Abstract
Recombinant HSP70 chaperone exerts a profound anticancer effect when administered intratumorally. This action is based on the ability of HSP70 to penetrate tumor cells and extract its endogenous homolog. To enhance the efficacy of HSP70 cycling, we employed phloretin, a flavonoid that enhances the pore-forming activity of the chaperone on artificial membranes. Phloretin increased the efficacy of HSP70 penetration in B16 mouse melanoma cells and K-562 human erythroblasts; this was accompanied with increased transport of the endogenous HSP70 to the plasma membrane. Importantly, treatment with HSP70 combined with phloretin led to the elevation of cell sensitivity to cytotoxic lymphocytes by 16–18 % compared to treatment with the chaperone alone. The incubation of K-562 cells with biotinylated HSP70 and phloretin increased the amount of the chaperone released from cells, suggesting that chaperone cycling could trigger a specific anti-tumor response. We studied the effect of the combination of HSP70 and phloretin using B16 melanoma and a novel method of HSP70-gel application. We found that the addition of phloretin to the gel reduced tumor weight almost fivefold compared with untreated mice, while the life span of the animals extended from 25 to 39 days. The increased survival was corroborated by the activation of innate and adaptive immunity; interestingly, HSP70 was more active in induction of CD8+ cell-mediated toxicity and γIFN production while phloretin contributed largely to the CD56+ cell response. In conclusion, the combination of HSP70 with phloretin could be a novel treatment for efficient immunotherapy of intractable cancers such as skin melanoma.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1778-1) contains supplementary material, which is available to authorized users.
Keywords: Phloretin, HSP70, Hydrogel, Melanoma B16, K-562 human erythroblasts, Anti-tumor immunity
Introduction
Intratumoral delivery of HSP70 chaperone has demonstrated immunomodulatory efficacy in several tumor models including mouse B16 melanoma and rat C6 glioblastoma [1–3]. This activity relates to the ability of the exogenous chaperone (exo-HSP70) to penetrate a living cell and to push its endogenous analogue (endo-HSP70) to the cell surface [4]. Once exposed on the membrane of a tumor cell, HSP70 or its TKD peptide becomes a target for natural killer cells (NK cells) [5]. Furthermore, HSP70 released from cancer cells into the extracellular matrix has been found to carry tumor antigens to dendritic cells and thus to activate CD4+- and CD8+-mediated responses (see [6] for a review).
Although the therapy based on intratumorally delivered HSP70 is effective [2, 3], there are ways to increase its efficacy. Ito and colleagues administered HSP70 into B16 melanoma tumor together with magnetic particles [1]. Using local hyperthermia concentrated on the tumor lesion, they achieved significant tumor regression and development of a generalized immune response. Another group, using HSP70 supplemented with the plasmid encoding protein inhibitor of the anti-tumor response, found a twofold increase in survival accompanied by elevated tumor-specific production of γ-interferon (γIFN) and interleukin-2 [7].
The immunomodulatory effect of exo-HSP70 may be increased by the enhanced transport of endo-HSP70 to the cell surface. This effect will depend on the ability of exo-HSP70 to penetrate a target cell. This penetrative activity and its molecular mechanisms are well documented. First, the chaperone has been found to have affinity to certain membrane structures, lipid rafts enriched with phosphatidylserine [8]. Second, HSP70 has been shown to form channels in model lipid membranes [9]. Recently, HSP70 was found to employ different intracellular transport pathways in a cell species-dependent manner [10].
Membrane-modulating activity characterizes flavonoids, amphiphilic molecules that easily integrate into plasma membrane and can change their physicochemical properties such as dipole potential [11–13]. Particularly, the dihydrochalcone, phloretin, has been found to increase the membrane channel-forming activity of antimicrobial peptides and lipopeptides [14–17]. Recently, we found that phloretin can interact directly with the channel-forming molecules on a cell membrane [18]. This prompted us to explore the potential of phloretin combined with HSP70 in in vitro and in vivo models of B16 mouse melanoma.
Materials and methods
Measurement of channel-forming activity of HSP70 and phloretin
Synthetic 1,2-dioleoyl-sn-glycero-3-phospho-l-serine (DOPS) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) were obtained from Avanti Polar Lipids, Inc. (Pelham, USA). Phloretin (3-(4-hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)-1-propanone) was purchased from Sigma-Aldrich (USA).
Lipid bilayers were made from equimolar mixtures of DOPS and DOPE as described elsewhere [16]. HSP70 was added to the cis-chamber in concentrations of 0.3–2.0 mg/ml. Phloretin was added to both cis- and trans-chambers in final concentrations of 20 µM. The channel-forming activity of HSP70 with and without phloretin was measured by HSP70-induced steady-state trans-membrane conductance (G).
HSP70 hydrogel composition
Recombinant human HSP70 was purified from bacteria transformed with a pMSHsp70 plasmid, as described elsewhere [19]. The HSP70 solution was further detoxified by incubation with polymyxin B-agarose gel (Sigma-Aldrich, USA) and sterilized by filtration through a 0.2-µm filter (Millipore, USA). According to the E-Toxate assay (Sigma-Aldrich, USA), the level of lipopolysaccharide in the final HSP70 preparation was lower than 0.25 U/ml. For microscopy, HSP70 was conjugated to Alexa555 dye (Invitrogen, USA) according to the manufacturer’s protocol. For biochemical experiments, HSP70 was biotinylated using Succinimide (NHS)-biotin (Sigma-Aldrich, USA).
The hydrogel was composed of carbopol (1 %), glycerol (1 %) and dimethylsulfoxide (10 %). HSP70 and phloretin were introduced into gel to obtain final concentrations of 0.7 mg/ml and 20 μM, respectively.
Cells and animals
Human erythroid leukemia K-562 cells were obtained from the Russian Cell Culture Collection at the Institute of Cytology, Russian Academy of Sciences (St. Petersburg, Russia). Mouse melanoma B16 cells were kindly provided by Prof. L. Sistonen (Turku Centre for Biotechnology, Finland). B16 cells were grown in DMEM media and K-562 cells in RMPI-1640, both supplemented with 10 % fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin and 0.1 mg/ml streptomycin. All media and sera were purchased from PanEco, Russia.
C57Bl/6 female mice subcutaneously engrafted with 106 B16 cells were divided into five groups, which were treated in different ways starting at day 7 after tumor cell inoculation. The animals in group 1 were untreated (n = 25), group 2 animals (the placebo group) were treated with clear hydrogel (n = 25), and the animals of groups 3, 4 and 5 were treated with hydrogel with phloretin only (group 3, n = 25), HSP70 alone (group 4, n = 25) and HSP70 + phloretin (group 5, n = 25), respectively. The application of drug-containing gel or control gel (placebo) was performed onto preliminarily shaven areas of skin surrounding a seventh day B16 tumor; the application was repeated every second day until the end of experiments.
