Abstract
IL-17A, produced by Th17 cells, may play a dual role in antitumor immunity. Using the GL261-glioma model, we investigated the effects of Th17 cells on tumor growth and microenvironment. Th17 cells infiltrate mouse gliomas, increase significantly in a time-dependent manner similarly to Treg and do not express Foxp3. To characterize the direct effects of Th17 cells on GL261 murine gliomas and on tumor microenvironment, we isolated IL-17-producing cells enriched from splenocytes derived from naïve (nTh17) or glioma-bearing mice (gTh17) and pre-stimulated in vitro with or without TGF-β. Spleen-derived Th17 cells co-expressing IL-17, IFN-γ and IL-10, but not Treg marker Foxp3, were co-injected intracranially with GL261 in immune-competent mice. Mice co-injected with GL261 and nTh17 survived significantly longer than gTh17 (P < 0.006) and gliomas expressed high level of IFN-γ and TNF-α, low levels of IL-10 and TGF-β. In vitro IL-17 per se did not exert effects on GL261 proliferation; in vivo gliomas grew equally well intracranially in IL-17 deficient and wild-type mice. We further analyzed relationship between Th17 cells and Treg. Treg were significantly higher in splenocytes from glioma-bearing than naïve mice (P = 0.01) and gTh17 produced more IL-10 than IFN-γ (P = 0.002). In vitro depletion of Treg using PC61 in splenocytes from glioma-bearing mice causes increased IL-17/IFN-γ cells (P = 0.007) and decreased IL-17/IL-10 cells (P = 0.03). These results suggest that Th17 polarization may be induced by Treg and that Th17 cells in gliomas modulate tumor growth depending on locally produced cytokines.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-011-1069-4) contains supplementary material, which is available to authorized users.
Keywords: Th17 cells, Regulatory T cells, Glioma microenvironment, IL-17A
Introduction
Th17 cells are a subset of T helper cells producing IL-17. They play a role in inflammation and tissue injury [1] and their dysregulation may cause autoimmune diseases [2, 3]. Simultaneous presence of TGF-β and IL-6 may drive the differentiation of murine Th17 cells [4]. Mechanisms causing the formation of human Th17 cells are not well defined. Recent observations suggest that IL-1 in combination with IL-6 and IL-23 provide an optimal cytokine cocktail for Th17 differentiation [5–7]. Other studies suggest that TGF-β is also important for human Th17 generation [8–10]. The involvement and function of Th17 cells in cancer must be elucidated. Th17 cells have been investigated in several human cancer such as ovarian [11, 12] and prostate cancer, [13] both in peripheral blood of patients, where they represent a small population, and in tumor tissues, where they are more abundant. In gastric cancer [14], blood Th17 cells increase with tumor stage. In other types of cancers, including melanoma, breast and colon carcinoma, increased percentages of tumor-infiltrating Th17 cells have been found compared with normal tissues [15], suggesting mechanisms for the accumulation of Th17 cells in tumor microenvironment. More recently, Th17 cells have been shown to favor the regression of established tumors in mouse models [16, 17], and a recent work has identified the presence of Th17 cells in human and murine malignant gliomas co-expressing the regulatory T cells (Treg) lineage marker Foxp3 [18].
In this study, we confirmed the infiltration of Th17 cells in a mouse malignant glioma, demonstrating in addition that Th17 cells increase during tumor development in correlation with Treg. We also observed that Th17 cells are involved in glioma development and in modulation of tumor microenvironment and that are able to influence both tumor inhibition and tumor progression. We further provide novel insights into the role of Treg in modulating the Th17 cell phenotype and cytokine profile. In particular, Treg may contribute to Th17 cell tumor-promoting activity, inducing IL-10 rather than IFN-γ secretion.
Materials and methods
Cell cultures
GL261 cells were grown in DMEM/F12 (Life Technologies, Gaithersburg, MD), supplemented with penicillin/streptomycin sulfate, B-27 (Life Technologies), human recombinant fibroblast growth factor 2 (FGF-2; 20 ng/ml; Peprotech, Rocky Hill, NJ), epidermal growth factor (EGF; 20 ng/ml; Chemicon, Germany) and heparin (Sigma-Aldrich, St. Louis, MO).
Spleens were surgically removed from 6 weeks old naïve mice or from mice 20 days after intracranial injection of GL261 cells.
Briefly, splenocytes depleted of red cells were cultured in 25 cm2 flasks at 40:1 E/T (effector/target) ratio with 20 Gy irradiated GL261 cells in RPMI 1640, 10% fetal bovine serum, 2% penicillin/streptomycin, 2% l-glutamine, 50 μM beta mercaptoethanol, 100 mM sodium pyruvate, 100× nonessential amino acids. Irradiation of GL261 cells was performed on a 43855F Cabinet X-ray System (Faxitron® X-ray corporation, Whelling Illinois, USA). Splenocytes and irradiated GL261 cells were co-cultured in the presence or absence of 5 ng/ml of recombinant human TGF-β1 (R&D Systems, Minneapolis, MN) for 24 and 48 h. Interleukin-17 secreting cells were immunomagnetically isolated and enriched with the Mouse IL-17 Secretion Assay—Cell Enrichment & Detection Kit (PE) (Miltenyi Biotec, Auburn, CA).
