Abstract
Objective
We report a truly quantitative technology for PD-L1 expression in non-small cell lung cancer (NSCLC). In addition, we present a non-enzymatic technology that creates a cell suspension from fresh tumor tissue so that either fine-needle aspiration (FNA) or fresh tissue can be used in this assay.
Methods
Non-enzymatic tissue homogenization (IncellPREP; IncellDx, Menlo Park, California) was performed on 4-mm punch biopsies. An FNA was taken from the same tumor to create matched sample sets. Cells were labeled with antibodies directed against CD45, PD-1, and PD-L1 and then stained with DAPI to identify intact, single cells, and to analyze cell cycle.
Results
Comparing the IncellPREP homogenization and FNA demonstrated a strong correlation (r 2 − 0.8) for expression of PD-L1. We compared PD-L1 expression by flow cytometry using a 1 % cutoff for positivity in the tumor cell population and a 1 % cutoff of cells with at least 1+ intensity in immunohistochemically stained tissue sections as positive. Ten of 12 lung tumor samples were concordant while 2 were discordant. PD-L1 expression by flow cytometry varied widely (1.2–89.4 %) even in the positive concordant cases. In addition, PD-L1 expression in the aneuploid tumor population did not necessarily agree with the expression in the diploid tumor population.
Summary
Fine, unequivocal quantification of PD-L1 on tumor and immune cells in NSCLC may allow for better prediction of response to therapies. The present study also offers a technology that can create a universal sample type from either FNA or fresh tissue.
Keywords: PD-L1, Non-small cell lung cancer, Immune cells, PD-1, Cell cycle
Introduction
Recently, a number of solid tumors including renal cell carcinoma (RCC), melanoma, ovarian cancer, and non-small cell lung cancer (NSCLC) have been shown to express programmed cell death ligand 1 (PD-1) which, through its receptor PD-L1, creates a local immunosuppressive microenvironment by evading immune checkpoints and results in a poorer prognosis [1–3]. A number of therapies block the PD-L1/PD-1 engagement and also activate the immune system to attack tumors [4–6], but the onus is now to determine which patients would benefit from these new pharmacologic agents. Furthermore, using diagnostic technology to quantify the expression of PD-L1 has the potential to spare patients from ineffective therapy and possible adverse autoimmune effects from these agents.
PD-1 is a protein encoded by the PDCD1 gene and is a cell surface receptor expressed on T cells. PD-1 binds two ligands, PD-L1 and PD-L2. PD-1 functions as an immune checkpoint by preventing the activation of T cells, which reduces autoimmunity and promotes self-tolerance [3, 7]. Expression of PD-L1 in NSCLC has diagnostic and prognostic significance given the favorable response of tumors expressing this marker to immune checkpoint inhibitor therapy [8]. PD-L1 expression is currently determined by immunohistochemistry (IHC) to identify patients who would respond to immune checkpoint inhibition. PD-L1 IHC as a companion diagnostic to therapy has been problematic in determining which patients will be responsive to therapy. Issues with this method include subjectivity of the reviewer; processing variability; differences in semiquantitative cutoffs; and staining of tumor, immune cell, and even stromal cells. The range of PD-L1 IHC expression ranges from 14 to 100 % making predictive drug response decisions difficult [9–13]. Even more significant, PD-L1 consists of 2 hydrophilic binding sites [14], which makes the use of anti-PD-L1 antibodies in formalin-fixed, paraffin-embedded (FFPE) specimens challenging. All these variables contribute to variability in quantification of the PD-L1 expression on tumor samples.
Other factors present in the tumor microenvironment such as the quantity of tumor-infiltrating lymphocytes (TIL) may also contribute to therapeutic response [15, 16]; thus, a multiparameter approach to personalizing therapy is highly desirable. In the present study, we demonstrate a novel assay consisting of non-enzymatic sample preparation that creates a single-cell suspension of fresh cells from NSCLC tumors. From this single-cell suspension, we then quantified total number of immune cells and PD-L1/PD-1 expression on both immune cell subsets and on tumor cells. These data are compared to PD-L1 IHC.
Materials and methods
Samples
Fresh tissues were obtained from 12 NSCLC cases by Spectrum Health (Grand Rapids, Michigan) following informed consent. Date of collection, age, sex, ethnicity, diagnosis, primary tumor size, and American Joint Committee on Cancer (AJCC) classification were recorded. Tissues were excised and then stored in Roswell Park Memorial Institute (RPMI) 1640 medium at 2–8 °C prior to overnight shipment to IncellDx on cold packs.
