Abstract
The extrinsic apoptosis pathway is triggered by the binding of death ligands of the tumor necrosis factor (TNF) family to their appropriate death receptors (DRs) on the cell surface. One TNF family member, TNF-related apoptosis-inducing ligand (TRAIL or Apo2L), seems to preferentially cause apoptosis of transformed cells and can be systemically administered in the absence of severe toxicity. Therefore, there has been enthusiasm for the use of TRAIL or agonist antibodies to the TRAIL DR4 and DR5 in cancer therapy. Nonetheless, many cancer cells are very resistant to TRAIL apoptosis in vitro. Therefore, there is much interest in identifying compounds that can be combined with TRAIL to amplify its apoptotic effects. In this review, I will provide a brief overview of apoptosis signaling by TRAIL and discuss apoptosis-sensitizing agents, focusing mainly on the proteasome inhibitor bortezomib (VELCADE) and some novel sensitizers that we have recently identified. Alternative ways to administer TRAIL or DR agonist antibodies as therapeutic agents will also be described. Finally, I will discuss some of the gaps in our understanding of TRAIL apoptosis signaling and suggest some research directions that may provide additional information for optimizing the targeting of the extrinsic apoptosis pathway for future cancer therapy.
Keywords: TRAIL, Bortezomib, Extrinsic apoptosis, Proteasome inhibition, Death receptors
Introduction
Multicellular organisms maintain homoeostasis by the controlled elimination of cells that are no longer needed or are damaged by a cell suicide pathway known as apoptosis. Aberrant regulation of apoptotic cell death mechanisms is an important pathological factor in a variety of major human diseases. Failure to appropriately engage this pathway is one of the hallmarks of cancer development and progression, and many cancer cells exhibit significant resistance to apoptosis signaling. However, although cancer cells have acquired molecular modifications that restrain apoptosis, they are nevertheless constantly driven to initiate this pathway by genomic and other aberrations. Thus, it is hoped that if these molecular blocks on apoptosis can be bypassed or evaded, cancer cells may be more sensitive to apoptosis than are normal cells. In mammalian cells, apoptosis occurs through two distinct molecular pathways. The intrinsic or mitochondrial pathway is activated by intracellular events and depends on the release of proapoptotic factors from the mitochondria. Standard chemotherapy and radiotherapy for cancer predominately initiate apoptosis via the intrinsic pathway and thus may positively select for cancer cells that can evade intrinsic apoptosis signaling. By contrast, the extrinsic apoptosis pathway receives signals through the binding of extracellular protein death ligands to proapoptotic death receptors (DRs). In some cancer cells following extrinsic apoptosis signaling, cell death can still occur in the absence of intrinsic apoptosis. Both pathways lead to the hierarchical activation of specialized proteases called caspases. The activation of the caspase enzyme cascade leads to the unique morphological and biochemical features of apoptosis such as plasma membrane “blebbing,” cell shrinkage, chromatin condensation, and internucleosomal DNA fragmentation.
The intrinsic apoptosis pathway
The intrinsic apoptosis pathway is instigated by the release of apoptogenic factors such as cytochrome c and second mitochondrial activator of caspases/direct IAP-binding protein with low pI (Smac/DIABLO) from the mitochondria into the cytoplasm following mitochondrial outer membrane permeabilization (MOMP). Cell intrinsic stress sensors control MOMP through modifying the interactions of proteins of the Bcl-2 family [1]. Members of this family can be divided into three subgroups, according to the number and structure of their Bcl-2-homology domains (designated BH1-4). The BH3-only proapoptotic proteins are activated in response to various cell stress conditions. Once activated, they promote oligomerization of the proapoptotic proteins Bax and Bak in the mitochondrial outer membrane, thereby promoting MOMP. By contrast, other multi-BH domain proteins such as Bcl-2, BclXL, and Mcl-1 inhibit MOMP induction by neutralizing specific proapoptotic family members. The release of apoptogenic factors from the mitochondria causes numerous cellular changes that can result in a “point of no return” whereby the subsequent death of the cell becomes inevitable [2]. In the cytosol, cytochrome c induces the formation of the apoptosome complex containing the adaptor protein Apaf-1 and caspase-9. This results in the activation of caspase-9, which then goes on to process and activate effector caspases. In addition, release of mitochondrial Smac/DIABLO into the cytoplasm allows it to interact with the antiapoptotic protein X chromosome-linked inhibitor of apoptosis protein (XIAP). Normally, XIAP binds to caspase-3, caspase-7, and caspase-9 through its IAP repeat domain to provide an important checkpoint to prevent inadvertent caspase activation. However, on interaction with Smac/DIABLO, the effects of XIAP are antagonized and this checkpoint is overridden [3]. Many mutations associated with cancer, such as loss of proapoptotic factors p53 and Bax, or the overexpression of antiapoptotic factors like Bcl-2 and Mcl-1, will reduce intrinsic apoptosis signaling, thus preventing the efficient elimination of transformed cells.
The extrinsic apoptosis pathway
The extrinsic apoptosis pathway transmits signals from extracellular death ligands through the appropriate DRs to the cells’ apoptotic machinery [4]. The best-described death ligands belong to the TNF family of proteins. These TNF family death ligands comprise TNF, FasL, and TRAIL, which are predominantly produced by cells of the immune system such as T cells, NK cells, NKT cells, macrophages, and dendritic cells. Although all death ligands can trigger apoptosis under appropriate circumstances, TRAIL currently presents the most promising candidate for clinical use. TRAIL appears to preferentially trigger apoptosis in cancer cells, whereas normal nontransformed cells are relatively resistant. Also, in contrast to FasL or TNF, increased signaling through TRAIL DRs is not associated with unacceptable levels of toxicity in vivo [5, 6]. Thus, most work on therapeutic targeting of the extrinsic apoptosis pathway for cancer therapy has focused on targeting TRAIL DRs through the administration of TRAIL or agonist antibodies to the TRAIL DRs.
