Abstract
The infiltration of tumors by lymphocytes is a prognosis factor in colorectal cancer (CRC). The magnitude and quality of this infiltration have emerged as important component of the clinical outcome in these patients. Specifically, markers associated with functional cell-mediated immunity, i.e., a Th1 immune response, are independent markers of better prognosis, whereas Th17-associated components are deleterious and correlate with poorer survival. Mucosal-associated invariant T (MAIT) cells are a recently described T cell subset with tissue-homing properties. They display a restricted TCR repertoire specific for widely conserved microbial ligands, and display anti-bacterial properties upon release of Th1-like, Th17-like, and/or cytotoxic granules. MAIT-cell-specific transcripts have been found in kidney and brain cancer, but have not been studies in other sites. In this study, we retrospectively analyzed by confocal microscopy the presence of MAIT cells within colorectal tumors as compared with paired healthy tissues. We observed a significant although variable increase, both in density and in proportion of overall tumor-infiltrating T lymphocytes inside the tumors. Importantly, survival curves as well as multivariate analysis showed that patients displaying a higher recruitment of MAIT cells in their tumor, as compared with the neighboring healthy tissue, showed a less favorable clinical outcome. This study suggests that including MAIT-cell-specific markers or transcripts in the analysis of tumor-infiltrating lymphocytes could be a benefit to the diagnosis and follow-up of CRC patients.
Keywords: Colorectal cancer, Tumor-infiltrating lymphocytes, Mucosal-associated invariant T cells, MAIT cells, Prognosis, IL-17
Introduction
Solid tumors can be infiltrated by lymphocytes at variable levels. The presence of tumor-infiltrating lymphocytes (TIL), mostly T cells, has been historically interpreted as the demonstration of the immunogenicity of cancer cells and a subsequent anti-tumoral immune response. Major studies from many groups have then demonstrated the existence of tumor-associated antigens recognized by T cells, uncovering the mechanisms by which the adaptive immune system can detect and destroy cancer cells. However, the presence of TIL is seldom associated with cancer regression. Numerous studies, mostly in the past decade, have addressed this apparent contradiction and have provided a highly complex and dynamic picture of the relationship between the immune system and tumors [1]. Schematically, the presence of lymphocytes by itself is not sufficient to drive tumor regression; the outcome depends upon the precise composition, as well as the location, of these lymphocytes inside tumors [2]. An effective anti-tumoral response requires a Th1-like differentiation of T cells and an infiltration by both CD4+ and cytolytic CD8+ T cells [3], however, TIL often include ineffective Th2 T cells, as well as regulatory T cells (Tregs), which may prevent the action of anti-tumoral T cells [4–9]. IL-17-producing cells apparently show a dual effect on tumor prognosis, depending on the cancer type; they are seemingly deleterious in lung or colorectal cancer (CRC), whereas they may promote or help cancer regression in other situations such as ovarian or esophageal cancers [10–13].
Seminal studies by Galon et al. in CRC have shown that it may be possible to use several immune system markers to improve prognosis of patients, an analysis called the immunoscore. They elegantly showed that an abundance of Th1/Tc1/cytolytic T cells, detected by markers such as CD8, CD45RO, IFNγ, and granzyme B, is an independent factor of better prognosis in CRC, whereas numerous Th17-associated transcripts are associated with a poor outcome [2, 10, 14]. Therefore, it is of great importance to deepen our analysis of in situ immune responses when new T cell subsets, not yet studied in this context, are identified.
MAIT cells are innate-like T cells with a restricted antigenic repertoire, specific for highly conserved microbial antigens presented by a monomorphic MHC class I-like molecule, MHC-related 1 (MR1) [15]. They display potent anti-bacterial and anti-fungal functions and probably represent an important first line of defense against microbial infections at mucosal surfaces [16, 17]. Indeed, they are abundant in the human blood but also in the liver and the gut lamina propria; furthermore, they may be rapidly recruited at sites of inflammation [18]. Activation and, in some cases, recruitment of MAIT cells have been shown in infectious diseases [19–21], but also in autoimmune/inflammatory pathologies, such as inflammatory bowel diseases, multiple sclerosis, and psoriasis [22–25]. Interestingly, one publication also reported the presence of MAIT-cell-specific transcripts in brain and kidney tumors [26]. MAIT cells display a mixed Tc1/Tc17 phenotype and function: They are CD8+, effector/memory T cells, and they mostly produce IFNγ and TNFα upon stimulation; however, they can be cytolytic and secrete IL-17 after specific stimuli; furthermore, they express Th17-associated markers, such as RAR-related orphan receptor gamma t (RORγt) and CD161 [18]. MAIT cells have never been specifically studied in the context of CRC. Interestingly, at least in the blood of healthy subjects, they express both markers identified as good (CD8, granzyme, CD45RO) and bad prognosis (RORγt) in CRC.