Tissue dissociation using IncellPREP
Tumor biopsies of at least 2 cm were placed in RPMI for transport after which 4-mm punches were taken from each tissue, and placed in 2-mL Eppendorf tubes containing 800 μL Dulbecco’s phosphate-buffered saline (DPBS). IncellPREP (IncellDx, Inc.) tissue homogenizers were inserted into each tube and set to run at 1 V until supernatant appeared cloudy (5–10 min). After tissue homogenization, a Cellometer (Auto T4, Nexcelom Bioscience, Inc.) was used to calculate cellular concentration, and cell counts were recorded. Supernatant was then removed and transferred to a separate tube before centrifugation at 300×g for 5 min. Following centrifugation, supernatant was aspirated, and cell pellet was resuspended in IncellPREP reagent at a concentration of 1 × 106 cells/mL and incubated at room temperature for 1 h prior to staining.
PD-L1/PD-1 staining of single-cell suspensions
Following fixation for 1 h in IncellFP (IncellDx, Inc.), 200 µL of sample equivalent to 200,000 cells was aliquoted to 12 × 75 mm tubes and subsequently washed with 1 mL of DPBS. Samples were then stained with PD-1 Alexa 488 (clone EH12.2H7, Biolegend, Inc.), PD-L1 PE (clone 29E.2A3, Biolegend, Inc.), and CD-45 PE/Cy7 (clone H130, Biolegend, Inc.)-conjugated antihuman antibodies in DPBS + 2 % bovine serum albumin (BSA) and then incubated for 30 min at room temperature in the dark. Next, 1 mL of DPBS + 2 % BSA was added to each tube and incubated at room temperature for 5 min, prior to centrifugation at 600×g for 5 min. Supernatant was aspirated, and a wash with DPBS + 2 % BSA was repeated once. Following this wash, 200 µL of 4′,6-diamidino-2-phenylindole (DAPI) at 1 µg/mL was added to each sample and incubated at room temperature for 30 min in the dark.
Flow cytometry
Cells were first analyzed on an EC800 Flow Cytometer (Sony Biosciences, Inc.) using a DAPI-Lin by DAPI-Peak-Lin density plot to set a gate on nucleated single cells. That gate was then applied to a CD45 by side scatter density plot to separate CD45+ cells (immune cells) from CD45− cells (putative tumor cells). Once those two populations were determined, each was analyzed for PD-1 and PD-L1 expression by forward scatter. Normal expression was determined by the level of expression on the CD45+ population, and gating was set. That gating was applied to the CD45− population, and PD-1 and PD-L1 expression above the normal cutoff was recorded.
Immunohistochemistry
FFPE blocks were sectioned at 5 μm thickness. Slides were deparaffinized in a series of xylenes and progressively diluted alcohols to water. After rinsing with DI water, the slides were treated with citrate-based antigen retrieval solution that was preheated to 65 °C. Slides were incubated in the preheated citrate buffer and heated for 20 min at 99 °C and cooled down for 20 min before commencing IHC staining on the Dako autostainer. Endogenous peroxidase was quenched with KPL solution, followed by protein blocking and rabbit anti-PD-L1 antibody (clone SP142, Ventana, Inc.) incubation for 1 h at room temperature. Relevant non-specific isotype control was used as negative control. After rinsing the primary antibodies, the slides were incubated with biotin-conjugated secondary antibody and HRP-enzyme-conjugated streptavidin, followed by incubation with diaminobenzidine (DAB) to visualize the signal. Upon completion of the IHC staining, the slides were dehydrated in a series of alcohols and xylenes followed by coverslipping for microscopic evaluation.
Results
Cell recovery using IncellPrep
To determine the advantages of non-enzymatic cell homogenization compared with standard fine-needle aspiration, we performed counts of cell suspensions derived from a French technique fine-needle aspiration (FNA) and from IncellPREP non-enzymatic homogenization using a Cellometer cell counter. As demonstrated in Table 1, both techniques generated greater than 106 cells/mL using standardized techniques with the IncellPREP yields being 2.5 times the number of cells per preparation compared with FNA with the difference being statistically significant (Mann–Whitney, p = 0.003). The yields from both techniques were satisfactory for the downstream assays performed in this study.
