Abstract
We have demonstrated previously that the inoculation of murine mammary tumor cells genetically modified to express high levels of secretory leukocyte protease inhibitor (2C1) do not develop tumors in immunocompetent mice and these cells are more prone to apoptosis than control cells. The aim of the present study was to evaluate the role of the adaptive immune response in the lack of tumor growth of 2C1 cells and the possibility of using these cells for immunotherapy. The s.c. administration of mock transfected F3II cells induces tumor in BALB/c and Nude mice. However, the inoculation of 2C1 cells develops tumor in Nude but not in BALB/c mice. The inoculation of mock transfected F3II cells to 2C1 immunized BALB/c mice by repeated administration of 2C1 cells (once a week for 3 weeks) developed significantly smaller tumors than those observed in non-immunized mice. Remarkably, survival of tumor-bearing immunized mice was higher than non-immunized animals. Herein, we demonstrate that an immunotherapy with SLPI over-expressing non-irradiated tumor cells which do not develop tumor in immunocompetent mice, partially restrain the tumor growth induced by F3II cells and increase the survival of the mice.
Keywords: Immunotherapy, SLPI, Cancer, Mammary tumor
Introduction
Several different vaccines derived from whole tumor cells or tumor cell lysates have been evaluated in preclinical models and clinical trials. For example, tumor cells extracted from surgical resection or biopsy specimens have been used to develop these vaccines [1–5]. Moreover, tumor cells were infected with viruses transduced with genes expressing cytokines, HLA molecules, or co-stimulatory molecules in order to increase the immunogenicity of these cells [6, 7]. Regardless the strategies developed to generate tumor cell-based vaccines; cells need to be irradiated before the inoculation to avoid their proliferation and dissemination. For instance, tumor irradiated cells have been shown to improve the survival of patients with metastasis [1]. These vaccines tend to boost an immune response by activating dendritic cells and eliciting a proper tumor cell-specific T-cell response.
Secretory leukocyte protease inhibitor (SLPI) is an 11.7 kDa non-glycosylated serine protease inhibitor synthesized and secreted by inflammatory and stroma cells in the local microenvironment of the respiratory, digestive, and genital mucosa [8]. Moreover, SLPI expression has been described in several tumor processes. However, the role of this protein in cancer is not completely elucidated. An increased or a decreased SLPI expression was described in different tumors, and this has been associated with either higher or lower tumorigenicity [9–16].
In previous studies, we have demonstrated that the inoculation of mammary tumor cells genetically modified to express high levels of SLPI does not develop tumors in BALB/c mice. Although, these cells proliferate in vitro, they are more prone to apoptosis than mock transfected cells. The aim of the present study was to evaluate the role of the adaptive immune response in the lack of tumor growth of SLPI over-expressing cells and the possibility of using these cells for immunotherapy. Herein, we demonstrate that SLPI over-expressing cells grow in Nude mice. Furthermore, the pretreatment of BALB/c mice with repeated administration of SLPI over-expressing mammary tumor cells reduced the tumor growth generated by mock transfected control cells.
Materials and methods
Animals
Eight weeks old female BALB/c and Nude (Swiss) mice were obtained from the animal facility of the Faculty of Veterinary, University of La Plata, Buenos Aires, Argentina. Animals were maintained at Animal Facility (Microbiology Department, School of Medicine, University of Buenos Aires) in accordance with the experimental ethics committee guidelines.
Tumor cell lines and culture conditions
F3II is a BALB/c murine mammary carcinoma hormone-independent cell line described previously [17]. 2C1 were obtained by transfecting F3II cells with the pcDNA3 plasmid encoded the hSLPI gene as previously described (not shown).
Both cells were cultured in RPMI 1640 (Gibco, Grand Island, New York), supplemented with heat inactivated 10% FBS, 2 mM l-Glutamine, and 40 μg/ml gentamicin at 37°C in a 5% CO2 atmosphere. For some experiments, 2C1 cells were irradiated with 3000 rad single dose of gamma-rays at CEBIRSA S.A. (Buenos Aires, Argentina).
