Abstract
Background
Malignant pleural effusion (MPE) is a common complication caused by malignant diseases. However, subjectivity, poor sensitivity, and substantial false-negative rates of cytology assay hamper accurate MPE diagnosis. The aim of this study was to assess whether CD163+CD14+ tumor-associated macrophages (TAMs) could be used as a biomarker for enabling sensitive and specific MPE diagnosis.
Methods
Pleural effusion samples and peripheral blood samples were collected from 50 MPE patients and 50 non-malignant pleural effusion (NMPE) patients, respectively. Flow cytometry was performed to analyze cell phenotypes, and RT-qPCR was used to detect cytokine expression in these monocytes and macrophages. A blinded validation study (n = 40) was subsequently performed to confirm the significance of CD163+CD14+ TAMs in MPE diagnosis. Student’s t test, rank sum test, and receiver operating characteristic curve analysis were used for statistical analysis.
Results
Notably, CD163+CD14+ cell frequency in MPE was remarkably higher than that in NMPE (P < 0.001). In a blinded validation study, a sensitivity of 78.9 % and a specificity of 100 % were obtained with CD163+CD14+ TAMs as a MPE biomarker. In total (n = 140), by using a cutoff level of 3.65 %, CD163+CD14+ cells had a sensitivity of 81.2 % and a specificity of 100 % for MPE diagnosis. Notably, MPE diagnosis by estimating CD163+CD14+ cells in pleural effusion could be obtained one week earlier than that obtained by cytological examination.
Conclusions
CD163+CD14+ macrophages could be potentially used as an immune diagnostic marker for MPE and has better assay sensitivity than that of cytological analysis.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1701-9) contains supplementary material, which is available to authorized users.
Keywords: Malignant pleural effusion, Tumor-associated macrophage, CD163, Biomarker, Diagnosis
Introduction
Pleural effusion is a common clinical condition caused by malignant tumors, as well as some non-malignant diseases [1–3]. Malignant pleural effusion (MPE) is associated with high cancer-related mortality and morbidity rates and confers a reduced quality of life for patients [4, 5]. A method for accurate MPE diagnosis is required to overcome the limitations of the current diagnostic methods and to determine the best treatment course. Currently, subjectivity in diagnostic methods, poor assay sensitivity, and substantial false-negative rates in cytological examinations hamper early diagnosis of MPE [6]. Hyperplasia of benign mesothelial cells in pleural effusion makes it difficult to distinguish the mesothelial cells from malignant cells and can lead to false-positive results for MPE. Currently, pleural fluid biomarkers cannot be efficiently used to diagnose MPE [7]. Therefore, a novel biomarker is required for an accurate and objective diagnosis of MPE.
The cellular microenvironment of MPE is crucial for the metastasis of malignant tumors [8, 9]. Tumor-infiltrating macrophages are referred to as tumor-associated macrophages (TAMs). Macrophage can differentiate into a classically activated phenotype (M1) or an alternatively activated phenotype (M2) in tumor tissues [10–12]. M1 macrophages are characterized by the expression of pro-inflammatory cytokines, whereas factors produced by alternatively activated M2 macrophages act in favor of tumor progression [13]. Although increased frequency of CD68+ macrophages has been correlated with increased tumor size, lymph node metastasis, and TNM stage in lung carcinomas [14], CD68 is not a specific marker for M2 macrophages [10, 15]. Our present study has confirmed this as well (Supplementary Figure S1). Cell frequencies of CD68+CD163+ cells and CD14+CD163+ cells were assessed, and the data showed that the frequencies were similar (P = 0.10, Supplementary Figure S1). Neither CD68 nor CD14 was determined to be a specific marker for M2 macrophages.
CD163 is a surface receptor on cells of the monocytic lineage, and CD163+CD14+ TAMs have been shown to be a hallmark of the tumor microenvironment and have been associated with poor prognosis in different types of cancer [15, 16]. In ovarian cancer, the expression of CD163 on TAMs in the ascites was inversely associated with relapse-free survival [15]. Additionally, CD163+CD14+ TAMs were closely involved in the progression of adult T cell leukemia/lymphoma [16]. However, the significance of CD163+CD14+ TAMs in MPE has not been reported.
In this study, we analyzed the phenotype and function of CD163+CD14+ TAMs in both MPE and non-malignant pleural effusion (NMPE). The purpose of this study was to determine whether CD163+CD14+ TAMs could be used as an effective and objective biomarker for the identification of MPE.
Materials and methods
Patient demographics and sample collection
One hundred patients with pleural effusion were recruited at The First Affiliated Hospital of Zhengzhou University from May 2011 to December 2013. All samples were obtained with the approval from the ethics committee of the hospital (No. 2011-17). For the MPE group, patients aged 18 years or older that had MPE and were diagnosed with lung cancer proven by histopathological examination of lung biopsy material, and those that did not have diseases of the immune system were included. For the NMPE group, patients with pneumonia, tuberculosis, or heart failure/hypoproteinemia were included. In addition, patients with a history of malignant disease within the last five years and patients with solid organ or bone marrow transplantation were not recruited for the NMPE group. The pleural effusions from all patients were confirmed by cytological analysis in the Department of Pathology (Supplementary Figure S2). Pleural effusion and peripheral blood were collected from 50 patients with lung cancer (Supplementary Table S1). The other 50 patients were previously diagnosed with NMPE (Supplementary Table S2). Additional 40 pleural effusion samples were collected for the blinded validation study (see below). The correlation between clinicopathological characteristics of MPE patients and NMPE patients is shown in Table 1. The results showed that there was no relationship with clinicopathological characteristics between MPE and NMPE (Table 1). We excluded the effects of gender, age, and smoking on the percentage of CD163+CD14+ TAMs in MPE.
Table 1.
