Abstract
Survivin is overexpressed in major types of cancer and is considered an ideal “universal” tumor-associated antigen that can be targeted by immunotherapeutic vaccines. However, its anti-apoptosis function raises certain safety concerns. Here, a new truncated human survivin, devoid of the anti-apoptosis function, was generated as a candidate tumor vaccine. Interleukin 2 (IL-2) has been widely used as an adjuvant for vaccination against various diseases. Meanwhile, the DNA prime and recombinant adenovirus (rAd) boost heterologous immunization strategy has been proven to be highly effective in enhancing immune responses. Therefore, the efficacy of a new cancer vaccine based on a truncated form of survivin, combined with IL-2, DNA prime, and rAd boost, was tested. As prophylaxis, immunization with the DNA vaccine alone resulted in a weak immune response and modest anti-tumor effect, whereas the tumor inhibition ratio with the DNA vaccine administered with IL-2 increased to 89 % and was further increased to nearly 100 % by rAd boosting. Moreover, complete tumor rejection was observed in 5 of 15 mice. Efficacy of the vaccine administered therapeutically was enhanced by nearly 300 % when combined with carboplatin. These results indicated that vaccination with a truncated survivin vaccine using DNA prime–rAd boost combined with IL-2 adjuvant and carboplatin represents an attractive strategy to overcoming immune tolerance to tumors and has potential therapeutic benefits in melanoma cancer.
Keywords: Survivin, Tumor vaccine, Prime-boost, Immunoadjuvant, Chemotherapeutics
Introduction
Cancer ranks as the number one cause of death in economically developed countries and the number two cause of death in developing countries [1]. Vaccines are emerging as a promising treatment approach for cancer patients. Many tumor-associated antigens (TAA) have been found as good targets for immunotherapy and vaccine design. An optimized cancer vaccine and therapeutic strategy should combine immunogenic tumor antigens with the most effective immunotherapy agents and/or delivery strategies to ensure successful clinical results [2].
Among the large number of tumor antigens identified to date, survivin has many characteristics that render it an attractive target for anti-cancer therapy. As a novel member of the inhibitor of apoptosis (IAP) protein family, survivin is overexpressed in most types of cancers and embryonic tissues, while it is not expressed in most normal adult tissues [3]. Survivin expression is also positively correlated with chemoresistance, unfavorable prognosis, and shortened patient survival, making it a potential target for cancer treatment. Multiple strategies have recently utilized survivin as a cancer immunotherapeutic target. Immunization with a modified vaccinia Ankara (MVA) vector expressing full-length murine survivin combined with the chemical drug gemcitabine resulted in significant tumor regression and prolonged survival in a murine pancreatic carcinoma model [4]. Efficient co-transduction of adenoviral vectors encoding carcinoembryonic antigen and survivin into dendritic cells by the CAR-TAT adaptor molecule to enhance anti-tumor immunity has also been demonstrated in a murine colorectal cancer model [5]. Furthermore, a human survivin DNA vaccine delivered by intradermal electroporation was verified to induce antigen-specific cytotoxic T lymphocytes (CTLs), suppress angiogenesis, and confer protection against mouse melanoma [6].
However, survivin functions as an IAP and its overexpression in a wild-type form may be harmful. In the present study, constructs expressing novel truncations of survivin, S8, and S38, with deletions of 7 and 37 N-terminal amino acids residues, respectively, were made based on the protein structure, function, and immunological characteristics. These truncated forms of survivin were expected to lose their anti-apoptosis function and, therefore, would both be safer vaccine candidate antigens than the full-length protein. In order to preserve immunogenicity, S8 with the smaller deletion was chosen as the candidate antigen for the construction of a tumor gene-specific vaccine. Several factors other than the antigen contribute to the efficacy of a vaccine, such as the immunoadjuvant and delivery strategy. In this study, the cytokine interleukin-2 (IL-2), which can activate many immune cell types, including T and B lymphocytes, macrophages, and NK cells, was chosen as an adjuvant [7].
DNA vectors are currently accepted as ideal vaccines to induce primary immunity and have certain advantages, such as mimicking natural immunity, the option of giving multiple immunizations, as well as the capability of inducing high levels of memory CTLs [6]. Previous efforts in targeting tumors using naked DNA vaccines have shown anti-tumor effects in vivo [8–10]. However, no or only modest tumor protection was reported for this type of vaccine. At present, the recombinant adenovirus (rAd) vector is one of the most widely used virus vectors in gene therapy research and clinical trials, since it can express exogenous antigens efficiently. rAd can be used to enhance (boost) immunity with robust responses by inducing production of various cytokines [11]. Since repeated vaccination will induce an immune response to the adenovirus vector itself and decrease efficacy of the vaccine, a DNA prime–rAd boost strategy is presently considered an effective strategy for vaccine immunotherapy [12].
Despite evidence of primed effector T cells, many studies have indicated that the failure of tumor vaccines in clinical trials is likely due to the heterogeneity of tumor cells, that is, not every tumor cell expresses the antigen targeted by the vaccine. Therefore, the combination of a vaccine with other therapies, such as chemotherapy [13], may be more effective. In this study, we chose a widely used anti-tumor chemotherapy drug carboplatin to test in combination with our cancer vaccine.
The aim of this study, therefore, was to construct and evaluate the immunogenicity and anti-tumor activity of a DNA vaccine and rAd vaccine expressing a novel truncation of survivin. At the same time, the DNA prime–rAd boost immunization strategy, IL-2 adjuvant, and carboplatin were used to enhance the immunogenicity and effectiveness of the anti-tumor vaccine.
Materials and methods
Primers
The sequences of the oligonucleotides (synthesized by Sangon Biotech Co. Ltd., Shanghai, China) used in this study for amplifying survivin and its truncates and producing the modified IL-2 by overlapping PCR are as follows:
P1: 5′-CTGCAGTCGACGCCGCCACCATGGGTGCCCCGACGTTG-3′; P2: 5′-GGATCCGGT ACCAAGCTTAATCCATGGCAGCCAGC-3′; P3: 5′-AGGGCTGCGCCTGCGCTCCGGAACG GATG-3′; P4: 5′-CATCCGTTCCGGAGCGCAGGCGCAGCCCT-3′; P5: 5′-TCTAGAGTCGA CATGCCTGCCTGGCAGCCC-3′; P6: 5′-TCTAGAGTCGACATGGCTGAGGCTGGCT-3′; P7: 5′-TTCAAGTTTTACATGCCTAAGAAAGCTACCGAGCTGAAACATCTGCAGTGTCTGG-3′; P8: 5′-GTTCAGCACTTCTTCCAGAGGCTTCAGTTCTTCTTCCAGACACTGCAGATGTTTC-3′; P9: 5′-CAACTACAAGAACCCTAAGCTGACAAGAATGCTGACCTTCAAGTTTTACATG CCTA-3′; P10: 5′-GTCTCTGGGTCTCAAGTGGAAATTCTTACTTTGGGCCAAGTTCAGCA CTTCTTCCAGA-3′; P11: 5′-TACTCCTGGACTTGCAGATGATATTGAATGGCATTAACAA CTACAAGAACCCTAAGC-3′; P12: 5′-TCAGTTCGAGGACGATCACGTTAATGTTGCTAA TCAAGTCTCTGGGTCTCAAGTGG-3′; P13: 5′-CAGCTCCACCAAGAAGACACAGCTACAG CTCGAACACTTACTCCTGGACTTGCAGAT-3′; P14: 5′-GTCAGCGTACTCACACATGAAT GTTGTCTCAGATCCCTTCAGTTCGAGGACGATCAC-3′; P15: 5′-CTCTGAGTCTGGCTCTG GTGACAAACAGTGCT CCTACATCCAGCTCCACCAAGAAGACA-3′; P16: 5′-TGATCCACC TGTTCAGAAACTCCACAATGGTAGCGGTCTCGTCAGCGTACTCACACATG-3′; P17: 5′-G GATCCGCCATGTATAGAATGCAGCTGCTGTCTTGTATCGCTCTGAGTCTGGCTCTGGT-3′; P18: 5′-AGATCTTCAAGTCAGGGTAGAGATGATGCTCTGACAGAATGTGATCCACCTGTT CAGAA-3′. The underlined nucleotides indicate sites of mutagenesis.