Table 1.
Comparison of cell yields from FNA and IncellPREP homogenization of fresh tumor tissue
| Sample ID | FNA (cells/mL) | IncellPrep (cells/mL) |
|---|---|---|
| 751 | 4.57E+05 | 4.17E+06 |
| 772 | 2.58E+05 | 7.83E+06 |
| 42 | 1.01E+06 | 3.40E+06 |
| 56 | 9.20E+05 | 5.02E+06 |
| 88 | 1.27E+06 | 5.78E+06 |
| 251 | 1.02E+06 | 4.13E+06 |
| 287 | 2.06E+06 | 3.76E+06 |
| 343 | 1.67E+06 | 5.79E+06 |
| 419 | 1.14E+06 | 8.37E+06 |
| 478 | 2.53E+06 | 4.77E+06 |
| 486 | 6.81E+06 | 5.07E+06 |
| 496 | 6.46E+06 | 3.79E+06 |
| Mean | 1.21E+06 | 4.90E+06 |
On average, greater than 106 cells/mL were recovered from both specimen types with a statistically significant increase in yield from the IncellPrep specimens
Quantification of cell-type-specific PD-L1 and PD-1
To address the difficulty of quantifying immuno-oncology markers in tissue with heterogeneous cell populations, we labeled cells in suspension from both FNA and IncellPREP homogenization with biomarkers that distinguish between immune cells and tumor cells. We further multiplexed PD-L1 and PD-1 markers to unequivocally quantify the expression of these markers on the pre-identified immune or tumor cells (See Fig. 1). As demonstrated in Table 2, we found a range of expression levels between cell types and between samples using IncellPrep-prepared cell suspensions. PD-L1 expression ranged from 0 to 31 % on immune cells and from 0 to 89 % on tumor cells. The expression of PD-L1 was independent of tumor histology. PD-1 expression ranged from 0 to 10 % on immune cells and from 0 to 47 % on tumor cells. Similarly, PD-1 expression was independent of tumor histology. Comparing the IncellPREP homogenization to cells obtained by FNA yielded a strong correlation (r 2 − 0.8) for expression of the immuno-oncology response marker PD-L1 (see Fig. 2). Interestingly, the expression of PD-L1 in the aneuploidy population of a particular tumor was higher in all six aneuploidy cases as identified in Table 2 with a numeric DNA index for the aneuploid population. In the six cases, the range of PD-L1 expression on the aneuploidy tumor cell population was 9–70 % higher than the expression on all tumor cells taken together (data not shown).
Fig. 1.
PD-L1 quantification of IncellPREP-prepared cell suspension by flow cytometry. a Immune cells were electronically separated from tumor cells by CD45 antibody staining and side scatter (ss). Tumor cells (red box) were gated for PD-L1 expression in b, c. PD-L1 expression in tumor cells varied from low (b) to high (c) as listed in Table 2
Table 2.
Expression of PD-L1 and PD-1 in tumor and immune cells from IncellPrep-prepared cell suspensions
| Sample ID | Histology | % CD45+ | % CD45− (tumor) | DNA Index CD45− | DNA Index CD45− aneuploid | CD45+ (immune) PD-L1 % | Tumor PD-L1 % | CD45+ (immune) PD-1 % | Tumor PD-1 % |
|---|---|---|---|---|---|---|---|---|---|
| 751 | SCC | 74.07 | 12.17 | 0.94 | 1.64 | 2.63 | 3.97 | 0.56 | 0 |
| 772 | AC | 19 | 49.55 | 0.97 | None | 3.98 | 1.22 | 1.06 | 0.31 |
| 42 | AC | 22.61 | 65.58 | 0.9 | 2.18 | 30.69 | 63.98 | 2.04 | 6.39 |
| 56 | AC | 59.45 | 30.06 | 0.96 | 1.4 | 26.18 | 69.81 | 1.24 | 12.53 |
| 88 | SCC | 46.54 | 45.53 | 0.95 | 1.34 | 23.65 | 89.44 | 1.71 | 0.88 |
| 251 | SCC | 15.07 | 28.75 | 1.01 | None | 0.18 | 13.74 | 0 | 0 |
| 287 | AS | 23.34 | 58.62 | 0.93 | None | 2.42 | 0.77 | 0 | 0 |
| 343 | SCC | 25.72 | 47.84 | 0.94 | None | 0.97 | 0.52 | 0 | 0 |
| 419 | AC | 32.03 | 30.53 | 1.2 | None | 0.8 | 5.63 | 0 | 0 |
| 478 | AC | 20.8 | 70.8 | 0.87 | None | 0.47 | 0 | 0.54 | 0.86 |
| 486 | AC | 88.52 | 8.81 | 0.91 | None | 5.07 | 25.77 | 3.97 | 47.31 |
| 496 | AC | 37.25 | 44.74 | 0.96 | 1.48 | 0 | 0.06 | 0 | 0.06 |
SCC squamous cell carcinoma, AC adenocarcinoma, AS adenosquamous carcinoma
Fig. 2.