TRAIL apoptosis signaling
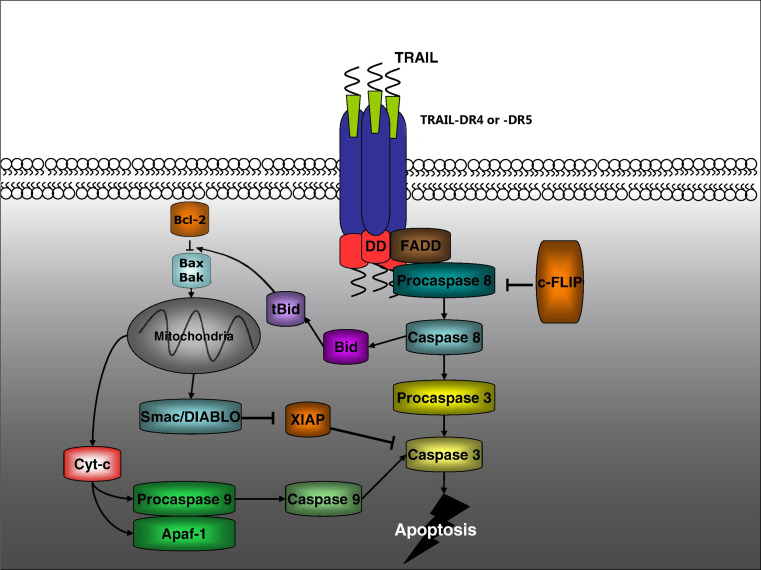
TRAIL was discovered independently by two laboratories as a novel proapoptotic member of the TNF family [7, 8]. It binds to five identified receptors [4]. Two of these receptors DR4 and DR5 contain protein motifs known as death domains in their cytoplasmic region. These death domains are crucial for the ability of DR4 and DR5 to transmit apoptotic signals. TRAIL also interacts with three “decoy” receptors that are unable to transmit apoptotic signals DcR1, DcR2, and osteoprotegerin (OPG). The precise physiological role in TRAIL signaling of these decoy receptors remains unclear. DR4 and DR5 are preassembled into timers before arriving at the cell surface. Ligation of DR4 or DR5 by TRAIL promotes further clustering of the receptors into high-molecular-weight complexes that drives the formation of a death-inducing signaling complex (DISC). Thus, on ligand stimulation, DR4 and DR5 recruit the adaptor protein Fas-associated death domain (FADD) through death domain interactions (Fig. 1). FADD then recruits procaspase-8 and procaspase-10 or FLICE-inhibitory protein (c-FLIP), resulting in the formation of the death-inducing signaling complex (DISC). The recruitment of procaspase-8 to the DISC results in its autocatalytic processing to the active caspase-8 enzyme, which on subsequent release from the DISC activates effector caspase-3, caspase-6, and caspase-7 [9]. The recruitment of high levels of the antiapoptotic protein c-FLIP to the DISC is thought to limit caspase-8 activation [10]. Three forms of c-FLIP protein have been detected: two short variants c-FLIPS and c-FLIPR and one long splice variant c-FLIPL. More recent studies indicate that glycosylation of both DR4 and DR5 seemed to promote TRAIL apoptosis signaling not by increasing the cell surface expression levels of the receptors but rather by producing a more efficient ligand-induced clustering of DR4 and DR5 [11]. In addition, it has been proposed that procaspase-8 in the DISC is subsequently ubiquitinated by the E3 ligase Cullin 3. This ubiquitination of caspase-8 then results in association with the protein p62 that promotes the translocation of caspase-8 into ubiquitin-rich foci within the cell. This focal concentration of caspase-8 seems to facilitate its full activation [12]. DR glycosylation, as well as procaspase-8 ubiquitination, may be molecular pathways that can be targeted in the future to enhance extrinsic apoptosis signaling.
Fig. 1.
TRAIL apoptosis signaling. On binding of TRAIL to death receptors DR4 or DR5, the apoptosis signal can be transmitted in two ways, depending on the particular cell type. In type I cells, caspase-8 activation can directly activate downstream effector caspases such as caspase-3, and there is no requirement for any participation of the mitochondria. In type II cells, caspase-8 activation needs to cleave Bid, resulting in mitochondrial perturbation, release of cytochrome c and Smac/DIABLO, and additional activation of the intrinsic apoptosis signaling pathway. In type II cells, this mitochondrial amplification loop is required for apoptosis to occur (figure adapted from Holoch and Griffith, European Journal of Pharmacology [49])
Following the activation of caspase-8 by death ligands, some cells can directly activate downstream effector caspases (type I cells), and apoptosis can proceed in the absence of any contribution from the mitochondria. However, in other cells (type II cells), levels of caspase-8 activation are insufficient to directly promote apoptosis [13, 14]. In these cells, there is convergence between the extrinsic and intrinsic pathways (Fig. 1). This link is provided by the caspase-8-mediated cleavage and the activation of proapoptotic BH3 family member Bid [15, 16]. Following caspase-8 cleavage, the truncated active form of Bid (tBid) engages Bax or Bak to induce MOMP. The importance of engaging this intrinsic amplification loop for apoptosis to proceed is probably cell type dependent. Clearly, in some cells, this mitochondrial amplification loop is not required for the instigation of apoptosis following extrinsic apoptosis signaling. Thus, some cancer cells resistant to standard chemotherapy or radiotherapy may still be killed following amplification of extrinsic apoptosis signaling, even if they possess mutations (such as p53 mutations) that limit intrinsic apoptosis signaling. Nonetheless, when large panels of cancer cells are examined in vitro, the majority are only partially sensitive to TRAIL-mediated apoptosis or are completely resistant. As such, agents that amplify TRAIL apoptosis signaling need to be identified in order to maximize any potential therapeutic application of TRAIL or agonist antibodies to DR4 or DR5.
