Abstract
Expression of monocyte chemoattractant protein-1 (MCP-1) and CC chemokine receptor 2 (CCR2) and its significance has been demonstrated in some cancer cells in recent clinical studies. However, the role of tumor MCP-1 and CCR2 expression in non-small cell lung cancer (NSCLC) remains unknown. The aim of the present study was to investigate the prognostic significance of MCP-1 and CCR2 expression in NSCLC cells. The relationship between MCP-1 and CCR2 expression in NSCLC cancer cells was examined by immunohistochemical staining of surgical specimens from 134 patients. Sixty-five of these patients had follow-up records. Kaplan–Meier analysis and Cox regression model were used to assess overall survival according to the presence or absence of MCP-1 and CCR2 expression in tumor cells. MCP-1 was detected in cancer cells of 107 NSCLC (79.9 %) and CCR2 was detected in cancer cells of 39 NSCLC (29.1 %). MCP-1 expression was correlated with sex, smoking habits, histology, and tumor size. Presence of MCP-1 in tumor cells was associated with better overall survival (P = 0.018). By multivariate analysis, MCP-1 expression in cancer cells showed an independent prognostic factor for overall survival (P = 0.002, hazard ratio [HR] = 0.256, 95 % confidence interval [CI] = 0.106–0.616). There was no significant relationship between CCR2 expression in tumor cells and clinical and pathological characteristics. Also, no significant positive correlation between MCP-1 and CCR2 expression was revealed by Spearman correlation analysis. Our data indicate that MCP-1 is overexpressed in NSCLC cells. Its expression in cancer cells is associated with better survival in NSCLC patients.
Keywords: Non-small cell lung cancer, Monocyte chemoattractant protein-1, CC chemokine receptor 2, Expression, Prognosis
Introduction
Lung cancer is the world’s leading cause of cancer-related death and the second most common malignant tumor [1]. Epidemiologically, there has been an marked elevation in morbidity and mortality of lung cancer in China over the past decade [2]. At least 75 % of lung cancer patients have evidence of metastasis at diagnosis [3]. Lung cancer can be divided into two main types: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Approximately 85 % of lung cancers are NSCLC and their prognosis is poor [4]. Therefore, surgery plays a critical role in the treatment for clinical stage I–IIIA NSCLC. Because of local recurrence or distant metastasis, even patients with pathologic stage I or patients who have undergone R0 resection do not have good outcomes. Some pathologic stage IA patients may receive adjuvant chemotherapy to minimize the risk of recurrence [5]. Although adjuvant chemotherapy is beneficial to pathologic stage IB–IIIA patients for improving their prognosis, it may not be necessary for all patients [6]. Therefore, new prognostic markers are needed to screen patients at high risk of relapse.
MCP-1 (monocyte chemoattractant protein-1) was first purified by Matsushima et al. [7] from serum-free culture supernatant of human myelomonocytic cells. It is a member of the C–C chemokine family and is a polypeptide containing 76 amino acids. The receptor of MCP-1 is CCR2 and it is expressed in various cell types, such as monocytes and memory T lymphocytes. When MCP-1 combines with CCR2, the allosteric effect and G-protein binding will occur to activate signal transduction pathway. MCP-1 is produced by a variety of activating cells (fibroblasts, endothelial cells, smooth muscle cells, lymphocytes, and macrophages) [8, 9]. Many recent studies have reported that MCP-1 is expressed by several malignant tumor cells, including prostate cancer, breast cancer, esophageal carcinoma, colon cancer, pancreatic cancer, cervical cancer, and ovarian cancer [10–16]. MCP-1 participates in the migration and infiltration of monocytes/macrophages toward inflammatory areas. It also regulates the recruitment and activation of tumor-associated macrophages (TAM) in tumor infiltration, including release of lysosomal enzyme and tumoricidal activity [13]. However, some studies have indicated that MCP-1 could promote tumor invasion and metastasis [12, 17, 18]. The role of MCP-1 in NSCLC is also controversial. It was reported that MCP-1 production was greater in NSCLC than in normal lung tissue, and the level of MCP-1 positively correlated with lung cancer-induced bone resorptive lesions in vivo [19, 20]. However, transfection of NSCLC cell lines by MCP-1 did not change the invasion behavior of lung cancer cell lines or inhibit cancer cell distant metastasis [21, 22]. The aim of the present study was to explore the expression and significance of MCP-1 and CCR2 in NSCLC.
Materials and methods
Tissue specimens
This study was retrospective and was approved by the institutional ethics board of Huadong Hospital, China. A total of 134 patients who underwent surgical resection for NSCLC at Huadong Hospital from January 1999 to December 2009 and who had complete clinicopathologic data were enrolled in the study, of which 65 patients had follow-up data. None of the patients received radiotherapy or chemotherapy before surgery. Histologic type was defined according to World Health Organization classification. TNM classification of NSCLC was made according to the seventh edition published in 2009 [23], with the agreement of both the International Union Against Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Clinicopathological information of the patients is shown in Table 1.
