = 1.5 RU/c with wild-type H-1PV (H-1PV) or Chi-H1/-based vectors, empty (∆800) or transducing IP-10, MCP-3 or IL-2, and s.c. implanted into female Balb/c mice (n = 8, wild-type H-1PV: n = 5). The two-dimensional protrusions of the developing tumours were measured twice a week using an electronic calliper and tumour volumes calculated using the following formula: V = ½ × L × B2. Data represent mean tumour volumes ± SEM. b 2.5 × 106 MiaPaCa-2 cells were buffer-treated (Mock) or infected (MOI = 2 RU/c) with Chi-H1/∆800, Chi-H1/MCP-3 or Chi-H1/IL-2, s.c. implanted into nude Balb/c mice (n = 10, Chi-H1/IL-2: n = 6), and tumours were monitored as described in a. c 1.5 × 105 Panc-1 and MiaPaCa-2 cells were infected with Chi-H1/MCP-3 (MOI = 3 RU/c), and the daily secretion of MCP-3 was analysed in cell supernatants using specific ELISA. Data represent means ± SD of three measurements of a representative infection
Taken together, these results show strong abilities of Chi-H1/IL-2 and Chi-H1/MCP-3 viruses to abrogate the growth of human pancreatic tumours, supporting the potential of a parvovirus-mediated delivery of these immunostimulatory molecules for the therapy of PDAC.
Parvoviral delivery of MCP-3 or IL-2 induces leucocyte infiltration into PDAC tumours
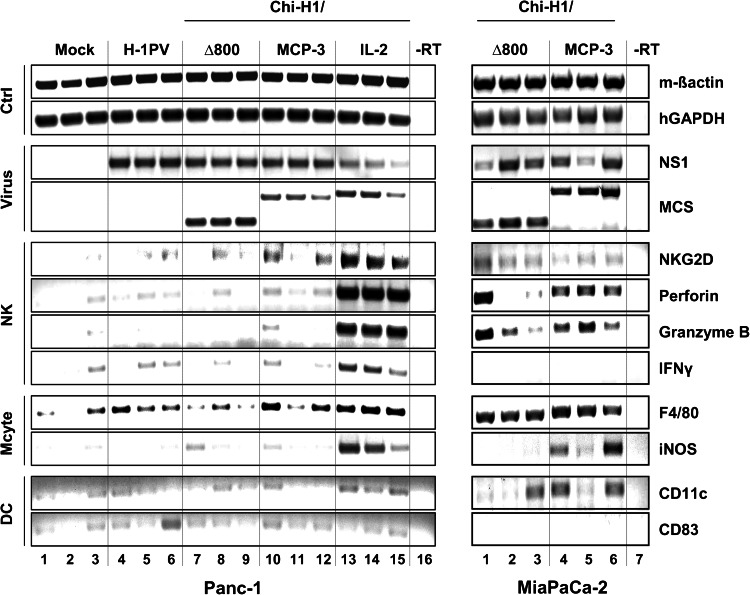
To analyse the leucocyte populations that contributed to the anti-tumour effects seen after ectopic expression of IL-2 or MCP-3, 5 × 106 Panc-1 cells were buffer-treated (Mock) or infected with 1.5 RU/c of Chi-H1/MCP-3, Chi-H1/IL-2, wild-type H-1PV or control Chi-H1/∆800. Developing tumours were resected at day 5 p.imp, and RT-PCR analysis was performed on total RNA extracts using primers specific for leucocyte markers and viral transcripts. Mouse (m) β-actin and human (h) GAPDH were included as internal controls for matching total RNA supply (Fig. 5, Ctrl panels). No amplification products were obtained when reverse transcriptase was omitted from the reaction (-RT, Fig. 5 lane 16), indicating the absence of contaminating genomic DNA. As expected, viral NS transcripts were found in all tumours treated with wild-type or recombinant viruses, whereas the signal characteristic of the transgene transcript, detected by means of primers flanking the multiple cloning site (MCS), was restricted to tumours infected with recombinant vectors (Fig. 5, virus panels). No expression of the markers CD177 and ELANE was detected, suggesting the absence of neutrophils in the tumour infiltrates (data not shown). In contrast, H-1PV-mediated chemo/cytokine transduction had an effect on tumour-associated NK cells and monocytes.