In this study, we retrospectively performed a descriptive analysis of MAIT cells inside a series of colorectal tumors and paired healthy intestinal tissue. We show that MAIT cells are specifically recruited inside tumors in CRC, but the level of infiltration is highly variable. Importantly, we found that an important infiltration by MAIT cells is correlated with a poorer outcome in patients, suggesting that they could represent an additional easy-to-use marker of prognosis in the context of CRC.
Materials and methods
Patients
This retrospective study included 35 patients undergoing surgery for CRC in the period 2005–2011. All patients gave prior informed consent. Relevant demographic and clinical data were collected and are listed in Table 1. The TNM stage was estimated using guidelines from the Union for International Cancer Control (UICC). Disease-free survival was defined as the time (in months) between surgery and any disease-related event, i.e., relapse or death. Overall survival was defined as the time between surgery and cancer-related death. We analyzed for each patients paired biopsies collected within the tumor and in the adjacent healthy tissue.
Table 1.
Demographic, clinical, and histological characteristics of the patients analyzed in the study
| Total number | 35 |
|---|---|
| Sex (n) | |
| Male | 18 |
| Female | 17 |
| Mean age at diagnosis | 72 |
| Min | 51 |
| Max | 89 |
| Histology (n) | |
| Glandular | 33 |
| Mucinous | 2 |
| Tumor grade (n) | |
| Well differentiated | 21 |
| Moderately differentiated | 11 |
| Poorly differentiated | 3 |
| Tumor localization (n) | |
| Right | 13 |
| Left | 12 |
| Sigmoid | 5 |
| Unknown | 5 |
| Tumor infiltration (n) | |
| T3 | 15 |
| T4 | 20 |
| TNM stage (n) | |
| I | 0 |
| II | 10 |
| III | 17 |
| IV | 8 |
| DFS median (months) | 24 |
| OS median (months) | 80 |
Reagents
The detection of lymphocytes in biopsies was performed on 10-μm formaldehyde-fixed frozen sections. The antibody panel included polyclonal rabbit anti-CD3 (Dako), polyclonal goat anti-CD26 (R&D Systems), and monoclonal anti-Vα7.2 (Biolegend) detected by the respective secondary antibodies: donkey anti-rabbit AlexaFluor 546, donkey anti-goat AlexaFluor 488, and donkey anti-mouse A647 (Invitrogen).
Confocal microscopy
The analyses were performed by a trained technician without knowledge of the origin (tumoral or healthy) of the biopsies. Fields were chosen randomly but according to the T cells density, such that a minimum of 300 CD3+ T cells/biopsy could be counted. Three-to-six fields were analyzed per biopsies. MAIT cells were identified and counted as CD3+CD26+Vα7.2+ cells. The frequency of MAIT cells was calculated as follow: % MAIT cells = (number of CD3+CD26+Vα7.2+ cells/number of CD3+). The number of cells/mm2 was extrapolated from the number of fields analyzed. Images were acquired at magnification 40×. Analyses were performed on the LSM 780 (Zeiss).
Statistical analysis
Statistical analyses for paired healthy and tumor samples were performed with a Wilcoxon test. Unpaired analyses according to clinical data used a Mann–Whitney test. In both cases, a value of p < 0.05 was considered significant. The Pearson’s coefficient (r) enabled to analyze linear correlations. The Kaplan–Meier method was used for calculation of survival probabilities and the log-rank test for comparison of survival curves. Multivariate analysis was performed with a Cox proportional hazards model including the following parameters: age (4 groups), TNM stage (II, III or IV), tumor differentiation (poor, moderate, or well differentiated), CD3 infiltration index (calculated as the ratio between the number of CD3+ cells in the tumoral vs. healthy tissue), and the MAIT cell infiltration index (with a cutoff at 3). A value of p < 0.05 was considered significant. Univariate analysis and survival probabilities were performed with GraphPad Prism; multivariate analysis was performed with Stata13.
