Abstract
The intratumoral injection of cytokines, in particular IL2, has shown promise for cutaneous melanoma patients with unresectable disease or continuous recurrence despite surgery. We recently reported that the intralesional injection of L19-IL2, an immunocytokine combining IL2 and the human monoclonal antibody fragment L19, resulted in efficient regional control of disease progression, increased time to distant metastasis and evidence of effect on circulating immune cell populations. We have also shown in preclinical models of cancer a remarkable synergistic effect of the combination of L19-IL2 with L19-TNF, a second clinical-stage immunocytokine, based on the same L19 antibody fused to TNF. Here, we describe the results of a phase II clinical trial based on the intralesional administration of L19-IL2 and L19-TNF in patients with stage IIIC and IVM1a metastatic melanoma, who were not candidate to surgery. In 20 efficacy-evaluable patients, 32 melanoma lesions exhibited complete responses upon intralesional administration of the two products, with mild side effects mainly limited to injection site reactions. Importantly, we observed complete responses in 7/13 (53.8 %) non-injected lesions (4 cutaneous, 3 lymph nodes), indicating a systemic activity of the intralesional immunostimulatory treatment. The intralesional administration of L19-IL2 and L19-TNF represents a simple and effective method for the local control of inoperable melanoma lesions, with a potential to eradicate them or make them suitable for a facile surgical removal of the residual mass.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1704-6) contains supplementary material, which is available to authorized users.
Keywords: Melanoma, Intralesional delivery, Immunocytokines, Neoadjuvant, Bystander effect
Introduction
In patients with locally advanced melanoma, i.e., stage IIIB/C or stage IVM1a, intralesional injection of accessible metastases has been shown to represent an attractive therapeutic avenue with distinctive advantages over other approaches [1]. Agents, which have been investigated for the intralesional treatment of melanoma, include bacillus Calmette–Guerin (BCG) and cytokines such as granulocyte–macrophage colony-stimulating factor (GM-CSF) [2], interferon (IFN)-α [3] and interferon-β [4] and interleukin 2 (IL2) [5–9]. In contrast to systemic treatment, the local application of drugs results in higher concentration of active species at the site of disease, leading to an improved therapeutic outcome with fewer systemic side effects. In contrast to surgery, which is normally limited to few operable lesions, intralesional interventional procedures may allow the treatment of diffuse metastatic spread distributed over large anatomical regions.
In the studies of the Garbe group with recombinant IL2 (Proleukin™), 72 melanoma patients in stages IIIB, IIIC and IVM1a received intratumoral injections of the cytokine three times weekly, with a maximum daily dose of 16 million international units (MIU) IL2 or variable, according to the individual tumor burden of patients [6, 7]. New lesions appearing during treatment were also injected, and treatment was continued until all lesions, including those that appeared during treatment, finally regressed. As a consequence, the overall length of treatment varied between 1 and 57 weeks (median 7.5 weeks). Complete responses of injected metastases, according to adapted RECIST (Response Criteria In Solid Tumors) criteria, were observed in more than 60 % of patients [6, 7]. The results of the follow-up study [10] clearly show that patients who mostly benefited, in terms of overall survival, were those in stage IIIB of the disease at the screening visit (86.8 % 5-year survival rate), as opposed to patients in stage IIIC and IVM1a, who only showed a 31.4 and 16.7 % 5-year survival rate, respectively.
In an attempt to improve on this approach, some of us have recently published the first study to address the use of an IL2-based immunocytokine for the intralesional treatment of stage IIIB/C melanoma patients [11]. Immunocytokines are recombinant fusion proteins in which cytokines of interest are expressed C- or N-terminally to antibody fragments and used as delivery vehicles. The antibody moiety favors the preferential accumulation and prolonged residence of the active payload at the site of disease, thus leading to an improved therapeutic activity while sparing normal organs [12]. L19-IL2 is an immunocytokine, consisting of IL2 fused to the monoclonal antibody L19 in diabody format. L19 recognizes the alternatively spliced extra-domain B (EDB) of fibronectin (FN), a marker of tumor angiogenesis [13]. EDB-containing FN is present in the newly formed vasculature of most solid tumors and hematological malignancies [13] but absent from almost all healthy adult tissues (with the exception of tissues of the female reproductive cycle). We reasoned that a targeted form of IL2 injected intralesionally into melanoma metastases would exhibit prolonged residence time in the lesions [14], as compared to the untargeted form, therefore allowing an extended immunological action, reduction in the frequency of administrations and a shorter duration of the treatment.
A complete response of all treated metastases was achieved in 6/24 evaluable patients (25 %), long-lasting in most cases (5 patients ≥24 months). Objective responses were documented in 53.9 % of all index lesions. We also recorded a slower progression to distant metastases in this cohort of patients as compared to historical controls [11].
Preclinical data collected by our group [15] have shown that a combination of L19-IL2 with a second clinical-stage immunocytokine (L19-TNF) in a syngeneic immunocompetent mouse model of cancer induced complete remissions when administered as a single intratumoral injection, whereas the two components did not lead to cures when administered separately. These results have been recently confirmed in two additional mouse models of cancer: the K1735M2 melanoma and Wehi-164 sarcoma. When used alone, immunocytokines did not exhibit complete cures but only tumor growth retardation. However, combination therapies resulted in complete and long-lasting tumor eradication that could not be achieved by conventional chemotherapy with dacarbazine or paclitaxel [14].
These observations, together with the fact that IL2 is an approved drug for the treatment of melanoma and TNF is widely used (although off-label) in melanoma in the ILP setting, provided the rationale for the clinical trial presented in this manuscript. Here, we describe the results of a phase II clinical trial based on the intralesional administration of L19-IL2 in combination with L19-TNF in patients with stage IIIB/C and IVM1a metastatic melanoma. The intralesional administration of two pro-inflammatory immunocytokines facilitated the local control of the disease and mediated a systemic anti-tumor effect in a proportion of non-injected lesions.