Correlation with clinical characteristics between MPE and NMPE (n = 140)
| Pleural effusion | Chi-square values | ||
|---|---|---|---|
| Malignant | Non-malignant | ||
| Gender | |||
| Male | 38 | 39 | 0.0003* |
| Female | 31 | 32 | |
| Age | |||
| ≤60 years | 28 | 37 | 1.436# |
| >60 years | 41 | 34 | |
| Smoking | |||
| Yes | 33 | 26 | 1.372& |
| No | 36 | 45 | |
* P = 0.99 > 0.05 for the comparison of gender in patients with MPE and NMPE; # P = 0.23 > 0.05 for the comparison of age in patients with MPE and NMPE; & P = 0.24 > 0.05 for the comparison of smoking in patients with MPE and NMPE
Isolation of mononuclear cells from pleural effusion and peripheral blood
Mononuclear cells were isolated using Ficoll-Hypaque (Huajing Biology Co., Shanghai) density gradient centrifugation. Briefly, 50–300 ml of pleural effusion collected from each patient was centrifuged at 1500 rpm for 10 min. After the removal of supernatant, the cells were resuspended in 30 ml of normal saline (NS). Each sample of peripheral blood (5 ml) was mixed with 25 ml of NS. Next, 30 ml of resuspended cells from pleural effusion or 30 ml of diluted blood was carefully added to 15 ml of lymphocyte separation medium, and the tubes were then centrifuged at 2500 rpm for 25 min at room temperature. After centrifugation, the interphase containing mononuclear cells was carefully aspirated, washed twice, and finally resuspended with NS for flow cytometry analysis or for isolation of monocytes using magnetic cell sorting system (MACS system, Miltenyi Biotec) as described below.
Flow cytometry analysis
Isolated mononuclear cells (1 × 105 cells) were stained with APC-Cy7-labeled antihuman CD14 antibody, PE-Cy7-labeled antihuman CD3 antibody, and PE-labeled antihuman CD163 antibody (Biolegend). Dead cells were stained using 7-AAD (BD Biosciences). After incubation on ice in the dark for 15 min, the cells were analyzed using FACSCanton II (BD). CD14 was used as a specific monocyte/macrophage marker [17, 18]. Previously, de Vos van Steenwijk et al. [17] isolated CD14 monocytes by using MACS cell separation to induce M2 macrophages, which were shown to belong to the CD163+CD14+ population. In this study, we used antihuman CD163 antibody and antihuman CD14 antibody to label M2 macrophages.
To determine whether CD163 staining enables specific labeling and whether tumor cell autofluorescence influenced the findings, mononuclear cells from pleural effusion and from peripheral blood were stained with human anti-CD45 antibody and human anti-CD163 antibody (Biolegend).
Monocyte isolation
CD14+ cell-enriched population was purified from mononuclear cells by using MACS system. Mononuclear cells were passed through 30-μm nylon mesh to remove cell clumps, which may clog the MACS column. Next, 1 × 107 cells were centrifuged at 300×g for 10 min, and then, the supernatant was aspirated completely. Cells were resuspended in 80 μl of MACS buffer (Miltenyi Biotec), added with 20 μl of CD14 MicroBeads (Miltenyi Biotec), and then incubated in the dark for 15 min at 4 °C. Cell suspension was applied onto the column, and unlabeled cells passed through the column (Supplementary Figure S3A). The percentage of the isolated cells was evaluated using fluorescence-activating cell sorter (FACS) analysis (Supplementary Figure S3B).
CD163+CD14+ and CD163−CD14+ populations were enriched from mononuclear cells derived from MPE by FACS (Beckman, Moflo XDP; n = 6). The mononuclear cells (1 × 108) were added with 20 μl of one of the following antibodies: antihuman CD163 antibody, antihuman CD14 antibody, antihuman CD3 antibody, or antihuman 7-AAD antibody. Next, cells were incubated in the dark for 15 min at 4 °C. Cells were resuspended with 1 ml of NS for sorting. The purities of CD163+CD14+ and CD163−CD14+ cells were analyzed using FACS.
RNA isolation and real-time quantitative PCR
Total RNA extracted from purified CD14+ macrophages by using Trizol reagent (Sigma-Aldrich) was used as the template for performing reverse transcription using cDNA synthesis kit (TaKaRa) according to the manufacturer’s instructions. Primers for real-time PCR were purchased and had the following sequences: human TGF-β, forward, 5′GCCAGAGTGGTTATCTTTTGATG3′ and reverse, 5′AGTGTGTTATCCCTGCTGTCAC3′; human TNF-α, forward, 5′CTGTAGCCCATGTTGTAGCAAAC3′ and reverse, 5′GCTGGTTATCTCTCAGCTCCAC3′; and human iNOS, forward, 5′GCCAAGCTGAAATTGAATGAGGA3′ and reverse, 5′ TTCTGTGCCGGCAGCTTTAAC3′. Real-time PCR was performed using cDNA as the template and using SYBR Premix ExTaq II (TaKaRa) on ABI PRISM 7300 Sequence Detection System (Applied Biosystems). Samples were amplified using the following cycling conditions: 40 cycles of 95 °C for 30 s, 95 °C for 5 s, and 60 °C for 30 s. Expression of each gene was normalized to GAPDH expression, and the data were expressed as relative expression in the form of fold increase ([2−∆CT]), where ∆CT = CT (target gene) − CT (GAPDH) [19].
The mRNA expression of iNOS, TNF-α, arginase-1, IL-10, and TGF-β in the isolated CD163+CD14+ cells and CD163−CD14+ cells was analyzed using real-time PCR described above. The primer sequences used were as follows: arginase-1, forward, 5′TCCCTGTATATCTGCCAAGGATATT3′ and reverse, 5′TTCCTAGTCTGTCCACTTCAGTCAT3′; IL-10, forward, 5′TTTAAGGGTTACCTGGGTTGC3′ and reverse, 5′TTGATGTCTGGGTCTTGGTTC3′.