Construction and preparation of plasmids
The cDNA of human survivin and its truncated fragments S8 and S38 were amplified from total RNA of HEK293 cells by RT-PCR using primer pairs P1/P2, P5/P2, and P6/P2. After DNA sequencing, the amplified fragments were cloned into the SalI/BamHI sites of the VR1012 vector (VR), generating VR-S, VR-S8, and VR-S38. The survivin mutant VR-S-T34A was obtained by PCR-mediated mutagenesis using the primer pair P3/P4 with VR-S as template. The point mutation, ACC (Thr) to GCT (Ala), was further verified by DNA sequencing. The human IL-2 cDNA (GenBank, BC070338) was human codon-optimized and synthesized by overlapping PCR to increase expression using six pairs of primers, P7–P18. The modified IL-2 cDNA was cloned into the VR vector at BamHI/BglII sites to generate VR-IL2. All plasmids were amplified in Escherichia coli Top10 cells and extracted using a Qiagen Maxi purification kit with the purity of 90 % for supercoiled DNA and 1 EU/mg of endotoxins.
Construction and preparation of rAd vectors
The rAd vector expressing truncated survivin was obtained using the AdMax™ Adenovirus Vector Creation System (Microbix Biosystems). The S8 fragment with EcoRI/BamHI sites was subcloned into the EcoRI/BglII sites of the pDC316 shuttle vector. rAd was produced by homologous recombination between pBHGloxΔE1,3Cre, and pDC316-S8 at the loxP or frt position when co-transfected into HEK293 cells. Plaque formation observed about 10 days post-transfection indicated successful generation of the rAd. After verification by PCR or Western blot analysis, the rAd containing S8 (AD-S8) was amplified and purified from cell lysates twice in CsCl density gradients, as described previously [14]. Viral products were desalted and stored at −80 °C in PBS containing 10 % glycerol (v/v). The titer of the viral stock was determined using the Reed and Muench method and expressed as 50 % tissue culture infectious doses (TCID50).
Reagents
The following reagents were obtained commercially: caspase-3 cellular activity assay kit (Cal Biochem), fetal bovine serum (FBS) (Gibco), paclitaxel (PTX) and Lipofectamine™ 2000 (Invitrogen), restriction enzymes (New England Biolabs), Trypan blue (Takara), non-radioactive cytotoxicity assay kit (Promega), ELISPOT kit (BD Biosciences), VR empty vector (Vical), and mouse monoclonal antibody against human survivin (Novus-Biologicals).
Cell culture and transfection
Cell lines Hela, COS-7, HEK293, Chang liver (human normal liver cell), HepG2 (human liver cancer cell), C2C12 (mouse skeletal muscle cell), and B16 (mouse melanoma cell derived from C57/BL/6 murine, H-2 Db) purchased from the Cell Bank, Chinese Academy of Sciences were maintained in DMEM containing 10 % heat-inactivated FBS. Cultured cells were incubated in a humidified atmosphere containing 5 % CO2 at 37 °C. Transfection of cells with various mammalian expression constructs by Lipofectamine™ 2000 was performed according to the manufacturer’s specifications.
Stable cell line and mouse tumor model
The S+fB16 cell line stably expressing full-length human survivin was established in our laboratory. Female C57BL/6 (H-2 Db) mice (age, 6–8 weeks; weight, 18–22 g) were purchased from the Beijing laboratory animal center and hosted in appropriate animal care facilities. Three independent experiments were performed to test the tumorigenicity of S+fB16 cells, and we confirmed that all mice injected with at least 105 cells developed tumors.
Cell death and apoptosis assay
At 20-h post-transfection with various plasmids, Hela, C2C12, Chang liver, and HepG2 cells were incubated with PTX (80 nM) for another 24 h. The viability of Hela cells was measured by the standard trypan blue exclusion method by counting blue dead cells. Apoptosis of C2C12, Chang liver, and HepG2 cells was determined by staining with DAPI and counting the bright blue cells that contained aberrant nuclei with large-scale chromatin condensation, chromatin fragmentation, or apoptotic bodies. Data are expressed as percentages of the control and are the means of three independent experiments.
Capase-3 activity assay
Twenty hours after transfection, cells were treated with PTX (80 nM). At 48 h after transfection, cells were harvested and disrupted in cell lysis buffer (50 mM HEPES, 1 mM DTT, 0.1 mm EDTA 0.1 % chaps, pH 7.4). Caspase-3 activity in the cell lysate was then assayed according to the instructions provided by the manufacturer of the kit (Cal Biochem).
Analysis of apoptotic DNA fragments
Twenty hours after transfection, cells were treated with PTX (80 nM). Cells were harvested after another 24 h, and then low-molecular-weight DNA was collected to analyze apoptotic DNA fragmentation by agarose gel electrophoresis as previously described [15].
Epitope prediction and synthesis
T cell epitopes from human survivin sequences were determined as previously described by prediction of the optimal proteasomal cleavage sites using the PAProC algorithm (www.paproc.de) and by the prediction of binding to H-2 Db MHC molecules using the SYFPEITHI algorithm (www.syfpeithi.de) [6, 16]. The three highest scoring SYFPEITHI peptides, S20–28 (STFKNWPFL), S46–54 (CPTENEPDL), and S88–96 (SVKKQFEEL), were selected and synthesized by Shanghai GL peptide Ltd. at > 90 % purity.
Plasmid DNA and rAd immunization in C57BL/6 mice
Five groups of C57BL/6 mice were used (5 mice per group). Immunizations were performed by intramuscular injection with 100 μg of plasmid or 1 × 108 plaque-forming units (pfu) rAd into the tibialis anterior muscles of both legs (50 μg each) at 2-week intervals. Two weeks after the final immunization, all mice were killed to collect blood and spleens for the evaluation of humoral and cellular immune responses.
Cytotoxicity assay
Cytotoxicity was measured by a standard lactate dehydrogenase (LDH) release assay with a non-radioactive cytotoxicity assay kit as previously described [17]. Briefly, splenocytes from the immunized mice were incubated at different effector cell-to-target cell ratios (E:T) with 1 × 104 target cells labeled with the H-2 Db-restricted survivin peptide or S+fB16 cells for at least 4 h. The released LDH was assayed to analyze CTL activity according to the manufacturer’s instructions.