In vivo experiments
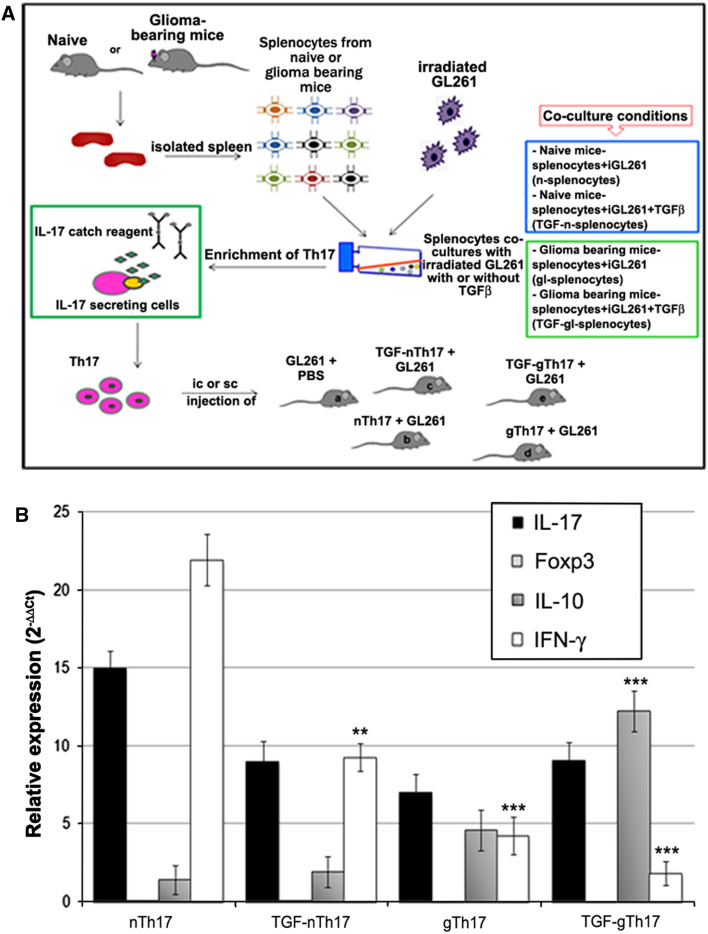
A total of 50 C57BL6N mice (5/group for survival and 5/group for MRI) received brain injections of 1 × 105 GL261 cells and 5 × 104 enriched Th17 cells using five different combinations (see Fig. 3a): (a) GL261 cells alone (n = 10); (b) GL261 cells and Th17 cells from naïve splenocytes (nTh17, n = 10); (c) GL261 cells and Th17 cells from naïve splenocytes cultured in the presence of rhTGF-β (TGF-nTh17, n = 10); (d) GL261 cells and Th17 cells from glioma-bearing splenocytes (gTh17, n = 10); and (e) GL261 cells and Th17 cells from glioma-bearing splenocytes cultured in the presence of rhTGF-β1 (TGF-gTh17, n = 10). The stereotactic coordinates with respect to the bregma are: 0.7 mm posterior, 3 mm left lateral, 3.5 mm deep into the nucleus caudatum. A total of 50 C57BL6N mice received subcutaneous co-injection of 4 × 105 enriched Th17 cells and 8 × 105 GL261 cells using the same different combinations as described previously.
Fig. 3.
Spleen-derived Th17 cells express IL-17, IFN-γ and IL-10 and do not express Foxp3. a Schematic experimental procedure. Spleen from naïve C57BL6N and glioma-bearing mice was surgically removed, and splenocytes were co-cultured in vitro with irradiated GL261 tumor cells in the presence or absence of rhTGF-β1. IL-17 secreting cells from different co-culture conditions (see the box below) were isolated and enriched immunomagnetically. Th17 cells were co-injected intracranially (ic) and subcutaneously (sc) with GL261 cells in immune-competent mice. b Th17 cells enriched by immunomagnetic separation were characterized by RT-PCR. All fractions express high levels of IL-17 and different expression of IFN-γ and IL-10 (*P < 0.01; **P < 0.0001; ***P < 0.00005). As shown in histogram, Foxp3 has not been detected
Animals were monitored every day until killed, in accordance with current directives of the IFOM-IEO campus animal house facility, Ethics Committee of the Institution and Minister of Health.
MRI studies
Magnetic resonance imaging (MRI) was performed on a 7 Tesla Bruker BioSpec 70/30 USR Preclinical System at 8 and 16 days after tumor implantation.
T2-weighted RARE sequence and T1-weighted spin echo sequences had been performed before and after intraperitoneal injection of contrast medium (Gadolinium DTPA).
Intracellular staining
Intracellular staining and FACS analysis were performed using the following antibodies: Anti-IL-17A FITC (Miltenyi Biotec), anti-IL-10 PE (eBioscence, San Diego, USA), anti-IFN-γ PE (Miltenyi Biotec) and anti-Foxp3 PE (eBioscience). Briefly, 1.5 × 106 cells were surface stained for 30 min at 4°C with anti-CD4 PE-Cy™5 (BD Pharmingen) and anti-CD25 FITC (BD Pharmingen) antibodies in PBS; for intracellular staining of cytokines, T cells were fixed and permeabilized with the BD Cytofix/Cytoperm™ Fixation/Permeabilization kit. Flow cytometry acquisition was performed on a FACSCalibur and analyzed with CellQuest software.
Real-time PCR (RT-PCR)
RNA for RT-PCR from paraffin-embedded material was extracted using the Absolutely RNA FFPE kit (Stratagene, S. Diego, CA). cDNA was synthesized from the RNA using oligo (dT) and M-MLV Reverse Transcriptase (Life Technologies, Carlsbad, CA).
Specific primers for the target genes were designed for Fast SYBR® Green chemistry (Applied Biosystems) and then purchased from Primm (Primm s.r.l., San Raffaele Biomedical Science Park—Milan, Italy). TaqMan® Gene Expression Assays were purchased from Applied Biosystems. cDNA templates were analyzed on a 7500 Real-Time PCR System (Applied Biosystems). The sequences of primers for target genes are shown in Supplemental Fig. 2. The relative mRNA expression level of all genes investigated was calculated using the ΔΔCt method and normalized with respect to the beta-2-microglobulin (b2m).
Histology and immunohistochemistry
Immunohistochemical analysis of Ki67 (BD Pharmingen), CD4 (R&D System), IL-17A (Santa Cruz Biotechnology, Santa Cruz, CA) and Foxp3 (eBioscience) was performed on paraffin-embedded sections.
For double immunofluorescence, tumor sections were incubated with anti-IL-17A, anti-CD4 and anti-Foxp3 antibodies overnight at 4°C and then with Alexa Fluor 594-conjugated anti-rat antibody for the detection of CD4 and Foxp3 and with Alexa Fluor 488-conjugated anti-rabbit antibody for the detection of IL-17. Quantitative analysis was made on three to five independent fields per tumor by counting the number of cells in the photographed fields using a 40× objective of Leica DM-LB microscope. Statistical comparisons of data sets were performed by a two-tailed Student’s t test. The data were considered to be significantly different when P < 0.05.