Tumor growth rate was estimated by weighing tumors taken from all groups of animals on day 18 after inoculation with B16 cells (n = 5 in each group). All animal experiments were in accordance with the guidelines for the welfare of animals of the Institute of Cytology, Russian Academy of Sciences.
Confocal microscopy
K-562 cells and B16 mouse melanoma cells were settled on poly-l-lysine-coated glass slides and incubated with 50 μg/ml Alexa555-labeled HSP70 alone or with HSP70 and 20 μM phloretin for 18 h at 37 °C. After incubation with the chaperone, the cells were washed with ice-cold PBS, incubated with polyclonal antibodies RSIII generated in our laboratory and known to recognize human, rat and mouse HSP70 and then fixed with 4 % paraformaldehyde in PBS followed by incubation with secondary Alexa488-conjugated goat anti-mouse antibody (Invitrogen, USA). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
Fluorescence images were captured using a Leica TCS SP2 confocal microscope (Leica, Germany). To avoid possible cross-interference of various fluorochromes, images for DAPI, Alexa488 and Alexa555 were acquired using the sequential image recording method.
Flow cytometry
To detect HSP70 localized to a cell surface, K-562 cells were incubated with 50 μg/ml HSP70 labeled with Cy5 with and without 20 μM phloretin for 18 h. After washing with ice-cold PBS, the cells were stained with cmHsp70.1-FITC monoclonal antibodies at 4 °C for 30 min. After three washes with ice-cold PBS, viable cells were gated and analyzed with the aid of CyFlow Space (Partec GmbH, Germany) apparatus equipped with two lasers (λ = 488 and 635 nm).
Analysis of HSP70 release from living cells
K-562 cells (1.5 × 107 in each probe) were incubated with 50 µg/ml biotinylated HSP70 alone or HSP70 and 20 μM phloretin. After 24 h, the cells were washed with PBS and placed into serum-free media for the next 90 min. The conditioned medium was supplemented with Tween-20, Tris–HCl buffer pH 7.5 and MgCl2 to reach final concentrations of 0.1 %, 20 and 5 mM, respectively. The first cycle of affinity precipitation was performed by adding NeutrAvidin-agarose gel slurry (Pierce, USA) to each sample of conditioned medium. The affinity resin was washed with 20 mM Tris–HCl, pH 7.5, 5 mM MgCl2 and 0.1 % Tween-20 and mixed with an equal volume of twofold buffer for electrophoretic samples containing 2 % sodium dodecylsulfate (SDS). Material unbound to the NeutrAvidin-agarose was mixed with ATP-agarose gel (Sigma-Aldrich, USA), and the suspension was rotated overnight at 4 °C. The gel probes were subjected to treatment with SDS as described above. Both groups of probes were subjected to electrophoresis in 11 % polyacrylamide gel and immunoblotting; the membranes were stained with avidin-peroxidase (Pierce, USA) or with RSIII anti-HSP70 polyclonal antibody. HSP70 from cell lysates was also precipitated using the procedure described above.
Cytotoxicity assay
B16 and K-562 cells were treated with 50 μg/ml HSP70 alone or in combination with 20 μM phloretin for 18 h. The concentration of phloretin was chosen in the preliminary experiments (data not shown). The effector cells were isolated from the spleen of C3HA mice; the incubation of effector–target cells was done in ratios 50:1 and 100:1 and lasted 4 h. We used the CytoTox 96 assay (Promega, USA) to measure the number of dying target cells.
To estimate specific cytotoxic activity, spleens of mice with B16 melanomas were sampled on day 21 after tumor injection and the splenocytes of untreated, placebo (saline solution), phloretin-treated, HSP70-treated and HSP70 + phloretin-treated mice were added to B16 target cells in ratios as above.
For the precise analysis of the NK or CD8+ cell response to the phloretin-, HSP70- and HSP70 + phloretin-containing gels, we firstly isolated spleen cells from animals belonging to appropriate treatments and divided them into two groups, one of which was incubated with Dynabeads FlowComp™ Mouse CD8 to remove CD8+ cells and the other with Dynabeads FlowComp™ Mouse CD56 (Invitrogen, USA) to remove NK cells. The remaining cell population after removal of CD8+ or NK cells was used as effector cells which were added to B16 cells at a ratio of 100:1.
Gamma-interferon measurement
Splenocytes were isolated from controls and treated with HSP70-, phloretin- and HSP70± phloretin gel-treated animals (n = 3 per group) on days 14 and 20 after grafting of B16 cells using a standard protocol. The level of γIFN in the medium of splenocytes incubated with B16 cells for 48 h, treated with concanavalin A (reference control) and in pure medium (spontaneous induction) was measured with the aid of a mouse γIFN ELISA kit (BD Biosciences, USA). All values were equalized to the level of the cytokine in reference control samples; the latter were quite similar in all groups of animals. The concentration of spontaneously released γIFN was less than 10 pg/ml in all groups and was subtracted from the experimental data.
To estimate the input of CD8+ and NK cells to γIFN production, the same fraction of lymphocytes taken from HSP70-, phloretin- and HSP70 + phloretin gel-treated animals were added to B16 cells, and γIFN production was measured as described above.
Statistics
Observations are generally reported as mean ± SE. One- or two-tailed unpaired Student’s t tests were used to evaluate the differences between the control and treated groups; differences were considered to be statistically significant when p < 0.05 or p < 0.01. The survival curves were estimated by the Kaplan–Meier method and compared using Wilcoxon’s test.
Results
Phloretin enhances the membrane-crossing activity of HSP70
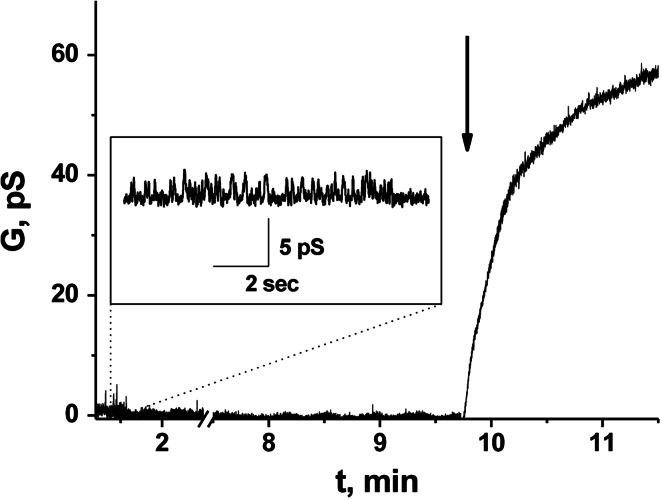
HSP70 was shown to exert channel-forming activity in several simulations. To understand whether phloretin was able to increase HSP70 capacity, we used a negatively charged lipid bilayer and an electrophysiological recording technique. The addition of HSP70 to the cis-compartment resulted in incorporation of the protein into the DOPS/DOPE bilayer and the emergence of single channel-like currents of different magnitude (Fig. 1). The addition of phloretin led to an increase in HSP70-induced steady-state membrane conductance, proving the HSP70-assisting effect of the flavonoid (Fig. 1). Notably, the same channel-forming capacity was found if HSP70 was labeled with low molecular weight tags such as biotin (Supplementary Fig. 1S). This proves the feasibility of the employment of labeled chaperone in further experiments.