Results
MAIT cells represent a significant proportion of CD3+ T cells in the healthy lamina propria of the gut, but there is no data available regarding their frequency inside tumoral intestinal tissue. Therefore, we aimed at quantifying these cells inside colorectal tumors. Blood MAIT cells can be identified as CD3+TCRVα7.2+ cells co-expressing high levels of CD161, IL-18Rα or CD26 [18]. Although CD161 may be the best marker for MAIT cell identification by flow cytometry, IL-18 Rα has been used with success for confocal microscopy analysis [18, 24]. In this study, we chose to identify MAIT cells as CD3+TCRVα7.2+CD26+ cells by immunofluorescence microscopy, because we obtained better images with CD26 as compared with IL-18Rα or CD161. We first counted the number of triple-positive cells in a retrospective series of biopsies from colorectal tumors and paired healthy tissues (Fig. 1). We noted a significantly higher number of MAIT cells in the core of the tumors as compared to the healthy mucosa (6.1/mm2 ±1.3 vs. 2.6/mm2 ±0.6; p < 0.0001; Fig. 2a). This increased number of MAIT cells could be the direct reflection of an increase in the number of total T cells inside tumors, as compared with healthy intestine. However, we found no such difference between paired tissues for CD3+ T cells (247/mm2 ±28 in tumors vs. 290/mm2 ±51; p = 0.69) (Fig. 2b). We therefore estimated the proportion of MAIT cells among total CD3+ T cells (Fig. 2c) and observed a significant increased frequency of MAIT cells within the tumor (2 % ±0.25 vs. 0.98 % ±0.15; p = 0.003). Although the number of MAIT cells per mm2 somewhat paralleled the number of total CD3+ T cells (Fig. 2d), their frequency was poorly correlated with the level of T cell infiltration (Fig. 2e). Therefore, the tumoral tissue harbors a higher number of MAIT cells than the healthy colon, resulting mostly from their increased frequency within infiltrating T cells.
Fig. 1.
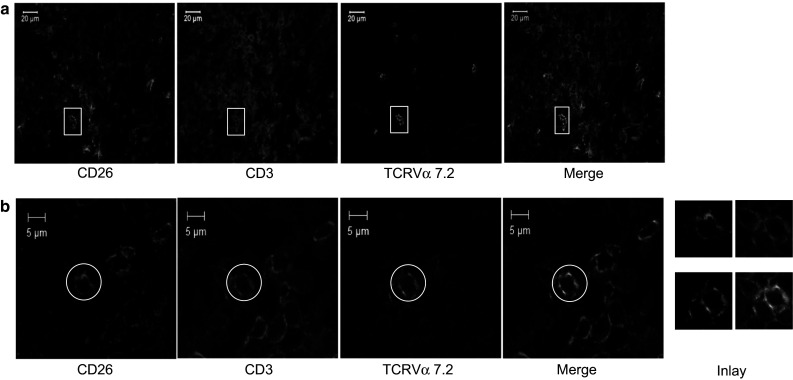
Immunofluorescent staining of tissue sections from a healthy colon with anti-CD3, anti-CD26, and anti-TCRVα7.2. Cells staining positive for the three markers are counted as MAIT cells. Images are acquired on a LSM780 confocal microscope (Zeiss) at magnification 40×. Two examples are shown. In (a), a panorama of a large field was obtained by computerized combination of separate pictures of the whole area, by a process referred to as stitching. White squares identify triple-positive cells. b Example of one microscopy field, with one triple-positive MAIT cell marked by a white circle. The inlay on the right side of the panel shows a zoom on this triple-positive cell
Fig. 2.