IFN-γ ELISPOT assay
Survivin peptide-specific IFN-γ release of effector CD8+ T cells was detected with an ELISPOT kit according to the instructions provided by the manufacturer. The reaction was terminated upon the appearance of dark purple spots, which were quantitated using the AlphaImager System.
Anti-survivin antibody detection
The sera were incubated at 1:50 dilution on blocked Western blot strips, which were prepared by separating survivin-transfected cell lysates by SDS–PAGE and transferring to nitrocellulose membranes for Western blot analysis.
Tumor protection in C57BL/6 mice
C57BL/6 mice (8–15 per group) were immunized four times with 100 μg of plasmid or 1 × 108 pfu rAd into the tibialis anterior muscles of both legs at 2-week intervals. The immunization schedule was the same as that outlined in Fig. 4a. One week after the final immunization, the mice were challenged subcutaneously with 5 × 105 S+fB16 viable cells in the right lower flank and monitored daily for tumor development. Tumor width and length were measured periodically with a caliper and estimated using the formula: (length × width2)/2 (cm3). Tumors > 2 mm in diameter with progressive growth were recorded as positive. The mice were monitored for approximately 4 weeks after tumor challenge, and the tumor-bearing mice were then killed for tumor weight measurement. In survival studies, the animals were monitored for approximately 50 days after tumor challenge.
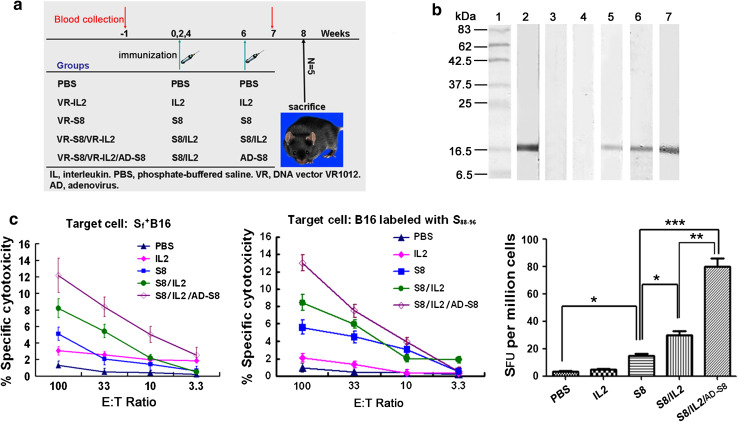
Fig. 4.
Survivin-based vaccine elicits specific humoral and cellular immune responses. a Immunization schedule. b Western blot to verify specific anti-survivin antibody responses in mouse sera collected from immunized mice 1 week after the last immunization. The lysate of COS-7 cells transfected with VR-S8 was used as the detection antigen. Lane 1, prestained protein marker; lane 2, positive control with the anti-survivin mAb; lane 3, PBS group; lane 4, VR-IL2 group; lane 5, VR-S8 group; lane 6, VR-S8/VR-IL2 group; lane 7, VR-S8/VR-IL2/AD-S8 group. c Survivin-specific functional CD8+ T cells induced by vaccination in C57BL/6 mice (left and middle) (E:T ratio, effector:target ratio). Splenocytes were pooled from five mice per group immunized with survivin DNA vaccine alone, combined with IL-2 adjuvant, or combined with a rAd boost. PBS and VR-IL2 groups were negative controls. The CTL lytic activity was assessed by the LDH assay with target cells S+fB16 (left) or B16 cells labeled with the S88–96 CTL epitope peptide (middle). Data points represent mean values of triplicate wells ± SEM. Released IFN-γ was detected by ELISPOT (right) and expressed as the average number of peptide-specific spots formed in response to S88–96 among 1 × 106 cells from each group (SFU, spot-forming units)
Tumor therapy in C57BL/6 mice
C57/BL/6 mice (8–15 per group) were injected subcutaneously with 5 × 105 S+fB16 viable cells per mouse on day 0. The vaccine group was then immunized intramuscularly with 100 μg VR-S8 plus 100 μg VR-IL2 per mouse on day 1 and 8 and with 1 × 108 pfu AD-S8 per mouse on day 15. Negative control mice were given 100 μl PBS, and positive control mice were given carboplatin intraperitoneally four times at 4-day intervals (0.5 mg/100 μl per mouse each time). The combination group was immunized with the vaccine and treated with carboplatin simultaneously.
Statistical analysis
All in vivo and in vitro experiments were performed at least 3 times. For all statistical methods, values of P < 0.05 were considered significant and those of P < 0.01 were considered highly significant. All statistical analyses were performed with SPSS software.
Results
Construction and expression of survivin and truncated mutants
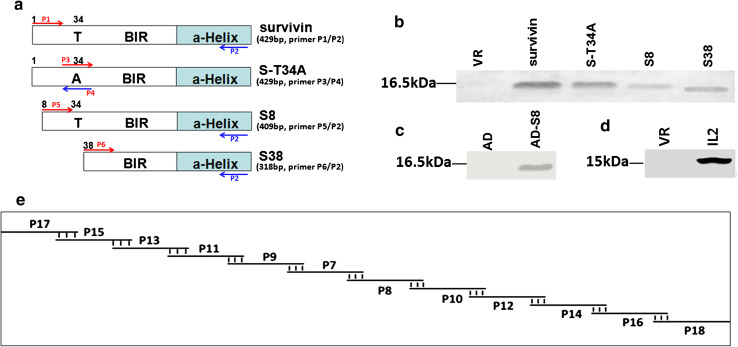
Several point mutations such as T34A, D53A, and C84A have been reported to eliminate the ability of survivin to inhibit apoptosis [18–21]. However, whether the N-terminal deletion of 7 or 37 amino acids in survivin (S8 and S38, respectively) can affect its anti-apoptosis function has not been studied. Therefore, we constructed expression vectors for S8 and S38 as novel survivin mutants and used the dominant negative Thr34 to Ala mutant of survivin (S-T34A) as a control (Fig. 1a) [22, 23]. The expression levels of survivin and these mutants in Hela cells are shown in Fig. 1b. S8 was also subcloned into the adenovirus vector, and the expression of this rAd in HEK293 cells is shown in Fig. 1c.
Fig. 1.