Apoptosis measurement
Tumor cells were seeded in a 6-well plate and incubated in RPMI 1640 (Gibco) with 2 mM l-glutamine, 40 μg/ml gentamicin, supplemented with heat inactivated 10% FBS (37°C, 5% CO2). After 24 h, cells were harvested with 0.05% trypsin–EDTA (Gibco) and apoptosis was measured by ethidium bromide/acridine orange staining [18]. Cell staining was analyzed using an ultraviolet fluorescence microscope (Nikon Eclipse TS100, Nikon, Tokio, Japan). Cells were scored into two categories: (1) non-apoptotic: cells with large, bright green, non-condensed nuclei; and (2) apoptotic cells: with red/orange nuclei that showed signs of nuclear bead formation or without bright green nuclei with signs of nuclear condensation and membrane blebbing. At least 200 cells/samples were counted and scored.
In vitro cell proliferation assay
2C1 clone cells and F3II control cells (3 × 104/well) were seeded in a 96-well plate and they were incubated in RPMI 1640 (Gibco), supplemented with heat inactivated 10% FBS, 2 mM l-Glutamine, and 40 μg/ml gentamicin at 37°C in a 5% CO2 atmosphere. After 24 h, cells were pulsed with 5 μCi/ml [methyl-3H] thymidine (specific activity 20 Ci/mmol; Perkin Elmer, Boston, MA) for the last 18 h of culture. Finally, the cells were harvested and radioactivity was measured in a liquid scintillation β-counter (Wallac 1214 Rackbeta, Turku, Finland).
Tumor cells administration
Mice were inoculated (s.c.) with F3II or 2C1 cells (5 × 106 cells) into a flank of BALB/c or Nude mice. The tumor length (L) and width (W) were measured with a caliper three times a week, and the tumor volume (V) was calculated as V = (L × W 2)/2. No animals died because of tumor growth. The interval between tumor cell inoculation and the endpoint was defined as the survival time. The endpoint for survival curves was taken into account when the tumor reached a volume of 1,100 mm3 for BALB/c and 400 mm3 for Nude. Animals were killed following the guidelines of the ethics committee for experimentation of the Animal Facility.
In another set of experiments, 3 × 106 2C1 irradiated or non-irradiated cells and 8 × 105 F3II cells were inoculated at the same time into the left flank (LF) and the right flank (RF) of BALB/c mice, respectively.
Immunization
Mice were inoculated with 8 × 105 2C1 cells into the left flank, three times once a week. After the last 2C1 inoculation, mice were challenged 2 weeks later with 8 × 105 cells into the RF. Tumor growth and survival were monitored as described above.
Statistical analysis
Comparisons between groups were made using unpaired or paired Student’s t test as indicated in each figure legend. Statistical analysis was performed using GraphPad InStat (Version 3.06; GraphPad Software Inc., La Jolla, CA) with differences considered significant at P < 0.05. Kaplan–Meier plots were used for survival analysis.
Results
The administration of 2C1 cells induces tumor growth in Nude but not in BALB/c mice