Table 1.
Clinicopathologic characteristics and frequency of non-small cell lung cancer patients (with or without follow-up records, 134 cases) according to the presence or absence of monocyte chemoattractant protein-1 (MCP-1) or CC chemokine receptor 2 (CCR2) in tumor cells
| Characteristics | With follow-up records (n = 65) |
Without follow-up records (n = 69) |
MCP-1 (+) |
MCP-1 (−) |
P | CCR2 (+) |
CCR2 (−) |
P |
|---|---|---|---|---|---|---|---|---|
| No. | No. | |||||||
| Gender | ||||||||
| Male | 41 | 38 | 55 | 24 | <0.001a | 26 | 53 | 0.245a |
| Female | 24 | 31 | 52 | 3 | 13 | 42 | ||
| Age (years) | ||||||||
| ≤60 | 29 | 33 | 48 | 14 | 0.515a | 18 | 44 | 0.986a |
| >60 | 36 | 36 | 59 | 13 | 21 | 51 | ||
| Smoking habits | ||||||||
| Yes | 26 | 25 | 34 | 17 | 0.003a | 18 | 33 | 0.216a |
| No | 39 | 44 | 73 | 10 | 21 | 62 | ||
| Histological type | ||||||||
| Squamous carcinoma | 19 | 20 | 23 | 16 | 0.001a | 15 | 24 | 0.305a |
| Adenocarcinoma | 41 | 47 | 78 | 10 | 22 | 66 | ||
| Others | 5 | 2 | 6 | 1 | 2 | 5 | ||
| Tumor size (cm) | ||||||||
| ≤3 | 38 | 43 | 69 | 11 | 0.034a | 19 | 61 | 0.172a |
| 3–7 | 23 | 23 | 31 | 15 | 16 | 30 | ||
| >7 | 4 | 3 | 7 | 1 | 4 | 4 | ||
| Differentiation | ||||||||
| High-moderate | 50 | 52 | 84 | 18 | 0.197a | 31 | 71 | 0.558a |
| Low | 15 | 17 | 23 | 9 | 8 | 24 | ||
| Grade | ||||||||
| T1-2 | 58 | 21 | 97 | 21 | 0.065a | 31 | 87 | 0.05a |
| T3-4 | 7 | 48 | 10 | 6 | 8 | 8 | ||
| Lymph node metastasis | ||||||||
| No | 46 | 53 | 77 | 22 | 0.314a | 30 | 69 | 0.607a |
| Yes | 19 | 16 | 30 | 5 | 9 | 26 | ||
| Distant organ metastasis | ||||||||
| No | 61 | 68 | 104 | 25 | 0.259a | 39 | 90 | 0.321b |
| Yes | 4 | 1 | 3 | 2 | 0 | 5 | ||
| Tumor stage | ||||||||
| I–II | 50 | 57 | 87 | 20 | 0.402a | 28 | 79 | 0.136a |
| III–IV | 15 | 12 | 20 | 7 | 11 | 16 | ||
MCP-1 (+) was defined as MCP-1-positive expression in NSCLC cells. MCP-1 (−) was defined as MCP-1-negative expression in NSCLC cells. CCR2 (+) was defined as CCR2-positive expression in NSCLC cells. CCR2 (−) was defined as CCR2-negative expression in NSCLC cells
aPearson’s chi-square test (two-sided), b Fisher’s exact probability test (two-sided)
Immunohistochemical analysis
MCP-1 and CCR2 expression was analyzed immunohistochemically on 4-μm-thick, formalin-fixed, paraffin-embedded specimen sections. Sections were deparaffinized in xylene and rehydrated in a series of grade alcohols. They were then protreated in ethylenediamine tetraacetic acid antigen retrieval solution (pH 9.0) using heat-induced epitope retrieval technique. After inhibiting internal peroxidase activity with 3 % hydrogen peroxide, the sections were incubated with primary antibodies (MCP-1, Cat No. ab9669, diluted × 80 [Abcam, USA]; CCR2, Cat No. ab32144, diluted × 1,100 [Abcam]). Next, EnVision Detection System kit (DAKO, Denmark) was used on sections with diaminobenzidine (DAB) as a chromogen. Finally, sections were counterstained with hematoxylin, then dehydrated and mounted. Positive staining controls were carried out with paraffin-embedded spleen and breast cancer sections using CCR2 and MCP-1 antibodies. Two of the authors reviewed the slides independently twice at two-week intervals.