The mRNA expression of CD14 and CD163 was used as control for the cell phenotypes in both experiments involving cell sorting. Primer sequences used for real-time PCR were as follows: human CD14, forward, 5′ACGCCAGAACCTTGTGAGC3′ and reverse, 5′GCATGGATCTCCACCTCTACTG3′; human CD163, forward, 5′ACTTGAAGACTCTGGATCTGCT3′ and reverse, 5′CTGGTGACAAAACAGGCACTG3′. In addition, the negative and positive controls for assessing mRNA expression were used in the PCR analysis. Water was used as the template for the negative control, and DNA from human peripheral blood mononuclear cells was used as a positive control for performing RT-PCR for TNF-α, IL-10, and TGF-β; DNA from human tumor-infiltrating lymphocytes was used as a positive control for both iNOS and arginase-1.
Blinded validation study
The blinded validation study was performed as previously described by Pass et al. [20]. In the validation study, additional 40 pleural effusion samples were obtained from 19 cancer patients with MPE and 21 cancer-free controls with NMPE with the approval of the ethics committee of our hospital. All of the 40 patients provided signed informed consent. All samples were specially numbered by one investigator. The frequency of CD163+CD14+ TAMs in pleural effusions was measured using flow cytometry, which was performed by another investigator who was not provided the MPE/NMPE patient status for the samples. The results were unblinded and analyzed by a third investigator.
Statistical analysis
Results were analyzed for determining statistical significance by using Chi-squared test and Student’s t test for normal distributions or by using rank sum test for non-normal distributions. The Statistical Program for Social Sciences (SPSS) 17.0 software was used to conduct the analyses. Data were expressed as mean ± SD.
Receiver operating characteristic curve (ROC) analysis, sensitivity, and specificity were also calculated using SPSS 17.0 software.
Results
Characterization of CD14+ monocytes obtained from MPE and NMPE
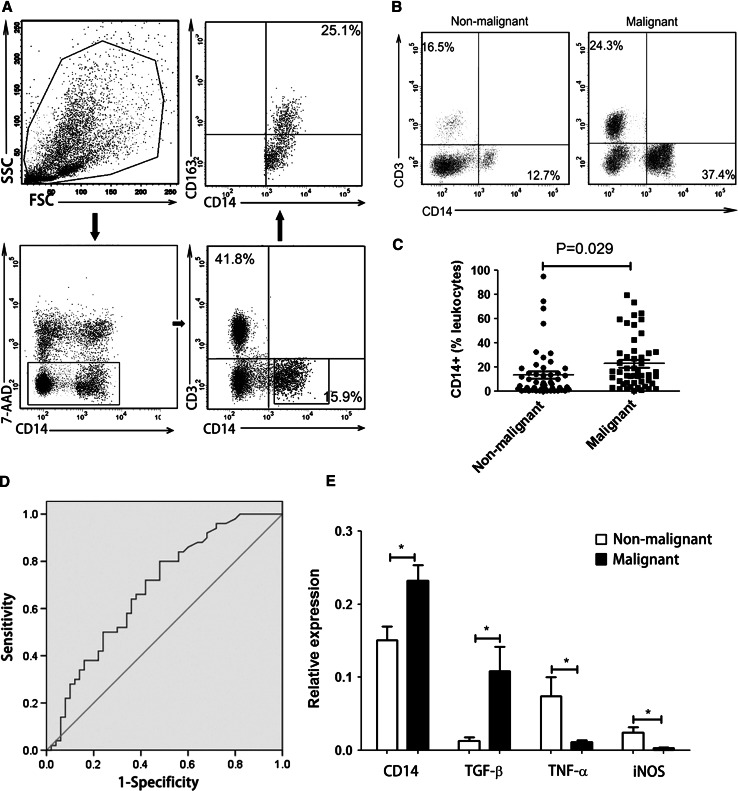
Mononuclear cells harvested from MPE and NMPE patient groups were assessed for characterizing the phenotypes of CD14+ cell populations by using flow cytometry (Fig. 1a, b). The CD14+ cell frequency of the MPE group was significantly higher than that of the NMPE group (MPE: 22.59 ± 21.35 %; NMPE: 13.37 ± 20.27 %; P = 0.029; Fig. 1c). The considerable difference of CD14+ cell frequency between MPE and NMPE groups indicated that CD14+ monocytes might serve as a diagnostic biomarker for MPE. Next, ROC analysis was used to evaluate the diagnostic value of CD14+ monocytes for MPE. The predictive ability of CD14+ cells was expressed as area under curve (AUC). For AUC values that ranged between 0.5 and 0.7, the accuracy of diagnosis was considered poor. For AUC value of 0.685, the increased percentage of CD14+ cells could not be identified as a specific marker for MPE (Fig. 1d).
Fig. 1.
Characterization of CD14+ monocytes derived from MPE and NMPE. a Representative phenotypic analysis of 7-AAD−CD3−CD14+CD163+ cells in pleural effusion by using flow cytometry. b Comparison of CD14+CD3− monocyte frequency in MPE and NMPE by using flow cytometry. One representative analysis from MPE and NMPE cases is shown. c Comparison of CD14+ monocyte frequency in MPE and NMPE. Results presented as a scatter diagram. d ROC analysis of CD14+ cell frequency in MPE with AUC of 0.685. e RT-PCR was performed to assess the relative expression of TGF-β, TNF-α, and iNOS in purified CD14+ cells derived from MPE versus NMPE, normalized by . Results presented as histogram. *P < 0.05
To evaluate the pro- and anti-inflammatory cytokines produced by CD14+ monocytes, we purified CD14+ cells from mononuclear cells using MACS system (Supplementary Figure S3A). The purity of CD14+ cells was higher than 95 % after cell sorting (Supplementary Figure S3B). The high cell purity enabled functional assessment of pleural effusion-derived CD14+ monocytes. The relative expression levels of TGF-β, TNF-α, iNOS, and CD14 in purified CD14+ monocytes from MPE and NMPE were analyzed using real-time quantitative PCR. The CD14 expression in purified CD14+ cells obtained from MPE was significantly higher than that in CD14+ cells obtained from NMPE (P = 0.0034; Fig. 1e). There was also a significant difference in the relative expression of TGF-β in CD14+ monocytes obtained from MPE as compared to that in CD14+ monocytes obtained from NMPE (MPE: 0.109 ± 0.128; NMPE: 0.013 ± 0.017, P = 0.01; Fig. 1e). In addition, the relative expression of TNF-α and iNOS in CD14+ cells from MPE (TNF-α: 0.011 ± 0.010; iNOS: 0.003 ± 0.004) was significantly lower than that in CD14+ monocytes from NMPE (TNF-α: 0.074 ± 0.099, P = 0.03; iNOS: 0.024 ± 0.030, P = 0.02; Fig. 1e). These data indicate that expression of anti-inflammatory factors in CD14+ monocytes from MPE is elevated, whereas the pro-inflammatory factors expression is elevated in CD14+ monocytes from NMPE.