Comparison of matched FNA and IncellPREP-prepared cells for PD-L1 demonstrated strong correlation (r 2 − 0.8) independent of the starting sample type. Aneuploid tumors are indicated with red circles
Quantification of PD-L1 by flow cytometry compared to immunohistochemistry
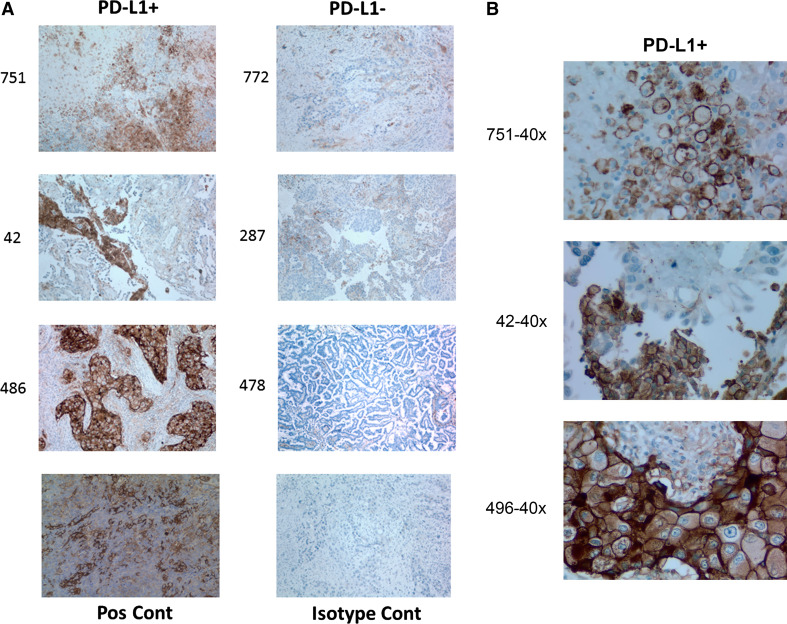
To directly compare PD-L1 expression on lung tumors homogenized with IncellPREP with PD-L1 expression in tissue sections from the same tumor, we used standardized methodology for PD-L1 expression on tissue. Specifically, a characteristic positive stain shows a brown, rim pattern as shown in Fig. 3. Table 3 compares PD-L1 expression by flow cytometry using a 1 % cutoff for positivity in the tumor cell population and a 1 % cutoff of cells with characteristic rim staining in immunohistochemically stained tissue sections as positive. Using these criteria, 11 of 12 samples were concordant with the one discordant case being a tumor with extensive necrosis giving a positive result by IHC and a negative result by flow cytometry.
Fig. 3.
a Immunohistochemical staining for PD-L1 in tissue blocks from matched flow cytometry samples. PD-L1-positive and PD-L1-negative tissues are shown (×200). Positive staining is indicated by a brown precipitate. A positive control tonsil sample and an isotype control (without primary antibody) are also shown. b High power view of characteristic rim pattern PD-L1 staining in tissues shown in a
Table 3.