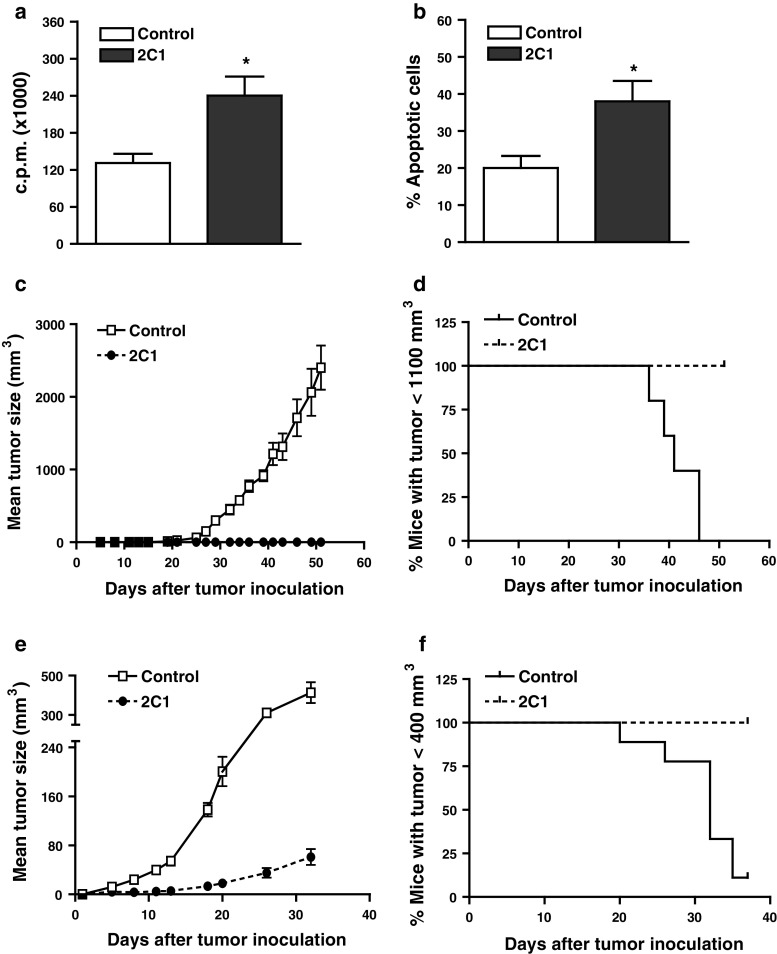
We have previously obtained SLPI over-expressing mammary tumor cell clones. Figure 1a shows that one of these clones, 2C1, presented in vitro a higher proliferation than F3II control cells (transfected with the empty plasmid). Also, in vitro, 2C1 cells were more prone to apoptosis than F3II control cells (Fig. 1b). Furthermore, we have observed that the s.c. administration of 8 × 105 2C1, but not F3II control cells, into BALB/c mice did not generate tumor at least up to 6 months after cells inoculation (not shown). The same was observed when more (5 × 106) 2C1 cells were inoculated to BALB/c (Fig. 1c). Moreover, 100% of these mice have survived until the end of the experiment (Fig. 1d). On the contrary, 5 × 106 2C1 and control cells generated tumors in Nude mice (Fig. 1e). However, the tumor developed by 2C1 cells in Nude mice was smaller than tumor generated by F3II cells. Moreover, none of the 2C1 inoculated Nude mice reached a tumor size >400 mm3 at day 38 (Fig. 1f). The later results suggest that cells of the adaptive immune response are required for avoiding the tumor growth.
Fig. 1.
SLPI over-expressing tumor cells (2C1 clone) growth in immunodeficient but not in immunocompetent mice. a In vitro proliferation of clones. 2C1 and F3II control clone were seeded in 96 wells plate and incubated for 24 h at 37°C. After, cells were pulsed with 5 μCi/ml (methyl-3H) thymidine for the last 18 h. Finally, the cells were harvested and the incorporation of radioactivity was measured in a liquid scintillation β-counter. Mean ± SEM of four independent experiments (*P < 0.05 2C1 vs. F3II Control; paired Student’s t test). b Percentage of apoptotic cells measured by ethidium bromide/acridine orange staining shown as mean ± SEM of five independent experiments (*P < 0.05 2C1 vs. F3II; paired Student’s t test). BALB/c (c) or Nude (e) mice were s.c. inoculated with 5 × 106 SLPI over-expressing tumor cells (2C1) or with control clone (F3II mock transfected). The tumor growth was measured with a caliper, and tumor volume was calculated as indicated in “Materials and methods.” It is shown the mean ± SD of one representative experiment of five. Each group contained five animals. Tumor growth was monitored for 52 (c) or 32 (e) days. Data on (d) and (f) are expressed as Kaplan–Meier survival curves of (c) and (e) experiments, respectively
Immunotherapy with 2C1 cells reduces mammary tumor growth
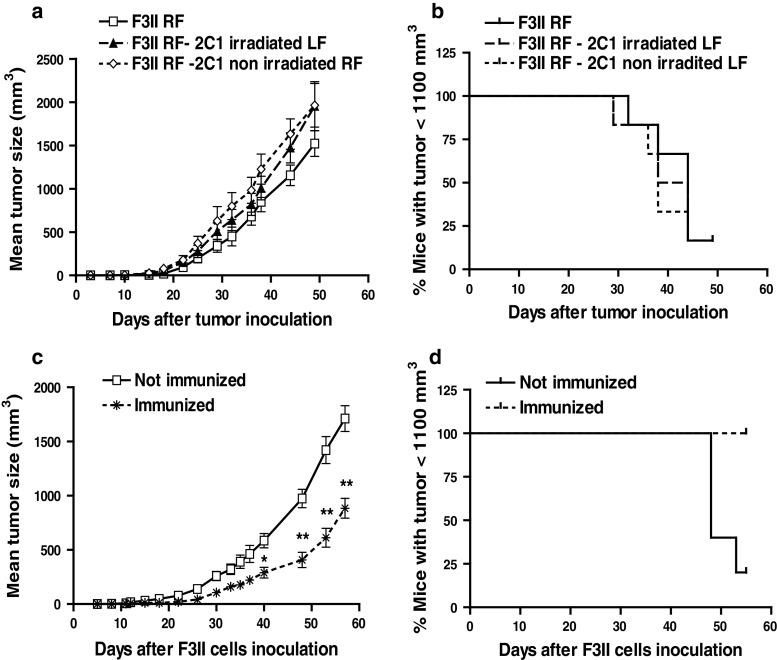