Materials and methods
Patients
This multicenter study (ClinicalTrials.gov Identifier: NCT02076633) was run at two clinical centers in Italy and approved by local ethic committees and national authorities. The study was conducted in accordance with the Declaration of Helsinki; all patients were included after signature of informed consent. Most relevant inclusion criteria were an histologically confirmed malignant melanoma of the skin in clinical stage III or stage IVM1a, presence of measurable and injectable cutaneous and/or subcutaneous lesions, male or female, age ≥ 18 years, Eastern Cooperative Oncology Group (ECOG)/World Health Organization (WHO) performance status ≤2, lactate dehydrogenase (LDH) serum level within normal range and a life expectancy of at least 12 weeks. Excluded were patients with uveal melanoma and mucosal melanoma, or those with evidence of visceral or brain metastases, previous or concurrent cancer distinct in primary site/histology or severe cardiac disease (New York Heart Association grade >2) at screening, history of human immunodeficiency virus (HIV) infection or infectious hepatitis B or C, presence of active infections, and pregnant or lactating women. Concurrent treatment for metastatic melanoma was not allowed.
Study design and treatment
The trial was a non-randomized, multicenter, prospective phase II study for patients with malignant melanoma of the skin in clinical stage III or stage IVM1a. Ten MIU of IL2 equivalents of L19-IL2 (Darleukin; Philogen, Siena, Italy) and 312 µg of L19-TNF (Fibromun; Philogen, Siena, Italy) were mixed (4.2 mL final volume) and immediately used for intralesional injection. One milligram of L19-IL2 corresponds to 6 MIU of IL2 equivalents; 312 µg of L19-TNF corresponds to ~100 µg of human recombinant tumor necrosis factor alpha (TNF-α). The maximum tolerated dose (MTD) for systemic administration of TNF-α is ~150–200 µg/m2 [16].
Twenty-two patients were treated with a mixture of L19-IL2 and L19-TNF once weekly for up to 4 weeks. The dose was shared among the lesions via multiple intralesional injections using 30-gauge needles for superficial injections and 27-gauge needles for deep injections. L19-TNF dose could be adjusted between 78 and 312 μg per administration according to size and number of lesions and at the investigator’s discretion. Newly occurring melanoma lesions in the regional field (in-transit metastases) within the 4-week treatment period were also treated as described. For the new lesions, however, the treatment period was not extended beyond the pre-defined 4-week treatment period with a clock start at the time of the first intralesional L19-IL2/L19-TNF injection.
Sonography was used to guide injections of deep soft tissue metastases. Patients received analgesic treatment with acetaminophen or metamizole 1 h before treatment with L19-IL2/L19-TNF and received 0.5–1.0 g acetaminophen 5 or 10 h after injection, as needed.
Tumor assessment was performed 2 weeks before start of treatment (baseline), at week 6 after first injection to check that no visceral metastases had developed under treatment and at week 12. Follow-up for recurrence and survival was performed every 12 weeks thereafter and up to 1 year after first injection. Adverse events were graded according to the Common Toxicity Criteria (version 3).
Response evaluation
Primary objective of this study was the assessment of efficacy of the treatment, measured as rate of patients with complete response (CR) of L19-IL2/L19-TNF-treated lesions at week 12. Secondary objectives included objective response rate [ORR; CR plus partial responses (PR)] and disease control rate [DCR; ORR plus stable disease (SD)] of L19-IL2/L19-TNF-treated lesions at week 12. Rate of patients with CR, PR and SD of all metastases (L19-IL2/L19-TNF-treated and non-treated) at week 12, 24 and 36, duration of ORR and DCR of all metastases and safety of intratumoral administration of L19-IL2/L19-TNF were also secondary objectives of this study. Progression-free survival (defined as time between first injection and occurrence of new skin, lymph node or visceral lesions) and overall survival were assessed, follow up was, however, limited to 1 year after first treatment.
Tumor assessment was performed on a locoregional basis and overall according to RECIST (vs 1.1). However, for the locoregional tumor assessment, the following changes to RECIST applied. All lesions present at baseline and deemed injectable were considered target lesions. These had to be measurable in at least one dimension with one of the following techniques and relative lower limit:
caliper measurement possible by clinical examination (no limit)
digital photography (5 mm)
ultrasound (5 mm)
computerized tomography (CT) or magnetic resonance imaging (MRI) scan (10 mm)
chest X-ray (20 mm).
Distant and untreated lesions were not included in the calculation of the score (sum of the longest diameters of all target lesions). Occurrence before the end of week 4 of new lesions, which were subjected to at least one intralesional injection with L19-IL2/L19-TNF as per protocol, was not considered progressive disease (PD), but these lesions were included in all following tumor assessment calculations. In doubtful cases (e.g., lesions exhibiting residual pigmentation) or after exeresis of any residual eschar, histopathology confirmation of response was conducted on biopsies or resected tissue samples.
Finally, each measurable lesion (both target and non-target) was assessed individually at week 12 and compared to baseline. Responses were classified either as CR (complete disappearance of the lesion) or non-complete responses if the lesion major axis at baseline was ≤5 mm.
Histopathological methods
For hematoxylin and eosin staining, formalin-fixed and paraffin-embedded sections from melanoma lesions were cut at 4 µm, mounted, heated (60 °C, 10 min), dewaxed, rehydrated in alcohol and rinsed in water before hematoxylin and eosin staining.
Immunohistochemistry was performed on 3-micron-thick paraffin sections by established protocols in the Section of Pathological Anatomy, with appropriate positive and negative controls. The following primary antibodies were used: CD8 (Novocastra, Leica Biosystems, Newcastle Upon Tyne, UK; dilution 1: 50); CD4 (NCL-L-CD4-368 Novocastra; dilution 1:80); forkhead box P3 (FOXP3, Ab22510 Abcam, Cambridge, UK; dilution 1:25); and granzyme B (Abcam; dilution 1: 500). Sections were stained automatically (Leica Microsystems, Wetzlar, Germany) according to the manufacturer’s instructions, with Leica Envision detection system. Slides were then counterstained with hematoxylin, dehydrated and mounted with DPX fluid (Sigma-Aldrich, St. Louis, MO).