Enhancing TRAIL apoptosis signaling
The combination of TRIAL DR agonists with numerous conventional and investigational anticancer drugs has been reported. Synergy has been described for the combination of TRAIL with a variety of cytotoxic agents including irinotecan, camptothecin, 5-fluorouracil, carboplatin, paclitaxel, doxorubicin, and gemcitabine in diverse preclinical models. Additional investigational drugs that show synergy with TRAIL have been identified, and these include histone deacetylase (HDAC) inhibitors, rituximab, synthetic triterpenoids, and sorafenib. These effects have been reported in more detail elsewhere [6, 17]. Therefore, I will focus on the combination of TRAIL DR agonists with the proteasome inhibitor bortezomib (VELCADE), as well as on our recent attempts to identify novel TRAIL-sensitizing compounds. Our group and others have reported that many human and mouse cancer cells lines can be sensitized by proteasome inhibitors such as bortezomib (VELCADE) to the apoptotic effects of TRAIL DR agonists [18]. Interestingly, nontransformed cells seem to be much more resistant to the apoptotic effects of bortezomib and TRAIL than are cancer cells [19–21]. This suggests that a therapeutic window may exist in vivo where this combination may have therapeutic benefit in the absence of accompanying toxicity. However, the molecular mechanism(s) of action whereby proteasome inhibition in cancer cells results in sensitization to TRAIL apoptosis remains unclear. Since proteasome function is required for normal cellular homeostasis, proteasome inhibition has multiple biological effects on cells. These include cell cycle arrest, inhibition of NF-kB activation, and direct triggering of apoptosis [22]. Following proteasome inhibition, increases in cell surface levels of DR4 and DR5 have also been observed in many cancer cells [23, 24]. This could contribute to the amplification of extrinsic apoptosis signaling, although this increase can often be quite subtle. Also, in some instances, this increase does not seem to be essential for the ability of bortezomib to promote TRAIL apoptosis [25, 26]. Thus, the increase in cell surface DR4 and/or DR5 following bortezomib treatment may help amplify the apoptotic signal, but may not be the crucial reason why proteasome inhibition sensitizes most cells to TRAIL apoptosis. In the majority of cancer cells following bortezomib treatment, there is a clear increase in caspase-8 activation following subsequent exposure of the cells to TRAIL. This could be due to either an increase in proximal apoptosis signaling or, alternatively, a consequence of cells undergoing apoptosis, when there is an extensive general increase in caspase activity. Recent studies using immunoprecipitation of the DISC suggest that bortezomib treatment increases recruitment of procaspase-8 to the DISC itself [19, 27]. This suggests that bortezomib quantitatively enhances DISC formation, which ultimately results in a stronger apoptotic signal. Other studies have reported that in some cells, bortezomib treatment can reduce cellular levels of c-FLIP, the negative regulator of caspase-8 activation [25, 28, 29]. Since c-FLIP can act as a brake on extrinsic apoptosis signaling, any reduction in levels would be anticipated to promote apoptosis. However, this drop in c-FLIP has not been observed in all bortezomib-sensitized cells [23]. As such, reduction in c-FLIP may be an important component of bortezomib’s mechanism of action in some, but not all, cancer cells. In addition to effects on components of the extrinsic apoptosis signaling pathway, there have also been reports that bortezomib can affect the intrinsic pathway by decreasing the levels of antiapoptotic components or increasing the levels of proapoptotic components [30]. In cells that require amplification of the TRAIL apoptosis signal by engagement of the mitochondrial pathway, any effects of bortezomib that could amplify intrinsic apoptosis signaling would likely promote apoptosis. Alternatively, if bortezomib induces a large increase in caspase-8 activation at the DISC, this may be sufficient to trigger apoptosis in the absence of any contribution from the mitochondria [27]. Thus, the dominant mechanism of action whereby bortezomib promotes TRAIL-mediated apoptosis may vary, depending upon the particular cancer cells being studied [31]. Indeed, bortezomib may be an efficient sensitizer to TRAIL apoptosis for many cancer cells because it can affect multiple components of apoptosis signaling pathways.