Determination of tumor expression of PD-L1 using published cutoffs for IHC and flow cytometric cutoffs as established in this study
| Sample ID | IHC qualitative call (1 % CO) | Flow cytometry quantitative call (1 % CO) |
|---|---|---|
| 751 | Pos | Pos |
| 772 | Neg | Neg |
| 42 | Pos | Pos |
| 56 | Pos | Pos |
| 88 | Pos | Pos |
| 251 | Pos | Pos |
| 287 | Neg | Neg |
| 343 | Pos | Neg |
| 419 | Pos | Pos |
| 478 | Neg | Neg |
| 486 | Pos | Pos |
| 496 | Neg | Neg |
Discussion
NSCLC accounts for the majority of lung cancer cases and includes various histologic types, including squamous cell carcinoma, adenocarcinoma, and large cell carcinoma [17]. The most common diagnostic sample types for NSCLC are FNA and biopsy [18, 19]. The majority of diagnoses are still performed by FNA because of the advanced stage and because of the widespread use of bronchoscopy [20–22]. The common use of FNA raises concern over the potential inequities of histologic typing of NSCLC and personalized diagnostics to inform treatment decisions that are based on immunohistochemistry on tissue blocks. Here, we present novel technology for creating equivalent cell suspension samples from both FNA samples and homogenized whole biopsy samples. We demonstrated a correlation for PD-L1 expression of >0.8 for matched FNA and solid tumor samples that have been converted to a cell suspension using the IncellPREP kit. As expected, the tumor suspension yielded 0.5 logs more cells than a standard French method FNA, though both techniques generated more than enough cells to perform the flow cytometry-based analysis.
A possible concern with the two sampling approaches (FNA vs. biopsy) is the ability to determine the histologic subtype of NSCLC (e.g., squamous cell or adenocarcinoma), which has been shown in some studies to exhibit different responses to therapies such as pemetrexed [23]. Recent data on the accuracy of FNA cytology, however, demonstrated that cytologic and histological typing was concordant in 88 % of cases with statistical significance [24]. Further, as it relates to PD-L1 as a biomarker for PD-L1 antagonists, PD-L1-directed therapy is equally effective on all histologic subtypes of NSCLC [23]. In particular, a meta-analysis looking at PD-L1 expression in NSCLC found no correlation with a squamous cell or adenocarcinoma histologic type [25]. Similarly, in the present study, we did not see a correlation between PD-L1 expression on tumor cells and histologic type.
Current immunotherapies directed against PD-L1 focus on the use of IHC, which has been used for decades in anatomic pathology for qualitative assessment of cell lineage-specific markers. The pitfalls of IHC for quantification of antigens are numerous and well documented [9]. The sources of variability start in the pre-analytical phase of IHC which include tissue procurement from the operating room, time, and temperature prior to fixation, and, most importantly, the type and length of fixation. Cross-linking, aldehyde-based fixatives are still the overwhelming source of tissue fixatives used to create slides for morphologic assessment and slides for additional stains including IHC. During fixation, tissue antigens are cross-linked by the aldehydes, which can destroy the epitopes of interest for some applications often requiring antigen retrieval reagents (in order to recover staining of the target antigen) [8].
In our study, we present an assay system that is automated, fully quantitative, and capable of distinguishing expression of important biomarkers on the major cell types present (including both tumor cells and immune cells) within the tumor microenvironment. CD45+ immune cells ranged in percentage from ~15 to 88 %.
We demonstrated that the 1 % cutoff for both IHC and our flow-based PD-L1 assay correlates in 11 out of 12 cases. However, the percentage of tumor cells positive for PD-L1 expression using the truly quantitative flow method ranged from 0.5 to 89 %. Using the 1 % cutoff for both IHC and flow cytometry, we demonstrated overall a >0.9 correlation of tumor PD-L1 compared to IHC with the added attribute of quantification on immune cells and on aneuploidy populations of the tumor. These data suggest that a more quantitative assay could allow for more precise and more quantitative prediction of response to therapeutic modalities.
Most disturbing concerning the use of PD-L1 IHC are the cases of individuals with PD-L1 tumors including melanoma, renal cell, and NSCLC who respond to PD-L1 antagonist when the IHC is negative for tumor expression of PD-L1 [9]. Fine quantification of PD-L1 expression may allow for more precise establishment and less subjective cutoffs. Though use of the approach to PD-L1 quantification as presented in the current study awaits outcome studies to test the relevance of more quantitative information for response to treatment, studies have shown that PD-L1 expression aside from its quantification as an indication for therapy may be an independent prognostic marker [8].
The present study used clinically available instrumentation without the need for sophisticated image analysis, making clinical utility straightforward. Unlike IHC, a method for standardization of complex flow cytometric assays has been described that allows for interinstitutional proficiency and quality assurance. This process will be implemented to ensure the current assay results apply across laboratories [26].
Acknowledgments
The authors thank Mark R. Vogel, M.A., a medical communications specialist in San Francisco, for his editorial assistance with the manuscript.