MCP-1 staining in tumor cells was positive when more than 10 % of cancer cells were stained in each section [12]. Semi-quantitative analysis of CCR2 staining in tumor cells was evaluated as grade 0, 1, 2, or 3 [24]. Grade 0 was defined as CCR2 staining in <1 % of tumor cells, grade 1 as 1–33 %, grade 2 as 34–66 %, and grade 3 as >67 %. Grade 0 corresponded to negative CCR2 expression, and grades 1–3 corresponded to positive CCR2 expression.
Statistical analysis
Overall survival was defined as the time from the date of operation until death or follow-up deadline. Comparisons between groups based on the presence of MCP-1 and CCR2 in tumor cells were analyzed using Pearson’s chi-square tests or Fisher’s exact probability tests as required. Spearman correlation analysis was used to detect the association between MCP-1 and CCR2 expression. Kaplan–Meier analyses and log-rank tests were used to evaluate the correlation between the presence of MCP-1 and CCR2 in cancer cells. Log-rank tests were also used for predictors in univariate analysis based on the Kaplan–Meier curves. Cox proportional hazards regression was used for multivariate analysis of overall survival. All predictors with P values <0.10 in univariate analysis were used in multivariate analysis, carried out by the forward stepwise (likelihood ratio) procedure. All analyses were performed using Statistical Package for Social Sciences version 16 (SPSS Inc., Chicago, IL, USA). For all comparisons, two-sided P values <0.05 were considered statistically significant.
Results
Expression of MCP-1 and CCR2 in NSCLC
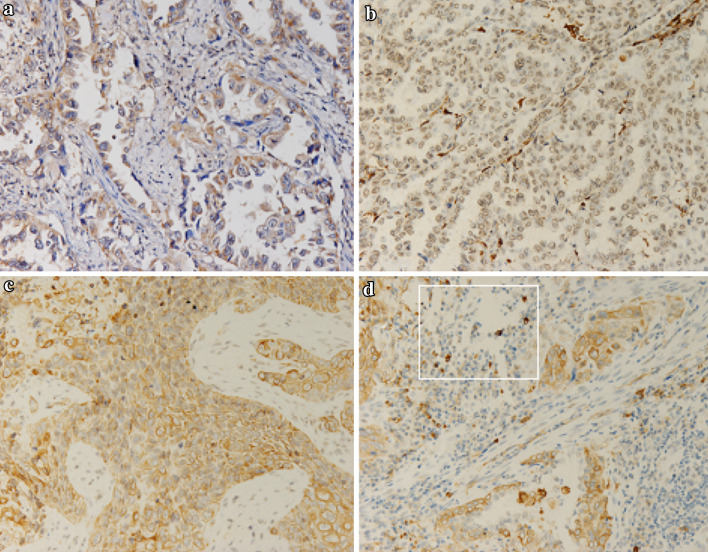
Of the 134 tumor surgical specimens, 107 (79.9 %) expressed MCP-1 in tumor cells. MCP-1 immunostaining was exclusively observed in cytoplasm of cancer cells. Expression of CCR2 in tumor cells was found in either cytoplasm or nucleus. In total, 39 of 134 cases (29.1 %) showed positive staining: 25 (18.7 %) in cytoplasm and 15 (11.2 %) in nucleus, scarcely in both cytoplasm and nucleus. Infiltrated lymphocytes of CCR2-positive staining were found in NSCLC (Fig. 1).
Fig. 1.
MCP-1 and CCR2 expression of non-small cell lung cancer(×200). a Positive cytoplasmic staining of MCP-1 in adenocarcinoma of the lung. b Positive nuclear staining of CCR2 in adenocarcinoma of the lung. c Positive cytoplasmic staining of CCR2 in squamous carcinoma of the lung. d Positive staining of tumor-infiltrating lymphocytes of CCR2
Association of clinicopathologic characteristics with expression of MCP-1 and CCR2 in NSCLC
MCP-1 expression in NSCLC was associated with gender or smoking habits and histological type of NSCLC. Tumor MCP-1 expression was observed more frequently in men than in women (P < 0.001), in never-smokers than in smokers (P = 0.003), and in smaller tumors. The presence of MCP-1-positive cancer cells was high in patients with adenocarcinoma of NSCLC relative to tumors of other histological types (P = 0.001). There was no significant association between MCP-1 expression and clinicopathological characteristics, including patient age, differentiation, T-grade, stage, lymph node metastasis, and distant organ metastasis. There was no relationship of tumor CCR2 expression with patient age, gender, smoking habits, histologic type of tumor, differentiation, tumor size, T-grade, tumor stage, lymph node metastasis, and distant organ metastasis (Table 1). No significant association was detected between MCP-1 and CCR2 expression in NSCLC tumor cells (Table 2, r = −0.047, P = 0.592).
Table 2.