Results
Patients and treatments
The first patient was enrolled in December 2012, and treatment of the last patient was completed by end of June 2014. Thirty-one patients were screened, and nine were found to meet at least one of the exclusion criteria. Twenty-two patients were enrolled and could be evaluated for safety, 20 patients were evaluable for efficacy (one patient was withdrawn from the study after the second intralesional injection because of grade 3 injection site reaction and one was replaced because effective intralesional injection of L19-IL2/L19-TNF could not be reliably achieved due to previous treatment with electrochemotherapy). Baseline data from the 22 enrolled patients are summarized in Table 1. The median patient age was 64.9 years (range 23.3–79.2 years). Most patients were treated in stage IIIC (18), and four patients were treated in stage IVM1a.
Table 1.
Patients’ characteristics
| Characteristic | No. of patient (n = 22) | Percent |
|---|---|---|
| Sex | ||
| Men | 10 | 45.4 |
| Women | 12 | 54.6 |
| Stage | ||
| IIIC | 18 | 81.8 |
| IVM1a | 4 | 18.2 |
| Site of treated metastases | ||
| Cutaneous | 11 | 50 |
| Subcutaneous | 8 | 36.4 |
| Both | 3 | 13.6 |
| Total No. of treated metastases | ||
| <10 | 19 | 86.4 |
| ≥10 | 3 | 13.6 |
| Previous therapies | ||
| Surgery | 21 | 95.4 |
| Systemic chemotherapy | 9 | 40.9 |
| Radiotherapy | 0 | 0.0 |
| Adjuvant interferon alpha | 3 | 13.6 |
| Immunotherapy | 4 | 18.2 |
| Targeted therapy | 1 | 4.5 |
Apart from surgical resection of the primary tumor, 21 out of 22 patients (95.4 %) had received surgery for melanoma recurrences. Seventeen of these 21 patients had undergone ≥2 previous surgeries, last surgery having been carried out ≤8 weeks before start of L19-IL2/L19-TNF therapy in three of them.
Nine patients out of 22 patients had received no previous systemic treatment for melanoma, six patients had received one line of treatment, two patients had received two (plus adjuvant treatment in one case), and one patient had received three. Four patients had received adjuvant treatment only (Supplementary Table 1). The average number of metastases treated per patient was 5.4 (range 1–14).
Safety
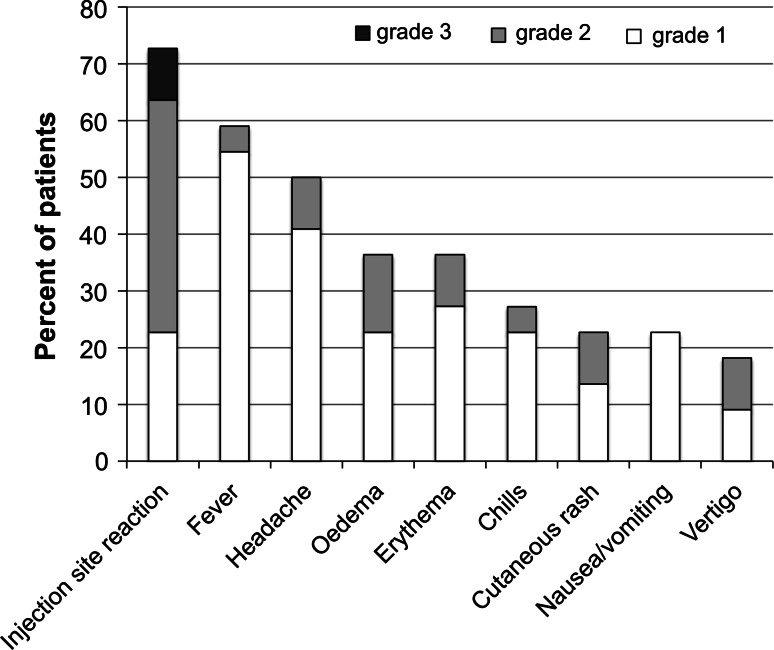
Twenty-two patients were evaluable for safety. Overall, the treatment was well tolerated, with mostly grade 1 and 2 drug-related adverse events recorded. As the only exception, two grade 3 injection site reactions were observed. The first, consisting in pain, erythema and edema at the injection site, occurred after the second administration and led to withdrawal of the patient from the study. The second, consisting in ulceration and necrosis at the injection site, was observed at tumor assessment at week 6 and was resolved by week 12. Intratumoral L19-IL2/L19-TNF therapy caused an inflammatory injection site reaction (local swelling and erythema) in 72.7 % of patients limited to the tumor tissue, followed by a selective tumor necrosis that did not affect the surrounding normal tissue. Injection site pain was present, manageable by application of a local anesthetic cream and oral metamizole. Fifty-nine percent of patients experienced low-grade fever, easily controlled by acetaminophen. Transient, low-grade headache was reported by 50.0 % of patients; edema and erythema (36.4 % of patients each) were also fairly common. These symptoms were usually mild and of short duration. A summary of frequent adverse events is presented in Fig. 1. Other common adverse events, possibly related to the treatment, included chills (27.3 % of patients), rash and nausea/vomiting (22.7 % each), and vertigo (18.2 %). Three patients had diarrhea, and one had a transient allergic reaction. None of the adverse effects recorded was considered serious.
Fig. 1.
Frequency of common adverse events. Percent of patients who experienced the most common adverse events, as related to all safety-evaluable patients, is indicated. For each patient, if an adverse event occurred more than once throughout the study, only the highest-grade adverse event was considered for the calculation
Clinical responses to L19-IL2/L19-TNF treatment
Twenty out of the 22 enrolled patients were evaluable for efficacy.
Among the patients, who could undergo tumor assessment at week 12, one (representing 5 % of efficacy-evaluable population) enjoyed a CR of all L19-IL2/L19-TNF-treated lesions (primary objective). Ten patients showed PR (50 %), while in another five patients the disease at week 12 was assessed to be stable with respect to baseline. Four patients (20 %) were considered to be in PD already at week 6 because of occurrence of new cutaneous lesions after end of treatment period. ORR of treated lesions was 55 %, and DCR of treated lesions was 80 % (secondary objective).