Construction and expression of survivin, N-terminal truncation mutants of survivin, and IL-2. a Schematic diagrams of constructs for survivin, S-T34A, S8, and S38. b Expression levels of survivin and its mutants transfected in Hela cells were verified by Western blot using an anti-survivin mAb. c Expression of AD-S8 in HEK293 cells. d Expression of VR-IL2 in COS-7 cells. e Schematic diagram of the synthesis of modified human IL-2 by overlapping PCR using six pairs of primers (BIR: baculovirus IAP repeat)
Overexpressed S8 and S38 do not inhibit apoptosis induced by treatment with PTX
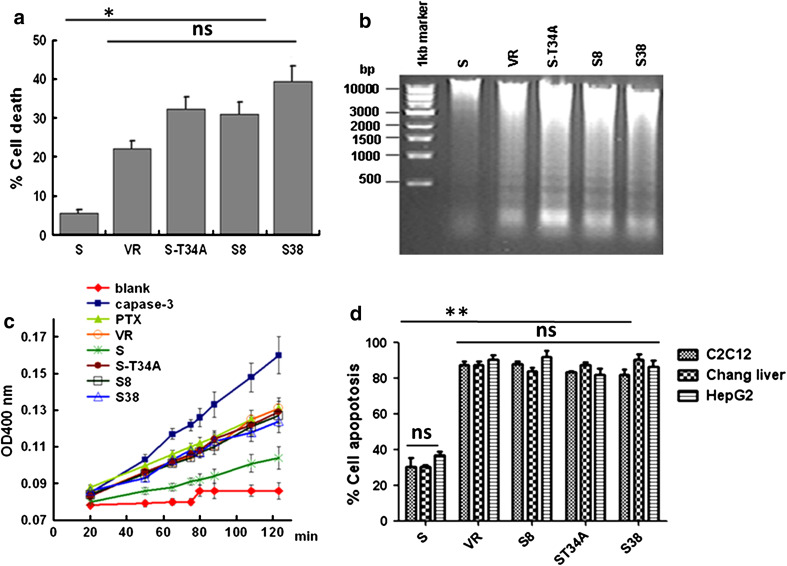
To investigate the function of our novel survivin truncations S8 or S38, we transfected Hela cells with various plasmids, including VR, VR-S, VR-S-T34A, VR-S8, or VR-S38. Twenty hours after transfection, cells were treated with PTX (80 nM). After 24 h of PTX treatment, S8, S38, and S-T34A transfected cells showed significant levels of apoptosis characterized by the typical morphological changes of apoptotic cell shapes from spindle-like to round. The results of cell viability, DNA fragmentation, and caspase-3 activity assays were similar between the Hela cells with S8 and S38 and those with S-T34A, which all had significantly greater levels of apoptosis than did cells expressing wild-type survivin (P < 0.05) (Fig. 2a–c). Similar results were also found in other cells, such as C2C12, Chang liver, and HepG2 cells (Fig. 2d).
Fig. 2.
Overexpression of S8 or S38 does not inhibit apoptosis in different types of cells induced by treatment with PTX. The ability of S8 and S38 to affect cell viability was assayed by transfecting cells (4 × 105 cells/well) in 6-well plates with 4 μg of each mammalian expression vector. Twenty-four hours after transfection, cells were incubated with PTX (80 nM) for another 24 h. a Viability of the Hela cells was then measured with the standard trypan blue exclusion method by counting blue dead cells. b Low-molecular-weight DNA was collected from Hela cells and analyzed on ethidium bromide-stained agarose gels. c Caspase-3 activity was detected using the lysed supernatant of Hela cells. d Apoptosis of C2C12, Chang liver, and HepG2 cells was determined by staining with DAPI and counting the bright blue cells that contained aberrant nuclei with large-scale chromatin condensation, chromatin fragmentation, or apoptotic bodies
Predicted epitope is targeted by CD8+ T cells in mice
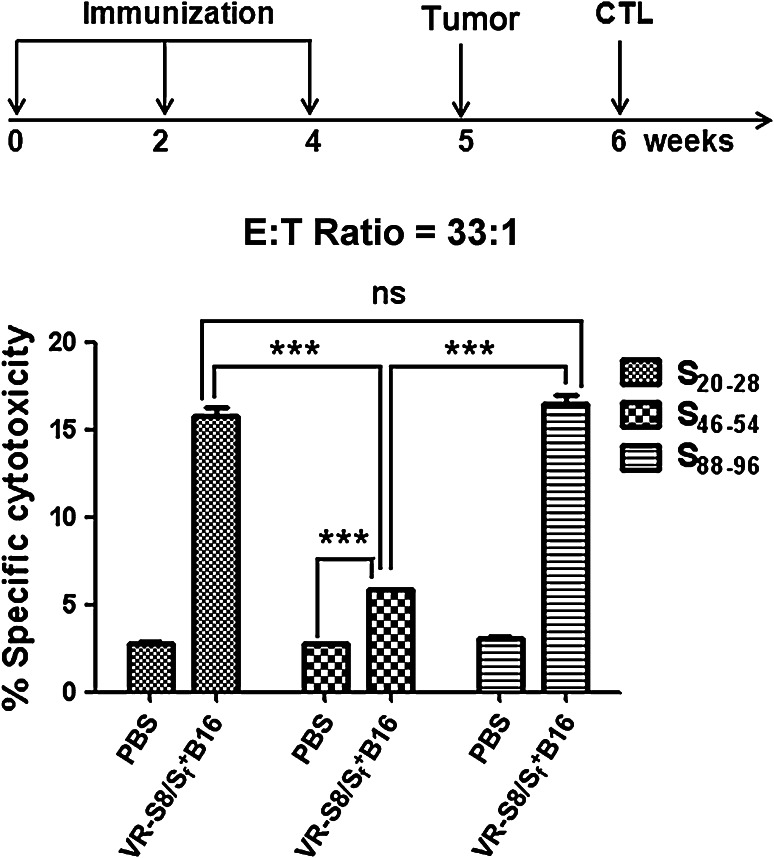
The predicted H-2 Db-restricted epitope peptides were tested in CTL assays in LDH release cytotoxicity assays. The cytolytic activities of splenocytes from VR-S8 immunized C57/BL/6 mice challenged with S+fB16 tumor cells were assessed against MHC-matched B16 tumor cells labeled with these peptides. The results showed that specific CTL responses were induced against S20–28, S46–54, and S88–96. The responses to S20–28 and S88–96 were stronger than that to S46–54, and the response to S88–96 was modestly stronger than that to S20–28. Therefore, we used the S88–96 peptide to measure CD8+ T cell responses in the subsequent experiment. The results of CTL assays using the E:T ratio of 33:1 are shown in Fig. 3.
Fig. 3.
Predicted survivin epitope peptides can be used to measure CD8+ T cell responses. Splenocytes were separated from five mice immunized with VR-S8 and challenged with S+fB16 tumor cells. CTL activity was assessed by the LDH assay with B16 target cells labeled with the predicted epitope peptides survivin S20–28, S46–54, and S88–96 at E:T ratio = 33:1 (**P < 0.01; ***P < 0.001)
Specific humoral responses to survivin
We examined the potential of the DNA vaccine VR-S8 alone to induce survivin-specific antibody responses, the efficiency of IL-2 as an adjuvant for the DNA vaccine, as well as the DNA prime–rAd boost strategy for enhancing humoral immunity using the immunization schedule shown in Fig. 4a. The results showed that the mice injected with VR-S8, VR-S8/VR-IL2, or VR-S8/VR-IL2/AD-S8 all produced survivin-specific antibodies, and the response in the VR-S8/VR-IL2 group was obviously stronger than that of the VR-S8 group, but obviously weaker than that of the VR-S8/VR-IL2/AD-S8 group (Fig. 4b).
Specific cellular immune responses to survivin
The CTL activities of splenocytes from immunized C57/BL/6 mice (Fig. 4c, left and middle) were assessed against MHC-matched B16 tumor cells labeled with an H-2 Db-restricted survivin peptide S88–96 and S+fB16 cells in LDH release cytotoxicity assays. The results showed that a weak specific CTL response was induced by the VR-S8 DNA vaccine alone. A higher specific CTL response was induced by the VR-S8/VR-IL2 DNA vaccine, while the specific lysis of the splenic lymphocytes from VR-S8/VR-IL2/AD-S8-vaccinated mice was significantly increased compared with that from VR-S8/VR-IL2-vaccinated mice (P < 0.05).