Recently, in an attempt to isolate novel compounds that might sensitize cancer cells to TRAIL via a different molecular mechanism of action, or be associated with less toxicity than bortezomib, we embarked on a high-throughput screening (HTS) to identify novel TRAIL-sensitizing compounds. More than 50,000 purified compounds, natural product extracts, and natural products were analyzed. Some compounds identified in this HTS were compounds already reported to sensitize cells to TRAIL, affirming the reliability of the HTS used. Furthermore, a number of novel hit compounds were identified [32]. Currently, we are focusing on TRAIL sensitization induced by cucurbitacins and withanolides, natural products isolated from different plants. There are many members of both the cucurbitacin and withanolide families that are very closely related in structure. However, the ability to sensitize cells to the apoptotic effects of TRAIL varies widely between family members. When assessing TRAIL sensitization using a panel of cancer cell lines in vitro, different patterns of apoptosis sensitization were obtained comparing the withanolides and cucurbitacins either with each other or with bortezomib. This suggests that different molecular mechanism of action may underlay their TRAIL-sensitizing effects. Indeed, it was recently reported that withaferin A, a member of the withanolide family, could sensitize the renal carcinoma cell line Caki-1 to TRAIL apoptosis. This effect was totally dependent on the ability of withaferin A to generate reactive oxygen species (ROS) within the cell [33]. We have confirmed this observation using the renal carcinoma ACHN and withanolide E, whereby inclusion of the antioxidant N-acetylcysteine (NAC) in the media completely abrogated the sensitizing effects of withanolide E. By contrast, NAC has no effect on bortezomib’s sensitizing activity. One potential mechanism of action could be that an increase in ROS leads to a drop in the levels of cellular c-FLIP.
Concerning bortezomib, it remains unknown how proteasome inhibition subsequently results in an increased activation of caspase-8 at the DISC on exposure to TRAIL. Interestingly, in contrast to some of our previous studies, we did not find a major drop in c-FLIP levels on bortezomib sensitization of ACHN cells to TRAIL [27], further suggesting that bortezomib and withanolide E sensitization of cells to TRAIL apoptosis may involve different molecular mechanisms of action. However, it is well established that proteasome inhibition causes a dramatic increase in ubiquitinated proteins that results in ER stress. On sensing ER stress, cells can initiate the unfolded protein response (UPR) to alleviate this stress and restore protein homeostasis. Indeed some of the direct proapoptotic effects of proteasome inhibition seem to involve an induction of the ER stress, but subsequent inhibition of the UPR, thus further increasing the stress of protein accumulation within cells [34, 35]. It seems likely that this increased ER stress may also result in a molecular cross-talk with apoptosis pathways, resulting in a sensitization of cells to TRAIL apoptosis. However, the precise molecular details as to how this could occur remains to be determined.
Specific molecular targeting of apoptosis pathways
Increased knowledge of some of the molecular components of the apoptosis signaling pathways has paved the way for the development of more specific agents that target one crucial signaling component. Such agents are early in development, yet the preliminary data seem very promising. To date, the best characterized of these compounds are those that target either various antiapoptotic Bcl-2 family members or IAP proteins. Various strategies have been developed recently to antagonize antiapoptotic Bcl-2 proteins with promising initial results. These include ABT-737 that binds to the surface grove of antiapoptotic Bcl-2 family members Bcl-2, Bcl-XL, and Bcl-w in a similar manner to the BH3 domain of Bax or Bak. ABT-737 and TRIAL have been found to synergize for inducing cell death in some cancer cells [36]. Obatoclax, another BH3 mimetic, antagonizes Mcl-1 as well as Bcl-2, Bcl-XL, and Bcl-w. Since high levels of Mcl-1 are often associated with resistance to TRAIL, obatoclax may sensitize a greater spectrum of cancer cells to TRAIL than ABT-737 [37]. Another approach to target antiapoptotic members of the Bcl-2 family has been the use of antisense oligonucleotides [38]. The family of IAPs comprises a family of endogenous cellular caspase inhibitors [3]. So far, most targeting strategies have focus on XIAP that seems to be the family member with the most potent antiapoptotic properties. Loss of XIAP protein upon administration of XIAP antisense oligonucleotides is known to increase TRAIL-mediated apoptosis [39]. Furthermore, the binding grove of the BIR3 domain of XIAP, which binds Smac/DIABLO, has served as a target for the design of compounds that inhibit XIAP. Smac mimetic peptides have demonstrated positive effects on apoptosis when combined with TRAIL [40], and more recently, small-molecule XIAP inhibitors have been demonstrated to synergize with TRAIL in promoting apoptosis [41]. Other small molecules that bind to the BIR2 domain of XIAP have been described, and these can also potentiate the proapoptotic effects of TRAIL [42]. Agents that block the activity of XIAP may be very useful in potentiating the apoptotic activity of TRAIL, if they can be administered in a way that limits their potential toxicity to normal cells in vivo [43].
Alternative ways to administer death ligands
The systemic administration of TRAIL DR agonists such as TRAIL itself or agonist antibodies to DR4 and DR5 is not the only way to deliver death ligands to tumors. Nonreplicative recombinant adenoviral vectors can deliver TRAIL directly into the tumor site [44]. Since this initial report, a number of recombinant viral vectors containing TRAIL cDNA have been described [45]. These formulations may prolong the systemic half-life of the TRAIL administered. They may offer additional advantages as therapeutic agents, since they may not only provoke apoptotic cells death of the tumor cells but may additionally stimulate an antitumor immune response. Indeed, subsequent combinations of Ad5-TRAIL and immunostimulatory CpG ODN have been shown to augment tumor antigen cross–presentation, resulting in enhanced cytotoxic T lymphocyte activity and increased animal survival [46]. Interestingly, similar findings have been reported using a combination of 3 monoclonal antibodies (TriMab). These were an anti-DR5 (MD5-1) to promote tumor cell apoptosis in combination with an agonist anti-CD40 mAb to enhance tumor antigen presentation, and an anti-CD137 (4-1BB) mAb to enhance T-cell activation. Such a strategy also resulted in the rejection of TRAIL-resistant tumor cells, probably due to the use of other cytolytic effector molecules by the activated tumor-specific T cells [47]. However, taking such a complex experimental combination into the clinic will have significant logistical hurdles.