Statistical analysis
Differences between groups were considered statistically significant for a P value < 0.05 calculated with the two-tailed Student’s t test.
Results
Infiltrating Th17 cells increase in GL261-gliomas in a time-dependent manner
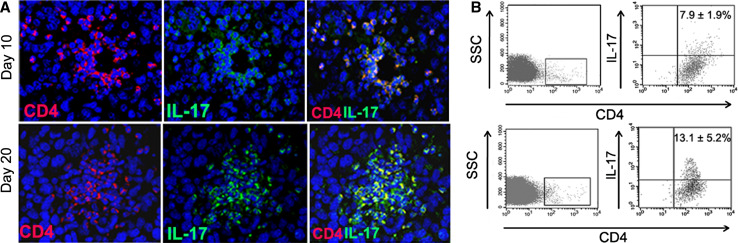
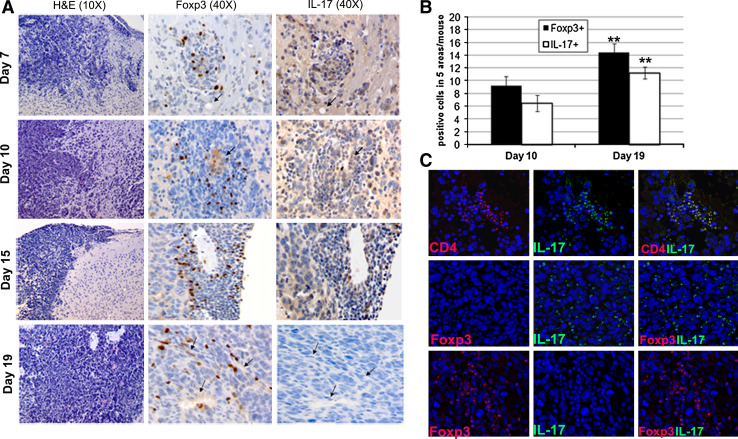
To evaluate the potential involvement of Th17 cells in glioma development, we first investigated their presence in the tumor microenvironment. Mice intracranially injected with GL261-glioma cells died by day 25. We examined the distribution of Th17 cells (CD4+ IL-17+ cells) in GL261-gliomas 10 and 20 days after tumor implantation (Fig. 1a). On day 10, Th17 cells were preferentially located in perivascular spaces, while on day 20, Th17 cells were distributed into the tumor mass. For quantitative determination of Th17 cells, we obtained TIL from fresh gliomas (n = 3 for each time point) and evaluated by flow cytometry-infiltrating Th17 cells as a percentage of CD4+ cells. Representative plots showed that Th17 cells in gated CD4+ cells increased significantly from 7.9 ± 1.9 on day 10 to 13.1 ± 5.2 on day 20 (Fig. 1b) (P = 0.03). No infiltration of CD4+ T cells was detected in a pool of normal brains used as control (data not shown). We further studied the distribution of Th17 cells in relationship with Treg at different time points. Staining for Foxp3 and IL-17 was performed on adjacent sections of paraffin-embedded brains at different time points. The same blood vessels in adjacent sections are shown in Fig. 2a. We found that both Foxp3 and IL-17 positive cells are preferentially located near blood vessels on day 7, 10 and 15. In contrast on day 19, Foxp3 and IL-17 positive cells are preferentially distributed into the tumor parenchyma. Foxp3 and IL-17 positive cells were differentially distributed, and double-positive cells were not found. Quantitative analysis evaluated on day 10 and 19 is summarized in histogram (Fig. 2b).
Fig. 1.
Increment of Th17 cells infiltration during glioma progression. a On day 10, only few CD4+ cells are detected inside the tumor area: most of double-positive lymphocytes, recruited from peripheral lymphoid organs, are concentrated around the tumor vessels. On day 20, after GL261 injection, the microenvironment immunocharacterization shows an increment of CD4+ IL-17+ population and double-positive cells are distributed as cellular cluster within the tumor area. Isotype control cells are not detectable in GL261-glioma (data not shown). b Infiltrating Th17 cells investigated by flow cytometry increased from 7.9 ± 1.9 on day 10 to 13.1 ± 5.2 on day 20 (P = 0.03). The panels show a representative dot plot and mean percentage value ± SD obtained from three different evaluations at each time point
Fig. 2.
Treg and Th17 cells are differentially distributed into the tumor mass. a Arrows indicate the same blood vessels in adjacent sections of tumors evaluated for the presence of infiltrating Foxp3 and IL-17 positive cells at different time points; 7, 10, 15 and 19 days after tumor implantation. Two representative mice for each time point have been investigated, and representative images are displayed. b Quantitative analysis is summarized in histogram, results are expressed as mean ± SD of positive cells in 5 areas/mouse/time point (number of positive cells/5 different 40× HPF). c Immunofluorescence of GL261-glioma on day 19 showed infiltrating CD4+ IL-17+ population double-positive cells, but negative for Treg marker Foxp3. Foxp3-positive cells do not co-express IL-17
We observed a significant increase in Treg and Th17 cells during tumor development (P = 0.001). Treg were significantly higher than IL-17 positive cells in tumors at both time points (P = 0.03, day 10; P = 0.006, day 19).
We failed to identify the concomitant expression of IL-17 and Foxp3 (Fig. 2c) suggesting that Th17- and Foxp3-positive cells were independently infiltrating the growing glioma.
Th17 cells play a dual role on tumor growth
To characterize the direct effects of Th17 cells on GL261-glioma growth, we isolated IL-17-producing cells from splenocytes of naïve (n) or glioma-bearing mice (gl). IL-17 cells were then pre-stimulated in the presence of irradiated GL261 cells for 24 and 48 h with or without TGF-β. (Fig. 3a).
Flow cytometry analysis showed that magnetic separation resulted in a fraction containing 68 ± 1.5% IL-17 positive cells, that was enriched for CD3/CD4+ T cells (73.1 ± 6.6%) and depleted for CD4+ CD25+ Foxp3+ cells (0.5 ± 0.3%) and for CD3/CD8+ T cells (3.4 ± 0.3%). In the IL-17 negative fraction, the percentage of CD3/CD4 positive T cells was significantly lower (15.8 ± 8.2, P < 0.00001), while CD4+ CD25+ Foxp3+ and CD3+ CD8+ cells were significantly higher (15.8 ± 8.2, P = 0.003; 6.7 ± 2.1, P = 0.02, respectively) (Supplemental Table 1).