Fig. 1.
Effect of phloretin on the channel-forming activity of HSP70. Addition of phloretin to the bilayer bathing solution is indicated by an arrow. Planar lipid bilayer was made from an equimolar mixture of DOPS and DOPE. Applied voltage was 100 mV. Bilayer bathing solution was 0.1 M KCl, 5 mM HEPES–KOH (pH 7.0). Concentration of HSP70 in cis-solution was equal to 1.3 mg/ml. Inset HSP70-induced ion channels
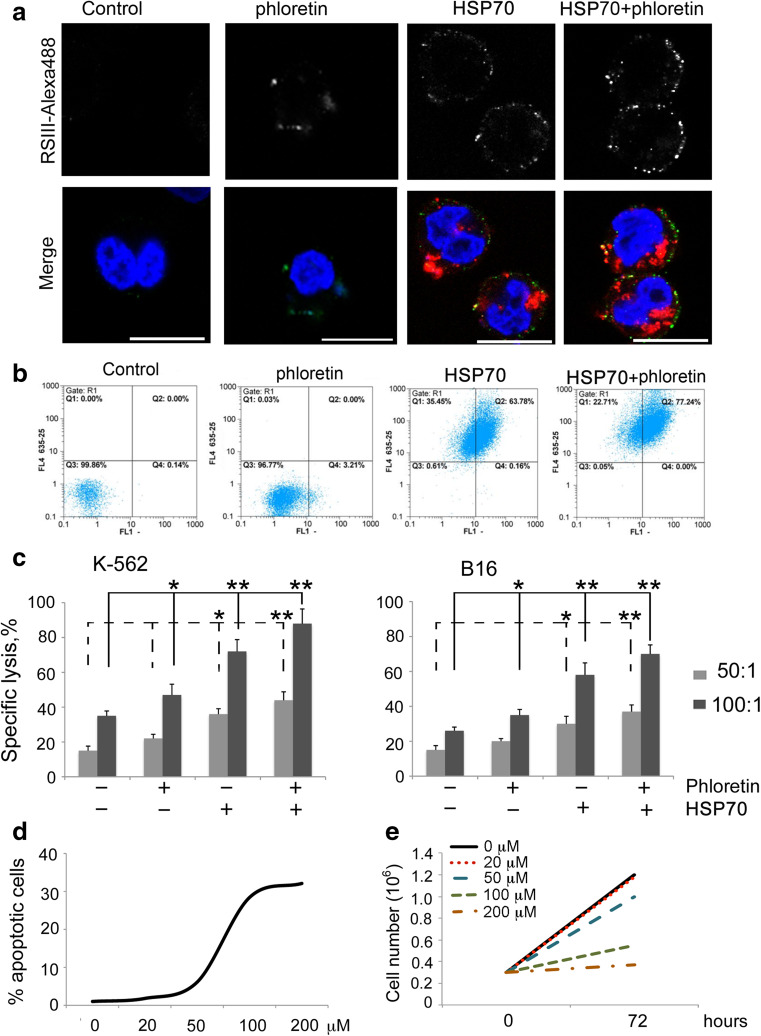
The ability of phloretin to enhance the membrane-crossing activity of HSP70 was confirmed in experiments with K-562 human erythroid cells. The cells were incubated with the HSP70 labeled with Alexa555 alone or its combination with phloretin. After the incubation, cells were stained with anti-HSP70 antibody. The pattern of combination of exo- and endo-HSP70 fully resembles that obtained in the previous work [4]: in nonpermeabilized K-562 cells exo-HSP70 quickly entered cells while its own intracellular analogue formed green spots near and inside the plasma membrane (Fig. 2a). The migration of HSP70 to the plasma membrane was associated with this effect because thorough washing did not prevent it (Supplementary Fig. 2Sa). Moreover, heat shock, which also leads to the release of endo-HSP70 [20], caused the chaperone to be anchored on the plasma membrane (Supplementary Fig. 2Sb). According to the flow cytometry data, approximately 85–98 % of tumor cells were able to incorporate exo-HSP70 within 1 h and the intensity of fluorescence increased with incubation time (Supplementary Fig. 3S). Phloretin increased both the intracellular transport of exo-HSP70 and the transport of endo-HSP70 to the cell membrane. This was shown with the aid of confocal microscopy and flow cytometry (Fig. 2a, b). Phloretin itself did not induce the transport of endo-HSP70 to the cell surface. This suggests that its role in the process is confined to assisting exo-HSP70.
Fig. 2.
Phloretin increases exposition of cellular HSP70 on the cell surface which leads to elevation of sensitivity to cytotoxic activity of splenocytes. a. K-562 cells were incubated with 50 μg/ml HSP70 labeled with Alexa555 alone (red) or in the presence of 20 μM phloretin overnight and stained with RSIII polyclonal antibody (green). Nuclei were visualized with DAPI (blue). Scale bar 10 μM. b. K-562 cells were incubated with 50 μg/ml HSP70 labeled with Cy5 alone or in the presence of 20 μM of phloretin during18 h, stained with cmHsp70.1 antibody and analyzed with flow cytometry. c. K-562 and B16 cells were incubated with phloretin (20 µM) and HSP70 (50 µg/ml) separately or in combination overnight and were incubated with splenocytes from naive C3HA mice in two ratios (100:1 and 50:1). * and ** denote a significant difference between treatment groups (p < 0.05 and p < 0.01, respectively). d. B16 cells were treated with phloretin in the concentrations indicated, and the proportion of cells with fragmented nuclei was calculated after 24 h. Representative data of three independent experiments are presented. e. B16 cells were seeded into a 12-well plate at a concentration of 2.5 × 105 cells/ml. The next day, they were treated with phloretin in the concentrations indicated. Cells were collected and counted after 72 h
Next, we checked whether the effect of endo-HSP70 expulsion could cause an increase in tumor cell sensitivity to cytotoxic lymphocytes as was demonstrated earlier [4]. We found that incubation with the combination of HSP70 and phloretin increased the proportion of dying K-562 cells to 88.2 ± 6.8 % in comparison with cells incubated with HSP70 alone (72.5 ± 8.4 %) (Fig. 2c, left panel). Phloretin itself also increased the proportion of dead cells to 47.6 ± 5.5 %, though the level of specific lysis of untreated cells was similar (35.2 ± 4.2 %).