Fig. 5.
Leucocyte infiltration into IL-2 and MCP-3-transduced PDAC tumours. Left panel 5 × 106 Panc-1 cells were buffer-treated (Mock) and infected (MOI = 1.5 RU/c) with wild-type (H-1PV) or recombinant vectors (Chi-H1/): control empty vector (∆800) and vector transducing MCP-3, IP-10 and IL-2. Right panel 2.5 × 106 MiaPaCa-2 were infected (MOI = 2 RU/c) with Chi-H1/Δ800 or Chi-H1/MCP-3. PDAC cells were s.c. implanted into female Balb/c mice (n = 3), and developing tumours were excised at day 5 (Panc-1) or 11 (MiaPaCa-2) p.imp. Three micrograms of total RNA were reverse-transcribed and cDNAs amplified using primers specific for control- (Ctrl), virus-, NK cell-, monocyte- (Mcyte) or dendritic cell (DC)-related transcripts and visualized on 1 % ethidium bromide-stained agarose gels
Indeed, H-1PV-mediated production of IL-2 (lanes 13–15) and MCP-3 (two out of three tumours, lanes 10 + 12) correlated with the infiltration of immune receptor NKG2D-positive NK cells into developing Panc-1 tumours. Expression of the markers perforin, granzyme B and interferon-γ (IFN-γ), indicative of the activation of the NK cell cytotoxicity programme, was markedly enhanced in IL-2-transduced Panc-1 tumours (lanes 13–15) whereas the other vectors used had little impact (lanes 1–12). Most likely because of the constitutive expression of MCP-1 in Panc-1 cells [25], the macrophage-specific marker (F4/80) was found to be present in all tumours, including the controls (∆800 and Mock), albeit to various levels. Yet, compared to Mock-, wild-type- and Chi-H1/∆800-treated cells, H-1PV-mediated expression of IL-2 (lanes 13–15) and MCP-3 (2 out of 3 tumours, lanes 10 + 12) resulted in increased levels of this marker, in keeping with the known features of IL-2 and the monocyte chemo-attractant properties of MCP-3 [41]. The induction of inducible nitric oxide synthase (iNOS), a marker for activated macrophages, was only observed in IL-2-transduced Panc-1 tumours (lanes 13–15). The expression of markers specific for resting (CD11c, [42]) and mature (CD83, [43]) dendritic cells (DC) was also analysed (Fig. 5, DC panels). Whereas CD11c induction was most prominent upon vector-mediated delivery of IL-2, no sign of mature DCs could be detected in the tumour infiltrates, as deduced from the weak signal corresponding to CD83-specific transcripts.
Leucocyte infiltration was also examined in MiaPaCa-2 tumours after infection of Chi-H1/MCP-3 versus the control vector Chi-H1/Δ800 (MOI = 2 RU/c). This analysis was carried out at two different time-points post-implantation (day 6 and 11), because of the different kinetics of appearance and tumour development between MiaPaCa-2 and Panc-1 cells. As no significant differences were observed in the expression of leucocyte markers at both time-points, only the results for tumours excised at day 11 are shown. As illustrated in Fig. 5 (right, NK and Mcyte panels), the average levels of perforin, granzyme B and iNOS transcripts were higher in MCP-3 (lanes 4–6) versus Δ800 (lanes 1–3)-transduced cells, revealing the presence of activated NK and monocytic cells in the tumour tissue.
Altogether these data show that the H-1PV-driven expression of IL-2—and to a more variable extent—of MCP-3 in PDAC xenotransplants, increases the infiltration of activated NK and monocytic cells.