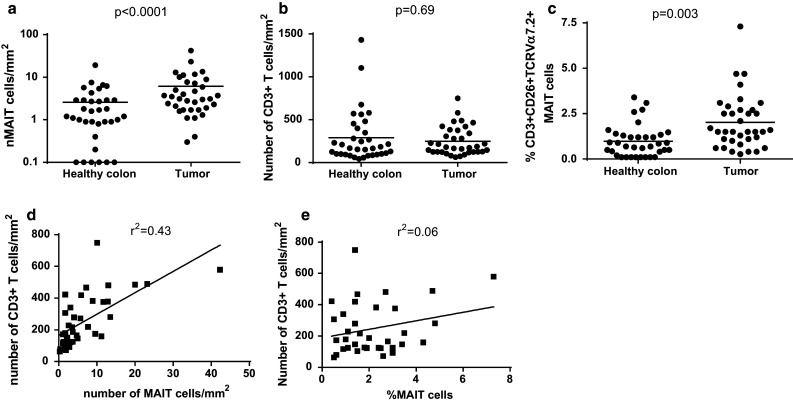
a Absolute values of MAIT cells per mm2 in paired samples from healthy and tumoral tissue. b Absolute values of CD3+ T cells per mm2 in paired samples from healthy and tumoral tissue. c Frequency of MAIT cells among CD3+ T cells in paired samples from healthy and tumoral tissue. d Correlation between the number of MAIT cells and the number of CD3+ T cells in tumoral tissue. e Correlation between the frequency of MAIT cells and the number of CD3+ T cells in tumoral tissue. Horizontal bars represent the mean values. Statistical analyses were performed with a matched Wilcoxon test. p values and Pearson’s coefficient are indicated. p values <0.05 are considered significant
Patients with CRC are stratified using the TNM score, which takes into account the tumor infiltration inside the tissue (T), the number of infiltrated lymph nodes (N), and distant metastases (M). This score is a useful prognosis marker, as it strongly correlates with the overall survival. We asked whether the degree of MAIT cell infiltration and specific recruitment was correlated with the TNM score. In our cohort, no patients scored at stage 1, whereas the great majority was at stage III (n = 17), and a smaller fraction at stage II (n = 10) or stage IV (n = 8). We first assessed the total number of T cells infiltrating the tumors according to the TNM score. As shown in Fig. 3a, similar numbers of T cells were found in stage II and III tumors, but this number dropped at stage IV (258.4/mm2 ±34.4, 296.2/mm2 ±46.1 and 145.3/mm2 ±24.5, respectively); however, the difference did not reach statistical significance, probably because of the low number of stage IV tumors in our cohort. With respect to MAIT cells, we mostly observed a trend toward an increased infiltration from stage II to stage III (3.8/mm2 ±1 and 8.3 mm2 ±2.6, respectively), but this difference did not reach statistical significance (Fig. 3b, c). We conclude that MAIT cells infiltrate colorectal tumors at various levels, and this increased infiltration is mostly independent of the TNM score.
Fig. 3.
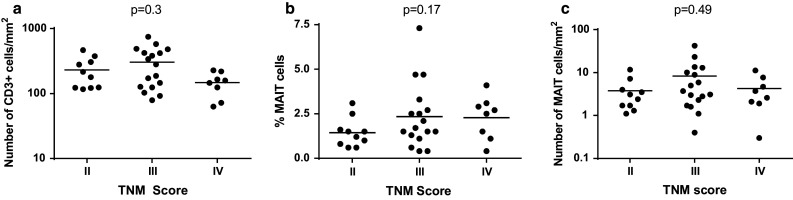
Number of CD3+ T cells/mm2 (a), the frequency of MAIT cells among CD3+ T cells (b) and the number of MAIT cells/mm2 (c) inside tumor samples were each analyzed according to the TNM score for comparison. Horizontal bars represent the mean values. Statistical analyses were performed a multiple comparison ANOVA test. A value of p < 0.05 was considered significant
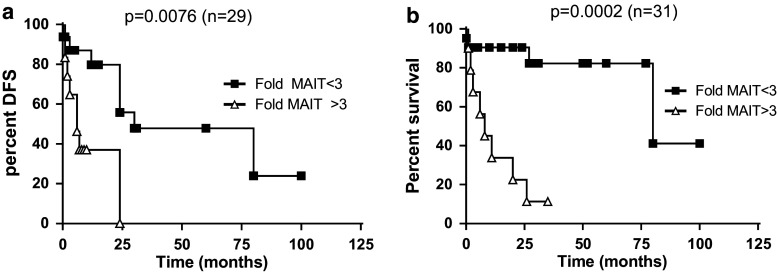
As shown in Fig. 2, the number and proportion of MAIT cells, both in healthy tissue and in tumors, is highly variable between individuals. Furthermore, because they are a significant subset of T cells in the normal mucosa, it is difficult to estimate from the above data to what extent they are recruited inside the tumor. However, when analyzing paired samples for each patient, it became clear that the level of specific recruitment inside tumors was highly variable and might be a better indicator of the behavior of MAIT cells (Fig. 4a). Indeed, when we calculated the specific recruitment of MAIT cells by the ratio between their number inside tumors and in healthy mucosa, there was a dramatic variation between individuals (Fig. 4b; range 0.6–77, median 2.4). We reasoned that this ratio might be a better marker to analyze