The ELISPOT results shown in Fig. 4c (right) indicate that survivin-specific CD8+ T cells releasing IFN-γ were induced in mice immunized with VR-S8, VR-S8/VR-IL2, and VR-S8/VR-IL2/AD-S8. Moreover, the average number of IFN-γ spots doubled in mice immunized with VR-S8/VR-IL2 compared with that of mice immunized with VR-S8 (P < 0.05). The average number of peptide-specific IFN-γ spots doubled in mice immunized with VR-S8/VR-IL2/AD-S8 compared with that of mice immunized with VR-S8/VR-IL2 (P < 0.01).
Tumor protective efficacy of the S8-based combination vaccine
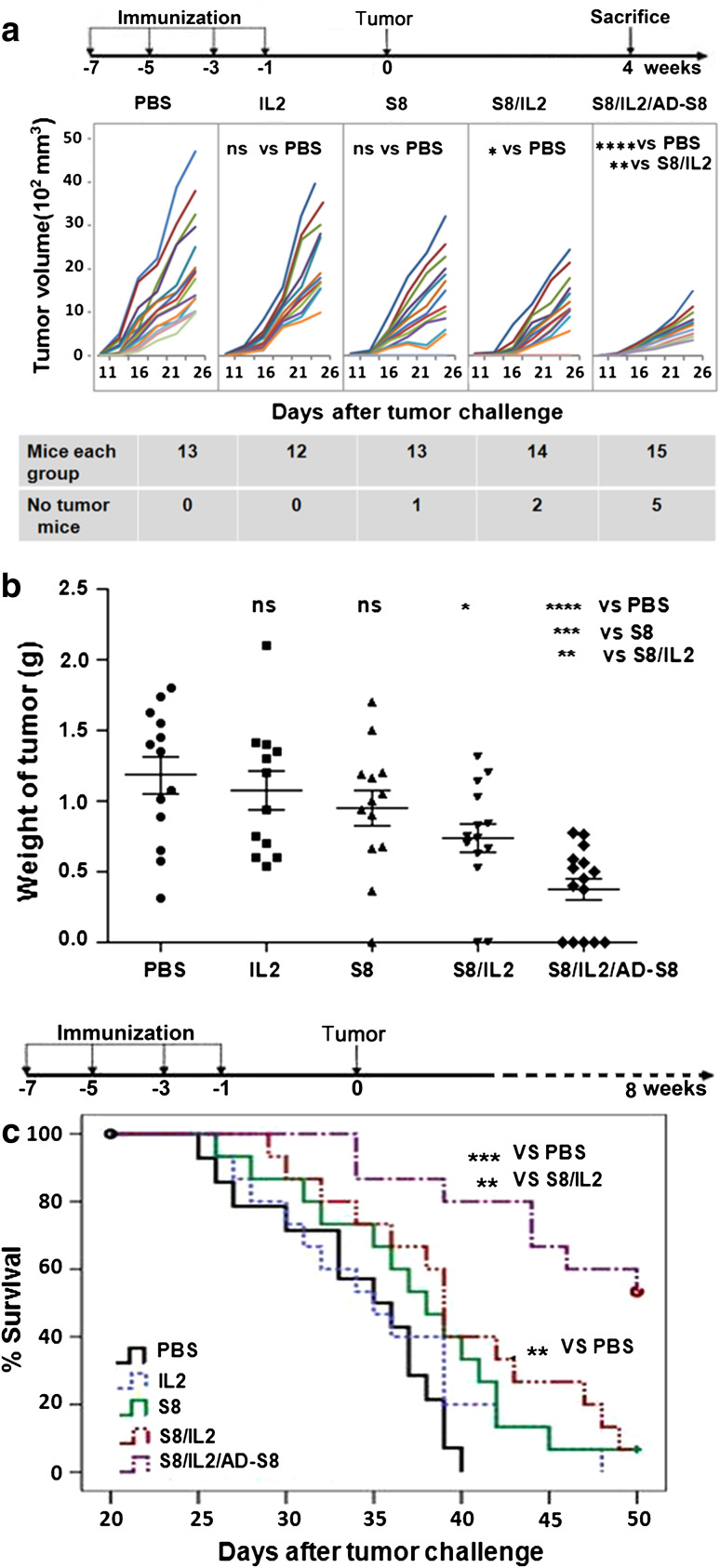
As shown in Fig. 5a and b, tumor growth was potently inhibited in mice vaccinated with VR-S8/VR-IL2 relative to mice immunized with VR-IL2 or VR-S8 alone, and the tumors were significantly inhibited in mice boosted with AD-S8 compared with those in mice given VR-S8/VR-IL2 (P < 0.01). Moreover, complete tumor rejection occurred in 5 of 15 mice, while no tumor rejection was observed in the control group. Survival (Fig. 5c) was monitored and illustrated using a Kaplan–Meier plot, which showed that 53.3 % of the mice in the AD-S8-boosted group were alive at 50 days with survival prolonged by 35.6 %, whereas only 6.7 % of the mice in the VR-S8/VR-IL2 and VR-S8 groups were alive with survival prolonged by 17 and 10.5 %, respectively. There was a significant difference in survival of the VR-S8/VR-IL2/AD-S8 group versus the VR-S8/VR-IL2 group (P < 0.01 by log-rank test).
Fig. 5.