In order to assess whether the administration of 2C1 cells could prevent or reduce the F3II tumor growth, we administered irradiated or not irradiated 2C1 cells into the left flank and F3II cells into the RF of BALB/c mice. Tumor growth induced by F3II was the same despite the presence or not of irradiated or non-irradiated 2C1 cells into the opposite flank (Fig. 2a). Furthermore, no significant differences were observed in survival curves (Fig. 2b). Moreover, it is important to note that non-irradiated 2C1 cells did not generate tumor in any case in immunocompetent mice; even if F3II were inoculated in the opposite flank. Then, we analyzed whether tumor protection could be achieved by several pre-administration of 2C1 cells into immunocompetent mice. Therefore, 8 × 105 2C1 cells were administrated into BALB/c mice, once a week for 3 weeks; 15 days after the last 2C1 inoculation, F3II cells were administered into the opposite flank. Figure 2c shows that the inoculation of F3II cells into 2C1 immunized mice developed significantly smaller tumors than non-immunized mice. Remarkably, survival of tumor-bearing immunized mice was higher than non-immunized animals (Fig. 2d).
Fig. 2.
Immunotherapy with 2C1 cells reduces mammary tumor growth. a BALB/c mice were inoculated with 8 × 105 F3II cells into the right flank (RF) and simultaneously with 3 × 106 2C1 irradiated (F3II RF—2C1 irradiated LF) or non-irradiated cells into the left flank (F3II RF—2C1 non-irradiated LF). Mice receiving only F3II cells into the RF were used as control (F3II RF). Tumors were monitored for 49 days. It is shown the mean ± SD of one representative experiment of two with similar results. Each group contained five animals. c BALB/c mice were immunized by the inoculation of 2C1 cells three times once a week with 8 × 105 cells into the LF. Two weeks later of the last treatment with 2C1 cells, mice were challenged with 8 × 105 F3II cells into the opposite side. Tumor growth was measured as described in Fig. 1. (*P < 0.05 and **P < 0.01, unpaired Student’s t test). Data on (b) and (d) are expressed as Kaplan–Meier survival curves of (a) and (c) experiments, respectively
Discussion
We have described that mammary tumor cells genetically modified to express high levels of SLPI do not generate tumor in BALB/c mice. In the present study, we found that these cells grow and generate tumor in Nude mice. Moreover, the repeated administration of SLPI over-expressing cells reduced the growth of tumor generated by mock transfected cells and increased the survival of BALB/c mice.
The standard goal for a successful cancer vaccine is to induce an effective antigen-specific CD8 T-cell immune response. In the last years, several different strategies have been used in order to elicit this immune response against tumor cells. For instance, dendritic cells have been loaded with tumor antigens or apoptotic tumor cells and used as adjuvants for cancer patients [19, 20]. These immunotherapies have produced variable results, some demonstrating tolerance and others priming of CD8 responses [19–21]. The kind of immune response generated by the administration of 2C1 cells to BALB/c mice has not been elucidated in this preliminary work. However, the adaptive immune response is involved because 2C1 cells grow in Nude mice.