differences between groups from a clinical viewpoint. However, using this index did not show any significant difference between tumors TNM stages (Fig. 4c). We then asked whether there was any link between this MAIT recruitment index and the patient’s disease-free and overall survival. We found no difference between patients in remission and those who relapsed (Fig. 4d). Then, we compared this index between patients who died because of their disease and the group of patients who survived (Fig. 4e). Again, we observed no significant difference overall; however, it was clear that the bulk of patients alive had a smaller index (geometric mean 2.1, 25/75 percentile 1.1/2.7), with only four patients showing higher ratios. By contrast, patients who succumbed from cancer had a highly variable MAIT infiltration index, with several patients showing very high scores (geometric mean 4.9, 25/75 percentile 1.1/25). We turned then to a survival analysis of the CRC patients according to the MAIT recruitment index calculated as before. It turned out that the geometric mean of this ratio on the whole population studied was 3.4; therefore, we chose to set the cutoff to 3 and analyzed the impact of this index on the disease-free survival (DFS) and the overall survival (OS) by a Kaplan–Meier analysis. As shown in Fig. 5a, there was a significant trend toward better DFS in the patient group with a MAIT index <3 (median DFS 30 vs. 6 months, p = 0.0076). More strikingly, the OS was clearly higher in the same group as compared patients with high index (median OS 80 vs. 8 months, p = 0.0002; Fig. 5b). This result was further confirmed by multivariate analysis that included several relevant parameters, such as age, TNM score, differentiation stage of the tumor, as well as a CD3 infiltration index. A Cox proportional hazards model including all these covariates showed a highly significant impact of the MAIT cell infiltration index on OS, with p = 0.008 and a hazard ratio of 8.8 (Table 2). We conclude from these data that patients with a high MAIT recruitment index, i.e., when the number of MAIT cells within the tumor is at least threefold higher than in the healthy mucosa, have shorter life time expectancy. Therefore, the infiltration of colorectal tumors by a high number of MAIT cells is a marker of bad prognosis.
Fig. 4.
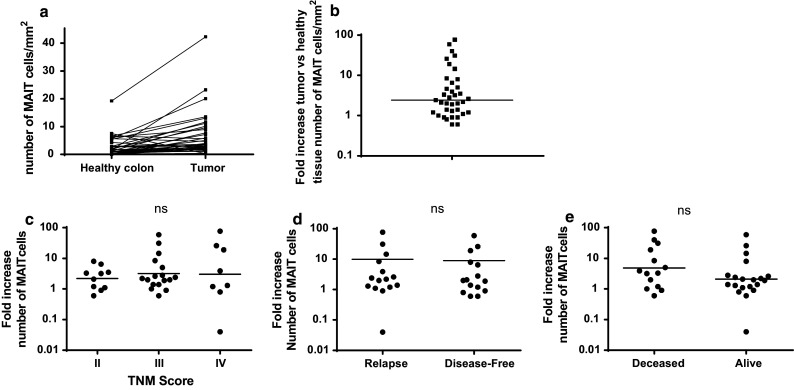
a Paired analysis of the absolute value of MAIT cells per mm2 in paired samples from healthy and tumoral tissue (same data as in Fig. 2a). b Fold increase in the number of MAIT cells/mm2 quantified in the tumor as compared to the paired healthy colon sample. Each symbol represents one patient. The horizontal bar delineates the geometric mean. c Fold increase in the number of tumor-infiltrating MAIT cells according to the TNM stage. Geometric means are shown. d Fold increase in the number of tumor-infiltrating MAIT cells in the disease-free group and the relapse group. Geometric means are shown. e Fold increase in the number of tumor-infiltrating MAIT cells according to the survival of the patients. Geometric means are shown
Fig. 5.
Kaplan–Meier analysis of survival probabilities between two groups of patients stratified according to the index of MAIT cell infiltration (<3 or >3). a Disease-free survival and b Overall survival. p values obtained with the log-rank test are indicated
Table 2.