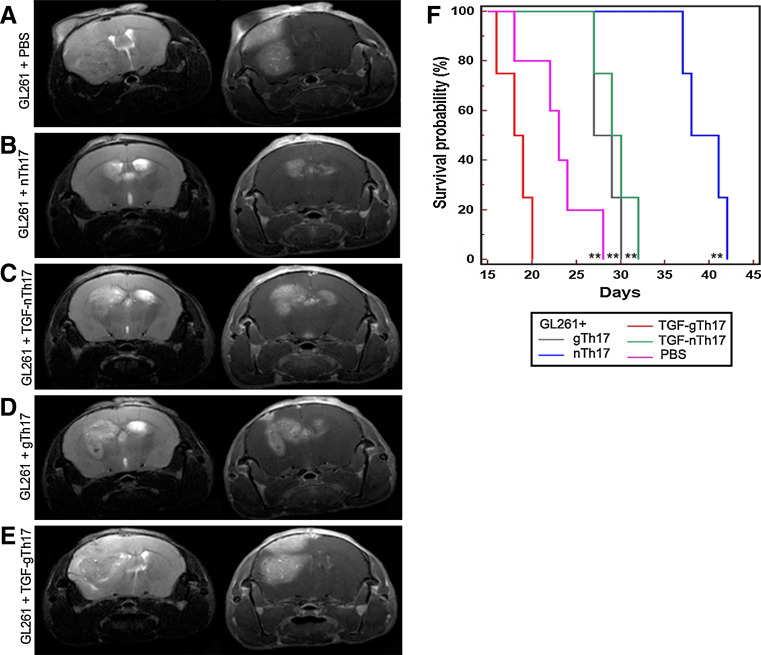
To verify whether Th17 cells derived from spleen resemble glioma infiltrating Th17 cells, we investigated the mRNA levels of IL-17, Foxp3, IFN-γ and IL-10 in IL-17 positive fractions (nTh17, TGF-nTh17, gTh17, TGF-gTh17, see scheme Fig. 3a). All Th17 fractions expressed high levels of IL-17 (Fig. 3b), variable levels of IFN-γ and IL-10 and do not express Foxp3. A significant increase in IL-10 expression in gTh17 and TGF-gTh17 compared to nTh17 was accompanied by a significant decrease in IFN-γ. Th17 cells derived from naïve (nTh17 and TGF-nTh17) or glioma-bearing mice splenocytes (gTh17 and TGF-gTh17) were co-injected intracranially (ic) or subcutaneously (sc) with GL261 cells into immune-competent mice. Mice injected ic were monitored by magnetic resonance imaging (MRI) 7 T. The different patterns of tumor growth were evaluated on day 16 after tumor implantation. The lesion derived from GL261 only was large (Fig. 4a), while small lesions derived from co-injection of GL261 with nTh17 visible on T2 and T1 with contrast medium (Fig. 4b). The lesion derived from co-injection with gTh17 was larger than that with nTh17 and diffused toward the midline (Fig. 4b, d). A large lesion from co-injection with TGF-gTh17 is shown in T1 and T2 images (Fig. 4e). Thus, MRI suggested that nTh17 provide significant delay on tumor engraftment and growth.
Fig. 4.
Th17 cells play a role on GL261-glioma growth. a MRI performed 16 days after tumor implantation shows that lesion derived from GL261 + PBS was large. b nTh17 cells provide a significant delay on tumor formation. T2 (b left, 20 slices; thickness 0.8 mm, no gap; TR: 2,658 ms/TE: 54 ms/RARE Factor 8/FOV: 2.20 × 2.20 cm2; averages 6; image matrix 256 × 256; in plane resolution 0.086 mm; acquisition time 8 min 30 s) and T1 with contrast medium representative images show the total absence of mass lesion compared to the other groups of co-injection (b right 18 slices; thickness 0.8 mm, no gap; TR: 435 ms/TE: 11 ms/FOV: 2.20 × 2.20 cm2; averages 4; image matrix 256 × 256: in plane resolution 0.086 mm; acquisition time 7 min 26 s). T2 and T1 images of gliomas from GL261 plus gTh17 (d) showed expanded and lesions compared to nTh17 or TGF-nTh17 (c). Lesion from co-injection with TGF-gTh17 (e) is shown both in T1 as a homogeneous enhancing mass and in T2 images as a strong mass effect toward lateral ventricle. Images refer to one representative animal for each group (five mice for each group). f Kaplan–Meier analysis curves represent the survival time (in days) of five mice for each group of co-injection. Survival rate of mice injected with nTh17 cells was significantly higher than that of control mice (GL261 plus PBS P = 0.005) and of mice injected with TGF-gTh17 cells (P = 0.0067). Mice injected with TGF-gTh17 cells survived significantly lower than controls (P = 0.02)
In agreement with this survival analysis showed that mice co-injected with GL261 and nTh17 survived significantly longer that controls (PBS, P = 0.005) or than the other groups (Fig. 4f): survival rate of mice co-injected with gTh17 was significantly lower than that of nTh17 mice (P = 0.006) and, interestingly, of control mice (P = 0.04). TGF-gTh17 co-injection had the worst effect in terms of survival compared to the other groups (P ≤ 0.02). A similar experimental scheme was used by co-injecting Th17 sc with GL261: in Supplemental Fig. 1, we show representative tumors for each experimental treatment. Gliomas in mice co-injected with nTh17 cells were significantly smaller than others.
These results demonstrate that Th17 cells may exert inhibitory or stimulatory effects on tumor growth depending on their origin and on cytokine conditioning, a suppressive effect on tumor growth was caused by nTh17 cells and a promoting effect by gTh17 cells.
Co-injection of GL261 and Th17 cells modulates tumor microenvironment
We then studied the effects of Th17 cells on tumor microenvironment by RT-PCR on paraffin-embedded ic- and sc-gliomas derived from co-injection of GL261 and Th17 cells investigating the expression of IFN-γ and TNF-α for immune activation, TGF-β and IL-10 for immune suppression.