CD163+CD14+ TAMs exhibit a stable anti-inflammatory phenotype
The different expression levels of pro- and anti-inflammatory cytokines in CD14+ monocytes obtained from MPE and NMPE indicate M2-type polarization of CD14+ macrophages obtained from MPE. CD163 has been reported as a specific marker for M2 macrophages [17]. Therefore, we next assessed the pro- and anti-inflammatory cytokines expression in CD163+CD14+ TAMs.
To determine the specificity of CD163 labeling, mononuclear cells were double-stained with human anti-CD45 antibody and anti-CD163 antibody. CD45 expression was used to distinguish leukocytes from tumor cells. The data showed that only CD45+ cells expressed CD163 and that CD45− cells did not show CD163 expression (Supplementary Figure S4). The results indicate that CD163 staining is specific for host-derived immune cells and is not indicative of autofluorescence in tumor cells.
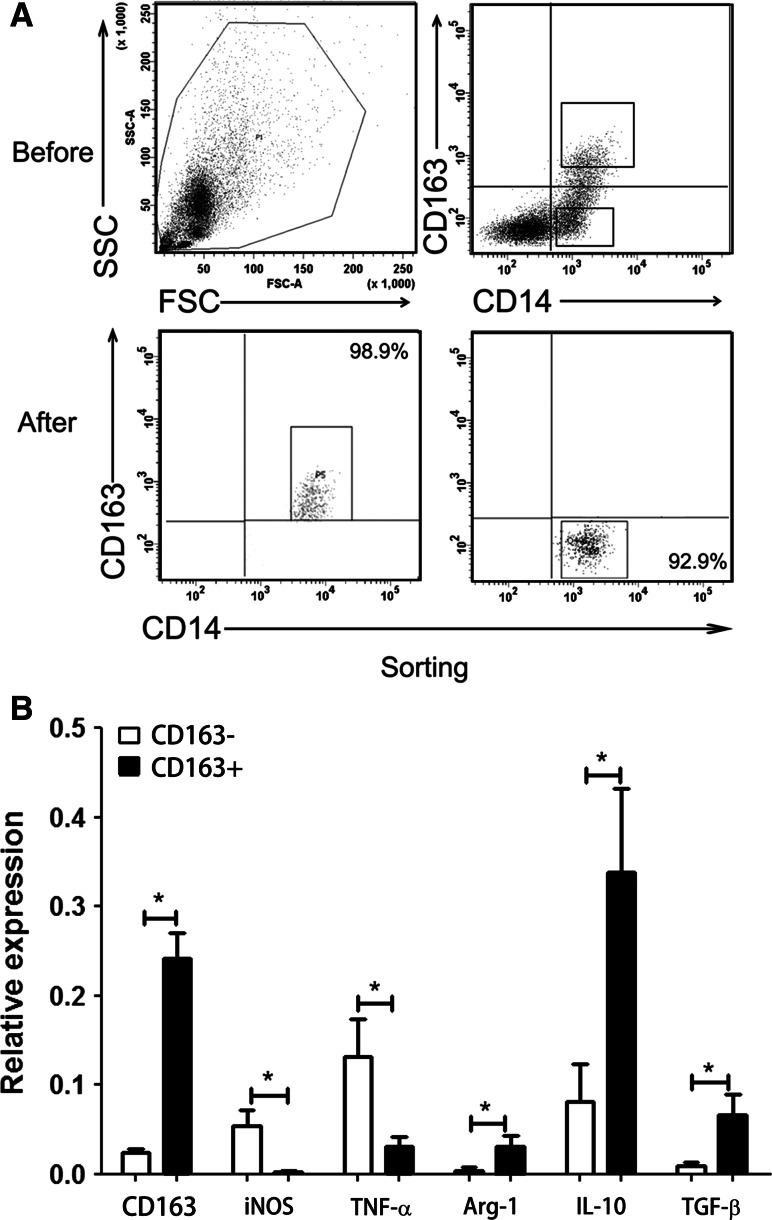
CD163+CD14+ and CD163−CD14+ TAMs isolated from MPE were enriched using FACS (Fig. 2a). We then used RT-qPCR to determine the relative expression levels of iNOS, TNF-α, arginase-1, IL-10, TGF-β, and CD163 in these purified cells. The expression of CD163 in CD163+CD14+ cells from MPE was significantly higher than that in CD163−CD14+ cells (P = 0.0022, Fig. 2b). The expression of both iNOS and TNF-α in CD163+CD14+ TAMs (iNOS: 0.0017 ± 0.0041; TNF-α: 0.0314 ± 0.0259) was significantly lower than that in CD163−CD14+ TAMs (iNOS: 0.0535 ± 0.0433, P = 0.04; TNF-α: 0.1319 ± 0.1021, P = 0.04; Fig. 2b). We also found significant differences in the relative expression of arginase-1, IL-10, and TGF-β in CD163+CD14+ TAMs as compared to that in CD163−CD14+ TAMs (arginase-1: 0.0315 ± 0.0288 vs. 0.0043 ± 0.0088, P = 0.03; IL-10: 0.3382 ± 0.2300 vs. 0.0811 ± 0.1033, P = 0.01; TGF-β: 0.0667 ± 0.0541 vs. 0.0092 ± 0.0085, P = 0.04; Fig. 2b). The results indicate that CD163+CD14+ TAMs (M2 type) from MPE show a suppressor or anti-inflammation phenotype, whereas pro-inflammation cytokines are mainly produced by CD163−CD14+ TAMs.