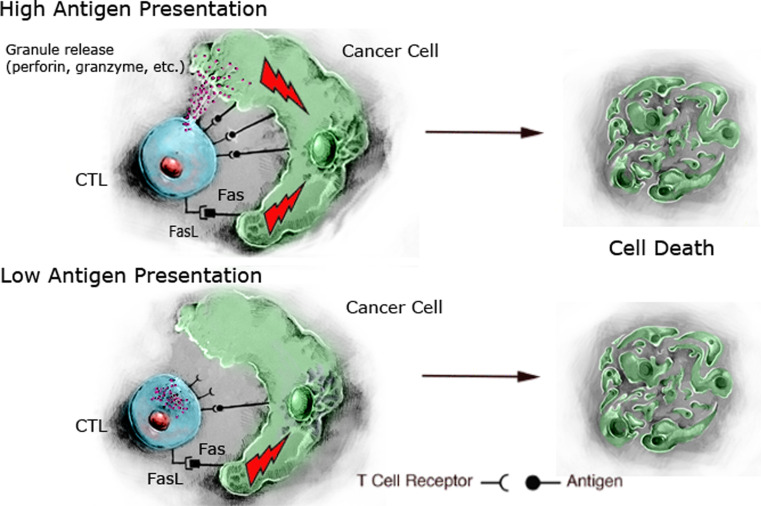
Since immune antitumor effector cells such as T cells, NK cells, macrophages, NKT cells, and dendritic cells can express multiple death ligands, these cells themselves could make optimal delivery vehicles for death ligands to tumor cells. In a mouse model of renal cancer Renca, we recently showed that the adoptive transfer of specific antitumor T cells could deliver FasL to the tumor metastases, and this was crucial for the therapeutic effects of the T cells [48]. Furthermore, FasL was the dominant lytic pathway used by these T cells to destroy the tumor cells. The reason for this dominance of the FasL pathway seemed to be due to low levels of antigen expression by the tumor cells. Such low levels of tumor antigen were sufficient to trigger the FasL but not the perforin-dependent lytic pathway of the T cells (Fig. 2). Since tumor antigens are often differentiation antigens, or mutated self-antigens, it is likely that they may not be efficient triggers of T-cell degranulation. Thus, the FasL pathway may be more important for the therapeutic effects of adoptive T cell than was previously thought. If such findings could be extrapolated into human patients, it could be anticipated that tumors highly sensitive to FasL apoptosis might be particularly responsive to adoptive T-cell transfer. Nonetheless, the recent findings that Fas can promote tumor growth under certain circumstances [49] suggest that caution would be necessary if employing such an approach. One advantage of using immune cells to deliver death ligands to tumors may also be their ability to traffic the tumor, and following specific tumor recognition, release high local concentrations of multiple death ligands. There has recently been much interest in the transfer of NK cells for the treatment of acute myeloid leukemia (AML). NK cells are lytic to tumor cells but their adoptive transfer may not pose many of the problems, such as graft-versus-host disease, that are associated with the transfer of allogeneic T cells [50] NK cells not only utilize the perforin-dependent lytic pathway but can also be a rich source of TRAIL and FasL. Combination of NK cell transfer, coupled with bortezomib treatment to sensitize cancer cells to extrinsic apoptosis, has been reported to provide greater efficacy than either individual treatment in preclinical cancer models [51–53]. It will be of interest to see whether such approaches can be adapted for clinical use.
Fig. 2.
Lytic pathways of cytotoxic T cells (CTL). On presentation by cancer cells of high levels of tumor antigen (as MHC class I/peptide complexes), the CTL can engage in both the granule exocytosis pathway and the FasL/Fas lytic pathways, both of which result in cancer cell apoptosis and death. On presentation of low levels of tumor antigen, only the FasL/Fas pathway is triggered. Therefore, under conditions where low levels of antigen are presented, the FasL/Fas pathway becomes the dominant lytic pathway
Future directions
Although much has been learned about the extrinsic apoptosis signaling pathway over the last 10 years, there are still many gaps in our knowledge. It seems likely that may agents that stress cancer cells also amplify extrinsic apoptosis signaling. Yet, precisely how ER or ROS stress might promote extrinsic apoptosis signaling at the molecular level remains to be determined. More recently, unbiased screening approaches using siRNAs have been employed in an attempt to identify novel proteins that control the extent of TRAIL-mediated apoptosis [54–56]. In addition, little is known as to how various miRNAs may affect the extrinsic apoptosis signaling pathway. Such information could help in the identification of novel therapeutic targets. However, a note of caution is required. For practical reasons, many studies on apoptosis sensitization utilize well-established human cancer cell lines propagated in vitro under standard tissue culture conditions. However, it has been reported that conditions that favor in vitro growth of cancer cells as spheroids can dramatically reduce their susceptibility to TRAIL-mediated apoptosis [57]. Clearly, more information is needed concerning how growth in three dimensions or changes in cell adhesion or differentiation may affect TRAIL apoptosis. Two recent interesting studies on tumor development in DR5 gene targeted mice, and both suggested that TRAIL could act as a tumor suppressor during certain stages of tumor development. In a mouse model of diethylnitrosamine-induced (DEN-induced) hepatocarcinogenesis, increased numbers of large tumors were observed in the livers of DEN-treated TRAIL-R −/− mice [58]. In another study of squamous cell carcinoma induced by DMBA/TPA treatment, TRAIL-R −/− mice did not exhibit any difference from wild-type mice in the growth of the primary tumor, but did show a significant increase in lymph node metastases [59]. This study suggested that loss of adhesion of the cancer cells during the metastatic process increased their sensitivity to TRAIL. More studies are clearly required to determine whether a natural window of TRAIL sensitivity occurs early in the metastatic process. Also, how the differentiation states of cancer cells, and/or different tumor microenvironments, influence TRAIL apoptosis is of major interest. Such studies are likely to have a major impact on the optimal utilization of TRAIL DR-based apoptosis signaling for cancer therapy in the future.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations implies endorsement by the US Government. This Research was supported [in part] by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. Thanks to Alan Brooks, Candace Thompson, Richard Pompei, Drs Nancy Booth, and Curtis Henrich for their assistance with this work and Andrew Sayers for help with the artwork.