Similar results were obtained with B16 cells. The combination of HSP70 with phloretin elevated specific death to 70.2 ± 6.2 % of the cell population, greater than for cells treated with HSP70 alone (58.2 ± 5.8 %). Incubation with pure phloretin also stimulated an increase in specific lysis compared to untreated cells (Fig. 2c, right panel). The combination of these data confirms the proposal that phloretin promotes the exo-HSP70-mediated exposition of its endogenous counterpart on the surface of tumor cells and hence elevates sensitivity to cytotoxic NK cells.
Since it was recently reported that phloretin is able to provoke programmed cell death in tumor cells [21], we investigated whether apoptosis could contribute to cell death caused by lymphocytes. B16 cells were incubated with phloretin in concentrations indicated in Fig. 2d and stained with DAPI to reveal apoptosis-specific changes in nuclear morphology. Phloretin in concentrations of 100 and 200 μM induced apoptosis in 28.4 ± 3.4 and 32.1 ± 2.7 % of cells, respectively. However, when phloretin was used in concentrations of 20 or 50 μM, the contribution of phloretin-mediated apoptosis comprised only 1.8 ± 0.4 and 6.1 ± 1.6 %. To study the effect of phloretin on the cell growth in more detail, we measured a number of B16 cells incubated with phloretin in various concentrations. We found that phloretin at concentrations of 100 and 200 μM inhibited the cell growth approximately twofold and threefold, respectively (Fig. 2e). Almost no inhibition was detected when phloretin was applied in concentrations of 20 or 50 μM. Finally, HSP70 does not impact the phloretin-mediated cytotoxicity (Supplementary Fig. 4S).
Phloretin enhances the HSP70 release into extracellular milieu
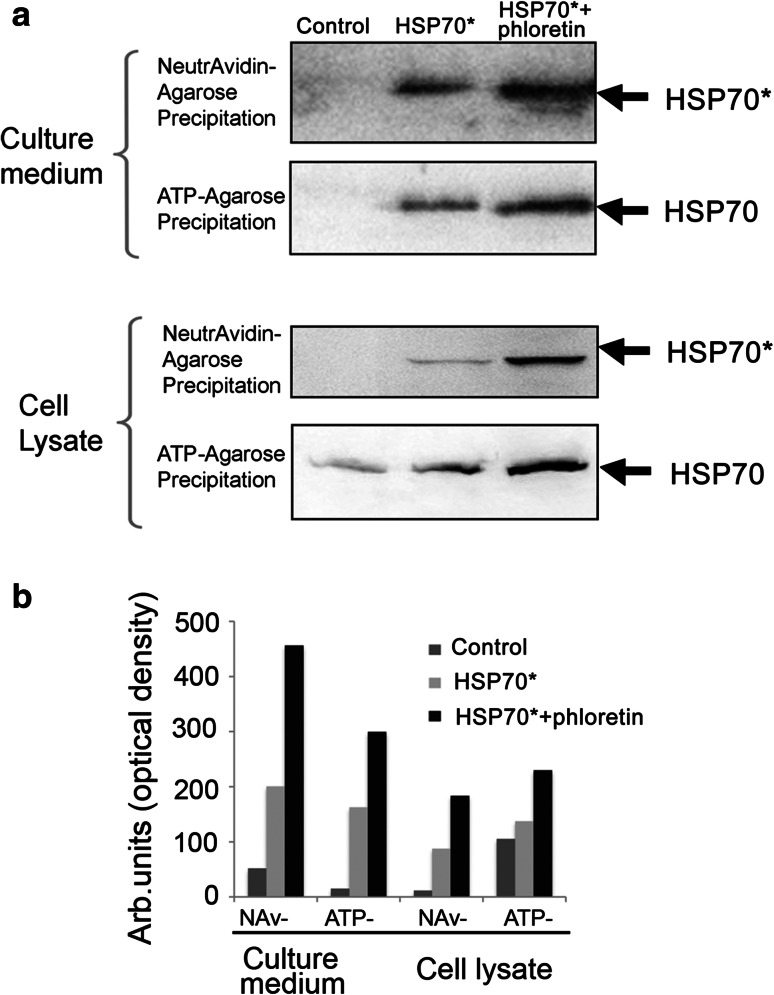
The HSP70 chaperone appearing in the extracellular matrix was able to carry tumor antigens and to participate in their presentation by dendritic cells giving rise to a burst of the specific immune response. To study how phloretin affects the extracellular transport of HSP70, we trapped exo- and endo-HSP70 using two affinity media. To discriminate between the exogenously introduced HSP70 and the self-chaperone, K-562 cells were incubated with biotinylated HSP70 for the 6 h (Fig. 3), then were carefully washed and were allowed to export the chaperone into serum-free culture media for 90 min. The conditioned medium was subjected to affinity precipitation with the aid of NeutrAvidin-agarose, and the unbound material was mixed with ATP-agarose gel. Two sets of samples, i.e., NeutrAvidin-agarose and ATP-agarose precipitates, were analyzed with the aid of blotting and staining with avidin-peroxidase or anti-HSP70 polyclonal antibody, recognizing both the exogenous and endogenous chaperones. It was found that the export of biotinylated HSP70 was considerably higher in cells treated with the combination of HSP70 and phloretin compared with cells treated with HSP70 alone, suggesting that the flavonoid enhanced the cycling of exo-HSP70 (Fig. 3a, upper panel). Both biotinylated HSP70 and unlabeled endo-HSP70 trapped with ATP-agarose were found in the cell medium. The amount of both types of chaperone released from cells treated with Hsp70 and phloretin was much greater than in cells treated only with Hsp70 (Fig. 3a, second panel from top). Interestingly, the amount of exo-HSP70 precipitated with NeutrAvidin-agarose from lysates of cells treated with HSP70 and phloretin was also much greater than from the cells treated with HSP70 alone (Fig. 3a, third panel from top). This clearly demonstrates that phloretin contributes to the efficacy of intra- and extracellular transport of HSP70. It is noteworthy that the mortality of K-562 cells affected by exo-HSP70 with or without phloretin did not exceed 3–7 %, indicating that living cells were mostly responsible for exporting both chaperones.
Fig. 3.
Phloretin increases both penetration and release of HSP70 from K-562 cells. a. HSP70 was labeled with biotin and added to a culture of K-562 cells alone or in combination with 20 μM phloretin. After 18 h incubation, cells were washed and fresh serum-free medium was added to flasks for the next 4 h. Medium and cells were collected, cells were lysed, and both cell lysate and medium were precipitated first with NeutrAvidin-agarose and then with ATP-agarose. Each precipitate was divided into two portions and subjected to western blotting. One portion was probed with avidin-peroxide (HSP70*) and other with anti-HSP70 antibody (HSP70). b. Western blot data were quantified with the aid of software TotalLab. Data of one of three independent experiments are presented
Phloretin added to HSP70-containing hydrogel assists the chaperone to impede tumor growth
The in vitro data prompted us to study whether the CTL-activating effect of combined HSP70 and phloretin took place in a tumor simulation in vivo. We used a B16 mouse melanoma model and a noninvasive method of HSP70 application; the chaperone alone or in combination with phloretin was incorporated in hydrogel as described previously [2].