In our study, we have used cells producing human SLPI instead of mouse SLPI. It is probable that a human protein may elicit an immune response in mouse. However, it is not clear why cells producing and secreting a human protein, such as SLPI, would not grow in immunocompetent animals. The reasons that sustain the lack of an immune response against the cells producing human SLPI are as follows: (1) human SLPI has a 76% degree of homology with the mouse protein [22]; (2) 2C1 cells produce a secreted protein since the transfection of F3II tumor cells has been done with the full length human SLPI cDNA including the sequence codifying for the signal peptide. To our knowledge, the protein is not expressed on the surface of 2C1 cells; (3) the administration of a clone that secretes 50% less SLPI than 2C1 cells, grows in immunocompetent mice (data not shown); and (4) chronic administration of rhSLPI in rat and murine models of inflammation does not generate an immune response against the protein [26]. Therefore, it is unlikely that a putative immune response against hSLPI would be the reason of the lack of growth of 2C1 cells.
Another issue is that the kinetic of growth of 2C1 cells was slower than F3II cells in immunodeficient mice. Therefore, it is probable that other mechanisms beside the adaptive immune response are involved in the lack of tumor growth in BALB/c mice. Moreover, the lack of differences on tumor growth in the bystander experiments (Fig. 2a, b), suggests that the adaptive immune response may be effective only at the first steps of tumor development. The effect observed by the repeated administration of 2C1 followed by F3II inoculation does not prevent the development but significantly decreases the rate of tumor growth. Overall, these results suggest that the adaptive immune response may not be very efficient to eradicate but to harness the tumor growth.
2C1 cells are SLPI over-expressing cells. Although, SLPI plays a role in several tumor models as a pro or antitumoral factor [13, 15, 23, 24], we do not believe that SLPI was responsible for shaping the immune response elicited by the administration of 2C1 cells. This is supported by the following: (1) the lack of growth of 2C1 cells in BALB/c mice along the experiments; and (2) the undetectable levels of SLPI in the serum of mice inoculated with 2C1 (not shown). This issue could be address by the repetitive administration of non-producing SLPI 2C1 cells, such as irradiated 2C1 cells. However, it is probable that the repetitive inoculation of irradiated F3II could also induce a protective effect on tumor growth. These experiments will be done in the future, but none of them will undermine the results present herein that used live tumor cells as an immunotherapy strategy.
Apoptosis of tumor cells have been associated with tolerance or no immune response. However, more recently it has been shown that some part of the apoptotic process is required for generating an immune reaction. The degree of apoptosis seems to be important. A high level of apoptosis is generally highly immunogenic [25]. In this regard, we have shown that 2C1 cells are more prone to apoptosis than F3II cells in vitro. The apoptosis was more marked under serum starving conditions (not shown). Since the induction of in vivo tumor cell apoptosis is likely to be critical for developing effective tumor immunotherapy approaches [26], we suggest that the in vivo apoptosis of 2C1 cells induces a putative protective adaptive immune response. Further studies are required in order to unravel the immune response elicited by the immunization with 2C1 cells.
To our knowledge, this is the first study that shows that genetically modified SLPI over-expressing non-irradiated tumor cells which do not develop tumor in immunocompetent mice, act as a vaccine that partially restrain the tumor growth, probably by apoptosis that boosts an adaptive immune response.
Acknowledgments
To Dr. D. Alonso (National University of Quilmes, Quilmes, Buenos Aires, Argentina) for providing F3II cell line. This work was supported by grants from Universidad de Buenos Aires (UBACYT2008-2010, M011), AGENCIA (BID 1728/OC-AR PICT 1476 and 2331, PAE-PID-2007-00127).