Fig. 2.
Expression of inflammatory cytokines in CD163+CD14+ and CD163−CD14+ cells. a CD163+CD14+ and CD163−CD14+ cells were purified from MPE using FACS. One representative analysis is shown. b Relative expression of iNOS, TNF-α, arginase-1, IL-10, and TGF-β in CD163+CD14+ cells and CD163−CD14+ cells derived from MPE, normalized by . Results presented as histogram. *P < 0.05
Percentage of CD163+CD14+ TAMs is elevated in MPE
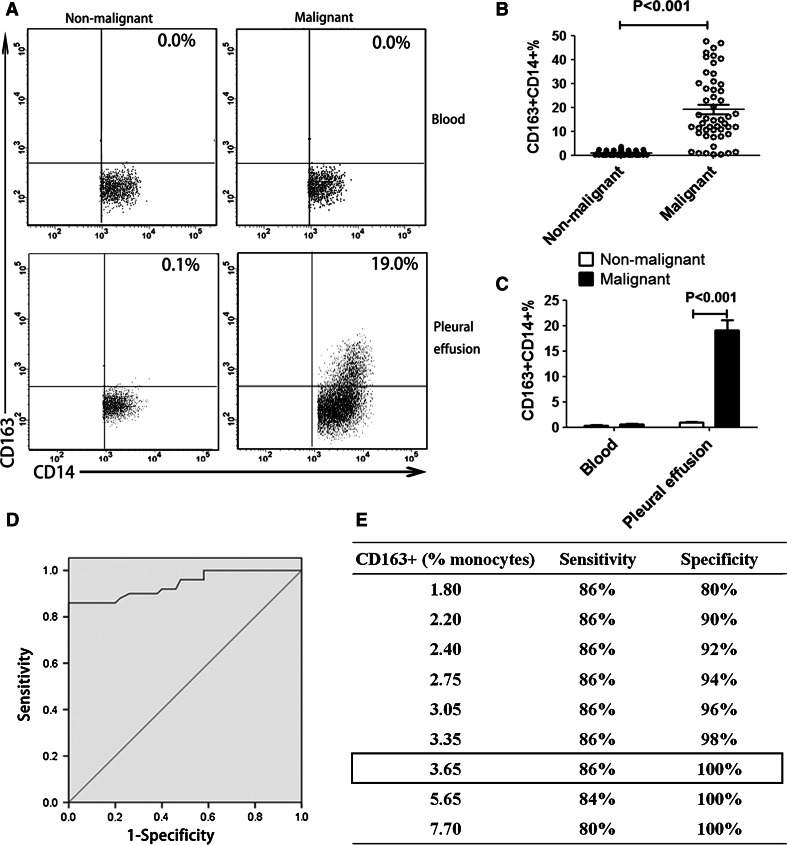
Flow cytometry was used to determine percentages of CD163+CD14+ TAMs in pleural effusion and peripheral blood obtained from patients with or without cancer. The results showed that CD163+CD14+ cell frequency of the MPE group was significantly higher than that of the NMPE group (MPE: 19.08 ± 13.96 %; NMPE: 0.92 ± 0.94 %; P < 0.001, Fig. 3a–c). In contrast, CD163 expression was barely detectable in peripheral blood mononuclear cells obtained from patients with or without cancer (Fig. 3a, c). These data demonstrate that the percentage of CD163+CD14+ TAMs is increased in MPE.
Fig. 3.
CD163+CD14+ cells in pleural effusion or peripheral blood from malignant or non-malignant patients. a CD163+CD14+ cells derived from pleural effusion and peripheral blood were detected in lung cancer patients and non-malignant patients by using flow cytometry. One representative analysis from MPE and NMPE cases is shown. b Comparison of CD163+CD14+ cell frequency in MPE and NMPE was performed. Results are presented as a scatter diagram. c The percentage of CD163+CD14+ cells in pleural effusion or peripheral blood from malignant or non-malignant patients. Results presented as histogram. d ROC analysis of CD163+CD14+ cell frequency in MPE with an AUC of 0.941 (P < 0.001). e Using a cutoff level of 3.65 %, CD163+CD14+ cell frequency-based MPE diagnosis for cancer patients had a sensitivity of 86 % and a specificity of 100 %
ROC analysis
The high level of CD163+CD14+ TAMs in MPE suggests that CD163+CD14+ TAMs could serve as a diagnostic marker for MPE. To evaluate the feasibility of using CD163+CD14+ cells as a diagnostic marker for MPE, ROC analysis was performed. The predictive ability of CD163+CD14+ TAMs was expressed as AUC, with an AUC of 0.941 [95 % confidence interval (CI), 0.895–0.987; P < 0.001; Fig. 3d]. The optimal cutoff points used were the peaks of the curve, where the sum of sensitivity and specificity is at maximum. Using a cutoff level of 3.65 %, a specificity of 100 % and a sensitivity of 86 % were achieved for using CD163+CD14+ macrophages as a biomarker for MPE (Fig. 3e).