To prove that HSP70 applied as hydrogel is able to penetrate animal tissues, the amount of the biotinylated chaperone in blood stream was measured after the treatment. We used ELISA-like assay depicted in Supplementary Fig. 5S and found that HSP70 amount in blood rose within 3 h up to 250 ng/ml and then dropped down.
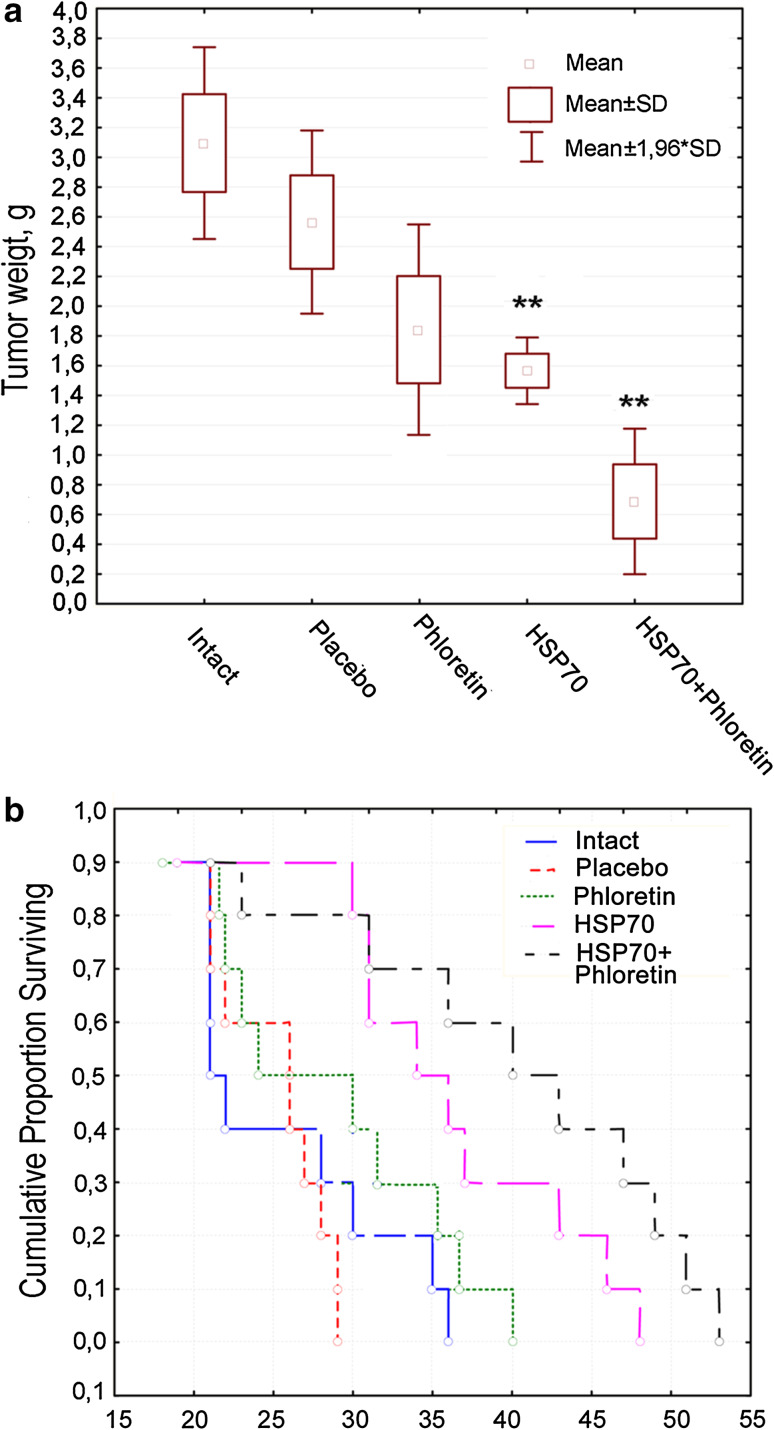
The rate of tumor growth was analyzed at the 18th day when tumors from five animals from each experimental group were extracted and weighted. The tumor weight from animals treated with HSP70 + phloretin was approximately four times lower than from tumors of untreated animals: 0.7 ± 0.2 versus 3.1 ± 0.2 g. Tumors of animals treated only with phloretin or only with HSP70 gels (1.6 ± 0.1 and 1.8 ± 0.4 g, respectively) were half the size of tumors from the control group, although the weight of control tumors varied widely. Interestingly, treatment with placebo gel also led to the reduction in tumor weight to 2.6 ± 0.02 g (Fig. 4a).
Fig. 4.
Phloretin increases anti-tumor activity of HSP70. C57Bl6 mice were inoculated with 106 B16 cells. Seven days later hydrogel with phloretin or HSP70 alone or in combination was applied every 2 days to the injected area. Tumor weight on day 18 (a) and life span of tumor-bearing mice (b) were estimated
The delay in tumor growth in HSP70- and HSP70 + phloretin-treated mice resulted in the prolonged survival of animals. The death of mice belonging to untreated and placebo groups occurred within 25.4 ± 4.6 and 25 ± 2.3 days, respectively, whereas in HSP70-treated and HSP70 + phloretin-treated groups, death was postponed until 35.5 ± 6.1 and 39.4 ± 7.7 days, respectively (Fig. 4b). Treatment with phloretin alone did not influence longevity (27.5 ± 4.7 days).
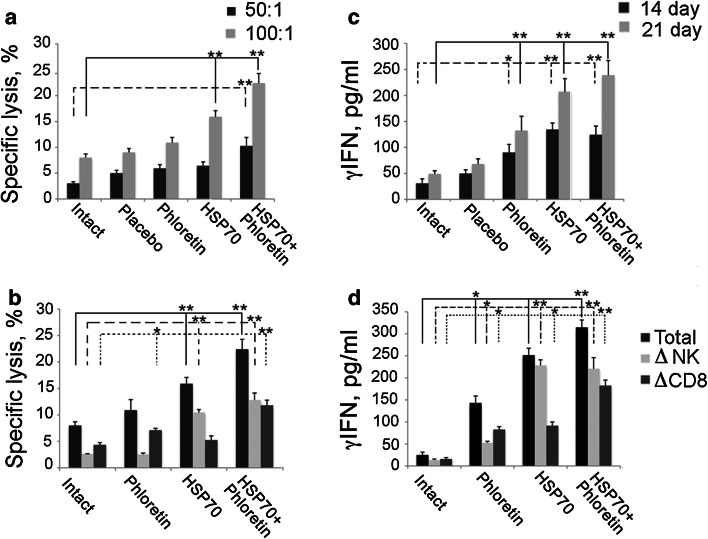
Next, we checked whether the addition of phloretin could promote HSP70-mediated activation of anticancer immunity. Two approaches were used. First, the mixed fraction of splenocytes was obtained from intact, placebo, HSP70-, phloretin- and HSP70 + phloretin-treated animals on day 21 after tumor cell grafting. Splenocytes were incubated with B16 melanoma cells, and cytotoxic activity was measured as the release of lactate dehydrogenase by dying cells using Promega CytoTox 96 assay. The cytotoxicity index of splenocytes of mice treated with a hydrogel with HSP70 or with HSP70 + phloretin was approximately 1.9- and 2.8-fold higher than that of splenocytes from the control animals (Fig. 5a).