Abbreviations
- TRAIL
TNF-related apoptosis-inducing ligand
- c-FLIP
Cellular FLICE-inhibitory protein
- IAP
Inhibitor of apoptosis protein
- FADD
Fas-associated death domain
- DISC
Death-inducing signaling complex
- Smac/DAIBLO
Second mitochondrial activator of caspases/direct IAP-binding protein with low pI
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Tenth International Conference on Progress in Vaccination against Cancer (PIVAC 10), held in St. Catharine’s College, Cambridge, UK, 27th–30th September 2010. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Coultas L, Strasser A. The role of the Bcl-2 protein family in cancer. Semin Cancer Biol. 2003;13:115–123. doi: 10.1016/S1044-579X(02)00129-3. [DOI] [PubMed] [Google Scholar]
- 2.Green DR, Marante-Mendes GP. The point of no return: mitochondria, caspases, and the commitment to cell death. Results Probl Cell Differ. 1998;24:45–61. doi: 10.1007/978-3-540-69185-3_3. [DOI] [PubMed] [Google Scholar]
- 3.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010;29:4752–4765. doi: 10.1038/onc.2010.221. [DOI] [PubMed] [Google Scholar]
- 5.Oldenhuis CNAM, Stegehuis JH, Walenkamp AME, de Jong S, de Vries EGE. Targeting TRAIL death receptors. Curr Opin Pharmacol. 2008;8:433–439. doi: 10.1016/j.coph.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Ashkenazi A, Herbst RS. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest. 2008;118:1979–1990. doi: 10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang C-P, Nicholl JK, Sutherland GR, Smith CA, Goodwin RG. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 8.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 9.Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 10.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 11.Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF, Totpal K, Huw L, Katta V, Cavet G, Hymowitz SG, Amler L, Ashkenazi A. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 12.Jin Z, Li Y, Pitti R, Lawrence D, Pham VC, Lill JR, Ashkenazi A. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–735. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH, Peter ME. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem. 1999;274:22532–22538. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- 14.Ozoren N, el Deiry WS. Defining characteristics of Types I and II apoptotic cells in response to TRAIL. Neoplasia. 2002;4:551–557. doi: 10.1038/sj.neo.7900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/S0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 16.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/S0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 17.Mahalingam D, Szegezdi E, Keane M, de Jong S, Samali A. TRAIL receptor signalling and modulation: are we on the right TRAIL? Cancer Treat Rev. 2009;35:280–288. doi: 10.1016/j.ctrv.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Sayers TJ, Murphy WJ. Combining proteasome inhibition with TNF-related apoptosis-inducing ligand (Apo2L/TRAIL) for cancer therapy. Cancer Immunol Immunother. 2006;55:76–84. doi: 10.1007/s00262-005-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganten TM, Koschny R, Haas TL, Sykora J, Li-Weber M, Herzer K, Walczak H. Proteasome inhibition sensitizes hepatocellular carcinoma cells, but not human hepatocytes, to TRAIL. Hepatology. 2005;42:588–597. doi: 10.1002/hep.20807. [DOI] [PubMed] [Google Scholar]
- 20.Koschny R, Ganten TM, Sykora J, Haas TL, Sprick MR, Kolb A, Stremmel W, Walczak H. TRAIL/bortezomib cotreatment is potentially hepatotoxic but induces cancer-specific apoptosis within a therapeutic window. Hepatology. 2007;45:649–658. doi: 10.1002/hep.21555. [DOI] [PubMed] [Google Scholar]
- 21.Shanker A, Brooks AD, Tristan CA, Wine JW, Elliott PJ, Yagita H, Takeda K, Smyth MJ, Murphy WJ, Sayers TJ. Treating metastatic solid tumors with bortezomib and a tumor necrosis factor-related apoptosis-inducing ligand receptor agonist antibody. J Natl Cancer Inst. 2008;100:649–662. doi: 10.1093/jnci/djn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 23.Liu X, Yue P, Chen S, Hu L, Lonial S, Khuri FR, Sun SY. The proteasome inhibitor PS-341 (bortezomib) up-regulates DR5 expression leading to induction of apoptosis and enhancement of TRAIL-induced apoptosis despite up-regulation of c-FLIP and survivin expression in human NSCLC cells. Cancer Res. 2007;67:4981–4988. doi: 10.1158/0008-5472.CAN-06-4274. [DOI] [PubMed] [Google Scholar]