Discussion
PDAC is a deadly disease with an extremely poor life expectancy. Therefore, there is urgent need for new alternative treatment options. The current study aims to evaluate the potential anti-PDAC efficacy of parvoviruses armed with immunostimulatory molecules. We show that, despite their sensitivity to PV-induced killing in vitro, PDAC tumours developed—yet at reduced rates—upon infection with low doses of wild-type H-1PV, in keeping with previous reports on different tumour models [44]. When armed with the immunostimulatory cytokines IL-2 or MCP-3, the capacities to control PDAC tumour development were strikingly enhanced, as indicated by the cytokine-mediated infiltration of a selective subset of leucocytes.
Although known to be endowed with strong anti-tumour properties, systemically applied IL-2 failed to develop into a first-line anti-cancer drug, mostly due to the risk of potentially life-threatening side effects. Therefore, efforts have been made aiming to develop strategies for targeting IL-2 directly to the tumour [reviewed in [45]]. Based on an orthotopic mouse model, the systemic application of a single chain Fv fragment-targeted IL-2 into established PDAC tumours was able to inhibit neoplastic progression [46]. However, the major disadvantage of such a strategy is the need of a marker, specifically expressed by cancer cells. Therefore, the oncospecificity of H-1PV makes this virus an especially attractive delivery vehicle for cytokine transduction at tumour sites. Although the H-1PV receptor(s) that is still unidentified is ubiquitously expressed, intracellular restrictions of normal tissue preventing parvovirus replication endow H-1PV with a striking oncotropism [9].
Recently, a pre- and post-operative application of recombinant IL-2, tested in PDAC patients, was shown to be able to increase the number of NK cells in the peripheral blood, but failed to induce their infiltration into the tumour [47]. Our approach, based on PV-mediated local expression of IL-2 (and/or MCP-3) by the transduced tumour cells themselves, appears to be advantageous in this regard, as the H-1PV-driven expression of the immunomodulators was found to recruit immune cells towards the tumour. The lytic properties of NK cells towards PDAC cells may further be enhanced, as a result of (recombinant) H-1PV infection of these cells, as we recently showed that wild-type H-1PV represses the expression of major histocompatibility class I [33].
The chemokine MCP-3 was shown to be endowed with a strong anti-tumour potential in different tumour models through the recruitment of activated NK and T cells [21, 48]. Here, we could extend MCP-3 efficacy to PDAC, as ectopic PV-mediated expression of this chemokine was able to induce the infiltration and activation of NK and monocytic cells. Although its anti-tumour efficacy was lower in the MiaPaCa-2 model compared to the Panc-1 model, MCP-3 was more potent in activating both types of leucocytes in the former model. Since only one early time-point (5 days p.imp.) had been analysed in the Panc-1 system, the activation of infiltrated NK and monocytic cells might occur at later time-points in this model. Alternatively, although the role of MCP-1 in cancer is controversial, the stronger anti-tumour effect of MCP-3 in the Panc-1 (compared to MiaPaCa-2) system may be traced back to the additive effects between this chemokine and MCP-1, which is constitutively secreted by Panc-1 cells, whereas MiaPaCa-2 cells are MCP-1-deficient. Indeed, both CC-chemokines have been reported to contribute to the recruitment of inflammatory monocytes through binding to their common receptor CCR2 [49].
In conclusion, recombinant parvoviral vectors may constitute new tools to overcome the strong immunosuppressive environment in pancreatic tumours by modulating the leucocyte infiltrate. These promising data expand our knowledge about the anti-cancer efficacy of recombinant H-1PV to the field of PDAC.