Fig. 5.
Phloretin increases immune response caused by HSP70-activating innate immunity. Splenocytes were extracted from spleens of tumor-bearing animals untreated or treated with hydrogels with HSP70 and phloretin separately or in combination 14 days (c) or 21 days (a, b, d) after inoculation. Mice treated with empty hydrogel are indicated as placebo. Sensitivity of naive B16 cells to cytotoxic activity of lymphocytes from mice (a, b) and their ability to induce γIFN release (c, d) were evaluated. Splenocytes from each group were divided into three groups. One was incubated with Dynabeads FlowComp™ Mouse CD8 to remove CD8+ cells (ΔCD8) and the second with Dynabeads FlowComp™ Mouse CD56 (ΔNK) to remove NK cells. The remaining splenocyte population was used as effector cells. The third group was left untreated (total). These lymphocytes were used for CTL assay (b) and for measurement of γIFN (d). * and ** denote a significant difference between treatment groups (p < 0.05 and p < 0.01, respectively)
Since numerous experiments proved that HSP70 could induce both innate and adaptive immunity, we checked which type of cells was responsible for the anti-tumor reaction of animals treated with phloretin, HSP70 and HSP70 + phloretin. For this purpose, we used Dynabeads FlowComp™ Mouse CD8 to remove CD8 positive cells from splenocyte population and Dynabeads FlowComp™ Mouse CD56 to deplete NK cells. After depletion, the remaining splenocyte populations were employed as effector cells.
Application of phloretin alone did not notably increase the cytotoxic activity of splenocytes toward B16 melanoma cells compared to control cells whereas the removal of NK cells reduced cytotoxicity 4.3-fold, suggesting that phloretin could sensitize tumor cells mainly to NK cells (Fig. 5b). Treatment of tumors with HSP70 alone increased cytotoxic activity of the splenocyte population depleted of NK cells, whereas the cytotoxic effect of splenocytes after depletion of NK cells remained similar to the control. This indicates that HSP70 particularly mediated the activation of the cells of adaptive immunity (Fig. 5b). Thus, the general response of mice to the B16 tumor was the result of the summation of impacts of HSP70-activated CD8+ cells and phloretin-initiated CD56+ lymphocytes (Fig. 5b).
The results of the CTL assay matched well the data on γIFN production by splenocytes obtained from tumor-bearing animals after their incubation with B16 cells. γIFN production by splenocytes increased over time: at day 14 production was 134.3 ± 12.5 pg/ml and by day 21 it was 207.2 ± 25.2 pg/ml in the group of animals treated with HSP70 alone, increasing 1.5-fold within a week (Fig. 5c). In the group treated with HSP70 + phloretin, the γIFN production increased from 124.3 ± 16.7 to 238.8 ± 28.2 pg/ml, almost doubling within the same time and demonstrating the HSP70-enforcing role of the flavonoid (Fig. 5c).
The data on γIFN production by splenocytes isolated from phloretin-treated mice and depleted of CD56+ or CD8+ cells show that their selective removal caused 2.7- and 1.7-fold reductions in the level of the cytokine, respectively (Fig. 5d). HSP70 demonstrated a much greater effect on γIFN production, and this effect was reduced 2.7-fold when the splenocyte population was depleted of CD8+ cells. These data are similar to results on CD56+- and CD8+-mediated cytotoxicity modulated by the chaperone + phloretin and suggest that each component of the HSP70 + phloretin combination was responsible for the cumulative effect.
Discussion
Studies of Srivastava, Multhoff and Calderwood et al. have shown that HSP70 persisting within a tumor cell and/or releasing from the cell has great immunogenic potential [22–24]. We have previously demonstrated that pure recombinant HSP70 displaced its cellular analogue and made it migrate to the cell surface and, further, to the extracellular milieu. This was accompanied by exo-HSP70 dose-dependent growth of tumor cell sensitivity to NK cells [4]. Based on these data, we suggested that the efficacy of exo-HSP70-based therapy could be increased by factors able to affect membrane structure and chose phloretin because it could affect the properties of an artificial lipid membrane [18].
Phloretin induced channels in the artificial lipid membrane and increased the membrane conductivity induced by the chaperone (Fig. 1); this increase was confirmed by records of the single channels formed by HSP70 in phospholipid and sphingolipid bilayers. Interestingly, phloretin showed similar assisting activity in experiments with the antifungal substance, amphotericin; its channel-forming capacity was dependent on the interactions between the dipole modifier, phloretin, amphotericin and the lipid components of the membrane [18]. The pore-forming activity of HSP70 has been demonstrated in several studies, and it has been found that the chaperone bonds preferentially with the phosphatidylserine moiety of a plasma membrane [25]. In more recent study, HSP70 was found to display a preference for less fluid lipid environments [26]. It has been suggested that HSP70, when being anchored to the membrane, exposes its TKD peptide that can be targeted by NK cells [5]; presumably, the role of phloretin is to enhance this process.
According to microscopy data, phloretin elevated the amount of HSP70 penetrating inside K-562 cells as well as the amount of endo-HSP70 exposed on the cell surface (Fig. 2a). Clearly, enhanced exposition of HSP70 on the membrane was associated with the sensitization of K-562 cells to cytotoxic NK cells, thus proving the benefit of phloretin addition to the chaperone (Fig. 2b).
Since the release of HSP70 from cancer cells causes tumor antigen cross-presentation by dendritic cells [27], we checked whether phloretin could enhance the capacity of K-562 erythroblasts to export HSP70 to the extracellular environment. We found that the combination of biotinylated HSP70 and phloretin was able to expel endogenous chaperone more efficiently than exo-HSP70 alone (Fig. 3).