Tumor assessment run adhering to RECIST versus 1.1 criteria revealed that ten evaluable patients (50 %) enjoyed a PR at week 12. The disease remained stable in six patients (30 %), while another four patients (20 %) were considered to be in PD already at week 6 (see above).
Seven patients with PR or SD underwent surgical resection of residual disease soon after tumor assessment at week 12 and were therefore withdrawn from the study. However, in three out of nine patients who could be followed up until end of study, CR could be recorded at tumor assessment performed at week 36 (both by local and RECIST criteria), indicating a long-lasting effect of the intralesional treatment
In the 20 patients evaluable for efficacy, a total of 110 individual lesions were selected by the investigators either as target lesions (n = 69) or as non-target lesions (n = 41) at baseline. One hundred and five out of these 110 lesions could be individually assessed (67 target, 38 non-target), and another five were considered not measurable. A CR was observed for 32 lesions (28.3 %; 21 target and 11 non-target lesions), and a non-complete response was assessed for the remaining 73 lesions (68.1 %; 46 target and 27 non-target lesions). Table 2 details the responses obtained at tumor assessment performed at week 12 (or week 6 for patients who showed PD) in the different lesions, as a function of major axis size at baseline.
Table 2.
Responses in individual lesions as a function of size at screening
| Length (mm) | No. of lesions | Target | Non-target | Injected | Non-injected | Outcome (% of total lesions) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CR | PR | SD | PD | Non-CR | ||||||
| x* > 20 | 9 | 8 | 1 | 9 | 0 | 1 (11.1) | 1 (11.1) | 5 (55.5) | 2 (22.2) | – |
| 10 > x ≥ 20 | 25 | 21 | 4 | 20 | 5 | 9 (36.0) | 4 (16.0) | 12 (48.0) | 0 (0.0) | – |
| 5 > x ≥ 10 | 39 | 32 | 7 | 37 | 2 | 12 (30.8) | 13 (33.3) | 10 (25.6) | 4 (10.2) | – |
| 0 > x ≥ 5 | 32 | 6 | 26 | 26 | 6 | 10 (31.2) | – | – | – | 22 (68.8) |
| Total§ | 105 | 67 | 38 | 92 | 13 | 32 (30.5) | 18 (17.1) | 27 (25.7) | 6 (5.7) | 22 (21.0) |
* x = length of major axis
§Five additional lesions were not measurable at tumor assessment performed at week 12 and were therefore not considered
Examples of different types of lesions, which were injected and responded with different kinetics to the treatment, are illustrated in Supplementary Figure 1. Supplementary Fig. 2 illustrates possible advantages of intralesional treatment with L19-IL2/L19-TNF with respect to repeated surgery of resectable lesions.
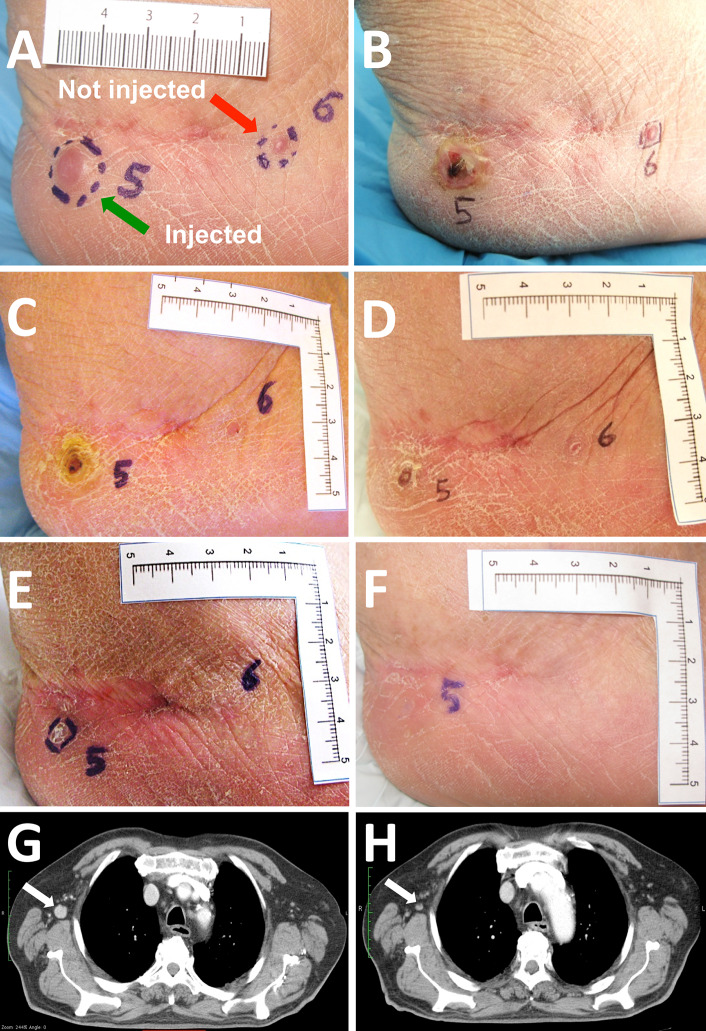
Ninety-seven of the 110 lesions present at baseline in the 20 evaluable patients were injected (including both target and non-target lesions). However, 13 lesions in 6 different patients (2, 1, 4, 2, 2 and 2 lesions per patient, respectively) were not considered injectable by the investigator (again including both target and non-target lesions). The thirteen non-injected lesions included cutaneous metastases (n = 6), subcutaneous metastases (n = 2) and distant lymph nodes (n = 5). We could observe CR in 7 out of 13 lesions (54 %; 4 cutaneous and 3 lymph nodes), a PR in 2 lesions and SD in another 4 lesions. Figure 2 shows examples of systemic bystander effect on both neighboring and distant lesions.
Fig. 2.