- 24.Kandasamy K, Kraft AS. Proteasome inhibitor PS-341 (VELCADE) induces stabilization of the TRAIL receptor DR5 mRNA through the 3′-untranslated region. Mol Cancer Ther. 2008;7:1091–1100. doi: 10.1158/1535-7163.MCT-07-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koschny R, Holland H, Sykora J, Haas TL, Sprick MR, Ganten TM, Krupp W, Bauer M, Ahnert P, Meixensberger J, Walczak H. Bortezomib sensitizes primary human astrocytoma cells of WHO grades I to IV for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Clin Cancer Res. 2007;13:3403–3412. doi: 10.1158/1078-0432.CCR-07-0251. [DOI] [PubMed] [Google Scholar]
- 26.Luster TA, Carrell JA, McCormick K, Sun D, Humphreys R. Mapatumumab and lexatumumab induce apoptosis in TRAIL-R1 and TRAIL-R2 antibody-resistant NSCLC cell lines when treated in combination with bortezomib. Mol Cancer Ther. 2009;8:292–302. doi: 10.1158/1535-7163.MCT-08-0918. [DOI] [PubMed] [Google Scholar]
- 27.Brooks AD, Jacobsen KM, Li W, Shanker A, Sayers TJ. Bortezomib sensitizes human renal cell carcinomas to TRAIL apoptosis through increased activation of caspase-8 in the death-inducing signaling complex. Mol Cancer Res. 2010;8:729–738. doi: 10.1158/1541-7786.MCR-10-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayers TJ, Brooks AD, Koh CY, Ma W, Seki N, Raziuddin A, Blazar BR, Zhang X, Elliott PJ, Murphy WJ. The proteasome inhibitor PS-341 sensitizes neoplastic cells to TRAIL-mediated apoptosis by reducing levels of c-FLIP. Blood. 2003;102:303–310. doi: 10.1182/blood-2002-09-2975. [DOI] [PubMed] [Google Scholar]
- 29.Kabore AF, Sun J, Hu X, McCrea K, Johnston JB, Gibson SB. The TRAIL apoptotic pathway mediates proteasome inhibitor induced apoptosis in primary chronic lymphocytic leukemia cells. Apoptosis. 2006;11:1175–1193. doi: 10.1007/s10495-006-8048-9. [DOI] [PubMed] [Google Scholar]
- 30.Nikrad M, Johnson T, Puthalalath H, Coultas L, Adams J, Kraft AS. The proteasome inhibitor bortezomib sensitizes cells to killing by death receptor ligand TRAIL via BH3-only proteins Bik and Bim. Mol Cancer Ther. 2005;4:443–449. doi: 10.1158/1535-7163.MCT-04-0260. [DOI] [PubMed] [Google Scholar]
- 31.Seki N, Toh U, Sayers TJ, Fujii T, Miyagi M, Akagi Y, Kusukawa J, Kage M, Shirouzu K, Yamana H. Bortezomib sensitizes human esophageal squamous cell carcinoma cells to TRAIL-mediated apoptosis via activation of both extrinsic and intrinsic apoptosis pathways. Mol Cancer Ther. 2010;9:1842–1851. doi: 10.1158/1535-7163.MCT-09-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Booth NL, Sayers TJ, Brooks AD, Thomas CL, Jacobsen K, Goncharova EI, McMahon JB, Henrich CJ. A cell-based high-throughput screen to identify synergistic TRAIL sensitizers. Cancer Immunol Immunother. 2009;58:1229–1244. doi: 10.1007/s00262-008-0637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee T-J, Um HJ, Min DS, Park J-W, Choi KS, Kwon TK. Withaferin A sensitizes TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of death receptor 5 and down-regulation of c-FLIP. Free Radic Biol Med. 2009;46:1639–1649. doi: 10.1016/j.freeradbiomed.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci USA. 2003;100:9946–9951. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nawrocki ST, Carew JS, Dunner K, Jr, Boise LH, Chiao PJ, Huang P, Abbruzzese JL, McConkey DJ. Bortezomib inhibits PKR-like endoplasmic reticulum (ER) kinase and induces apoptosis via ER stress in human pancreatic cancer cells. Cancer Res. 2005;65:11510–11519. doi: 10.1158/0008-5472.CAN-05-2394. [DOI] [PubMed] [Google Scholar]
- 36.Huang S, Sinicrope FA. BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer Res. 2008;68:2944–2951. doi: 10.1158/0008-5472.CAN-07-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy M, Sr, Goulet D, Viallet J, Belec L, Billot X, Acoca S, Purisima E, Wiegmans A, Cluse L, Johnstone RW, Beauparlant P, Shore GC. Small molecule obatoclax (GX15–070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci USA. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Z, Friess H, Solioz M, Aebi S, Korc M, Kleeff J, Buchler MW. Bcl-x(L) antisense oligonucleotides induce apoptosis and increase sensitivity of pancreatic cancer cells to gemcitabine. Int J Cancer. 2001;94:268–274. doi: 10.1002/ijc.1447. [DOI] [PubMed] [Google Scholar]
- 39.LaCasse EC, Cherton-Horvat GG, Hewitt KE, Jerome LJ, Morris SJ, Kandimalla ER, Yu D, Wang H, Wang W, Zhang R, Agrawal S, Gillard JW, Durkin JP. Preclinical characterization of AEG35156/GEM 640, a second-generation antisense oligonucleotide targeting X-linked inhibitor of apoptosis. Clin Cancer Res. 2006;12:5231–5241. doi: 10.1158/1078-0432.CCR-06-0608. [DOI] [PubMed] [Google Scholar]