Prophylactic immunization of mice with VRS8/VR-IL2 or VRS8/VR-IL2/AD-S8 inhibits growth of survivin-expressing tumors and prolongs survival of tumor-bearing mice. Female C57BL/6 mice were vaccinated four times at 2-week intervals as described in Fig. 4a and challenged with 5 × 105 S+fB16 tumor cells subcutaneously 1 week after the final vaccination. The tumor volume (a) was measured for 26 days after tumor challenge. The mice were killed and tumor weights were measured (b) on day 26 after tumor challenge. Tumor weights expressed as mean ± SD were as follows: PBS group = 1.18 ± 0.47 g, VR-IL2 group = 1.07 ± 0.47 g, VR-S8 group = 0.95 ± 0.45 g, VR-S8/VR-IL2 group = 0.73 ± 0.39 g, and VR-S8/VR-IL2/AD-S8 group = 0.37 ± 0.29 g. Survival (c) was monitored for 50 days, and the mean survival times were as follows: PBS group = 33.92 ± 1.36 days, VR-IL2 group = 35.40 ± 1.70 days, VR-S8 group = 37.47 ± 1.60 days, VR-S8/VR-IL2 group = 39.67 ± 1.70 days, and VR-S8/VR-IL2/AD-S8 group = 46.01 ± 1.60 days. (**P < 0.01; ***P < 0.001; ****P < 0.0001)
Synergistic effect of S8-based vaccine combined with carboplatin in tumor therapy
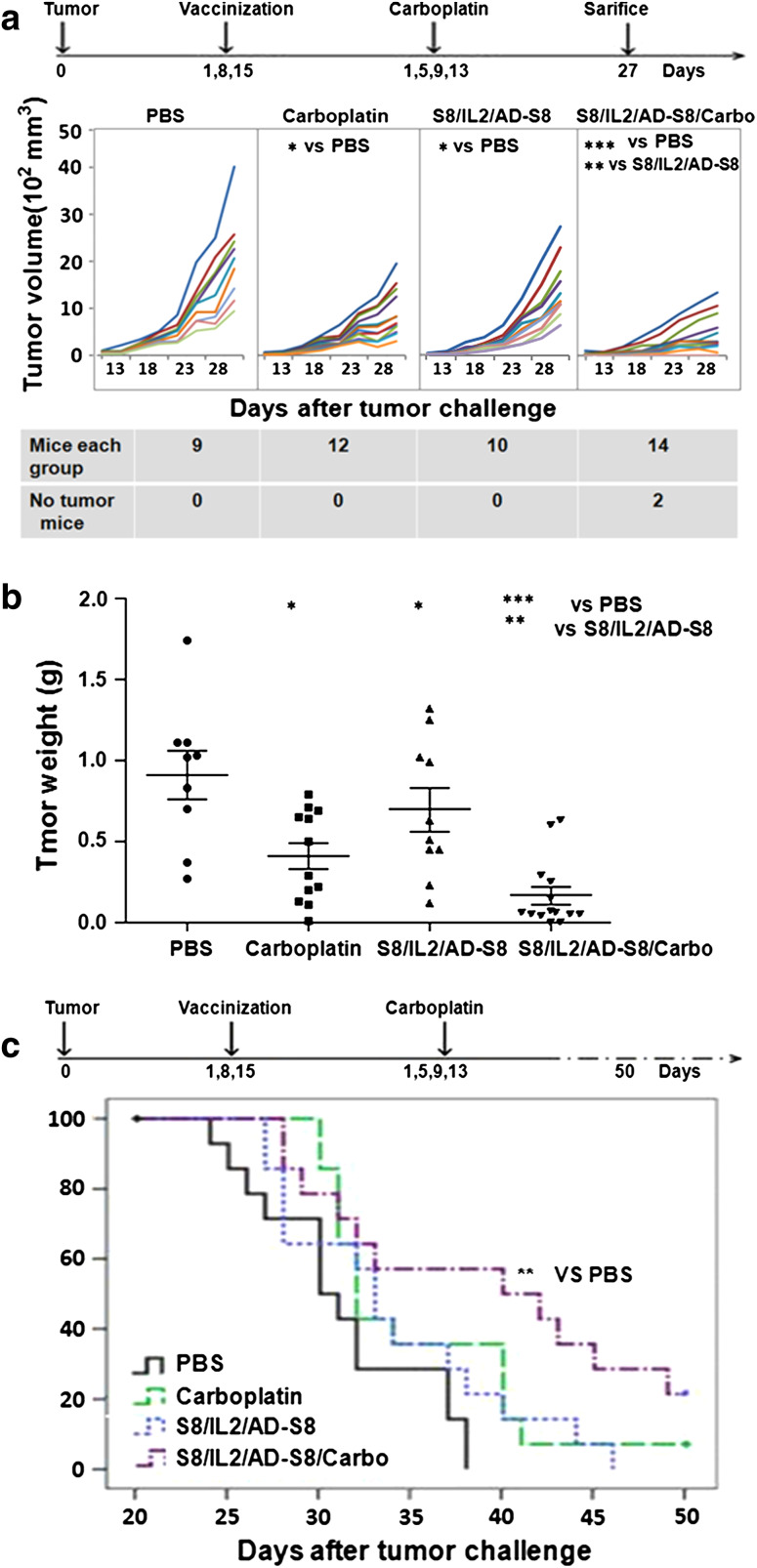
Prior to the tumor therapy assessment, we performed an independent experiment to compare the possible anti-tumor effects of VR-S8/VR-IL2, AD-S8, VR-IL2/AD-S8, VR-S8/VR-IL-2/AD-S8 and found that only VR-S8/VR-IL-2/AD-S8 showed an anti-tumor effect (data not shown). Therefore, only the VR-S8/VR-IL-2/AD-S8 combination vaccine was tested in this study. Results of the tumor inhibition experiment (Fig. 6a, b) showed that the vaccine or carboplatin treatment alone could potently delay tumor growth compared with PBS (both P < 0.05). Moreover, the combination of the vaccine with carboplatin could significantly inhibit tumor growth (P < 0.001), and complete tumor rejection was observed in 2 out of 15 mice. According to the survival analysis shown in Fig. 6c, all mice in the vaccine group were dead by day 44 after tumor inoculation, whereas 7.1 % of mice in the carboplatin-treated group remained alive, indicating that the survival was prolonged by 13.07 %. However, by day 50 after tumor implantation, 21.4 % of mice in the vaccine/carboplatin combination group remained alive, with survival significantly prolonged by 25.89 % (P < 0.01) compared with PBS treatment.
Fig. 6.
Therapeutic immunization of mice with survivin-based vaccine with or without chemotherapeutic treatment. Treatment with the VR-S8/VR-IL2/AD-S8 vaccine protocol, chemical drug carboplatin, or the combination of both could inhibit the growth of survivin-expressing tumors, and the combination treatment prolonged survival of the tumor-bearing mice. Female C57BL/6 mice were inoculated with 5 × 105 S+fB16 tumor cells on day 0 and then treated as follows: VR-S8/VR-IL2 on day 1 and day 8; AD-S8 on day 15; carboplatin on days 1, 5, 9, and 13. Tumor volumes (a) were measured for 27 days after tumor challenge. The mice were killed and tumor weights were measured (b) on day 27 after tumor challenge. Tumor weights denoted as mean ± SD were as follows: PBS group = 1.81 ± 0.88 g, VR-S8/VR-IL2/AD-S8 group = 1.39 ± 0.84 g, carboplatin group = 0.82 ± 0.56 g, and VR-S8/VR-IL2/Ad-S8/Carbo group = 0.53 ± 0.89 g. Survival (c) was monitored for 50 days, the mean survival times were as follows: PBS group = 31.21 ± 1.28 days, VR-S8/VR-IL2/AD-S8 group = 33.93 ± 1.67 days, carboplatin group = 35.29 ± 1.53 days, VR-S8/VR-IL2/AD-S8/Carbo = 39.29 ± 2.28 days (**P < 0.01)
Safety
The survivin-based vaccine was well tolerated in mice, and no adverse events were observed during or after vaccination. The major organs (such as heart, liver, kidney, lung, and spleen) of mice immunized with the DNA prime–rAd boost regimen were removed for toxicity testing by hematoxylin–eosin staining (data not shown), and the results showed no toxic effects.
Discussion
Survivin has many advantages that make it an attractive tumor antigen. Moreover, specific immune responses against survivin have been detected in cancer patients [24]. Hence, survivin is likely to be an ideal “universal” TAA for the development of cancer vaccines. At present, there are several ongoing phase I and II clinical trials using survivin-based immunotherapy. These studies are mostly focused on the specific epitopes that elicit the most potent immunodominant and immunoprevalent T cell responses against survivin, with the likelihood that those inducing both a CD8+ and CD4+ T cell response will be most effective. Most of these trials are testing peptide vaccines [25–29] or dendritic cell (DC)-based vaccines [30–33]. There are also many preclinical studies targeting survivin. Administration of survivin-based vaccines to experimental animals has been found to induce tumor regression in several types of malignancies, including lung cancer [34], pancreatic cancer [4], lymphoma, and neuroblastomas [10].