Correlation of monocyte chemoattractant protein-1 (MCP-1) or CC chemokine receptor 2 (CCR2) expression in NSCLC tumor cells
| MCP-1 | CCR2 | Total | r | P | |
|---|---|---|---|---|---|
| (+) | (−) | ||||
| (+) | 30 | 77 | 107 | −0.047 | 0.592 |
| (−) | 9 | 18 | 27 | ||
| Total | 39 | 95 | 134 | ||
MCP-1 (+) was defined as MCP-1-positive expression in NSCLC cells. MCP-1 (−) was defined as MCP-1-negative expression in NSCLC cells. CCR2 (+) was defined as CCR2-positive expression in NSCLC cells. CCR2 (−) was defined as CCR2-negative expression in NSCLC cells
Prognostic significance of MCP-1 and CCR2 expression in NSCLC
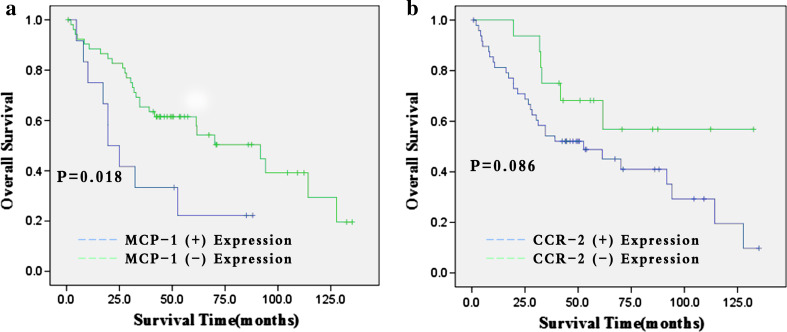
Expression of MCP-1 in NSCLC correlated with overall survival (P = 0.018) in Kaplan–Meier curves, but CCR2 expression of tumor cells had no association with overall survival (P = 0.086) (Fig. 2). Univariate analysis of clinicopathological characteristics indicated that histological type of tumor, tumor stage, lymph node metastasis, distant organ metastasis, and MCP-1 expression were significantly related with overall survival (P = 0.015,0.012, 0.020, 0.012, and 0.018, respectively). Gender, age, smoking habits, tumor size, differentiation, and T-grade were not associated with increased risk of death (Table 3). Cox proportional multivariate analysis using all predictors with P values <0.1 in the univariate analysis revealed that histological type of tumor, tumor stage, and presence of MCP-1 were correlated with overall survival (Table 3, P < 0.001 HR 3.270 95 % CI 1.758–6.082; P = 0.021 HR 2.635 95 % CI 1.156–6.008; P = 0.002 HR 0.256 95 % CI 0.106–0.616).
Fig. 2.
Kaplan–Meier estimates with log-rank tests for overall survival of 65 non-small cell lung cancer (NSCLC) patients. a MCP-1 expression status in NSCLC. b CCR2 expression status in NSCLC
Table 3.
Univariate and multivariate analyses of variates for overall survival in 65 non-small cell lung cancer patients with follow-up records
| Variates | Univariate P | Multivariate | ||
|---|---|---|---|---|
| Hazard ratio | 95 % CI | P | ||
| Gender: male versus female | 0.261 | |||
| Age (years): ≤60 versus >60 | 0.516 | |||
| Smoking habits: no versus yes | 0.407 | |||
| Tumor size (cm): ≤3 versus 3–7 versus >7 | 0.925 | |||
| Histological type: Squamous carcinoma versus adenocarcinoma versus other types | 0.015 | 3.270 | 1.758–6.082 | <0.001 |
| Differentiation: high-moderate versus low | 0.105 | |||
| Tumor grade: T1-2 versus T3-4 | 0.232 | |||
| Lymph node metastasis: no versus yes | 0.020 | |||
| Distant organ metastasis: no versus yes | 0.012 | 2.392 | 0.539–10.613 | 0.251 |
| Tumor stage: I–II versus III–IV | 0.012 | 2.635 | 1.156–6.008 | 0.021 |
| MCP-1 expression in tumor cells: negative versus positive | 0.018 | 0.256 | 0.106–0.616 | 0.002 |
Log-rank tests were used for predictors in univariate analysis based on the Kaplan–Meier curves. Cox proportional hazards regression was used for multivariate analysis of overall survival. All predictors with P values <0.10 in univariate analysis were used in multivariate analysis, carried out by the forward stepwise (likelihood ratio) procedure
Discussion
Chemokines represent a family of small-molecular-weight chemotactic cytokines with homologous structure and similar function, which bind to G-protein-coupled receptors [25]. According to the arrangement of four conserved amino-terminal cysteine residues in mature proteins, they can be classified into four major subfamilies: CXC, CC, CX3C, and C.MCP-1; this is the most representative member of the CC chemokine subfamily, also known as CC ligand 2 (CCL2). Besides recruitment of monocytes and memory T cells in peripheral blood, MCP-1 can induce influx of calcium, molecular adhesion, and monocyte secretion of pro-inflammatory factors [26]. Some studies reflected the unfavorable prognostic influence of MCP-1 on certain types of cancer. In one study, the serum level of MCP-1 in breast cancer and ovarian cancer was associated with tumor stage [11, 16]. Koide et al. [12] observed that MCP-1 expression in esophageal carcinoma cells was correlated with venous invasion, distant metastasis, and lymph node metastasis, and theorized that it might attribute to MCP-1 involvement of angiogenesis [17] and production of matrix metalloprotease-9 (MMP-9) [18]. However, other studies demonstrated that MCP-1 was a favorable prognostic marker in colon cancer and pancreatic cancer. Huang et al. [27] found that MCP-1 could reduce the metastatic potential of colon cancer, and Watanabe et al. [13] found that decreased MCP-1/normal ratio was associated with poor prognosis in colorectal cancer patients. Berencsi et al. [28] theorized that MCP-1 could inhibit the progression of colon cancer cells by regulating tumor-infiltrating cytotoxic T cells (CTL). Similarly, Monti et al. [14] observed that high serum levels of MCP-1 had a significant relation with improved prognosis in resected pancreatic cancer patients and considered that high circulating level of MCP-1 would predict antimalignant activity in pancreatic cancer.