- 40.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/T-or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8:808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 41.Vogler M, Walczak H, Stadel D, Haas TL, Genze F, Jovanovic M, Bhanot U, Hasel C, Moller P, Gschwend JE, Simmet T, Debatin KM, Fulda S. Small molecule XIAP inhibitors enhance TRAIL-induced apoptosis and antitumor activity in preclinical models of pancreatic carcinoma. Cancer Res. 2009;69:2425–2434. doi: 10.1158/0008-5472.CAN-08-2436. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Cuddy M, Samuel T, Welsh K, Schimmer A, Hanaii F, Houghten R, Pinilla C, Reed JC. Cellular, biochemical, and genetic analysis of mechanism of small molecule IAP inhibitors. J Biol Chem. 2004;279:48168–48176. doi: 10.1074/jbc.M405022200. [DOI] [PubMed] [Google Scholar]
- 43.Jost PJ, Grabow S, Gray D, McKenzie MD, Nachbur U, Huang DC, Bouillet P, Thomas HE, Borner C, Silke J, Strasser A, Kaufmann T. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature. 2009;460:1035–1039. doi: 10.1038/nature08229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffith TS, Anderson RD, Davidson BL, Williams RD, Ratliff TL. Adenoviral-mediated transfer of the TNF-related apoptosis-inducing ligand/Apo-2 ligand gene induces tumor cell apoptosis. J Immunol. 2000;165:2886–2894. doi: 10.4049/jimmunol.165.5.2886. [DOI] [PubMed] [Google Scholar]
- 45.Holoch PA, Griffith TS. TNF-related apoptosis-inducing ligand (TRAIL): a new path to anti-cancer therapies. Eur J Pharmacol. 2009;625:63–72. doi: 10.1016/j.ejphar.2009.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanoosten RL, Griffith TS. Activation of tumor-specific CD8+T Cells after intratumoral Ad5-TRAIL/CpG oligodeoxynucleotide combination therapy. Cancer Res. 2007;67:11980–11990. doi: 10.1158/0008-5472.CAN-07-1526. [DOI] [PubMed] [Google Scholar]
- 47.Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler RS, Gejyo F, Okumura K, Yagita H, Smyth MJ. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12:693–698. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 48.Shanker A, Brooks AD, Jacobsen KM, Wine JW, Wiltrout RH, Yagita H, Sayers TJ. Antigen presented by tumors in vivo determines the nature of CD8+T-cell cytotoxicity. Cancer Res. 2009;69:6615–6623. doi: 10.1158/0008-5472.CAN-09-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen L, Park SM, Tumanov AV, Hau A, Sawada K, Feig C, Turner JR, Fu YX, Romero IL, Lengyel E, Peter ME. CD95 promotes tumour growth. Nature. 2010;465:492–496. doi: 10.1038/nature09075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 51.Hallett WH, Ames E, Motarjemi M, Barao I, Shanker A, Tamang DL, Sayers TJ, Hudig D, Murphy WJ. Sensitization of tumor cells to NK cell-mediated killing by proteasome inhibition. J Immunol. 2008;180:163–170. doi: 10.4049/jimmunol.180.1.163. [DOI] [PubMed] [Google Scholar]
- 52.Lundqvist A, Yokoyama H, Smith A, Berg M, Childs R. Bortezomib treatment and regulatory T-cell depletion enhance the antitumor effects of adoptively infused NK cells. Blood. 2009;113:6120–6127. doi: 10.1182/blood-2008-11-190421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lundqvist A, Abrams SI, Schrump DS, Alvarez G, Suffredini D, Berg M, Childs R. Bortezomib and depsipeptide sensitize tumors to tumor necrosis factor-related apoptosis-inducing ligand: a novel method to potentiate natural killer cell tumor cytotoxicity. Cancer Res. 2006;66:7317–7325. doi: 10.1158/0008-5472.CAN-06-0680. [DOI] [PubMed] [Google Scholar]
- 54.Sudbery I, Enright AJ, Fraser AG, Dunham I. Systematic analysis of off-target effects in an RNAi screen reveals microRNAs affecting sensitivity to TRAIL-induced apoptosis. BMC Genomics. 2010;11:175. doi: 10.1186/1471-2164-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aza-Blanc P, Cooper CL, Wagner K, Batalov S, Deveraux QL, Cooke MP. Identification of modulators of TRAIL-induced apoptosis via RNAi-based phenotypic screening. Mol Cell. 2003;12:627–637. doi: 10.1016/S1097-2765(03)00348-4. [DOI] [PubMed] [Google Scholar]
- 56.Ovcharenko D, Kelnar K, Johnson C, Leng N, Brown D. Genome-scale microRNA and small interfering RNA screens identify small RNA modulators of TRAIL-induced apoptosis pathway. Cancer Res. 2007;67:10782–10788. doi: 10.1158/0008-5472.CAN-07-1484. [DOI] [PubMed] [Google Scholar]
- 57.Yang TM, Barbone D, Fennell DA, Broaddus VC. Bcl-2 family proteins contribute to apoptotic resistance in lung cancer multicellular spheroids. Am J Respir Cell Mol Biol. 2009;41:14–23. doi: 10.1165/rcmb.2008-0320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Finnberg N, Klein-Szanto AJ, El-Deiry WS. TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. J Clin Invest. 2008;118:111–123. doi: 10.1172/JCI29900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grosse-Wilde A, Voloshanenko O, Bailey SL, Longton GM, Schaefer U, Csernok AI, Schutz G, Greiner EF, Kemp CJ, Walczak H. TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development. J Clin Invest. 2008;118:100–110. doi: 10.1172/JCI33061. [DOI] [PMC free article] [PubMed] [Google Scholar]