However, using the wild-type survivin as a tumor antigen in a vaccine is potentially dangerous due to its ability to inhibit apoptosis. In order to improve the safety of a tumor vaccine based on survivin, two N-terminal truncations of survivin were designed in this study according to its structural and functional characteristics. Three residues (leu6, pro7, trp10) in the N-terminus of survivin have been shown to be key to its dimer formation [35] and highly important for its anti-apoptosis function [36, 37]. In addition, survivin5–13 is an HLA-A2-restricted epitope [38–40]. Based on this information, the novel truncation S8 was made with a 7 amino acid N-terminal deletion in survivin. This truncation was expected to also affect dimerization and thereby impact the function of the protein on cellular apoptosis.
Thr34 is a CDC2/cyclin b1-specific phosphorylation site, and it has been reported that the survivin point mutant S-T34A is able to spontaneously induce apoptosis [22, 23]. In addition, the 38th amino acid residue of the human survivin protein is methionine, which provides a translation initiation signal for the expression of a truncated human survivin protein. Accordingly, the second truncation tested in this study S38 was constructed with a 37 amino acid deletion in the N-terminal of survivin. We anticipated that both S8 and S38 would not have the anti-apoptosis function of the wild-type survivin. The abilities of S8 and S38 to affect cellular apoptosis after overexpression in Hela cells were assessed using trypan blue exclusion, DNA fragmentation assay, and caspase-3 activity assay, using wild-type survivin and S-T34A as negative and positive controls, respectively. The results of these three methods confirmed that neither S8 nor S38 could inhibit apoptosis induced by PTX in vitro (Fig. 2). In this study, we chose the larger mutant S8 as the antigen for the construction of a tumor recombinant DNA vaccine and adenovirus vaccine in order to retain as much of the protein as possible to maximize the potential immune responses.
Many studies have shown that a heterologous immunization strategy is an effective strategy for enhancing immune responses [41]. Here, in order to elicit a strong specific immune response and effective anti-tumor activity, DNA and rAd vectors expressing S8 were constructed for a heterologous prime-boost immunization strategy. Meanwhile, human codon-optimized IL-2 was used as an immunoadjuvant to further enhance the efficiency of the vaccine. The experimental results demonstrated that the S8 DNA vaccine alone elicited weak immune responses and modest anti-tumor activity, while the IL-2 adjuvant could obviously enhance the production of survivin-specific CTLs and antibodies and anti-tumor efficacy. As prophylaxis, immunization with VR-S8/VR-IL2 enhanced the tumor inhibition ratio by 89 % compared with VR-S8. Thus, the DNA prime–rAd boost immunization strategy could remarkably improve vaccine-induced immunity and anti-tumor activity. Furthermore, rAd boosting after DNA priming enhanced the IFN-γ-secreting cellular response by nearly twofold in the ELISPOT assays, while the prophylactic tumor inhibition ratio and the prolonging of survival in tumor-bearing mice was enhanced twofold.
Preliminary data on the combination of immunotherapy with chemotherapy suggest a synergistic effect, opening new avenues in cancer treatment [42]. In this study, administration of the widely used chemotherapy drug carboplatin in combination treatment obviously enhanced the anti-tumor effect over use of the tumor gene vaccine alone. In the therapeutic study, the tumor inhibition ratio was enhanced nearly threefold and the survival was prolonged nearly twofold in tumor-bearing mice treated with the vaccine and carboplatin compared with those given the vaccine alone.
In summary, our results showed that the VR-S8 DNA vaccine could induce both cellular and humor immune responses, and the anti-tumor efficacy was enhanced when combined with the IL-2 immunoadjuvant and boosted by the recombinant AD-S8 vaccine. The VR-S8/VR-IL2/AD-S8 combination vaccine is a potent formulation that may be effective as a prophylactic strategy. However, in order to obtain better therapeutic effects, the vaccine should be combined with a chemotherapy drug, such as carboplatin. Such a DNA prime–rAd boost immunization strategy in combination with IL-2 and carboplatin may become the new scheme in therapy for survivin-expressing tumors.
Acknowledgments
The authors thank the staff of the National Engineering Laboratory of AIDS Vaccine for reagent ordering and instrument management. The present work was supported by the Central University Basic Research Foundation of China (No. 200903255, 201103179).
Conflict of interest
The authors declare that there are no conflicts of interest in regard to this work.
Contributor Information
Xianghui Yu, Phone: +86-431-85167826, FAX: +86-431-85167674, Email: xianghui@jlu.edu.cn.
Wei Kong, Phone: +86-431-85167826, FAX: +86-431-85167674, Email: weikong@jlu.edu.cn.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Bolhassani A, Safaiyan S, Rafati S. Improvement of different vaccine delivery systems for cancer therapy. Mol Cancer. 2011;10:3. doi: 10.1186/1476-4598-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 4.Ishizaki H, Manuel ER, Song G-Y, et al. Modified vaccinia Ankara expressing survivin combined with gemcitabine generates specific antitumor effects in a murine pancreatic carcinoma model. Cancer Immunol Immunother. 2010;60:99–109. doi: 10.1007/s00262-010-0923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HS, Kim CH, Park MY, et al. Efficient co-transduction of adenoviral vectors encoding carcinoembryonic antigen and survivin into dendritic cells by the CAR-TAT adaptor molecule enhance anti-tumor immunity in a murine colorectal cancer model. Immunol Lett. 2010;131:73–80. doi: 10.1016/j.imlet.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Lladser A, Ljungberg K, Tufvesson H, et al. Intradermal DNA electroporation induces survivin-specific CTLs, suppresses angiogenesis and confers protection against mouse melanoma. Cancer Immunol Immunother. 2009;59:81–92. doi: 10.1007/s00262-009-0725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zwirner NW, Domaica CI. Cytokine regulation of natural killer cell effector functions. BioFactors. 2010;36:274–288. doi: 10.1002/biof.107. [DOI] [PubMed] [Google Scholar]
- 8.Decker WK, Qiu J, Farhangfar F, et al. A retrogen plasmid-based vaccine generates high titer antibody responses against the autologous cancer antigen survivin and demonstrates anti-tumor efficacy. Cancer Lett. 2006;237:45–55. doi: 10.1016/j.canlet.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 9.Lladser A, Parraga M, Quevedo L, et al. Naked DNA immunization as an approach to target the generic tumor antigen survivin induces humoral and cellular immune responses in mice. Immunobiology. 2006;211:11–27. doi: 10.1016/j.imbio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Zhu K, Qin H, Cha SC, et al. Survivin DNA vaccine generated specific antitumor effects in pancreatic carcinoma and lymphoma mouse models. Vaccine. 2007;25:7955–7961. doi: 10.1016/j.vaccine.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 11.Barouch DH, Nabel GJ. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum Gene Ther. 2005;16:149–156. doi: 10.1089/hum.2005.16.149. [DOI] [PubMed] [Google Scholar]