In the present study, we found a striking association between MCP-1 expression and prognosis in lung cancer patients. Namely, positive presence of MCP-1 predicted improved prognosis relative to negative in NSCLC. MCP-1 regulates the progression and invasion of malignant tumors through a series of mechanisms, including infiltration of inflammatory cells in tumor tissue, elevated cytotoxic activity of monocytes and natural killer cells, and mediation of macrophage-induced angiogenesis. Monti et al. [14] demonstrated that the expression of MCP-1 was significantly increased in a synergistic manner by IFN-γ, tumor necrosis factor (TNF), and interleukin 1 (IL-1). They also found that MCP-1 attracted tumor-associated macrophages in pancreatic tumors in vivo. In a mouse melanoma model, MCP-1 played a dual role in tumor growth [29]: low level of MCP-1 stimulated angiogenesis and promoted tumor growth, whereas high level of MCP-1 inhibited tumor growth by the recruitment of macrophages. The interaction between macrophages and tumor is very complicated. There were two phenotypes of macrophages: M1 and M2. Classical or M1 macrophages could be activated by interleukin 2 (IL-2) and IFN, and effect tumoricidal activity [30, 31]; M2 macrophages could promote tumor progression by producing various vasogenic and lymphatic factors, cytokines and protease [32]. To explain our findings, we highlighted on the role and contribution to tumor development by tumor-infiltrating lymphocytes and chemokines. We hypothesized that NSCLC tumor cells would produce MCP-1 to amplify monocytes/macrophages recruitment in tumor tissue; then, these monocytes/macrophages might kill tumor cells after activation by secreting pro-inflammatory cytokines, which in turn would stimulate MCP-1 production; meanwhile, cytokines produced by activated macrophages would change macrophage phenotypes. Although MCP-1 exerted angiogenic effects in vivo, it worked at certain doses but not at higher doses [17]. Moreover, chemokines had the potential to activate protease and promoted degradation of matrix regulating by macrophages [33]. This hypothesis might be partly confirmed by our observation since we found the smaller the tumor size, the more positive for MCP-1 expression in tumor cells. We will do more work to clarify the mechanisms in the future studies. Miotto et al. [34] reported MCP-1 plasma levels were similar in patients with NSCLC, healthy smokers, and non-smokers. However, in our study, tumor MCP-1 expression was observed less frequently in smokers than in non-smokers. We could not present definitive explanation for this finding, but we speculate that some possible mechanism dysregulate the expression of MCP-1 in smokers during malignant transformation in the lung induced by smoking.
Some researchers focused on functional single-nucleotide polymorphism (SNP) in the MCP-1 gene. Yang et al. [35] suggested the MCP-1 -2518A/G was correlated with genetic susceptibility to NSCLC in the Han nationality of North China, whereas polymorphism in the MCP-1 distal regulatory region -2518A/G and -2578A/G found to affect the transcriptional activity and regulate the level of MCP-1 expression [36, 37]. Ghilardi et al. [38] and Tse et al. [39] suggested that polymorphisms of MCP-1 SNP -2518 could be used to evaluate the risk of progression for early-staged breast cancer patients and for nasopharyngeal carcinoma patients after treatment. In our studies, tumor MCP-1 expression was observed more frequently in female patients than in males (94.5 and 69 %, respectively), in adenocarcinoma (88.6 %) than in squamous carcinoma (59 %). Although we could not explain this phenomenon, we hypothesized it might relate with polymorphism of MCP-1 gene. Validation researches are warranted in the future.