Multivariate Cox proportional hazard analysis of the overall survival of the patients included in the study
| p | HR | 95 % CI | |
|---|---|---|---|
| Age (1) | 0.35 | 1.96 | 0.47–8.11 |
| TNM stage (2) | 0.8 | 1.05 | 0.72–1.52 |
| Differentiation (3) | 0.65 | 0.77 | 0.24–2.41 |
| CD3 index (4) | 0.56 | 0.85 | 0.49–1.48 |
| MAIT index (5) | 0.008 | 8.82 | 1.77–43.98 |
(1) Patients were classified among 4 groups: <65 yo; >65 < 75 yo; >75 < 85 yo; >85 yo
(2) TNM stages: II, III or IV
(3) Poor, intermediate, well differentiated
(4) CD3 index was included as continuous variable
(5) Patients were classified among two groups: MAIT cell index <3 or >3
CI confidence interval, yo years old, HR hazard ratio
Discussion
MAIT cells are dedicated antimicrobial cells found in significant proportion in the healthy lamina propria of the intestine, where they are probably ideally located to promptly respond to bacterial and fungal infectious agents. They are also present in the peripheral blood, from where they can be recruited to infected tissue, such as the lung in pulmonary tuberculosis. More generally, MAIT cells behave as inflammatory cells, capable of invading most inflammatory peripheral tissue. Indeed, they are found in the central nervous system (CNS) lesions of patients with multiple sclerosis (MS), in the psoriatic lesions of the skin, or in the inflamed gut mucosa of patients with Crohn’s disease (CD) [22–24]. With respect to cancer, a previous study found an increased amount of transcripts for the MAIT cell-specific TCRα chain (Vα7.2-Jα33), in kidney and brain tumors, suggesting their potential to infiltrate tumoral tissues [26]. In this study, we show for the first time that MAIT cells constitute a substantial subset of TIL in CRC and that the importance of specific MAIT cell infiltration is a marker of bad prognosis in patients.
The difficulty in studying the recruitment of MAIT cells in cancer cells is related to their relative abundance in the normal mucosa. In this study, we found that an average of about 1 % of CD3+ T cells were MAIT cells as defined as triple-positive CD3+CD26+TCRVα7.2+ cells. This frequency is a bit lower but still similar to our previous observations on patients with CD, in which we observed an average of 1.5 ± 0.3 % MAIT cells among intestinal T cells [24]. It is also close but a bit lower than estimates obtained from another study [27], where mononuclear cells from the intestinal lamina propria were isolated and MAIT cells enumerated by flow cytometry. The minor differences obtained could be related to the greater mean age of the patients studied here, as the proportion of MAIT cells may be impacted by immune senescence [28]. Furthermore, it is possible than the various strategies in use, in terms of markers (CD161 vs. IL18Rα vs. CD26) as well as technology (in situ confocal microscopy vs. flow cytometry after cell extraction) somewhat impact on the precise results. Nevertheless, the comparison of those different data show that our strategy to enumerate MAIT cells in situ is valid and that they are found in higher numbers and proportion within the colorectal tumors. The paired analysis of samples in each patient enabled us to calculate a recruitment index of MAIT cells which demonstrated a prognostic value, as patients with a higher recruitment have a reduced OS.
In CRC, seminal studies by Galon et al. have demonstrated that the composition of TIL is variable and complex, and deeply influences prognosis. Indeed, the presence of high numbers of effector/memory cytotoxic T cells and Th1 CD4+ T cells is an independent factor of better outcome in these patients [10, 14]. Interestingly, an important infiltration by IL-17-producing cells is a marker of bad prognosis in CRC, whereas it has a positive impact on other cancer types [12]. In our study, we found that an increased infiltration by MAIT cells identifies patients with a lower DFS and OS. Our current knowledge on MAIT cells functions show they have a mixed phenotype and cytokine secretion pattern between Tc1 and Tc17 cells [18]. On the one hand, they express granzymes and perforin, which are cytolytic and secrete mostly IFNγ and TNFα upon in vitro stimulation. On the other hand, they express most Tc17 markers, such as the transcription factor RORγt and the surface molecule CD161 [18]. The parameters driving the functional differentiation of MAIT cells toward a Tc1, Tc17, or mixed Tc1/Tc17 phenotype are not known. Based on the results shown here, we hypothesize that in CRC, strong infiltration by MAIT cells may be accompanied by a differentiation of these cells toward a Tc17 phenotype. This hypothesis would be concordant with the aforementioned studies, where strong Th17 markers identified in patients with a poor prognosis would include MAIT cells. In addition, MAIT cells infiltrate several organs and tissues in chronic inflammatory diseases, and some studies suggest this is accompanied by a functional Tc17 differentiation [23, 24]. These results, together with this study, would be consistent with the idea that IL-17 production is maximal within tissues, as compared with blood cells. Il-17 production may be pro-oncogenic and therefore deleterious in the context of chronic inflammatory diseases [29, 30]. This further suggests that in diseases such as inflammatory bowel diseases, which are associated with the development of colorectal cancer [31], sustained infiltration by MAIT cells would increase the risk of cancer development. Future studies are required to confirm this hypothesis and to unravel the mechanisms involved in MAIT cells infiltration and differentiation in CRC.