References
- 1.Dillman RO, Beutel LD, Barth NM, de Leon C, O’Connor AA, DePriest C, Nayak SK. Irradiated cells from autologous tumor cell lines as patient-specific vaccine therapy in 125 patients with metastatic cancer: induction of delayed-type hypersensitivity to autologous tumor is associated with improved survival. Cancer Biother Radiopharm. 2002;17(1):51–66. doi: 10.1089/10849780252824073. [DOI] [PubMed] [Google Scholar]
- 2.Dillman RO, Nanci AA, Williams ST, Kim RB, Hafer RL, Coleman CL, Wang PC, Duma CM, Chen PV, Selvan SR, Cornforth AN, DePriest C. Durable complete response of refractory, progressing metastatic melanoma after treatment with a patient-specific vaccine. Cancer Biother Radiopharm. 2010;25(5):553–557. doi: 10.1089/cbr.2010.0819. [DOI] [PubMed] [Google Scholar]
- 3.Galili U. Autologous tumor vaccines processed to express alpha-gal epitopes: a practical approach to immunotherapy in cancer. Cancer Immunol Immunother. 2004;53(11):935–945. doi: 10.1007/s00262-004-0524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoenfeld J, Jinushi M, Nakazaki Y, Wiener D, Park J, Soiffer R, Neuberg D, Mihm M, Hodi FS, Dranoff G. Active immunotherapy induces antibody responses that target tumor angiogenesis. Cancer Res. 2010;70(24):10150–10160. doi: 10.1158/0008-5472.CAN-10-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnis ME, Rooney CM. Enhancement of dendritic cells as vaccines for cancer. Immunotherapy. 2010;2(6):847–862. doi: 10.2217/imt.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okada H, Attanucci J, Tahara H, Pollack IF, Bozik ME, Chambers WH, Lotze MT. Characterization and transduction of a retroviral vector encoding human interleukin-4 and herpes simplex virus-thymidine kinase for glioma tumor vaccine therapy. Cancer Gene Ther. 2000;7(3):486–494. doi: 10.1038/sj.cgt.7700140. [DOI] [PubMed] [Google Scholar]
- 7.Karpoff HM, Kooby D, D’Angelica M, Mack J, Presky DH, Brownlee MD, Federoff H, Fong Y. Efficient cotransduction of tumors by multiple herpes simplex vectors: implications for tumor vaccine production. Cancer Gene Ther. 2000;7(4):581–588. doi: 10.1038/sj.cgt.7700135. [DOI] [PubMed] [Google Scholar]
- 8.Williams SE, Brown TI, Roghanian A, Sallenave JM. SLPI and elafin: one glove, many fingers. Clin Sci. 2006;110(1):21–35. doi: 10.1042/CS20050115. [DOI] [PubMed] [Google Scholar]
- 9.Bouchard D, Morisset D, Bourbonnais Y, Tremblay GM. Proteins with whey-acidic-protein motifs and cancer. Lancet Oncol. 2006;7(2):167–174. doi: 10.1016/S1470-2045(06)70579-4. [DOI] [PubMed] [Google Scholar]
- 10.Cheng WL, Wang CS, Huang YH, Liang Y, Lin PY, Hsueh C, Wu YC, Chen WJ, Yu CJ, Lin SR, Lin KH. Overexpression of a secretory leukocyte protease inhibitor in human gastric cancer. Int J Cancer. 2008;123(8):1787–1796. doi: 10.1002/ijc.23746. [DOI] [PubMed] [Google Scholar]
- 11.Zhang D, Simmen RC, Michel FJ, Zhao G, Vale-Cruz D, Simmen FA. Secretory leukocyte protease inhibitor mediates proliferation of human endometrial epithelial cells by positive and negative regulation of growth-associated genes. J Biol Chem. 2002;277(33):29999–30009. doi: 10.1074/jbc.M203503200. [DOI] [PubMed] [Google Scholar]