As a high affinity receptor of MCP-1, CCR2 expression was considered relatively restricted to certain types of cells. According to the difference of C-terminal tails, CCR2 can be divided into two isoforms: CCR2A and CCR2B [40]. Mononuclear cells and vascular smooth muscle cells predominantly express CCR2A isoform, whereas monocytes and activated natural killer cells mainly express CCR2B isoform [41]. CCR2 has dual roles, including pro-inflammatory and anti-inflammatory activities [42]. Several recent studies have detected CCR2 expression in cancer cells, including multiple myeloma and prostate cancer cells [43, 44]. Using immunohistochemistry, we found CCR2 expression in NSCLC cells: 25 of 134 cases (18.7 %) showed positive staining in cytoplasm and 15 (11.2 %) in nucleus; only 1 case showed CCR2 localization in both cytoplasm and nucleus. We also observed CCR2 expression in infiltrating monocytes. Expression of CCR2 in multiple myeloma predicted improved survival [43], while positive CCR2 presence in prostate cancer cells was associated with tumor progression [44]. In our study, however, neither clinicopathological characteristics nor prognosis was correlated with expression status of CCR2 in cancer cells. Spearman correlation coefficient revealed no significant association between MCP-1 and CCR2 expression in NSCLC cells. Interestingly, when Monti et al. [14] demonstrated the potential mechanisms of antimalignant activity for MCP-1 in pancreatic progression, they failed to detect CCR2 expression in pancreatic cancer cells. Therefore, we supposed that MCP-1 might combine with CCR2 in various inflammatory cells, including monocytes/macrophages, memory T cells, natural killer cells, and endothelial cells rather than CCR2 in cancer cells to modulate progression in NSCLC. It is noteworthy that CCR2 mRNA and protein expressions in lung cancer A549 and H1299 cells were significantly higher compared with that in control cells [44]. Potential association between CCR2 expression of tumor cells and malignant transformation in the lung remains unknown and merits further study.
Taken together, in the present study, we demonstrated the expression of MCP-1 and CCR2 in NSCLC by simple immunohistochemistry. We observed the association between MCP-1 expression and prognosis of NSCLC patients. We believe that the expression of MCP-1 is one of the independent prognostic risk factors in NSCLC, suggesting that MCP-1 expression may reflect the malignant potential of a tumor. Nevertheless, this study is restricted by its retrospective nature and the patients gathered in one institute. Additional prospective large sample-sized studies are needed to confirm the role of MCP-1 in progression and invasion of NSCLC. And validation studies on actions of MCP-1 and CCR2 in lung cancer cells by others are awaited.
Conflict of interest
The authors made no disclosure.
Abbreviations
- NSCLC
Non-small cell lung cancer
- SCLC
Small cell lung cancer
- MCP-1
Monocyte chemoattractant protein-1
- CCR2
CC chemokine receptor 2
- TAM
Tumor-associated macrophages
- UICC
International Union Against Cancer
- AJCC
American Joint Committee on Cancer
- CCL2
CC ligand 2
- MMP-9
Matrix metalloprotease-9
- CTL
Cytotoxic T cells
- IFN
Interferon
- TNF
Tumor necrosis factor
- IL-1
Interleukin 1
- IL-2
Interleukin 2
- SNP
Single-nucleotide polymorphism
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Parkin DM, Li LD, Chen YD, Bray F. Estimation and projection of the national profile of cancer mortality in China: 1991–2005. Br J Cancer. 2004;90:2157–2166. doi: 10.1038/sj.bjc.6601813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulshine JL. Clinical practice. Lung cancer screening. N Engl J Med. 2005;352:2714–2720. doi: 10.1056/NEJMcp042630. [DOI] [PubMed] [Google Scholar]
- 4.Visbal AL, Williams BA, Nichols FC., 3rd Marks RS, Jett JR, Aubry MC, Edell ES, Wampfler JA, Molina JR, Yang P (2004) Gender differences in non-small-cell lung cancer survival: an analysis of 4618 patients diagnosed between 1997 and 2002. Ann Thorac Surg. 2004;78:209–215. doi: 10.1016/j.athoracsur.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 7.Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakashima E, Mukaida N, Kubota Y, Kuno K, Yasumoto K, Ichimura F, Nakanishi I, Miyasaka M, Matsushima K. Human MCAF gene transfer enhances the metastatic capacity of a mouse cachectic adenocarcinoma cell line in vivo. Pharm Res. 1995;12:1598–1604. doi: 10.1023/A:1016276613684. [DOI] [PubMed] [Google Scholar]