Acknowledgments
We are grateful to Ghislain Opdenakker for kindly providing human MCP-3 cDNA, Nathalie Salomé and Juerg Nuesch for helpful discussions, Chris Dillen, Alexandra Stroh-Dege and Claudia Plotsky for technical assistance. Laura Whiele is acknowledged for her help in the production of viral stocks. This work was supported by the Fund for Scientific Research of Flanders (F.W.O.-Vlaanderen, contract number G064809, J.V.D.), the Interuniversity Attraction Poles (I.A.P.) Program-Belgian Science Policy (J.V.D.) and the European Union 6FP EC contract INNOCHEM (J.V.D.).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Schneider G, Schmid RM. Genetic alterations in pancreatic carcinoma. Mol Cancer. 2003;2:15. doi: 10.1186/1476-4598-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, Kirn DH, Freeman SM. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9(2):555–561. [PubMed] [Google Scholar]
- 3.Petrulio CA, Kaufman HL. Development of the PANVAC-VF vaccine for pancreatic cancer. Exp Rev Vac. 2006;5(1):9–19. doi: 10.1586/14760584.5.1.9. [DOI] [PubMed] [Google Scholar]
- 4.Gordon EM, Chan MT, Geraldino N, Lopez FF, Cornelio GH, Lorenzo CC, 3rd, Levy JP, Reed RA, Liu L, Hall FL. Le morte du tumour: histological features of tumor destruction in chemo-resistant cancers following intravenous infusions of pathotropic nanoparticles bearing therapeutic genes. Int J Oncol. 2007;30(6):1297–1307. [PubMed] [Google Scholar]
- 5.Chawla SP, Chua VS, Fernandez L, Quon D, Blackwelder WC, Gordon EM, Hall FL. Advanced phase I/II studies of targeted gene delivery in vivo: intravenous Rexin-G for gemcitabine-resistant metastatic pancreatic cancer. Mol Ther. 2010;18(2):435–441. doi: 10.1038/mt.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu X, Tao L, Li M, Fisher WE, Zhang X. Effective treatment of pancreatic cancer xenografts with a conditionally replicating virus derived from type 2 herpes simplex virus. Clin Cancer Res. 2006;12(10):3152–3157. doi: 10.1158/1078-0432.CCR-06-0045. [DOI] [PubMed] [Google Scholar]
- 7.Kasuya H, Nishiyama Y, Nomoto S, Goshima F, Takeda S, Watanabe I, Nomura N, Shikano T, Fujii T, Kanazumi N, Nakao A. Suitability of a US3-inactivated HSV mutant (L1BR1) as an oncolytic virus for pancreatic cancer therapy. Cancer Gene Ther. 2007;14(6):533–542. doi: 10.1038/sj.cgt.7701049. [DOI] [PubMed] [Google Scholar]
- 8.Liu S-H, Davis A, Li Z, Ballian N, Davis E, Wang X-P, Fisher W, Brunicardi FC. Effective ablation of pancreatic cancer cells in SCID mice using systemic adenoviral RIP-TK/GCV gene therapy. J Surg Res. 2007;141(1):45–52. doi: 10.1016/j.jss.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 9.Rommelaere J, Geletneky K, Angelova AL, Daeffler L, Dinsart C, Kiprianova I, Schlehofer JR, Raykov Z (2010) Oncolytic parvoviruses as cancer therapeutics. Cytokine Growth Factor Rev. doi:10.1016/j.cytogfr.2010.02.011 [DOI] [PubMed]
- 10.Cornelis JJ, Becquart P, Duponchel N, Salome N, Avalosse BL, Namba M, Rommelaere J. Transformation of human fibroblasts by ionizing radiation, a chemical carcinogen, or simian virus 40 correlates with an increase in susceptibility to the autonomous parvoviruses H-1 virus and minute virus of mice. J Virol. 1988;62(5):1679–1686. doi: 10.1128/jvi.62.5.1679-1686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dempe S, Stroh-Dege AY, Schwarz E, Rommelaere J, Dinsart C. SMAD4: a predictive marker of PDAC cell permissiveness for oncolytic infection with parvovirus H-1PV. Int J Cancer. 2010;126(12):2914–2927. doi: 10.1002/ijc.24992. [DOI] [PubMed] [Google Scholar]