Evidence of bystander effect. Example of bystander effect on neighboring, non-injected lesions. Two cutaneous lesions were present at baseline on the right heel of patient 004 (a). Lesion 5 (green arrow) was injected with the immunocytokine combination, while lesion 6 (red arrow) was left uninjected. b To f illustrate the evolution of the two lesions at the end of treatment (day 22, b), week 6 (c), week 12 (d), week 18 (e) and week 24 (f). Both the injected and the not injected lesions appear to have disappeared 24 weeks after first treatment. Example of bystander effect on distant, non-injected lesions. Patient 006 presented at baseline one large cutaneous lesion and four enlarged mediastinal lymph nodes. Only the cutaneous lesion was treated as per protocol. g shows a CT scan of an axillary lymph node measuring 15 × 15 mm at baseline (white arrow). At tumor assessment performed at week 18 (h), the lymph node had shrunk (8 × 6 mm, white arrow). Normalization in size was also observed in two other lymph nodes of the same patient (not shown)
Progression-free survival and overall survival
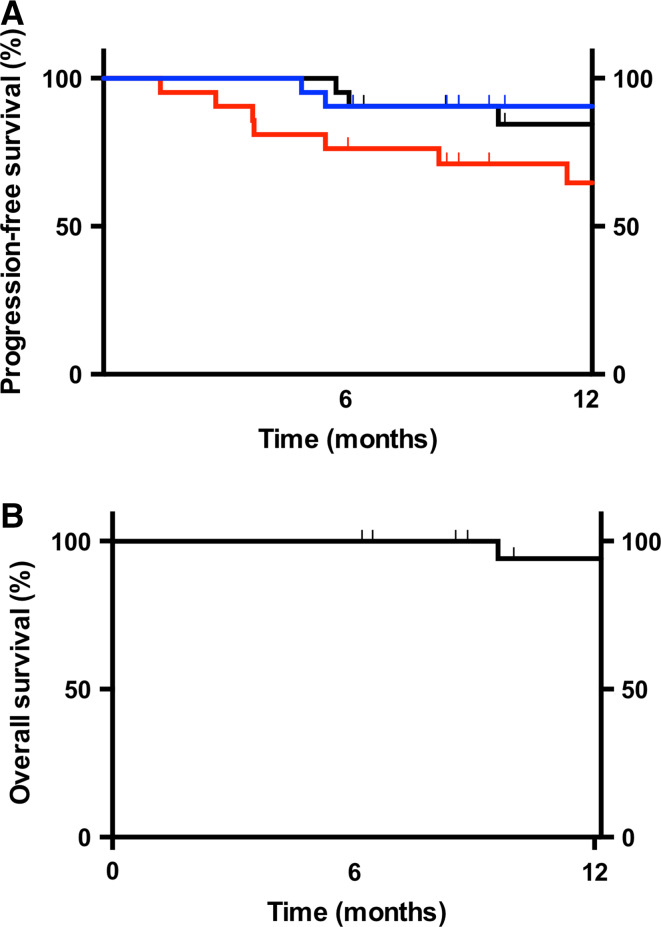
Preliminary data about progression and overall survival were collected in 19 out of 20 efficacy-evaluable patients (one patient was lost at follow-up). Figure 3a shows Kaplan–Meier curves of progression-free survival (PFS) to further cutaneous/subcutaneous, lymph node or distant metastases, respectively. Fifteen patients have already completed the 1-year follow-up period, and another four patients have been censored at the time of last follow-up visit.
Fig. 3.
Progression-free survival and overall survival a Kaplan–Meier plots of progression-free survival to new cutaneous/subcutaneous lesions (red line), lymph nodes (blue line) or distant organs (black line), respectively. b Kaplan–Meier plot of overall survival
This first site of recurrence was to new cutaneous/subcutaneous metastases in seven patients. The disease further progressed to lymph nodes in one out of these seven patients, while in another patient the disease spread to lymph nodes directly, without appearance of new cutaneous lesions. At the moment of writing, the disease had spread to distant organs in 3 out of 19 patients.
Figure 3b shows the overall survival (OS) data for the 19 evaluable patients. One patient died at 292 days after the date of first treatment. The other 18 patients had survived at the time point of last follow-up for periods ranging from 189 to ≥365 days.
Immunohistochemistry
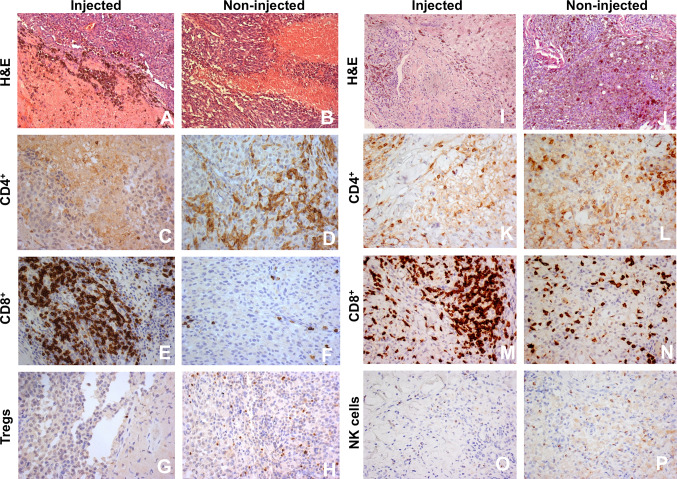
Immunohistochemical staining of biopsies of treated and not-treated lesions or of sections of tissue specimens after surgical exeresis of residual lesions was carried out in several patients with the scope of assessing the degree of necrosis induced by the treatment, presence of residual melanoma cells and eventual presence of infiltrating lymphocytes. Figure 4 shows examples of histological and immunohistochemical findings in two different patients. Hematoxylin/eosin staining of sections from treated lesions shows presence of extensive necrosis and melanophages (panels A and I), while in untreated lesions only small areas of necrosis are apparent (panel B and J). Infiltration by CD8+ T cells is markedly increased in treated lesions (panels E and M), while CD4+, Tregs and NK T cells infiltration does not appear to be significantly modulated by the intralesional treatment with the immunocytokine combination.
Fig. 4.