- 9.Zachariae CO, Anderson AO, Thompson HL, Appella E, Mantovani A, Oppenheim JJ, Matsushima K. Properties of monocyte chemotactic and activating factor (MCAF) purified from a human fibrosarcoma cell line. J Exp Med. 1990;171:2177–2182. doi: 10.1084/jem.171.6.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Y, Cai Z, Galson DL, Xiao G, Liu Y, George DE, Melhem MF, Yao Z, Zhang J. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006;66:1311–1318. doi: 10.1002/pros.20464. [DOI] [PubMed] [Google Scholar]
- 11.Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM, Barry FP, O’Brien T, Kerin MJ (200)7 Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res 13: 5020–5027 [DOI] [PubMed]
- 12.Koide N, Nishio A, Sato T, Sugiyama A, Miyagawa S. Significance of macrophage chemoattractant protein-1 expression and macrophage infiltration in squamous cell carcinoma of the esophagus. Am J Gastrogenterol. 2004;99:1667–1674. doi: 10.1111/j.1572-0241.2004.30733.x. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe H, Miki C, Okugawa Y, Toiyama Y, Inoue Y, Kusunoki M. Decreased expression of monocyte chemoattractant protein-1 predicts poor prognosis following curative resection of colorectal cancer. Dis Colon Rectum. 2008;51:1800–1805. doi: 10.1007/s10350-008-9380-7. [DOI] [PubMed] [Google Scholar]
- 14.Monti P, Leone BE, Marchesi F, Balzano G, Zerbi A, Scaltrini F, Pasquali C, Calori G, Pessi F, Sperti C, Di Carlo V, Allavena P, et al. The CC chemokine MCP-1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Res. 2003;63:7451–7461. [PubMed] [Google Scholar]
- 15.Lebrecht A, Hefler L, Tempfer C, Koelbl H. Serum cytokine concentrations in patients with cervical cancer: interleukin-4, interferon-gamma, and monocyte chemoattractant protein-1. Gynecol Oncol. 2001;83:170–171. doi: 10.1006/gyno.2001.6361. [DOI] [PubMed] [Google Scholar]
- 16.Hefler L, Tempfer C, Heinze G, Mayerhofer K, Breitenecker G, Leodolter S, Reinthaller A, Kainz C. Monocyte chemoattractant protein-1 serum levels in ovarian cancer patients. Br J Cancer. 1999;81:855–859. doi: 10.1038/sj.bjc.6690776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- 18.Robinson SC, Scott KA, Balkwill FR. Chemokine stimulation of monocyte matrix metalloproteinase-9 requires endogenous TNF-alpha. Eur J Immunol. 2002;32:404–412. doi: 10.1002/1521-4141(200202)32:2<404::AID-IMMU404>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Cai Z, Chen Q, Chen J, Lu Y, Xiao G, Wu Z, Zhou Q, Zhang J. Monocyte chemotactic protein 1 promotes lung cancer-induced bone resorptive lesions in vivo. Neoplasia. 2009;11:228–236. doi: 10.1593/neo.81282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Strom SR, Burdick MD, Iannettoni MD, Strieter RM. Macrophage infiltration in human non-small-cell lung cancer: the role of CC chemokines. Cancer Immunol Immunother. 2000;49:63–70. doi: 10.1007/s002620050603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai E, Ogawa T, Kielian T, Ikubo A, Suzuki T. Irradiated tumor cells adenovirally engineered to secrete granulocyte/macrophage-colony-stimulating factor establish antitumor immunity and eliminate preexisting tumors in syngeneic mice. Cancer Immunol Immunother. 1998;47:72–80. doi: 10.1007/s002620050506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nokihara H, Yanagawa H, Nishioka Y, Yano S, Mukaida N, Matsushima K, Sone S. Natural killer cell-dependent suppression of systemic spread of human lung adenocarcinoma cells by monocyte chemoattractant protein-1 gene transfection in severe combined immunodeficient mice. Cancer Res. 2000;60:7002–7007. [PubMed] [Google Scholar]
- 23.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 24.Pitkin L, Luangdilok S, Corbishley C, Wilson PO, Dalton P, Bray D, Mady S, Williamson P, Odutoye T, Rhys Evans P, Syrigos KN, Nutting CM, et al. Expression of CC chemokine receptor 7 in tonsillar cancer predicts cervical nodal metastasis, systemic relapse and survival. Br J Cancer. 2007;97:670–677. doi: 10.1038/sj.bjc.6603907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zlotnik A, Yoahie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/S1074-7613(00)80165-X. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y, Beller DI, Frendl G, Graves DT. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992;148:2423–2428. [PubMed] [Google Scholar]