The small number of patients included in our cohort does not permit to draw any definitive conclusion about the use of the MAIT cells infiltration index as a ready-to-use tool for prognosis assessment. However, our study suggests that it would be of great interest to include MAIT-cell-specific markers in the so-called tumor immunoscore, together with other already analyzed useful transcripts. Even if MAIT cells infiltration always comes together with a Th17-like differentiation of intra-tumoral T cells, and therefore does not represent an independent factor of outcome, they are easy to monitor and therefore may represent a useful tool as a marker of prognosis in CRC.
Acknowledgments
E. Treiner wishes to thank Dr. François Vergez for help with statistical analysis. This work was funded by Conseil Régional de Picardie and the Fonds Européen de Développement Economique et Régional (FEDER).
Abbreviations
- CD
Crohn’s disease
- CNS
Central nervous system
- CRC
Colorectal cancer
- DFS
Disease-free survival
- MAIT
Mucosal-associated invariant T cells
- MR1
MHC-related 1
- MS
Multiple sclerosis
- RORγt
RAR-related orphan receptor gamma t
- OS
Overall survival
- TIL
Tumor-infiltrating lymphocytes
Compliance with ethical standards
Conflict of interests
The authors declare no conflicts of interests.
References
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 2.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 3.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui SA, Frigola X, Bonne-Annee S, Mercader M, Kuntz SM, Krambeck AE, et al. Tumor-infiltrating Foxp3−CD4+CD25+ T cells predict poor survival in renal cell carcinoma. Clin Cancer Res. 2007;13(7):2075–2081. doi: 10.1158/1078-0432.CCR-06-2139. [DOI] [PubMed] [Google Scholar]
- 5.Sheu BC, Lin RH, Lien HC, Ho HN, Hsu SM, Huang SC. Predominant Th2/Tc2 polarity of tumor-infiltrating lymphocytes in human cervical cancer. J Immunol. 2001;167(5):2972–2978. doi: 10.4049/jimmunol.167.5.2972. [DOI] [PubMed] [Google Scholar]
- 6.Roussel E, Gingras MC, Grimm EA, Bruner JM, Moser RP. Predominance of a type 2 intratumoural immune response in fresh tumour-infiltrating lymphocytes from human gliomas. Clin Exp Immunol. 1996;105(2):344–352. doi: 10.1046/j.1365-2249.1996.d01-753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Ye DF, Xie X, Chen HZ, Lu WG. Proportion of CD4+CD25+ regulatory T cell is increased in the patients with ovarian carcinoma. Cancer Invest. 2005;23(5):399–403. [PubMed] [Google Scholar]
- 8.Kharkevitch DD, Seito D, Balch GC, Maeda T, Balch CM, Itoh K. Characterization of autologous tumor-specific T-helper 2 cells in tumor-infiltrating lymphocytes from a patient with metastatic melanoma. Int J Cancer. 1994;58(3):317–323. doi: 10.1002/ijc.2910580302. [DOI] [PubMed] [Google Scholar]
- 9.Asselin-Paturel C, Echchakir H, Carayol G, Gay F, Opolon P, Grunenwald D, et al. Quantitative analysis of Th1, Th2 and TGF-beta1 cytokine expression in tumor, TIL and PBL of non-small cell lung cancer patients. Int J Cancer. 1998;77(1):7–12. doi: 10.1002/(SICI)1097-0215(19980703)77:1<7::AID-IJC2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 10.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71(4):1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 11.Lv L, Pan K, Li XD, She KL, Zhao JJ, Wang W, et al. The accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinoma. PLoS ONE. 2011;6(3):e18219. doi: 10.1371/journal.pone.0018219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114(6):1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Wan J, Liu J, Xie W, Diao X, Xu J, et al. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69(3):348–354. doi: 10.1016/j.lungcan.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 15.Gapin L. Check MAIT. J Immunol. 2014;192(10):4475–4480. doi: 10.4049/jimmunol.1400119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11(8):701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 17.Gold MC, Lewinsohn DM. Co-dependents: MR1-restricted MAIT cells and their antimicrobial function. Nat Rev Microbiol. 2013;11(1):14–19. doi: 10.1038/nrmicro2918. [DOI] [PubMed] [Google Scholar]