- 12.Israeli O, Goldring-Aviram A, Rienstein S, Ben-Baruch G, Korach J, Goldman B, Friedman E. In silico chromosomal clustering of genes displaying altered expression patterns in ovarian cancer. Cancer Genet Cytogenet. 2005;160(1):35–42. doi: 10.1016/j.cancergencyto.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Hu Y, Sun H, Drake J, Kittrell F, Abba MC, Deng L, Gaddis S, Sahin A, Baggerly K, Medina D, Aldaz CM. From mice to humans: identification of commonly deregulated genes in mammary cancer via comparative SAGE studies. Cancer Res. 2004;64(21):7748–7755. doi: 10.1158/0008-5472.CAN-04-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugino T, Yamaguchi T, Ogura G, Kusakabe T, Goodison S, Homma Y, Suzuki T. The secretory leukocyte protease inhibitor (SLPI) suppresses cancer cell invasion but promotes blood-borne metastasis via an invasion-independent pathway. J Pathol. 2007;212(2):152–160. doi: 10.1002/path.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang N, Thuraisingam T, Fallavollita L, Ding A, Radzioch D, Brodt P. The secretory leukocyte protease inhibitor is a type 1 insulin-like growth factor receptor-regulated protein that protects against liver metastasis by attenuating the host proinflammatory response. Cancer Res. 2006;66(6):3062–3070. doi: 10.1158/0008-5472.CAN-05-2638. [DOI] [PubMed] [Google Scholar]
- 16.Kluger HM, Kluger Y, Gilmore-Hebert M, DiVito K, Chang JT, Rodov S, Mironenko O, Kacinski BM, Perkins AS, Sapi E. cDNA microarray analysis of invasive and tumorigenic phenotypes in a breast cancer model. Lab Invest. 2004;84(3):320–331. doi: 10.1038/labinvest.3700044. [DOI] [PubMed] [Google Scholar]
- 17.Alonso DF, Farias EF, Urtreger A, Ladeda V, Vidal MC, Joffe EBDK. Characterization of F3II, a sarcomatoid mammary carcinoma cell line originated from a clonal subpopulation of a mouse adenocarcinoma. J Surg Oncol. 1996;62(4):288–297. doi: 10.1002/(SICI)1096-9098(199608)62:4<288::AID-JSO14>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Duke RC. Morphological and biochemical assays of apoptosis. Current Protocols in Immunology. New York: Greene Publishing and Wiley-Interscience; 1992. [Google Scholar]
- 19.Zanetti M, Castiglioni P, Ingulli E. Principles of memory CD8 T-cells generation in relation to protective immunity. Adv Exp Med Biol. 2010;684:108–125. doi: 10.1007/978-1-4419-6451-9_9. [DOI] [PubMed] [Google Scholar]
- 20.Turtle CJ, Riddell SR. Artificial antigen-presenting cells for use in adoptive immunotherapy. Cancer J. 2010;16(4):374–381. doi: 10.1097/PPO.0b013e3181eb33a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang FP, Chen YX, To CK. Guiding the “misguided” - functional conditioning of dendritic cells for the DC-based immunotherapy against tumours. Eur J Immunol. 2010;41(1):18–25. doi: 10.1002/eji.201040543. [DOI] [PubMed] [Google Scholar]
- 22.Wright CD, Kennedy JA, Zitnik RJ, Kashem MA. Inhibition of murine neutrophil serine proteinases by human and murine secretory leukocyte protease inhibitor. Biochem Biophys Res Commun. 1999;254(3):614–617. doi: 10.1006/bbrc.1998.0108. [DOI] [PubMed] [Google Scholar]
- 23.Devoogdt N, Rasool N, Hoskins E, Simpkins F, Tchabo N, Kohn EC. Overexpression of protease inhibitor-dead secretory leukocyte protease inhibitor causes more aggressive ovarian cancer in vitro and in vivo. Cancer Sci. 2009;100(3):434–440. doi: 10.1111/j.1349-7006.2009.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura K, Takamoto N, Hongo A, Kodama J, Abrzua F, Nasu Y, Kumon H, Hiramatsu Y. Secretory leukoprotease inhibitor inhibits cell growth through apoptotic pathway on ovarian cancer. Oncol Rep. 2008;19(5):1085–1091. [PubMed] [Google Scholar]
- 25.Bonnotte B, Favre N, Moutet M, Fromentin A, Solary E, Martin M, Martin F. Bcl-2-mediated inhibition of apoptosis prevents immunogenicity and restores tumorigenicity of spontaneously regressive tumors. J Immunol. 1998;161(3):1433–1438. [PubMed] [Google Scholar]
- 26.Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, Frelinger JA, Robinson BW. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170(10):4905–4913. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]