Blinded validation study
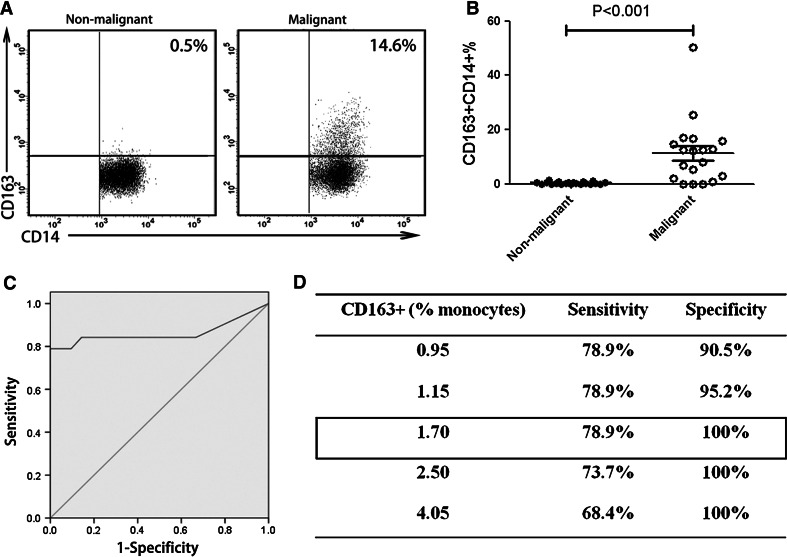
To further confirm the sensitivity and specificity of using CD163+CD14+ macrophages as a diagnostic marker for MPE, we performed a blinded validation study using pleural effusion samples collected from our hospital. The CD163+CD14+ macrophage frequency in MPE was significantly higher than that in NMPE (MPE: 11.357 ± 11.875 %; NMPE: 0.352 ± 0.387 %; P < 0.001; Fig. 4a, b). ROC analysis was performed, and AUC of 0.862 was obtained (95 % CI, 0.721–1.000; P < 0.001; Fig. 4c). At a specificity of 100 %, a sensitivity of 78.9 % was achieved under a cutoff level of 1.70 % (Fig. 4d).
Fig. 4.
Blinded validation study of CD163+CD14+ macrophages in MPE. a Phenotype analysis of CD163+CD14+ macrophages obtained from MPE and NMPE was performed. One representative analysis from MPE and NMPE cases is shown. b Comparison of CD163+CD14+ cell frequency in MPE and NMPE was performed. Results presented as a scatter diagram. c ROC analysis of CD163+CD14+ cell frequency was performed with an AUC of 0.862 (P < 0.001). d Using a cutoff level of 1.70 %, a specificity of 100 % and a sensitivity of 78.9 % were achieved
CD163+CD14+ TAMs as a potential diagnostic marker for MPE
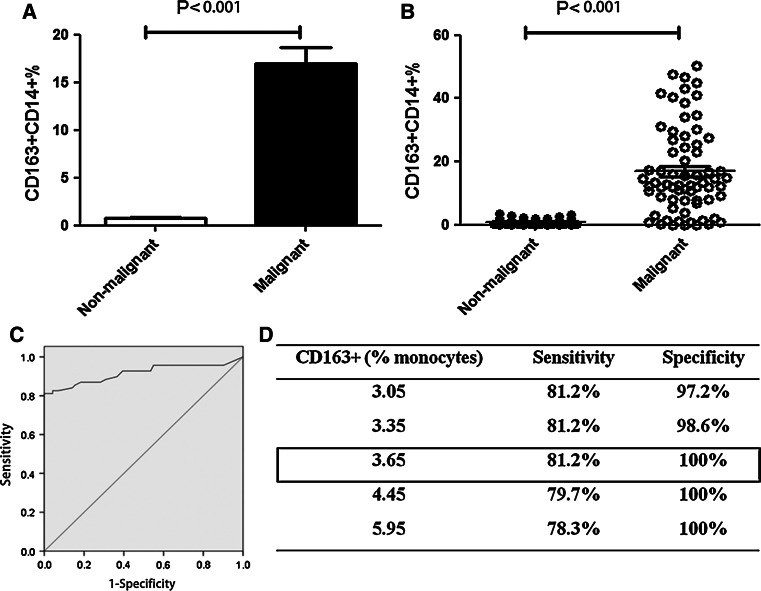
The percentage of CD163+CD14+ cells was tested in a total of 140 pleural effusion samples, which included 69 MPE and 71 NMPE. Of the 140, 100 were non-blinded pleural effusions, and 40 were blinded pleural effusions. These samples were analyzed together. We found that the CD163+CD14+ cell frequency in MPE (16.959 ± 13.782 %) was significantly higher than that in NMPE (0.751 ± 0.851 %; P < 0.001; Fig. 5a, b). High percentage of CD163+CD14+ cells was identified as a specific marker for MPE in patients with lung cancer to distinguish the subjects from NMPE, with an AUC of 0.916 (95 % CI, 0.895–0.987; P < 0.001; Fig. 5c). Using a cutoff level of 3.65 %, CD163+CD14+ cells had a sensitivity of 81.2 % and a specificity of 100 % in the diagnosis of cancer patients with MPE (Fig. 5d). These results indicate that CD163+CD14+ macrophages could be used as a highly sensitive and specific marker for MPE diagnosis in clinic.
Fig. 5.
CD163+CD14+ cell frequency in MPE and NMPE from total 140 cases. Histogram (a) and scatter diagram (b) of CD163+CD14+ cell frequency in MPE (n = 69) and NMPE (n = 71). c ROC analysis was performed with an AUC of 0.916 (P < 0.001). d Using a cutoff level of 3.65 %, the percentage of CD163+CD14+ cells was used to predict MPE prevalence, and the assay had a sensitivity of 81.2 % and a specificity of 100 %
Pleural effusions with high percentage of CD163+CD14+ cells are confirmed to be MPE by cytological analysis
In this study, first-visit patients with pleural effusion were analyzed using both cytological examination and flow cytometry concurrently. Twenty pleural effusions, of 140 cases, were determined to be primarily negative for malignancy by using cytological analysis (Supplementary Figure S5A). However, high-level expression of CD163 in CD14+ cells was detected by flow cytometry in pleural effusion from 20 cases (Supplementary Figure S5B). Next, these patients were further tested by performing cytological examination for a second time. A third cytological analysis was performed if the second examination yielded negative results. A sample was determined to be negative after three separate tests without the presence of malignant cells in the pleural effusion. The interval between consecutive cytological analyses was more than 3 days for patients with pleural effusion. Finally, malignant cell assessment for pleural effusion samples was conducted by performing cytological examination in our Department of Pathology (Supplementary Table S3, Supplementary Figure S5C). The data indicate that the sensitivity of CD163+CD14+ macrophage frequency assessment for diagnosis of MPE is better than that of cytological analysis.