Taken together, these results prove that phloretin enhances endo-HSP70 transport to the cell membrane causing an increase in cancer cell sensitivity to CTL, and the release of endo-HSP70 to the extracellular matrix that induces a burst of adaptive immunity. This led us to test the efficacy of combined administration of HSP70 and phloretin in vivo using a novel method of drug delivery, application of a chaperone-containing hydrogel on the surface of B16 mouse melanoma. The method was effective with HSP70 alone, and its application resulted in the significant reduction in the rate of tumor growth and the increase in the life span of animals [2]. In the present study, we observed an almost fivefold reduction in tumor weight when the combination of HSP70 and phloretin was applied to the tumor surface (Fig. 4). Interestingly, application of phloretin alone also halved the size of tumors, but did not increase the survival of animals.
The intratumoral delivery of HSP70 causes a profound response of cells constituting innate and adaptive immunity [1, 3, 7]. We found that HSP70 also elevated cytotoxic activity of the immune cells 1.9-fold; with the addition of phloretin cytotoxic activity increased by 2.6-fold (Fig. 5a). The impact of HSP70 on the general cytotoxic anti-tumor response mainly related to CD8+ cells, whereas the effect of phloretin was mainly related to CD56+ cells, indicating that the flavonoid contributed to NK-mediated cytotoxicity (Fig. 5b). A similar pattern of activation of γIFN-producing cells by HSP70 and/or phloretin was observed in the experiments on populations depleted of CD8+ or NK cells. Phloretin was able to activate γIFN release mainly from NK cells (Fig. 5c). We found only one other study proving the participation of phloretin in regulation of anticancer immunity; the flavonoid was shown to elevate the killing effect of γδ T cells on SW-1116 cells. The putative mechanism of the effect may lie in an enhanced proliferation of the T cells, activation of Wnt signaling and the increase in γIFN production [28]. Our results confirm this hypothesis.
Phloretin is also known to induce apoptosis in cancer cells of various origins [19, 29]. Particular targets for such pro-apoptotic activity are cells overexpressing GLUT1 or GLUT2 glucose transporters [30, 31]. Cell death induced by phloretin is accompanied by chromatin fragmentation and caspase-3 cleavage [29, 32]. Importantly, these studies used the flavonoid in concentrations of 100 and/or 200 µM compared with the 20 µM used in our experiments; at this concentration, phloretin had no effect on cell growth or viability (Fig. 2d). Indeed, when phloretin was administrated in combination with HSP70, the sensitivity of K-562 and B16 cells to NK cells was considerably elevated, although the effect of the flavonoid alone on cytotoxicity was not significant.
In conclusion, phloretin enhances anticancer activity of exo-HSP70 through the elevation of exo- and endogenous chaperone cycling leading to the activation of both innate and adaptive immunity in in vitro and in vivo tumor models. The drug is already used as an efficient antioxidant in numerous clinical protocols, which may facilitate its promotion as a co-stimulatory compound in HSP70 chaperone-based therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Authors thank Prof. Gabriele Multhoff for cmHsp70.1-FITC antibody and Katerina Pankratova for technical assistance. This study was funded by a grant from the Russian Science Foundation (No. 14-50-00068) for Elena Komarova, Maxim Shevtsov, Darya Meshalkina, Boris Margulis and Irina Guzhova and by a grant from the Russian Academy of Sciences “Molecular and Cell Biology” Program No 1.7P for Olga Ostroumova and Sergey Abkin.
Abbreviations
- CD
Cluster of differentiation
- CTL
Cytotoxic T lymphocyte
- DAPI
4′,6-Diamidino-2-phenylindole
- DOPE
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine
- DOPS
1,2-Dioleoyl-sn-glycero-3-phospho-l-serine
- ELISA
Enzyme-linked immunosorbent assay
- HSP
Heat-shock protein
- γIFN
Gamma-interferon
- GLUT1 and GLUT2
Glucose transporters of 1 and 2 type
- NK cell
Natural killer cell
- PBS
Phosphate-buffered saline
- PrG-Sepharose
ProteinG-Sepharose
- SDS
Sodium dodecylsulfate
- SE
Standard error
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Ito A, Matsuoka F, Honda H, Kobayashi T. Antitumor effects of combined therapy of recombinant heat shock protein 70 and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Immunol Immunother. 2004;53:26–32. doi: 10.1007/s00262-003-0416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abkin SV, Pankratova KM, Komarova EY, Guzhova IV, Margulis BA. Hsp70 chaperone-based gel composition as a novel immunotherapeutic anti-tumor tool. Cell Stress Chaperones. 2013;18:391–396. doi: 10.1007/s12192-012-0391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shevtsov MA, Pozdnyakov AV, Mikhrina AL, Yakovleva LY, Nikolaev BP, DobrodumovAV Komarova EY, Meshalkina DA, Ischenko AM, Pitkin E, Guzhova IV, Margulis BA. Effective immunotherapy of rat glioblastoma with prolonged intratumoral delivery of exogenous heat shock protein Hsp70. Int J Cancer. 2014;135:2118–2128. doi: 10.1002/ijc.28858. [DOI] [PubMed] [Google Scholar]
- 4.Shevtsov MA, Komarova EY, Meshalkina DA, Bychkova NV, Aksenov ND, Abkin SV, Margulis BA, Guzhova IV. Exogenously delivered heat shock protein 70 displaces its endogenous analogue and sensitizes cancer cells to lymphocytes-mediated cytotoxicity. Oncotarget. 2014;5:3101–3114. doi: 10.18632/oncotarget.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gastpar R, Gross C, Rossbacher L, Ellwart J, Riegger J, Multhoff G. The cell surface-localized heat shock protein 70 epitope TKD induces migration and cytolytic activity selectively in human NK cells. J Immunol. 2004;172:972–980. doi: 10.4049/jimmunol.172.2.972. [DOI] [PubMed] [Google Scholar]
- 6.Murshid A, Gong J, Stevenson MA, Calderwood SK. Heat shock proteins and cancer vaccines: developments in the past decade and chaperoning in the decade to come. Expert Rev Vaccines. 2011;10:1553–1568. doi: 10.1586/erv.11.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geng H, Zhang GM, Xiao H, Yuan Y, Li D, Zhang H, Qiu H, He YF, Feng ZH. HSP70 vaccine in combination with gene therapy with plasmid DNA encoding sPD-1 overcomes immune resistance and suppresses the progression of pulmonary metastatic melanoma. Int J Cancer. 2006;118:2657–2664. doi: 10.1002/ijc.21795. [DOI] [PubMed] [Google Scholar]