Immunohistochemical findings in two patients. Hematoxylin/eosin staining of sections from treated (a and i) or untreated (b and j) lesions. Frequency of CD8+ T cells in treated (e and m) as compared to untreated lesions (f and n). CD4+ T cells in treated lesions (c and k) as compared to untreated lesions (d and l). Tregs (g) and NK cells (o) infiltration of treated lesions as compared to untreated lesions (Tregs, h; NK cells, p). Magnification: a, b, i, and j 100×; c–h and k–p, 200×
Discussion
Immunocytokines promise to significantly enhance the therapeutic index of the corresponding cytokine payloads by mediating their preferential accumulation at the site of disease. Compared to more conventional approaches based on systemic or locoregional (e.g., isolated limb perfusion) delivery [17, 18], the intralesional administration of immunocytokines to patients with skin metastases of melanoma may allow to reach a sufficiently high local cytokine concentration which is required for efficacy, while minimizing side effects. The antibody-mediated prolonged residence time of cytokines on the injected lesions [14] is likely to contribute to the observed therapeutic benefit, allowing a reduced number of injections compared to non-targeted cytokines, which are typically injected three times per week for an extended period of time [6, 7].
This study describes, for the first time, the clinical use of a combination of two immunocytokines. The combination of immunostimulatory agents is gaining momentum for the treatment of metastatic conditions (reviewed in [19]). In this case, L19-IL2 and L19-TNF were used for the intralesional treatment of injectable lesions in stage IIIB/C and stage IVM1a metastatic melanoma patients. Treatment was in general well tolerated, with a limited number of drug-related adverse events, mostly of grade ≤2, which were normally rapidly resolved.
In terms of efficacy, 5 % of CR and 50 % of PR were recorded by local assessment of injected metastases at week 12. Although no CR was observed according to RECIST criteria at week 12, PR was recorded in 50 % of patients. However, three patients (who were classified as PR at week 12) enjoyed CR at later tumor assessment (week 36), both by RECIST and local criteria.
Progression and survival data analysis seems to confirm our previous observation [11] that intralesional injection of immunocytokines might be slowing down progression to distant organs.
Intralesional injection of IL2 had previously been shown to induce objective responses in a majority of injected lesions in patients evenly distributed between stages IIIB, IIIC and IV (32, 35 and 33 %, respectively) [6, 7]. Recently published meta-analyses of trials featuring intralesional injection of IL2 (mostly in stage IIIB patients) confirmed a pathologic CR rate ranging between 35 and 50 % [20, 21]. We have previously published that L19-IL2 yielded a somewhat lower ORR in stage IIIB and IIIC melanoma patients [11]. Differences in efficacy between the previous studies (IL2, L19-IL2) and the present study may be explained at least in part with a different composition of the patient populations (17 % stage IIIB and 83 % stage IIIC in the L19-IL2 study; 81 % stage IIIC and 19 % stage IVM1a in the present study), by a different schedule and duration of treatment for the IL2 studies with respect to L19-IL2 and L19-IL2/L19-TNF and by the size of the lesions at baseline, which were typically larger in the L19-IL2/L19-TNF combination trial. Since many patients (7 out of 20 evaluable for efficacy) underwent surgical ablation of residual scabs and were therefore withdrawn from the study after tumor assessment at week 12, it is possible that ORR in the present study may have been underestimated. Furthermore, the time course of responses in the present combination study seems to follow a slower kinetics (3 pathologic CR observed at week 36) as compared to the one recorded during the previous trial with L19-IL2 alone. This effect might be correlated to the onset of the systemic bystander effect discussed below.
The observation of disappearance or reduction in size of 8 out of 12 lesions, which were not injected, suggests that intralesional injection of the L19-IL2 in combination with L19-TNF mediates a systemic anticancer activity. To date, only a few of the several agents used for intralesional treatment of melanoma were found to be effective in inducing, beside local disease control, a systemic response evidenced by the regression of bystander, untreated lesions [22, 23]. Systemic administration of IL2 in vivo augments the activity of cytotoxic T lymphocytes [24] and also induces specific T helper cells, natural killer and lymphokine-activated killer (LAK) cells. On the other hand, systemic administration of TNF activates a number of pathways, which ultimately lead to extravasation of erythrocytes and lymphocytes that provoke hemorrhagic necrosis of the tumor (reviewed in [25]). TNF also targets the tumor-associated vasculature by inducing hyperpermeability and destruction of the vascular lining. In responder patients of the present study, lesions undergo necrosis, accompanied by ulceration and bleeding, which continues even in absence of additional administrations of immunocytokines.
In clinical studies, IL2 treatment was shown to increase the percent of circulating CD4+CD25highFoxp3+ Tregs in both melanoma and renal cell carcinoma patients [26, 27]. Recent studies [28, 29] have shown that myeloid-derived suppressor cells (MDSC) have strong prognostic impact in melanoma patients with distant metastases, and their levels are inversely correlated with the presence of functional antigen-specific T cells and survival. In our previous clinical trial [11], a translational study carried out on peripheral blood mononuclear cell samples (PBMCs) from 11 patients revealed a transient augmentation of Tregs and NK T lymphocytes, as well as a decrease in MDSCs over time and a sustained increase in the absolute number of CD4+ lymphocytes. No significant modulation in the proportion or the absolute number of CD8+ T cells could be detected. In the present study, immunohistochemical analysis of biopsies seems to point to a massive infiltration of CD8+ T cells into treated lesions, which is not apparent in not-treated ones or in lesions that did not respond. CD4+ T cells, Tregs or NK cells do not appear to be substantially increased in the lymphocytic infiltrates of treated lesions.
The combination of tumor cell lysis and a systemic induction/activation of melanoma antigen-specific T cells due to high cytokine concentration after injection might be at the basis of the observed systemic effect on neighboring and distant lesions.