Discussion
It has been well documented that various immune cells, including macrophages, infiltrate tumor tissue. With the emerging novel concept of macrophage differentiation into M1 and M2 phenotypes, the role of TAMs in cancer development and progression is gradually becoming clearer. Specifically, in human malignant tumors, M2 macrophages can act as “protumoral macrophages” and become involved in angiogenesis, immunosuppression, and activation of tumor cells [21–24].
CD163 is a surface receptor expressed on cells of monocytic lineage and has been shown to have sparse expression on peripheral blood monocytes, but is abundantly expressed on a majority of tumor-derived macrophages [25–27]. CD163+CD14+ TAMs are considered as “protumoral” macrophages. In lung tumor sections, the number of CD163+CD14+ cells is higher in malignant lesions than in benign lesions and correlates with histological grading of malignancy [28, 29]. Behnes et al. [30, 31] found that nearly all macrophages in papillary renal cell carcinoma (RCC) type II expressed CD163, whereas less than 30 % of macrophages expressed CD163 in type I papillary RCC. This may help explain the poor prognosis of papillary RCC type II as compared to that of type I. However, Komohara et al. [21] showed that CD163+ cells were in fact CD68+ TAMs by performing double immunostaining and also showed that CD68 is not a specific marker for M2 macrophages. Helm et al. [32] also confirmed these findings. Consistent with these results, we showed that neither CD68 nor CD14 is a specific marker for M2 macrophages (Supplementary Figure S1). Furthermore, high levels of anti-inflammatory cytokines and low levels of pro-inflammatory cytokines in CD163+CD14+ cells indicate that CD163+CD14+ cells are M2 macrophages.
Accurate and early diagnosis of MPE is important and critical for immediate intervention and better prognosis [33, 34]. Current methods of MPE diagnosis include imaging, cytological analysis, biomarker detection, needle pleural biopsy, and thoracoscopy [35, 36]. However, the false-negative rate is substantially high for these MPE diagnosis methods [37, 38]. Even though pleural effusion plays a crucial role in the spread of lung cancer, the contribution of cellular components in MPE is poorly understood. The role of CD163+CD14+ macrophages in the diagnosis of MPE is poorly characterized [39]. Therefore, in this study, we assessed the sensitivity and specificity of using CD163+CD14+ macrophages for diagnosis of MPE. The pleural effusions used in this study were derived from lung cancer patients. MPE induced by other types of cancer will be evaluated in our future studies.
Previously, Mundt et al. [40] found that soluble syndecan-1 was a promising candidate biomarker with a sensitivity of 74.9 % and a specificity of 61.1 % for the cytopathological diagnosis and prognostication of MPE, using enzyme-linked immunosorbent assays. Xu et al. [41] reported that measurement of IL-17 levels by using enzyme-linked immunosorbent assays might be a useful diagnostic test for MPE with a sensitivity of 79.5 % and a specificity of 91.1 %. Our study showed that flow cytometry-based detection of CD163+CD14+ cell frequency in pleural effusion samples had a sensitivity of 81.2 % and a specificity of 100 %. Thus, our assay has a higher sensitivity and specificity, and CD163+CD14+ TAMs may serve as a reliable marker for diagnosis of MPE. The correlation of CD163+CD14+ TAM frequency with soluble syndecan-1 or IL-17 for MPE diagnosis was not assessed in this study. Further investigations are required to determine whether assessment of a combination of CD163+CD14+ TAMs and soluble syndecan-1 or IL-17 for MPE diagnosis may have higher assay sensitivity, specificity, and reliability.
In this study, we found that 20 patients that were primarily diagnosed with NMPE showed high frequency of CD163+CD14+ cells in pleural effusion. Notably, these 20 patients were later diagnosed with MPE after performing second or third tumor cytological analysis. In support of using CD163+CD14+ cells as marker for MPE diagnosis, there have been a number of clinical cases that although had undetectable tumor cells, showed prevalence of CD163+CD14+ macrophages in pleural effusion, and were then diagnosed with lung cancer through bronchoscopy or lung biopsy assessment. Moreover, MPE patients presented high percentage of MPE-derived CD163+CD14+ macrophages in this study. Taken together, our data indicate that the diagnosis of MPE by performing tumor cytological analysis has low sensitivity and that the sensitivity of CD163+CD14+ cell detection appears to be superior to tumor cytological analysis for definitive diagnosis.
In this study, there were six MPE cases which the percentage of CD163+CD14+ TAMs in MPE was lower than the cutoff value of 3.65 %. Furthermore, we analyzed the relationship between CD163+CD14+ TAMs percentage and patient survival. The results showed that high percentage of CD163+CD14+ TAMs in MPE was closely correlated with poor progression-free survival rates (unpublished data). In the follow-up, all six cases with low level of CD163+CD14+ TAMs exhibited higher survival rates and better prognosis. Therefore, we propose that CD163+CD14+ TAMs could serve as a potential prognostic biomarker for MPE.
We also found that the percentage of CD3+ cells in MPE was higher than that in NMPE. Gong et al. showed that Tc17 cells may have a protective role in patients with MPE [42]. The higher frequency of Tc17 cells may serve as a biomarker for the prognosis of patients with MPE. We speculate that the higher numbers of CD3+ cells may be attributed to increased Tc17 infiltration. However, we did not focus on CD3+ cells in this study, and the relationship between Tc17 cells and CD163+CD14+ TAMs in MPE is unclear. We plan to further dissect this potential relationship in our future studies.