Abbreviation
- AJCC
American Joint Committee on Cancer
- DAB
Diaminobenzidine
- DAPI
4′,6-Diamidino-2-phenylindole
- DPBS
Dulbecco’s phosphate-buffered saline
- FFPE
Formalin-fixed, paraffin-embedded
- FNA
Fine-needle aspiration
- IHC
Immunohistochemistry
- NSCLC
Non-small cell lung cancer
- PD-L1
Programmed cell death ligand 1
- RCC
Renal cell carcinoma
- RPMI
Roswell Park Memorial Institute
- TIL
Tumor-infiltrating lymphocytes
Compliance with ethical standards
Conflict of interest
Amanda Chargin, Rian Morgan, Keith Shults, and Bruce K. Patterson are employees of IncellDx, Inc., Ellen Tsay was an uncompensated intern of IncellDx, Inc., Uma Sundram and Navneet Ratti declare that they have no conflict of interest.
References
- 1.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 2.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2010;28:682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 3.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velcheti V, Schalper KA, Caravajal DE. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2013;94:107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 10.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm0902-1039c. [DOI] [PubMed] [Google Scholar]
- 11.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips T, Simmons P, Inzunza HD, et al. Development of an automated PD-L1 immunohistochemistry (IHC) assay for non-small cell lung cancer. Appl Immunohistochem Mol Morphol. 2015;23:541–549. doi: 10.1097/PAI.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z, Schwartz J-CD, Guo X, et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity. 2004;20:337–347. doi: 10.1016/S1074-7613(04)00051-2. [DOI] [PubMed] [Google Scholar]
- 15.Avci N, Deligonul A, Tolunay S, et al. Prognostic impact of tumor lymphocytic infiltrates in patients with breast cancer undergoing neoadjuvant chemotherapy. J BUON. 2015;20:994–1000. [PubMed] [Google Scholar]
- 16.Pham CD, Flores C, Yang C, et al. Differential immune microenvironments and response to immune checkpoint blockade amongst molecular subtypes of murine medulloblastoma. Clin Cancer Res. 2015;22:582–595. doi: 10.1158/1078-0432.CCR-15-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jemal A, Siegel R, Xu J, et al. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 18.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 19.Travis W, Rekhtman N. Pathological diagnosis and classification of lung cancer in small biopsies and cytology: strategic management of tissue for molecular testing. Semin Respir Crit Care Med. 2011;32:22–31. doi: 10.1055/s-0031-1272866. [DOI] [PubMed] [Google Scholar]
- 20.Jones AM, Hanson IM, Armstrong GR, O’Driscoll BR. Value and accuracy of cytology in addition to histology in the diagnosis of lung cancer at flexible bronchoscopy. Respir Med. 2001;95:374–378. doi: 10.1053/rmed.2001.1051. [DOI] [PubMed] [Google Scholar]
- 21.Rivera MP, Mehta AC. Initial diagnostics of lung cancer. Chest. 2007;132:131S–148S. doi: 10.1378/chest.07-1357. [DOI] [PubMed] [Google Scholar]
- 22.Vazquez MF, Koizumi JH, Henschke CI, Yankelevitz DF. Reliability of cytologic diagnosis of early lung cancer. Cancer. 2007;111:252–258. doi: 10.1002/cncr.22767. [DOI] [PubMed] [Google Scholar]
- 23.Scagliotti G, Hanna N, Fossella F, Sugarman K, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two phase III studies. Oncologist. 2009;14:253–263. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 24.Nizzoli R, Tiseo M, Gelsomino F, et al. Accuracy of fine needle aspiration cytology in the pathological typing of non-small cell lung cancer. J Thorac Oncol. 2011;6:489–493. doi: 10.1097/JTO.0b013e31820b86cb. [DOI] [PubMed] [Google Scholar]
- 25.Pan Z-K, Ye F, Wu X, An H-X, Wu J-X. Clinicopathological and prognostic significance of programmed cell death ligand 1 (PD-L1) expression in patients with non-small cell lung cancer: a meta-analysis. J Thorac Dis. 2015;7:462–470. doi: 10.3978/j.issn.2072-1439.2015.02.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shults KE, Miller DT, Davis BH, et al. A standardized ZAP-70 assay-lessons learned in the trenches. Cytom B Clin Cytom. 2006;70:276–283. doi: 10.1002/cyto.b.20136. [DOI] [PubMed] [Google Scholar]