As mentioned above, 40 % of the patients enrolled in this study underwent surgical exeresis of lesion residues after tumor assessment and some of the patients could be rendered NED. Intralesional administration of L19-IL2/L19-TNF reduced local tumor burden and prevented progression of the disease prior to surgery, facilitating planning and management of the patient, gaining time for a more detailed immunohistochemical analysis of treated lesions and allowing in the end a less complex and/or disfiguring surgical intervention. In one case, a giant melanoma metastatic lesion of 11.2 cm in diameter could be substantially reduced, prior to successful surgery (not shown).
The advent of new effective agents against metastatic melanoma is driving a resurgence of the neoadjuvant approach also in localized melanoma, and reports of neoadjuvant therapy with recently approved agents like vemurafenib have recently been published [30–32].
The evaluation of the therapeutic benefit following intralesional administration of immunocytokines to patients with cutaneous melanoma metastases still presents challenges in terms of response assessment and endpoints for pivotal clinical trials. Neither RECIST [33] nor immune-related response criteria (irRC) [34] are well suited to describe tumor responses observed in studies based on intralesional delivery approaches as lesions are often converted into residual scabs of necrotic tissue, which require months before falling off and this may lead to underestimating the real therapeutic benefit for the patient. While it could be argued that “time-to-stage IV” could be used as a meaningful endpoint for the treatment of patients with stage IIIC melanoma, it is reasonable to assume that successful management of cutaneous lesions may provide a tangible benefit also to more advanced stage IV patients, measurable in terms of improved quality of life (lesions and surgery can be disfiguring), facilitated access to treatment (surgery planning can be time-consuming) and immediate control of disease.
In conclusion, intralesional administration of L19-IL2 and L19-TNF represents a simple and effective method for the local control of inoperable lesions in advanced stage melanoma, with a potential to eradicate them or make them suitable for a facile surgical removal of the residual mass.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Support from the EU Seventh Framework Programme “PRIAT” (Profiling Responders In Antibody Therapies), Grant agreement no: 305309, is gratefully acknowledged.
Conflict of interests
D. Neri is co-founder and shareholder of Philogen. R. Danielli, M. Santinami and M. Maio received compensation from the Philogen group for advisory services, patient treatment and/or as coordinating investigators of this trial. The other authors declare no competing interest.
List of abbreviations
- BCG
Bacillus Calmette–Guerin
- CD
Cluster of differentiation
- CR
Complete response
- DCR
Disease control rate
- ECOG
Eastern Cooperative Oncology Group
- EDB
Extra-domain B
- FN
Fibronectin
- FOXP3
Forkhead box P3
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- HIV
Human immunodeficiency virus
- HSV
Herpes simplex virus
- IFN-α
Interferon alpha
- IFN-β
Interferon beta
- IL2
Interleukin-2
- irRC
Immune-related response criteria
- LAK
Lymphokine-activated killer
- LDH
Lactate dehydrogenase
- MIU
Million of international units
- mOS
Median overall survival
- NED
No evidence of disease
- NK
Natural killer
- ORR
Objective response rate
- PBMC
Peripheral blood mononuclear cells
- PD
Progressive disease
- PR
Partial response
- RECIST
Response criteria in solid tumors
- SD
Stable disease
- TNF-α
Tumor necrosis factor alpha
- Tregs
Regulatory T cells
- WHO
World Health Organization
Footnotes
Riccardo Danielli, Roberto Patuzzo, Michele Maio and Mario Santinami contributed equally to the study.
References
- 1.Testori A, Faries MB, Thompson JF, Pennacchioli E, Deroose JP, van Geel AN, Verhoef C, Verrecchia F, Soteldo J. Local and intralesional therapy of in-transit melanoma metastases. J Surg Oncol. 2011;104(4):391–396. doi: 10.1002/jso.22029. [DOI] [PubMed] [Google Scholar]
- 2.Si Z, Hersey P, Coates AS. Clinical responses and lymphoid infiltrates in metastatic melanoma following treatment with intralesional GM-CSF. Melanoma Res. 1996;6(3):247–255. doi: 10.1097/00008390-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 3.von Wussow P, Block B, Hartmann F, Deicher H. Intralesional interferon-alpha therapy in advanced malignant melanoma. Cancer. 1988;61(6):1071–1074. doi: 10.1002/1097-0142(19880315)61:6<1071::AID-CNCR2820610603>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 4.Kubo H, Ashida A, Matsumoto K, Kageshita T, Yamamoto A, Saida T. Interferon-beta therapy for malignant melanoma: the dose is crucial for inhibition of proliferation and induction of apoptosis of melanoma cells. Arch Dermatol Res. 2008;300(6):297–301. doi: 10.1007/s00403-008-0841-6. [DOI] [PubMed] [Google Scholar]
- 5.Gutwald JG, Groth W, Mahrle G. Peritumoral injections of interleukin 2 induce tumour regression in metastatic malignant melanoma. Br J Dermatol. 1994;130(4):541–542. doi: 10.1111/j.1365-2133.1994.tb03397.x. [DOI] [PubMed] [Google Scholar]
- 6.Radny P, Caroli UM, Bauer J, Paul T, Schlegel C, Eigentler TK, Weide B, Schwarz M, Garbe C. Phase II trial of intralesional therapy with interleukin-2 in soft-tissue melanoma metastases. Br J Cancer. 2003;89(9):1620–1626. doi: 10.1038/sj.bjc.6601320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weide B, Derhovanessian E, Pflugfelder A, Eigentler TK, Radny P, Zelba H, Pfohler C, Pawelec G, Garbe C. High response rate after intratumoral treatment with interleukin-2: results from a phase 2 study in 51 patients with metastasized melanoma. Cancer. 2010;116(17):4139–4146. doi: 10.1002/cncr.25156. [DOI] [PubMed] [Google Scholar]