- 27.Huang S, Singh RK, Xie K, Gutman M, Berry KK, Bucana CD, Fidler IJ, Bar-Eli M. Expression of the JE/MCP-1 gene suppresses metastatic potential in murine colon carcinoma cells. Cancer Immunol Immunother. 1994;39:231–238. doi: 10.1007/BF01525986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berencsi K, Rani P, Zhang T, Gross L, Mastrangelo M, Meropol NJ, Herlyn D, Somasundaram R. In vitro migration of cytotoxic T lymphocyte derived from a colon carcinoma patient is dependent on CCL2 and CCR2. J Transl Med. 2011;9:33. doi: 10.1186/1479-5876-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci USA. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartoli C, Civatte M, Pellissier JF, Figarella-Branger D. CCR2A and CCR2B, the two isoforms of the monocyte chemoattractant protein-1 receptor are up-regulated and expressed by different cell subsets in idiopathic inflammatory myopathies. Acta Neuropathol. 2001;102:385–392. doi: 10.1007/s004010100394. [DOI] [PubMed] [Google Scholar]
- 31.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vande Broek I, Leleu X, Schots R, Facon T, Vanderkerken K, Van Camp B, Van Riet I. Clinical significance of chemokine receptor (CCR1, CCR2 and CXCR4) expression in human myeloma cells: the association with disease activity and survival. Haematological. 2006;2006(91):200–206. [PubMed] [Google Scholar]
- 33.Lu Y, Cai Z, Xiao G, Liu Y, Keller ET, Yao Z, Zhang J. CCR2 expression correlates with prostate cancer progression. J Cell Biochem. 2007;101:676–685. doi: 10.1002/jcb.21220. [DOI] [PubMed] [Google Scholar]
- 34.Miotto D, Boschetto P, Bononi I, Milani G, Legorini C, Cavallesco G, Lo Cascio N, Zeni E, Fabbri LM, Mapp CE. CC ligand 2 levels are increased in LPS-stimulated peripheral monocytes of patients with non-small cell lung cancer. Respir Med. 2007;101:1738–1743. doi: 10.1016/j.rmed.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Shi GL, Song CX, Xu SF. Relationship between genetic polymorphism of MCP-1 and non-small-cell lung cancer in the Han nationality of North China. Genet Mol Res. 2010;27:765–771. doi: 10.4238/vol9-2gmr740. [DOI] [PubMed] [Google Scholar]
- 36.Rovin BH, Lu L, Saxena R. A novel polymorphism in the MCP-1 gene regulatory region that influences MCP-1 expression. Biochem Biophys Res Commun. 1999;259:344–348. doi: 10.1006/bbrc.1999.0796. [DOI] [PubMed] [Google Scholar]
- 37.Page SH, Wright EK, Jr, Gama L, Clements JE. Regulation of CCL2 expression by an upstream TALE homeodomain protein-binding site that synergizes with the site created by the A-2578G SNP. PLoS One. 2011;6:e22052. doi: 10.1371/journal.pone.0022052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghilardi G, Biondi ML, La Torre A, Battaglioli L, Scorza R. Breast cancer progression and host polymorphisms in the chemokine system: role of the macrophage chemoattractant protein-1 (MCP-1) -2518 G allele. Clin Chem. 2005;51:452–455. doi: 10.1373/clinchem.2004.041657. [DOI] [PubMed] [Google Scholar]
- 39.Tse KP, Tsang NM, Chen KD, Li HP, Liang Y, Hsueh C, Chang KP, Yu JS, Hao SP, Hsieh LL, Chang YS. MCP-1 promoter polymorphism at 2518 is associated with metastasis of nasopharyngeal carcinoma after treatment. Clin Cancer Re. 2007;13:6320–6326. doi: 10.1158/1078-0432.CCR-07-1029. [DOI] [PubMed] [Google Scholar]
- 40.Nesbit M, Schaider H, Miller TH, Herlyn M. Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J Immunol. 2001;166:6483–6490. doi: 10.4049/jimmunol.166.11.6483. [DOI] [PubMed] [Google Scholar]
- 41.Brigati C, Noonan DM, Albini A, Benelli R. Tumors and inflammatory infiltrates: friends or foes? Clin Exp Metastasis. 2002;19:247–258. doi: 10.1023/A:1015587423262. [DOI] [PubMed] [Google Scholar]
- 42.Tsung K, Dolan JP, Tsung YL, Norton JA. Macrophages as effector cells in interleukin 12-induced T-cell-dependent tumor rejection. Cancer Res. 2002;62:5069–5075. [PubMed] [Google Scholar]
- 43.Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnsen M, Lund LR, Rømer J, Almholt K, Danø K. Cancer invasion and tissue remodeling: common themes in proteolytic matrix degradation. Curr Opin Cell Biol. 1998;10:667–671. doi: 10.1016/S0955-0674(98)80044-6. [DOI] [PubMed] [Google Scholar]