- 8.Arispe N, Doh M, Simakova O, Kurganov B, De Maio A. Hsc70 and Hsp70 interact with phosphatidylserine on the surface of PC12 cells resulting in a decrease of viability. FASEB J. 2004;18:1636–1645. doi: 10.1096/fj.04-2088com. [DOI] [PubMed] [Google Scholar]
- 9.Arispe N, De Maio A. ATP and ADP modulate a cation channel formed by Hsc70 in acidic phospholipid membranes. J Biol Chem. 2000;275:30839–30843. doi: 10.1074/jbc.M005226200. [DOI] [PubMed] [Google Scholar]
- 10.Komarova EY, Meshalkina DA, Aksenov ND, Pchelin IM, Martynova E, Margulis BA, Guzhova IV. The discovery of Hsp70 domain with cell-penetrating activity. Cell Stress Chaperones. 2015;20:343–354. doi: 10.1007/s12192-014-0554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen OS, Finkelstein A, Katz I, Cass A. Effect of phloretin on the permeability of thin lipid membranes. J Gen Physiol. 1976;67:749–771. doi: 10.1085/jgp.67.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franklin JC, Cafiso DS. Internal electrostatic potentials in bilayers: measuring and controlling dipole potentials in lipid vesicles. Biophys J. 1993;65:289–299. doi: 10.1016/S0006-3495(93)81051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cseh R, Hetzer M, Wolf K, Kraus J, Bringmann G, Benz R. Interaction of phloretin with membranes: on the mode of action of phloretin at the water-lipid interface. EurBiophys J. 2000;29:172–183. doi: 10.1007/s002490000082. [DOI] [PubMed] [Google Scholar]
- 14.Rokitskaya TI, Antonenko YN, Kotova EA. Effect of the dipole potential of a bilayer lipid membrane on gramicidin channel dissociation kinetics. Biophys J. 1997;73:850–854. doi: 10.1016/S0006-3495(97)78117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luchian T, Mereuta L. Phlorizin- and 6-ketocholestanol-mediated antagonistic modulation of alamethicin activity in phospholipid planar membranes. Langmuir. 2006;22:8452–8457. doi: 10.1021/la0613777. [DOI] [PubMed] [Google Scholar]
- 16.Ostroumova OS, Malev VV, Bessonov AN, Takemoto JY, Schagina LV. Altering the activity of syringomycin E via the membrane dipole potential. Langmuir. 2008;24:2987–2991. doi: 10.1021/la800206v. [DOI] [PubMed] [Google Scholar]
- 17.Ostroumova OS, Malev VV, Ilin MG, Schagina LV. Surfactin activity depends on the membrane dipole potential. Langmuir. 2010;26:15092–15097. doi: 10.1021/la102691y. [DOI] [PubMed] [Google Scholar]
- 18.Ostroumova OS, Efimova SS, Mikhailova EV, Schagina LV. The interaction of dipole modifiers with amphotericin-ergosterol complexes. Effects of phospholipid and sphingolipid membrane composition. Eur Biophys J. 2014;43:207–215. doi: 10.1007/s00249-014-0946-0. [DOI] [PubMed] [Google Scholar]
- 19.Guzhova IV, Lazarev VF, Kaznacheeva AV, Ippolitova MV, Muronetz VI, Kinev AV, Margulis BA. Novel mechanism of Hsp70 chaperone-mediated prevention of polyglutamine aggregates in a cellular model of huntington disease. Hum Mol Genet. 2011;20:3953–3963. doi: 10.1093/hmg/ddr314. [DOI] [PubMed] [Google Scholar]
- 20.Multhoff G, Botzler C, Wiesnet M, Müller E, Meier T, Wilmanns W, Issels RD. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61:272–279. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- 21.Kobori M, Shinmoto H, Tsushida T, Shinohara K. Phloretin-induced apoptosis in B16 melanoma 4A5 cells by inhibition of glucose transmembrane transport. Cancer Lett. 1997;119:207–212. doi: 10.1016/S0304-3835(97)00271-1. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava PK, Udono H, Blachere NE, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 23.Multhoff G, Pfister K, Gehrmann M, Hantschel M, Gross C, Hafner M, Hiddemann W. A 14-mer Hsp70 peptide stimulates natural killer (NK) cell activity. Cell Stress Chaperones. 2001;6:337–344. doi: 10.1379/1466-1268(2001)006<0337:AMHPSN>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calderwood SK, Theriault JR, Gong J. How is the immune response affected by hyperthermia and heat shock proteins? Int J Hyperthermia. 2005;21:713–716. doi: 10.1080/02656730500340794. [DOI] [PubMed] [Google Scholar]
- 25.Arispe N, Doh M, De Maio A. Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp70. Cell Stress Chaperones. 2002;7:330–338. doi: 10.1379/1466-1268(2002)007<0330:LIDTCA>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armijo G, Okerblom J, Cauvi DM, Lopez V, Schlamadinger DE, Kim J, Arispe N, De Maio A. Interaction of heat shock protein 70 with membranes depends on the lipid environment. Cell Stress Chaperones. 2014;19:877–886. doi: 10.1007/s12192-014-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noessner E, Gastpar R, Milani V, Brandl A, Hutzler PJ, Kuppner MC, Roos M, Kremmer E, Asea A, Calderwood SK, Issels RD. Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J Immunol. 2002;169:5424–5432. doi: 10.4049/jimmunol.169.10.5424. [DOI] [PubMed] [Google Scholar]
- 28.Zhu SP, Liu G, Wu XT, Chen FX, Liu JQ, Zhou ZH, Zhang JF, Fei SJ. The effect of phloretin on human γδ T cells killing colon cancer SW-1116 cells. Int Immunopharmacol. 2013;15:6–14. doi: 10.1016/j.intimp.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Kim MS, Kwon JY, Kang NJ, Lee KW, Lee HJ. Phloretin induces apoptosis in H-Ras MCF10A human breast tumor cells through the activation of p53 via JNK and p38 mitogen-activated protein kinase signaling. Ann NY Acad Sci. 2009;1171:479–483. doi: 10.1111/j.1749-6632.2009.04692.x. [DOI] [PubMed] [Google Scholar]
- 30.Cao X, Fang L, Gibbs S, Huang Y, Dai Z, Wen P, Zheng X, Sadee W, Sun D. Glucose uptake inhibitor sensitizes cancer cells to daunorubicin and overcomes drug resistance in hypoxia. Cancer Chemother Pharmacol. 2007;59:495–505. doi: 10.1007/s00280-006-0291-9. [DOI] [PubMed] [Google Scholar]
- 31.Yang KC, Tsai CY, Wang YJ, Wei PL, Lee CH, Chen JH, Wu CH, Ho YS. Apple polyphenol phloretin potentiates the anticancer actions of paclitaxel through induction of apoptosis in human hep G2 cells. Mol Carcinog. 2009;48:420–431. doi: 10.1002/mc.20480. [DOI] [PubMed] [Google Scholar]
- 32.Wu CH, Ho YS, Tsai CY, Wang YJ, Tseng H, Wei PL, Lee CH, Liu RS, Lin SY. In vitro and in vivo study of phloretin-induced apoptosis in human liver cancer cells involving inhibition of type II glucose transporter. Int J Cancer. 2009;124:2210–2219. doi: 10.1002/ijc.24189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.