- 8.Boyd KU, Wehrli BM, Temple CL. Intra-lesional interleukin-2 for the treatment of in-transit melanoma. J Surg Oncol. 2011;104(7):711–717. doi: 10.1002/jso.21968. [DOI] [PubMed] [Google Scholar]
- 9.Dehesa LA, Vilar-Alejo J, Valeron-Almazan P, Carretero G. Experience in the treatment of cutaneous in-transit melanoma metastases and satellitosis with intralesional interleukin-2. Actas Dermosifiliogr. 2009;100(7):571–585. doi: 10.1016/S0001-7310(09)71905-2. [DOI] [PubMed] [Google Scholar]
- 10.Weide B, Eigentler TK, Pflugfelder A, et al. Survival after intratumoral interleukin-2 treatment of 72 melanoma patients and response upon the first chemotherapy during follow-up. Cancer Immunol Immunother. 2011;60(4):487–493. doi: 10.1007/s00262-010-0957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weide B, Eigentler TK, Pflugfelder A, et al. Intralesional treatment of stage III metastatic melanoma patients with L19-IL2 results in sustained clinical and systemic immunologic responses. Cancer Immunol Res. 2014;2(7):668–678. doi: 10.1158/2326-6066.CIR-13-0206. [DOI] [PubMed] [Google Scholar]
- 12.Pasche N, Neri D. Immunocytokines: a novel class of potent armed antibodies. Drug Discov Today. 2012;17(11–12):583–590. doi: 10.1016/j.drudis.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Carnemolla B, Borsi L, Balza E, et al. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood. 2002;99(5):1659–1665. doi: 10.1182/blood.V99.5.1659. [DOI] [PubMed] [Google Scholar]
- 14.Pretto F, Elia G, Castioni N, Neri D. Preclinical evaluation of IL2-based immunocytokines supports their use in combination with dacarbazine, paclitaxel and TNF-based immunotherapy. Cancer Immunol Immunother. 2014;63(9):901–910. doi: 10.1007/s00262-014-1562-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwager K, Hemmerle T, Aebischer D, Neri D. The immunocytokine L19-IL2 eradicates cancer when used in combination with CTLA-4 blockade or with L19-TNF. J Invest Dermatol. 2013;133(3):751–758. doi: 10.1038/jid.2012.376. [DOI] [PubMed] [Google Scholar]
- 16.Roberts NJ, Zhou S, Diaz LA, Jr, Holdhoff M. Systemic use of tumor necrosis factor alpha as an anticancer agent. Oncotarget. 2011;2(10):739–751. doi: 10.18632/oncotarget.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eigentler TK, Weide B, de Braud F, et al. A dose-escalation and signal-generating study of the immunocytokine L19-IL2 in combination with dacarbazine for the therapy of patients with metastatic melanoma. Clin Cancer Res. 2011;17(24):7732–7742. doi: 10.1158/1078-0432.CCR-11-1203. [DOI] [PubMed] [Google Scholar]
- 18.Papadia F, Basso V, Patuzzo R, et al. Isolated limb perfusion with the tumor-targeting human monoclonal antibody-cytokine fusion protein L19-TNF plus melphalan and mild hyperthermia in patients with locally advanced extremity melanoma. J Surg Oncol. 2013;107(2):173–179. doi: 10.1002/jso.23168. [DOI] [PubMed] [Google Scholar]
- 19.Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013;19(5):997–1008. doi: 10.1158/1078-0432.CCR-12-2214. [DOI] [PubMed] [Google Scholar]
- 20.Temple-Oberle CF, Byers BA, Hurdle V, Fyfe A, McKinnon JG. Intra-lesional interleukin-2 therapy for in transit melanoma. J Surg Oncol. 2014;109(4):327–331. doi: 10.1002/jso.23556. [DOI] [PubMed] [Google Scholar]
- 21.Hassan S, Petrella T, Zhang T, et al. Pathologic complete response to intralesional interleukin-2 therapy associated with improved survival in melanoma patients with in-transit disease. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-4199-z. [DOI] [PubMed] [Google Scholar]
- 22.Thompson JF, Hersey P, Wachter E. Chemoablation of metastatic melanoma using intralesional Rose Bengal. Melanoma Res. 2008;18(6):405–411. doi: 10.1097/CMR.0b013e32831328c7. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17(3):718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 24.Lotze MT. Biologic therapy with interleukin-2: preclinical studies. In: DeVita VTJ, Hellman S, Rosenberg SA, editors. Biologic therapy of cancer. Philadelphia: Lippincott; 1995. pp. 207–233. [Google Scholar]
- 25.van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11(4):397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- 26.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107(6):2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berntsen A, Brimnes MK, thor Straten P, Svane IM. Increase of circulating CD4+ CD25highFoxp3+ regulatory T cells in patients with metastatic renal cell carcinoma during treatment with dendritic cell vaccination and low-dose interleukin-2. J Immunother. 2010;33(4):425–434. doi: 10.1097/CJI.0b013e3181cd870f. [DOI] [PubMed] [Google Scholar]
- 28.Martens A, Zelba H, Garbe C, Pawelec G, Weide B. Monocytic myeloid-derived suppressor cells in advanced melanoma patients: indirect impact on prognosis through inhibition of tumor-specific T-cell responses? Oncoimmunology. 2014;3(1):e27845. doi: 10.4161/onci.27845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weide B, Martens A, Zelba H, et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin Cancer Res. 2014;20(6):1601–1609. doi: 10.1158/1078-0432.CCR-13-2508. [DOI] [PubMed] [Google Scholar]
- 30.Fadaki N, Cardona-Huerta S, Martineau L, et al. Inoperable bulky melanoma responds to neoadjuvant therapy with vemurafenib. BMJ Case Rep 2012. 2012 doi: 10.1136/bcr-2012-007034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koers K, Francken AB, Haanen JB, Woerdeman LA, van der Hage JA. Vemurafenib as neoadjuvant treatment for unresectable regional metastatic melanoma. J Clin Oncol. 2013;31(16):e251–e253. doi: 10.1200/JCO.2012.45.3845. [DOI] [PubMed] [Google Scholar]
- 32.Kolar GR, Miller-Thomas MM, Schmidt RE, Simpson JR, Rich KM, Linette GP. Neoadjuvant treatment of a solitary melanoma brain metastasis with vemurafenib. J Clin Oncol. 2013;31(3):e40–e43. doi: 10.1200/JCO.2012.43.7061. [DOI] [PubMed] [Google Scholar]
- 33.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.