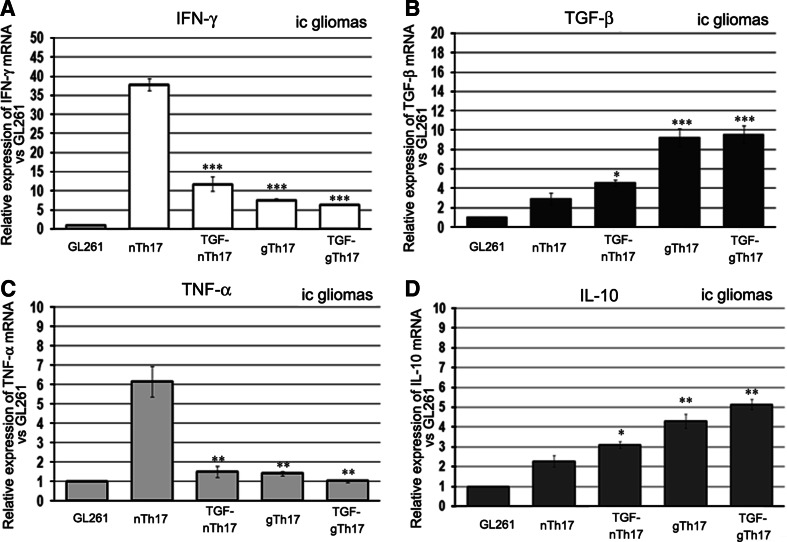
The relative expression of these cytokines in ic-gliomas is shown in Fig. 5a–d. Microenvironment characterization in sc-gliomas is reported in Supplemental Fig. 2. Opposite patterns of cytokine expression were obtained in tumors derived from GL261 co-injected ic with either nTh17 or gTh17. In nTh17 gliomas, we found enhanced expression of IFN-γ (6.0 folds vs. TGF-gTh17 tumors; P < 0.0001) and TNF-α (4.3 fold higher; P < 0.001) (Fig. 5a, c). On the contrary, IL-10 and TGF-β strongly increased in gTh17. In particular, IL-10 was 2.0 fold higher (P < 0.001) and TGF-β 3.1 fold higher than ic nTh17 gliomas. (P < 0.0001) (Fig. 5b, d).
Fig. 5.
Th17 cells modulate tumor microenvironment. RT-PCR analysis of different gene expression performed on paraffin-embedded ic-gliomas. mRNA levels in each group were normalized to the relative quantity of b2m; relative expression of different cytokines was evaluated versus the GL261 plus PBS. a and c nTh17 tumors show an enhanced expression in IFN-γ and TNF-α compared to GL261 and TGF-gTh17 tumors. On the contrary, TGF-β and IL-10 (b and d) are strongly increased in gTh17-treated mice (P value: *P = 0.01; **P < 0.001; ***P < 0.0001)
IL-17 does not affect GL261 cells proliferation in vitro and in vivo
To investigate whether IL-17 has a direct effect on GL261 proliferation, we first verified the expression of IL-17 receptor on GL261 cells by semi-quantitative PCR. We then assessed the effects of IL-17A on tumor proliferation of GL261 cells. Proliferation kinetics and MTT analysis showed that IL-17 did not exert activity on GL261 cells proliferation (Supplemental Fig. 3).
We also injected GL261 in IL-17 KO mice and immune-competent mice (wt) and did not observe differences on tumor formation by MRI performed 8 days after tumor implantation (Fig. 6a). A small lesion is located in the left hemisphere, as confirmed by histological analysis performed on mice WT and IL-17 KO 5 days after MRI (Fig. 6b upper). The proliferation index was also similar (Ki67 positive cells 87.1 ± 0.8 and 87.4 ± 0.8 in wt and IL-17 KO mice, respectively, Fig. 6b lower), as well as the infiltration of Treg cells in wt and IL-17 KO mice (data not shown). The MRI analysis on day 16 showed tumors of similar size of both WT and IL-17 KO, (Fig. 6c), and the overall survival of the two groups of mice was also similar (Fig. 6d).
Fig. 6.
IL-17 has no effect on the GL261-glioma formation. a MRI 7 T images on a frontal plane. T2 (Left) and T1 after contrast medium (Right) images on day 8 after GL261 implantation performed on a wild-type (WT) and IL-17 KO (KO) mouse show a similar tumor engraftment. b Brain pictures of wt and IL-17 KO 12 days after tumor implantation (Upper) and Ki-67 staining (magnification 40×, Lower) show a similar proliferation ratio in wt and IL-17 KO mice. c MRI 7 T images on a frontal plane. T2 (Left) and T1 after contrast medium (Right) images 16 after GL261 implantation performed on a wild-type and IL-17 KO mouse. d Kaplan–Meier analysis curves represent the survival time (in days) of IL-17 KO compared to WT mice injected ic with GL261 cells. All mice died by day 29. The median survival of IL-17 KO was 27 days (n = 5, mean ± SD is 26.8 ± 2.2); the median of WT mice was 26.5 (n = 5, mean ± SD is 26.8 ± 1.7) (P = 0.9)
Increase in CD4+ CD25+ Foxp3+ T cells is associated with different Th17 cytokine profiles
To gain hints on the direct contribution of Th17 cells to tumor development, we studied cytokine profiles investigating key factors relevant to tumor microenvironment.
Naïve and gl-splenocytes were stimulated in the presence of irradiated GL261 cells with or without TGF-β, and Treg were evaluated by flow cytometry for Foxp3 expression on CD4+ CD25+ cells; the percentage of double-positive IL-17/IFN-γ or IL-17/IL-10-producing CD4+ T cells was determined by intracellular staining.
Splenocytes from naïve and glioma-bearing mice monitored before stimulation had a similar proportion of IFN-γ- (2.3 ± 0.6% and 1.2 ± 0.6%, respectively) or IL-10-producing CD4+ Th17 cells (1.9 ± 0.5% and 2.4 ± 0.7%, respectively).