- 18.Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117(4):1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 19.Leung DT, Bhuiyan TR, Nishat NS, Hoq MR, Aktar A, Rahman MA, et al. Circulating mucosal associated invariant T cells are activated in Vibrio cholerae O1 infection and associated with lipopolysaccharide antibody responses. PLoS Negl Trop Dis. 2014;8(8):e3076. doi: 10.1371/journal.pntd.0003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimaldi D, Le Bourhis L, Sauneuf B, Dechartres A, Rousseau C, Ouaaz F, et al. Specific MAIT cell behaviour among innate-like T lymphocytes in critically ill patients with severe infections. Intensive Care Med. 2014;40(2):192–201. doi: 10.1007/s00134-013-3163-x. [DOI] [PubMed] [Google Scholar]
- 21.Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8(6):e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willing A, Leach OA, Ufer F, Attfield KE, Steinbach K, Kursawe N, et al. CD8(+) MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL-18 serum levels in multiple sclerosis. Eur J Immunol. 2014;44(10):3119–3128. doi: 10.1002/eji.201344160. [DOI] [PubMed] [Google Scholar]
- 23.Teunissen MB, Yeremenko NG, Baeten DL, Chielie S, Spuls PI, de Rie MA, et al. The IL-17A-producing CD8+ T-cell population in psoriatic lesional skin comprises mucosa-associated invariant T cells and conventional T cells. J Invest Dermatol. 2014;134(12):2898–2907. doi: 10.1038/jid.2014.261. [DOI] [PubMed] [Google Scholar]
- 24.Serriari NE, Eoche M, Lamotte L, Lion J, Fumery M, Marcelo P, et al. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014;176(2):266–274. doi: 10.1111/cei.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazaki Y, Miyake S, Chiba A, Lantz O, Yamamura T. Mucosal-associated invariant T cells regulate Th1 response in multiple sclerosis. Int Immunol. 2011;23(9):529–535. doi: 10.1093/intimm/dxr047. [DOI] [PubMed] [Google Scholar]
- 26.Peterfalvi A, Gomori E, Magyarlaki T, Pal J, Banati M, Javorhazy A, et al. Invariant Valpha7.2-Jalpha33 TCR is expressed in human kidney and brain tumors indicating infiltration by mucosal-associated invariant T (MAIT) cells. Int Immunol. 2008;20(12):1517–1525. doi: 10.1093/intimm/dxn111. [DOI] [PubMed] [Google Scholar]
- 27.Greathead L, Metcalf R, Gazzard B, Gotch F, Steel A, Kelleher P. CD8+/CD161++ mucosal-associated invariant T-cell levels in the colon are restored on long-term antiretroviral therapy and correlate with CD8+ T-cell immune activation. AIDS. 2014;28(11):1690–1692. doi: 10.1097/QAD.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 28.Novak J, Dobrovolny J, Novakova L, Kozak T. The decrease in number and change in phenotype of mucosal-associated invariant T cells in the elderly and differences in men and women of reproductive age. Scand J Immunol. 2014;80(4):271–275. doi: 10.1111/sji.12193. [DOI] [PubMed] [Google Scholar]
- 29.Wang K, Kim MK, Di Caro G, Wong J, Shalapour S, Wan J, et al. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity. 2014;41(6):1052–1063. doi: 10.1016/j.immuni.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491(7423):254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jess T, Gamborg M, Matzen P, Munkholm P, Sorensen TI. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100(12):2724–2729. doi: 10.1111/j.1572-0241.2005.00287.x. [DOI] [PubMed] [Google Scholar]