- 12.Toolan HW, Rhode SL, 3rd, Gierthy JF. Inhibition of 7,12-dimethylbenz(a)anthracene-induced tumors in Syrian hamsters by prior infection with H-1 parvovirus. Cancer Res. 1982;42(7):2552–2555. [PubMed] [Google Scholar]
- 13.Angelova AL, Aprahamian M, Grekova SP, Hajri A, Leuchs B, Giese NA, Dinsart C, Herrmann A, Balboni G, Rommelaere J, Raykov Z. Improvement of gemcitabine-based therapy of pancreatic carcinoma by means of oncolytic parvovirus H-1PV. Clin Cancer Res. 2009;15(2):511–519. doi: 10.1158/1078-0432.CCR-08-1088. [DOI] [PubMed] [Google Scholar]
- 14.Geletneky K, Kiprianova I, Ayache A, Koch R, Herrero YCM, Deleu L, Sommer C, Thomas N, Rommelaere J, Schlehofer JR (2010) Regression of advanced rat and human gliomas by local or systemic treatment with oncolytic parvovirus H-1 in rat models. Neuro Oncol. doi:10.1093/neuonc/noq023 [DOI] [PMC free article] [PubMed]
- 15.Cornelis JJ, Salome N, Dinsart C, Rommelaere J. Vectors based on autonomous parvoviruses: novel tools to treat cancer? J Gene Med. 2004;6(Suppl 1):S193–S202. doi: 10.1002/jgm.502. [DOI] [PubMed] [Google Scholar]
- 16.Parker JC, Collins MJ, Cross SS, Rowe WP. Minute virus of mice. II. Prevalence, epidemiology, and occurrence as a contaminant of transplanted tumors. J Natl Cancer Inst. 1970;45(2):305–310. [PubMed] [Google Scholar]
- 17.Kestler J, Neeb B, Struyf S, Van Damme J, Cotmore SF, D’Abramo A, Tattersall P, Rommelaere J, Dinsart C, Cornelis JJ. Cis requirements for the efficient production of recombinant DNA vectors based on autonomous parvoviruses. Hum Gene Ther. 1999;10(10):1619–1632. doi: 10.1089/10430349950017626. [DOI] [PubMed] [Google Scholar]
- 18.Olijslagers S, Dege AY, Dinsart C, Voorhoeve M, Rommelaere J, Noteborn MH, Cornelis JJ. Potentiation of a recombinant oncolytic parvovirus by expression of Apoptin. Cancer Gene Ther. 2001;8(12):958–965. doi: 10.1038/sj.cgt.7700392. [DOI] [PubMed] [Google Scholar]
- 19.Enderlin M, Kleinmann EV, Struyf S, Buracchi C, Vecchi A, Kinscherf R, Kiessling F, Paschek S, Sozzani S, Rommelaere J, Cornelis JJ, Van Damme J, Dinsart C. TNF-alpha and the IFN-gamma-inducible protein 10 (IP-10/CXCL-10) delivered by parvoviral vectors act in synergy to induce antitumor effects in mouse glioblastoma. Cancer Gene Ther. 2009;16(2):149–160. doi: 10.1038/cgt.2008.62. [DOI] [PubMed] [Google Scholar]
- 20.Haag A, Menten P, Van Damme J, Dinsart C, Rommelaere J, Cornelis JJ. Highly efficient transduction and expression of cytokine genes in human tumor cells by means of autonomous parvovirus vectors; generation of antitumor responses in recipient mice. Hum Gene Ther. 2000;11(4):597–609. doi: 10.1089/10430340050015789. [DOI] [PubMed] [Google Scholar]
- 21.Wetzel K, Struyf S, Van Damme J, Kayser T, Vecchi A, Sozzani S, Rommelaere J, Cornelis JJ, Dinsart C. MCP-3 (CCL7) delivered by parvovirus MVMp reduces tumorigenicity of mouse melanoma cells through activation of T lymphocytes and NK cells. Int J Cancer. 2007;120(6):1364–1371. doi: 10.1002/ijc.22421. [DOI] [PubMed] [Google Scholar]
- 22.Giese NA, Raykov Z, DeMartino L, Vecchi A, Sozzani S, Dinsart C, Cornelis JJ, Rommelaere J. Suppression of metastatic hemangiosarcoma by a parvovirus MVMp vector transducing the IP-10 chemokine into immunocompetent mice. Cancer Gene Ther. 2002;9(5):432–442. doi: 10.1038/sj.cgt.7700457. [DOI] [PubMed] [Google Scholar]