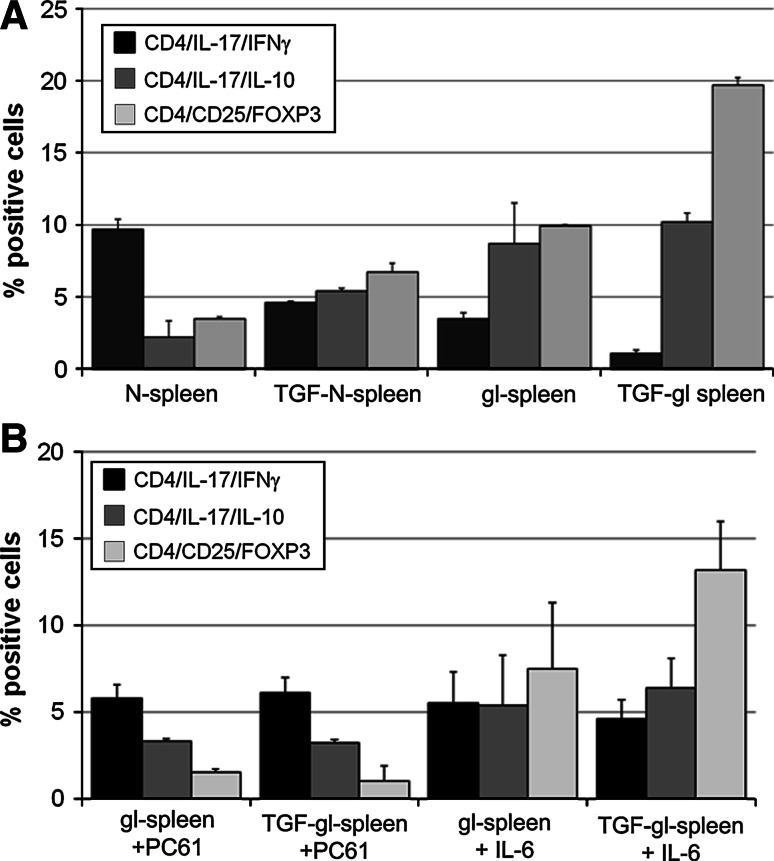
In naïve splenocytes, in the presence of 3.5 ± 0.05% Treg, we found that nTh17 produced higher levels of IFN-γ than IL-10 (9.7 ± 0.7% vs. 2.2 ± 1.5%, respectively; P = 0.001). TGF-β stimulation significantly increased the Treg fraction (from 3.5 ± 0.05% in n-splenocytes to 6.7 ± 0.6 in TGF-n-splenocytes%; P = 0.02) resulting in a significant reduction in IL-17/IFN-γ-producing CD4 T cells (P = 0.009, TGF-nTh17 vs. nTh17). The Treg fraction was significantly higher in g- than n-splenocytes (9.9 ± 0.1% vs. 3.5 ± 0.05%, respectively, P = 0.01) and gTh17 cells produced more IL-10 than IFN-γ (8.7 ± 2.8% vs. 3.5 ± 0.4%, respectively; P = 0.002). TGF-β induced a further increase in Treg cells from 9.9 ± 0.1% to 19.7 ± 0.5% (P = 0.001) (Fig. 7a). Notably, Treg promoted a significant decrease in IFN-γ (1.1 ± 0.2; P = 0.01) and increase in IL-10-producing T cells (10.2 ± 0.3; P = 0.03).
Fig. 7.
CD4+ CD25+ Foxp3+ cells (Treg) modulate the Th17 cytokine profile. a Intracellular FACS analysis of naïve and tumor bearing CD4+ lymphocytes. Treg are significantly higher in glioma-bearing mice versus naïve splenocytes (P = 0.01), and they increase significantly in the presence of TGF-β (P = 0.001 in gl-splenocytes; P = 0.02 in n-splenocytes). nTh17 produced higher levels of IFN-γ than IL-10 (P = 0.001) that appeared reduced by TGF-β (P = 0.01). On the contrary, gTh17 produced more IL-10 than IFN-γ (P = 0.002). The presence of TGF-β promoted a relevant decrease in IFN-γ (P = 0.01) and a significant increase in IL-10-producing T cells (P = 0.03). Notably, the absolute number of Th17 cells obtained from the same number of splenocytes did not change significantly (nTh17 8.1 × 105; TGF-nTh17 8 × 105; gTh17 7.9 × 105; TGF-gTh17 9.2 × 105). Data are relative of three independent experiments. b Intracellular FACS analysis of tumor bearing CD4+ lymphocytes. PC61 antibody (0.5 μg/1 × 106 cells) decreased Treg population from gl- and TGF-gl-splenocytes (P < 0.001), increasing IL-17/IFN-γ production (gTh17 P = 0.05; TGF-gTh17 P = 0.01) and decreasing IL-17/IL-10 secretion (gTh17 P = 0.0004; TGF-gTh17, P = 0.003). Data are statistically significant when they are compared to the same culture condition without PC61 (Fig. 7a). No significant differences (P > 0.05) were found in the same experiments performed with IL-6. In vitro data are represented as mean ± SD obtained combining percentage obtained from 24 to 48 h pre-stimulation of splenocytes
These results indicate that the relative abundance of Treg is associated with a Th17 polarization to either an IFN-γ or an IL-10-producing phenotype and that TGF-β seems particularly effective at directing the Th17 cytokine profile.
The results show that the percentage of IL-17/IL-10 CD4+ T cells and Treg synchronically increased in gl-splenocytes during in vitro pre-stimulation.
Reduced frequency of Treg correlates positively with the increase in IFN-γ-producing Th17 cells
To assess whether Treg drive the Th17 cytokine profile, we depleted the Treg fraction in gl- and TGF-gl-splenocytes by using the anti-CD25 monoclonal antibody PC61.
Treg from gl- and TGF-gl-splenocytes significantly decreased (from 9.9 ± 0.1% to 19.7 ± 0.5% without PC61 to 1.5 ± 0.2% and 1.0 ± 0.9% with PC61, respectively; P < 0.001).
The ability of gTh17 and TGF-gTh17 to produce IFN-γ increased in the presence of PC61 (from 3.5 ± 0.3% to 5.8 ± 0.8%, P = 0.05 and from 1.1 ± 0.2% to 6.1 ± 0.9%, P = 0.01). Conversely, their capacity to secrete IL-17/IL-10 decreased from 8.7 ± 0.1% to 3.3 ± 0.14% (P = 0.0004) and from 11.3 ± 0.2 to 3.2 ± 0.2% (P = 0.003), respectively.