- 12.Schulte R, Suh YS, Sauermann U, et al. Mucosal prior to systemic application of recombinant adenovirus boosting is more immunogenic than systemic application twice but confers similar protection against SIV-challenge in DNA vaccine-primed macaques. Virology. 2009;383:300–309. doi: 10.1016/j.virol.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Andersen MH, Sorensen RB, Schrama D, et al. Cancer treatment: the combination of vaccination with other therapies. Cancer Immunol Immunother. 2008;57:1735–1743. doi: 10.1007/s00262-008-0480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu B, Zhang Y, Zhan Y, et al. Co-expression of herpes simplex virus thymidine kinase and Escherichia coli nitroreductase by an hTERT-driven adenovirus vector in breast cancer cells results in additive anti-tumor effects. Oncol Rep. 2011;26:255–264. doi: 10.3892/or.2011.1285. [DOI] [PubMed] [Google Scholar]
- 15.Herrmann M, Lorenz HM, Voll R, et al. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 1994;22:5506–5507. doi: 10.1093/nar/22.24.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagaraj S, Pisarev V, Kinarsky L, et al. Dendritic cell-based full-length survivin vaccine in treatment of experimental tumors. J Immunother. 2007;30:169–179. doi: 10.1097/01.cji.0000211329.83890.ba. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S, Zhang H, Shi H, et al. Induction of immune response and anti-tumor activities in mice with a DNA vaccine encoding human mucin 1 variable-number tandem repeats. Hum Immunol. 2008;69:250–258. doi: 10.1016/j.humimm.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Li F, Ackermann EJ, Bennett CF, et al. Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nat Cell Biol. 1999;1:461–466. doi: 10.1038/70242. [DOI] [PubMed] [Google Scholar]
- 19.Grossman D, McNiff JM, Li F, Altieri DC. Expression and targeting of the apoptosis inhibitor, survivin, in human melanoma. J Invest Dermatol. 1999;113:1076–1081. doi: 10.1046/j.1523-1747.1999.00776.x. [DOI] [PubMed] [Google Scholar]
- 20.Grossman D, McNiff JM, Li F, Altieri DC. Expression of the apoptosis inhibitor, survivin, in nonmelanoma skin cancer and gene targeting in a keratinocyte cell line. Lab Invest. 1999;79:1121–1126. [PubMed] [Google Scholar]
- 21.O’Connor DS, Grossman D, Plescia J, et al. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci USA. 2000;97:13103–13107. doi: 10.1073/pnas.240390697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Connor DS. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Nat Acad Sci. 2000;97:13103–13107. doi: 10.1073/pnas.240390697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grossman D, Kim PJ, Schechner JS, Altieri DC. Inhibition of melanoma tumor growth in vivo by survivin targeting. Proc Natl Acad Sci USA. 2001;98:635–640. doi: 10.1073/pnas.98.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yagihashi A, Ohmura T, Asanuma K, et al. Detectio n of autoantibodies to survivin and livin in sera from patients with breast cancer. Clin Chim Acta. 2005;362:125–130. doi: 10.1016/j.cccn.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Kameshima H, Tsuruma T, Torigoe T, et al. Immunogenic enhancement and clinical effect by type-I interferon of anti-apoptotic protein, survivin-derived peptide vaccine, in advanced colorectal cancer patients. Cancer Sci. 2011;102:1181–1187. doi: 10.1111/j.1349-7006.2011.01918.x. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki A, Kobayashi J, Torigoe T, et al. Phase I clinical trial of survivin-derived peptide vaccine therapy for patients with advanced or recurrent oral cancer. Cancer Sci. 2011;102:324–329. doi: 10.1111/j.1349-7006.2010.01789.x. [DOI] [PubMed] [Google Scholar]
- 27.Honma I, Kitamura H, Torigoe T, et al. Phase I clinical study of anti-apoptosis protein survivin-derived peptide vaccination for patients with advanced or recurrent urothelial cancer. Cancer Immunol Immunother. 2009;58:1801–1807. doi: 10.1007/s00262-009-0691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuruma T, Torigoe T, Hata F, et al. Anti-apoptosis protein, survivin-2B-derived peptide vaccine therapy. Gan To Kagaku Ryoho. 2004;31:1634–1636. [PubMed] [Google Scholar]
- 29.Tsuruma T, Hata F, Torigoe T, et al. Phase I clinical study of anti-apoptosis protein, survivin-derived peptide vaccine therapy for patients with advanced or recurrent colorectal cancer. J Transl Med. 2004;2:19. doi: 10.1186/1479-5876-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trepiakas R, Berntsen A, Hadrup SR, et al. Vaccination with autologous dendritic cells pulsed with multiple tumor antigens for treatment of patients with malignant melanoma: results from a phase I/II trial. Cytotherapy. 2010;12:721–734. doi: 10.3109/14653241003774045. [DOI] [PubMed] [Google Scholar]
- 31.Hirschowitz EA, Foody T, Hidalgo GE, Yannelli JR. Immunization of NSCLC patients with antigen-pulsed immature autologous dendritic cells. Lung Cancer. 2007;57:365–372. doi: 10.1016/j.lungcan.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pisarev V, Yu B, Salup R, et al. Full-length dominant-negative survivin for cancer immunotherapy. Clin Cancer Res. 2003;9:6523–6533. [PubMed] [Google Scholar]
- 33.Fuessel S, Meye A, Schmitz M, et al. Vaccination of hormone-refractory prostate cancer patients with peptide cocktail-loaded dendritic cells: results of a phase I clinical trial. Prostate. 2006;66:811–821. doi: 10.1002/pros.20404. [DOI] [PubMed] [Google Scholar]
- 34.Xiang R, Mizutani N, Luo Y, et al. A DNA vaccine targeting survivin combines apoptosis with suppression of angiogenesis in lung tumor eradication. Cancer Res. 2005;65:553–561. [PubMed] [Google Scholar]
- 35.Verdecia MA, Huang H, Dutil E, et al. Structure of the human anti-apoptotic protein survivin reveals a dimeric arrangement. Nat Struct Biol. 2000;7:602–608. doi: 10.1038/77929. [DOI] [PubMed] [Google Scholar]
- 36.Chantalat L, Skoufias DA, Kleman JP, et al. Crystal structure of human survivin reveals a bow tie-shaped dimer with two unusual alpha-helical extensions. Mol Cell. 2000;6:183–189. [PubMed] [Google Scholar]
- 37.Shi Y. Survivin structure: crystal unclear. Nat Struct Biol. 2000;7:620–623. doi: 10.1038/77904. [DOI] [PubMed] [Google Scholar]
- 38.Bachinsky MM, Guillen DE, Patel SR, et al. Mapping and binding analysis of peptides derived from the tumor-associated antigen survivin for eight HLA alleles. Cancer Immunol. 2005;5:6. [PubMed] [Google Scholar]
- 39.Andersen MH, Pedersen LO, Becker JC, Straten PT. Identification of a cytotoxic T lymphocyte response to the apoptosis inhibitor protein survivin in cancer patients. Cancer Res. 2001;61:869–872. [PubMed] [Google Scholar]
- 40.Andersen MH, Pedersen LO, Capeller B, et al. Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res. 2001;61:5964–5968. [PubMed] [Google Scholar]
- 41.Pitisuttithum P, Rerks-Ngarm S, Bussaratid V, et al. Safety and reactogenicity of canarypox ALVAC-HIV (vCP1521) and HIV-1 gp120 AIDSVAX B/E vaccination in an efficacy trial in Thailand. PLoS ONE. 2011;6:e27837. doi: 10.1371/journal.pone.0027837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gabrilovich DI. Combination of chemotherapy and immunotherapy for cancer: a paradigm revisited. Lancet Oncol. 2007;8:2–3. doi: 10.1016/S1470-2045(06)70985-8. [DOI] [PubMed] [Google Scholar]