- 23.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52(1):145–176. [PubMed] [Google Scholar]
- 24.Kuehn R, Lelkes PI, Bloechle C, Niendorf A, Izbicki JR. Angiogenesis, angiogenic growth factors, and cell adhesion molecules are upregulated in chronic pancreatic diseases: angiogenesis in chronic pancreatitis and in pancreatic cancer. Pancreas. 1999;18(1):96–103. doi: 10.1097/00006676-199901000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Monti P, Marchesi F, Reni M, Mercalli A, Sordi V, Zerbi A, Balzano G, Di Carlo V, Allavena P, Piemonti L. A comprehensive in vitro characterization of pancreatic ductal carcinoma cell line biological behavior and its correlation with the structural and genetic profile. Virchows Arch. 2004;445(3):236–247. doi: 10.1007/s00428-004-1053-x. [DOI] [PubMed] [Google Scholar]
- 26.Hussain F, Wang J, Ahmed R, Guest SK, Lam EW, Stamp G, El-Bahrawy M. The expression of IL-8 and IL-8 receptors in pancreatic adenocarcinomas and pancreatic neuroendocrine tumours. Cytokine. 2010;49(2):134–140. doi: 10.1016/j.cyto.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP (2005) CXC chemokines in angiogenesis. Cytokine & growth factor reviews 16 (6):593-609. doi:10.1016/j.cytogfr.2005.04.007 [DOI] [PubMed]
- 28.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184(3):963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taub DD, Sayers TJ, Carter CR, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155(8):3877–3888. [PubMed] [Google Scholar]
- 30.Penna G, Sozzani S, Adorini L. Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J Immunol. 2001;167(4):1862–1866. doi: 10.4049/jimmunol.167.4.1862. [DOI] [PubMed] [Google Scholar]
- 31.Luster AD, Leder P. IP-10, a -C-X-C- chemokine, elicits a potent thymus-dependent antitumor response in vivo. J Exp Med. 1993;178(3):1057–1065. doi: 10.1084/jem.178.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pross HF, Lotzova E. Role of natural killer cells in cancer. Nat Immun. 1993;12(4–5):279–292. [PubMed] [Google Scholar]
- 33.Bhat R, Dempe S, Dinsart C, Rommelaere J. Enhancement of NK cell anti-tumour responses using an oncolytic parvovirus. Int J Cancer. 2011;128(4):908–919. doi: 10.1002/ijc.25415. [DOI] [PubMed] [Google Scholar]
- 34.Wrzesinski C, Tesfay L, Salome N, Jauniaux JC, Rommelaere J, Cornelis JJ, Dinsart C. Chimeric and pseudotyped parvoviruses minimize the contamination of recombinant stocks with replication-competent viruses and identify a DNA sequence that restricts parvovirus H-1 in mouse cells. J Virol. 2003;77(6):3851–3858. doi: 10.1128/JVI.77.6.3851-3858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B. Activation of NK cells by CC chemokines. Chemotaxis, Ca2 + mobilization, and enzyme release. J Immunol. 1996;156(1):322–327. [PubMed] [Google Scholar]
- 36.Polentarutti N, Allavena P, Bianchi G, Giardina G, Basile A, Sozzani S, Mantovani A, Introna M. IL-2-regulated expression of the monocyte chemotactic protein-1 receptor (CCR2) in human NK cells: characterization of a predominant 3.4-kilobase transcript containing CCR2B and CCR2A sequences. J Immunol. 1997;158(6):2689–2694. [PubMed] [Google Scholar]
- 37.Natuk RJ, Welsh RM. Chemotactic effect of human recombinant interleukin 2 on mouse activated large granular lymphocytes. J Immunol. 1987;139(8):2737–2743. [PubMed] [Google Scholar]