Accumulating evidence indicates that TGF-β and IL-6 promote Th17 differentiation and that IL-6 down-regulates [4, 19] Treg development. For this reason, we tried to define the effect of IL-6 per se or in combination with TGF-β on Th17 differentiation and Treg modulation in vitro. IL-6 per se contributed to a slight and not significant decrease in Treg (from 9.9 ± 0.1% to 7.5 ± 3.8 in gl- and from 19.7 ± 0.5% to 13.2 ± 2.8% in TGF-gl-splenocytes) and a trend to increased production of IFN-γ Th17 cells (from 3.5 ± 0.4% to 5.5 ± 1.8% in gl- and from 1.1 ± 0.2% to 5.4 ± 2.9% in TGF-gl-cells) and decreased production of IL-10 Th17 cells (from 8.7 ± 0.1% to 6.4 ± 1.7% in gl- and from 11.3 ± 0.6% to 5.4 ± 2.9% in TGF-gl-cells (Fig. 7b).
Discussion
In this study, we showed that Th17 cells and Treg synchronically increase during glioma development with a similar distribution and that Treg are significantly more abundant than Th17 cells. However, we failed to identify Th17 cells co-expressing the Treg marker Foxp3 [18, 20], in agreement with a recent work by Viaud [21]. Starting from these observations, we hypothesized a close relationship between infiltrating Treg and Th17 cells. In order to investigate the Th17 role in glioma microenvironment and the potential effects of Treg on Th17 cell properties, we generated an enriched Th17 population exploiting different polarizing culture conditions in vitro. Irradiated GL261 were used as a source of multiple-specific tumor antigens to pre-stimulate splenocytes; these antigens were indeed essential to educate naïve splenocytes and to prime memory T cells in glioma-bearing splenocytes. We initially compared the effects of splenocyte stimulation using TGF-β compared to IL-6 plus TGF-β on Th17 and Treg cell differentiation. We found that TGF-β and IL-6 did not change the percentage of CD4+ IL-17+ (data not shown), but induced an increase in IFN-γ production and a decrease in IL-10 secretion compared to TGF-β stimulation. The Treg percentage evaluated as CD4+ CD25+ Foxp3+ decreased slightly compared to the percentage mediated by TGF-β only.
It is known that TGF-β plus IL-6 stimulates Th17 differentiation, whereas TGF-β only promotes Treg differentiation. However, TGF-β has been proposed as a link between Th17 and Treg cells in mouse autoimmune diseases where Treg-derived TGF-β supports Th17 cell differentiation [22, 23]. High levels of TGF-β also seem involved in the development of Treg [7, 24]. In addition, it was reported that TGF-β plus IL-6 strongly stimulates IL-17+ CD8+ cells [25] and it is also known that IL-6 may suppress Treg [19].
In murine gliomas, we have recently characterized the presence of infiltrating Treg [26] and their correlation with the amount of TGF-β, a cytokine abundant in glioma and responsible for immune suppressive microenvironment (Supplemental Fig. 4a–d). Our in vitro conditions using TGF-β stimulation have been necessary to verify an important and previously unappreciated effect of Treg on Th17 cells, resulting in their polarization toward high IFN-γ or IL-10-producing cells. Emerging data indicate that Th17 cells might be converted into Th1/Th17 cells characterized by the co-expression of cytokines IFN-γ and IL-17 and the transcription factors T-bet and RORγt [27]. In this regard, our results show that ex vivo but not in vitro nTh17 cells express T-bet, while TGF-nTh17 cells show decreased T-bet expression in agreement with the observation that TGF-β blocks T-bet induction and IFN-γ expression [28] (Supplemental Fig. 4e–f). In vivo Th17 cells could undergo an evolution in their ability to secrete cytokines; in addition their antitumor or tumor-promoting effect might not be mutually exclusive, but dependent on timing and tumor microenvironment. These conditions are supported by opposite results obtained using intracranial co-injection of GL261 and nTh17 or gTh17 cells. nTh17 cells produce higher quantities of IFN-γ than the other fractions inducing antitumor effect in early stages of glioma; on the contrary, gTh17 cells might be generated at later stages under the effect of immunosuppressive microenvironment and the interaction with Treg.
The other key issue that our experiments have addressed is represented by the controversial role of IL-17 in tumors. In particular, IL-17 can have a pro-tumorigenic activity in immune-deficient mice [29, 30] or an antitumorigenic activity in immune-competent mice [31]. Recently, opposite results were obtained from two groups on the same experimental tumor, MC38 [32, 33]. When we injected GL261 in IL-17 KO or wild-type mice, we did not find differences in tumor initiation and growth, suggesting that IL-17 per se has no significant effect on tumor progression.
It has been reported that, in experimental murine tumors, peripheral Treg can be converted into Th17 under the influence of inflammation [23, 34, 35]. In addition, IL-17 has been shown to exert pro-inflammatory effects on astrocytes and microglial cells into the CNS through direct interaction with their constitutively expressed IL-17 receptor (IL-17R). Above all that, the response of glial cells to IL-17 depends on the interaction between IL-17 and other cytokine signaling systems. Only one report showed that few established glioma cell lines express IL-17R and that IL-17 can stimulate the secretion of IL-6 and IL-18 [36]. GL261 cells express IL-17R, but exogenous IL-17 does not provide a direct effect on GL261-cell proliferation, supporting the idea that Th17 cells may contrast or favor tumor development depending on the immunological context, as demonstrated by the presence of IFN-γ or IL-10.
The tumor inhibitory activity of Th17 cells depends on the production of IFN-γ that in this setting seems to function as an anti-proliferative factor rather than as an immune stimulatory factor. We previously suggested that high concentrations of IFN-γ are able to reduce GL261 proliferation [26], and now, we found that the simultaneous injection of GL261 with IFN-γ-producing Th17 cells could favor such anti-proliferative effect. Interestingly, Muranski et al. [17] reported that the therapeutic effect of Th17 cells on a murine model of melanoma critically depends on IFN-γ production and that IL-17 depletion has a little influence on this effect. Th17 cells also seem to indirectly mediate an antitumor activity, by promoting the recruitment of other effector cells [37, 38] or by eliciting pro-inflammatory conditions that can promote CD8+ effector cells [16]. On the contrary, Th17 might favor tumor development by inhibiting CD8+ cell infiltration and enhancing myeloid-derived suppressor cells at tumor site [39].