- 38.Allavena P, Bianchi G, Zhou D, van Damme J, Jilek P, Sozzani S, Mantovani A. Induction of natural killer cell migration by monocyte chemotactic protein-1, -2 and -3. Eur J Immunol. 1994;24(12):3233–3236. doi: 10.1002/eji.1830241249. [DOI] [PubMed] [Google Scholar]
- 39.Maghazachi AA, Skalhegg BS, Rolstad B, Al-Aoukaty A. Interferon-inducible protein-10 and lymphotactin induce the chemotaxis and mobilization of intracellular calcium in natural killer cells through pertussis toxin-sensitive and -insensitive heterotrimeric G-proteins. FASEB J. 1997;11(10):765–774. doi: 10.1096/fasebj.11.10.9271361. [DOI] [PubMed] [Google Scholar]
- 40.Tannous BA, Kim D-E, Fernandez JL, Weissleder R, Breakefield XO. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol Ther. 2005;11(3):435–443. doi: 10.1016/j.ymthe.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Van Damme J, Proost P, Lenaerts JP, Opdenakker G. Structural and functional identification of two human, tumor-derived monocyte chemotactic proteins (MCP-2 and MCP-3) belonging to the chemokine family. J Exp Med. 1992;176(1):59–65. doi: 10.1084/jem.176.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amano H, Amano E, Santiago-Raber ML, Moll T, Martinez-Soria E, Fossati-Jimack L, Iwamoto M, Rozzo SJ, Kotzin BL, Izui S. Selective expansion of a monocyte subset expressing the CD11c dendritic cell marker in the Yaa model of systemic lupus erythematosus. Arthritis Rheum. 2005;52(9):2790–2798. doi: 10.1002/art.21365. [DOI] [PubMed] [Google Scholar]
- 43.Lechmann M, Berchtold S, Hauber J, Steinkasserer A. CD83 on dendritic cells: more than just a marker for maturation. Trends Immunol. 2002;23(6):273–275. doi: 10.1016/S1471-4906(02)02214-7. [DOI] [PubMed] [Google Scholar]
- 44.Cornelis JJ, Lang SI, Stroh-Dege AY, Balboni G, Dinsart C, Rommelaere J. Cancer gene therapy through autonomous parvovirus-mediated gene transfer. Curr Gene Ther. 2004;4(3):249–261. doi: 10.2174/1566523043346228. [DOI] [PubMed] [Google Scholar]
- 45.Shaker MA, Younes HM. Interleukin-2: evaluation of routes of administration and current delivery systems in cancer therapy. J Pharm Sci. 2009;98(7):2268–2298. doi: 10.1002/jps.21596. [DOI] [PubMed] [Google Scholar]
- 46.Wagner K, Schulz P, Scholz A, Wiedenmann B, Menrad A. The targeted immunocytokine L19-IL2 efficiently inhibits the growth of orthotopic pancreatic cancer. Clin Cancer Res. 2008;14(15):4951–4960. doi: 10.1158/1078-0432.CCR-08-0157. [DOI] [PubMed] [Google Scholar]
- 47.Degrate L, Nobili C, Franciosi C, Caprotti R, Brivio F, Romano F, Leone BE, Trezzi R, Uggeri F. Interleukin-2 immunotherapy action on innate immunity cells in peripheral blood and tumoral tissue of pancreatic adenocarcinoma patients. Langenbecks Arch Surg. 2009;394(1):115–121. doi: 10.1007/s00423-008-0393-4. [DOI] [PubMed] [Google Scholar]
- 48.Fioretti F, Fradelizi D, Stoppacciaro A, Ramponi S, Ruco L, Minty A, Sozzani S, Garlanda C, Vecchi A, Mantovani A. Reduced tumorigenicity and augmented leukocyte infiltration after monocyte chemotactic protein-3 (MCP-3) gene transfer: perivascular accumulation of dendritic cells in peritumoral tissue and neutrophil recruitment within the tumor. J Immunol. 1998;161(1):342–346. [PubMed] [Google Scholar]
- 49.Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, Pamer EG. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol. 2008;180(10):6846–6853. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]