In summary, Th17-mediated effects in vivo might depend on the accumulation of Treg during glioma development. The ability of these cells to secrete IFN-γ as previously demonstrated [40] might result in a change of plasticity or in the ability to secrete IL-10 or IFN-γ, due to the presence of Treg. These results should be considered for further evaluations of the Th17 cell involvement in human antitumor activity.
A question that remains open is whether tumor-infiltrating Th17 cells are recruited from the periphery or induced in tumor microenvironment. Many factors released by tumor cells, such as TGF-β, IL-6, PGE2 and TNF-α, play a role in the induction of Th17 differentiation [41]. Moreover, recent observations showed that dendritic cells, derived from monocytes and migrating across the blood–brain barrier, secrete IL-12, TGF-β and IL-6, thus promoting the proliferation and expansion of IL-17-secreting Th17 CD4+ T lymphocytes [42].
Th17 cell regulation could provide useful insights in immunotherapy protocols. For example, in prostate cancer, a higher frequency of Th17 cells before immunotherapy is correlated with a shorter time to metastatic progression [43]. In gliomas, the role of Th17 cells could vary during tumor development: IFN-γ-producing Th17 cells could be inversely related to Treg and thus considered as predictors of stronger antitumor reactivity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Ileana Zucca for collaboration in Magnetic Resonance Imaging, Dr. Alberto Gobbi and Emiko Kazama for help with mice shipment. This work has been supported by a grant from Il Fondo di Gio, a charity affiliated to the International Brain Tumor Alliance and by funds from the Italian Minister of Health.
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 2.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 3.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Das J, Ren G, Zhang L, Roberts AI, Zhao X, Bothwell AL, Van Kaer L, Shi Y, Das G. Transforming growth factor beta is dispensable for the molecular orchestration of Th17 cell differentiation. J Exp Med. 2009;206:2407–2416. doi: 10.1084/jem.20082286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 6.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 8.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 10.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci USA. 2008;105:15505–15510. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, Xue X, Wei G, Liu X, Fang G. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374:533–537. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 15.Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- 16.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wainwright DA, Sengupta S, Han Y, Ulasov IV, Lesniak MS. The Presence of IL-17A and T Helper 17 Cells in experimental mouse brain tumors and human glioma. PLoS One. 2010;5:e15390. doi: 10.1371/journal.pone.0015390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+ CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 20.Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, Beckhove P, Gounari F, Khazaie K. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res. 2009;69:5490–5497. doi: 10.1158/0008-5472.CAN-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viaud S, Flament C, Zoubir M, Pautier P, LeCesne A, Ribrag V, Soria JC, Marty V, Vielh P, Robert C, Chaput N, Zitvogel L. Cyclophosphamide induces differentiation of Th17 cells in cancer patients. Cancer Res. 2011;71:661–665. doi: 10.1158/0008-5472.CAN-10-1259. [DOI] [PubMed] [Google Scholar]
- 22.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 24.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 25.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 26.Pellegatta S, Poliani PL, Stucchi E, Corno D, Colombo CA, Orzan F, Ravanini M, Finocchiaro G. Intra-tumoral dendritic cells increase efficacy of peripheral vaccination by modulation of glioma microenvironment. Neuro Oncol. 2010;12:377–388. doi: 10.1093/neuonc/nop024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boniface K, Blumenschein WM, Brovont-Porth K, McGeachy MJ, Basham B, Desai B, Pierce R, McClanahan TK, Sadekova S, de Waal Malefyt R. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185:679–687. doi: 10.4049/jimmunol.1000366. [DOI] [PubMed] [Google Scholar]
- 28.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 30.Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, Sasaki H. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 31.Hirahara N, Nio Y, Sasaki S, Minari Y, Takamura M, Iguchi C, Dong M, Yamasawa K, Tamura K. Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. Oncology. 2001;61:79–89. doi: 10.1159/000055357. [DOI] [PubMed] [Google Scholar]
- 32.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ngiow SF, Smyth MJ, Teng MW. Does IL-17 suppress tumor growth? Blood. 2010;115:2554–2555. doi: 10.1182/blood-2009-11-254607. [DOI] [PubMed] [Google Scholar]
- 34.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat Rev Immunol. 2009;9:883–889. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 35.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 36.Kehlen A, Thiele K, Riemann D, Rainov N, Langner J. Interleukin-17 stimulates the expression of IkappaB alpha mRNA and the secretion of IL-6 and IL-8 in glioblastoma cell lines. J Neuroimmunol. 1999;101:1–6. doi: 10.1016/S0165-5728(99)00111-3. [DOI] [PubMed] [Google Scholar]
- 37.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 38.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, Xu H. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184:2281–2288. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suryani S, Sutton I. An interferon-gamma-producing Th1 subset is the major source of IL-17 in experimental autoimmune encephalitis. J Neuroimmunol. 2007;183:96–103. doi: 10.1016/j.jneuroim.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 41.Nam JS, Terabe M, Kang MJ, Chae H, Voong N, Yang YA, Laurence A, Michalowska A, Mamura M, Lonning S, Berzofsky JA, Wakefield LM. Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008;68:3915–3923. doi: 10.1158/0008-5472.CAN-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ifergan I, Kebir H, Bernard M, Wosik K, Dodelet-Devillers A, Cayrol R, Arbour N, Prat A. The blood-brain barrier induces differentiation of migrating monocytes into Th17-polarizing dendritic cells. Brain. 2008;131:785–799. doi: 10.1093/brain/awm295. [DOI] [PubMed] [Google Scholar]
- 43.Derhovanessian E, Adams V, Hahnel K, Groeger A, Pandha H, Ward S, Pawelec G. Pretreatment frequency of circulating IL-17+ CD4+ T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int J Cancer. 2009;125:1372–1379. doi: 10.1002/ijc.24497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.