Recently, increasing research interest has developed for studying other myeloid populations in peripheral blood derived from MPE patients. Gonda et al. [43] reported that myeloid-derived suppressor cell (MDSC) numbers in malignant effusions altered with the number of MDSCs in peripheral blood and also changed in patients with clinical responses. Romano et al. [44] demonstrated that circulating MDSC numbers correlated with clinical outcome of Hodgkin lymphoma patients treated up front with a risk-adapted strategy. In this study, we have not focused on this cell population; however, it appears that MDSC numbers might provide valuable diagnostic information.
Our results indicate that MPE is indicative of an immunosuppressive state. Thus, treatments that involve “re-education” of CD163+CD14+ TAMs could be considered for palliative management of malignant effusion. Animal studies have shown that specific inhibition of NF-κB activity in TAMs was able to reverse their tumor-polarized phenotype and tumoricidal activity through IL-12-dependent NK cell recruitment [45]. Taken together, TAMs may be a promising therapeutic target for cancer treatment, and CD163+CD14+ macrophages might serve as a molecular target for treating MPE patients.
Conclusions
Taken together, a comparative analysis of phenotype and inflammatory gene expression of CD163+CD14+ macrophages obtained from MPE and NMPE can be used for distinguishing MPE from NMPE. Thus, CD163+CD14+ macrophages in pleural effusion is likely a promising immune diagnostic marker for lung cancer-associated MPE.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81171986, 81271815, 812111102), Research grant from the Ministry of Public Health (Grant No. 20110110001), the Basic and Advanced Technology Research Foundation from Science and Technology Department of Henan Province (Grant Nos. 112300410153, 122300410155), Funds for Creative Research Team of Henan Province, Creative Research Team of Higher Education of Henan Province, and the Innovation Team of The First Affiliated Hospital of Zhengzhou University.
Conflict of interest
All authors declare that they have no conflict of interest.
Abbreviations
- AUC
Area under curve
- CI
Confidence interval
- FACS
Fluorescence-activating cell sorter
- MACS
Magnetic cell sorting
- MDSC
Myeloid-derived suppressor cells
- MPE
Malignant pleural effusion
- NMPE
Non-malignant pleural effusion
- NS
Normal saline
- RCC
Renal cell carcinoma
- ROC
Receiver operating characteristic curve
- SPSS
Statistical program for social sciences
- TAMs
Tumor-associated macrophages
Footnotes
Fei Wang and Li Yang have contributed equally to this work.
Change history
8/4/2021
A Correction to this paper has been published: 10.1007/s00262-021-03023-1
References
- 1.Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc. 2008;83:235–250. doi: 10.1016/S0025-6196(11)60848-3. [DOI] [PubMed] [Google Scholar]
- 2.Stathopoulos GT. Translational advances in pleural malignancies. Respirology. 2011;16:53–63. doi: 10.1111/j.1440-1843.2010.01890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra EK, Davies RJ. Advances in the investigation and treatment of pleural effusions. Expert Rev Respir Med. 2010;4:123–133. doi: 10.1586/ers.09.67. [DOI] [PubMed] [Google Scholar]
- 4.Morgensztern D, Waqar S, Subramanian J, Trinkaus K, Govindan R. Prognostic impact of malignant pleural effusion at presentation in patients with metastatic non-small-cell lung cancer. J Thorac Oncol. 2012;7:1485–1489. doi: 10.1097/JTO.0b013e318267223a. [DOI] [PubMed] [Google Scholar]
- 5.Salah S, Tanvetyanon T, Abbasi S. Metastatectomy for extra-cranial extra-adrenal non-small cell lung cancer solitary metastases: systematic review and analysis of reported cases. Lung Cancer. 2012;75:9–14. doi: 10.1016/j.lungcan.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Rakha EA, Patil S, Abdulla K, Abdulkader M, Chaudry Z, Soomro IN. The sensitivity of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Diagn Cytopathol. 2010;38:874–879. doi: 10.1002/dc.21303. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Panadero F, Romero-Romero B. Current and future options for the diagnosis of malignant pleural effusion. Expert Opin Med Diagn. 2013;7:275–287. doi: 10.1517/17530059.2013.786038. [DOI] [PubMed] [Google Scholar]
- 8.Basak SK, Veena MS, Oh S, Huang G, Srivatsan E, Huang M, Sharma S, Batra RK. The malignant pleural effusion as a model to investigate intratumoral heterogeneity in lung cancer. PLoS ONE. 2009;4:e5884. doi: 10.1371/journal.pone.0005884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stathopoulos GT, Kalomenidis I. Malignant pleural effusion: tumor-host interactions unleashed. Am J Respir Crit Care Med. 2012;186:487–492. doi: 10.1164/rccm.201203-0465PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta. 2009;1796:11–18. doi: 10.1016/j.bbcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Rogers TL, Holen I. Tumour macrophages as potential targets of bisphosphonates. J Transl Med. 2011;9:177. doi: 10.1186/1479-5876-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edin S, Wikberg ML, Dahlin AM, Rutegard J, Oberg A, Oldenborg PA, Palmqvist R. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS ONE. 2012;7:e47045. doi: 10.1371/journal.pone.0047045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Zhu YB, Shen Y, Zhu YH, Zhang XG, Huang JA. Increase of circulating B7-H4-expressing CD68+ macrophage correlated with clinical stage of lung carcinomas. J Immunother. 2012;35:354–358. doi: 10.1097/CJI.0b013e31824212c4. [DOI] [PubMed] [Google Scholar]
- 15.Komohara Y, Niino D, Saito Y, Ohnishi K, Horlad H, Ohshima K, Takeya M. Clinical significance of CD163(+) tumor-associated macrophages in patients with adult T-cell leukemia/lymphoma. Cancer Sci. 2013;104:945–951. doi: 10.1111/cas.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinartz S, Schumann T, Finkernagel F, Wortmann A, Jansen JM, Meissner W, Krause M, Schworer AM, Wagner U, Muller-Brusselbach S, Muller R. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: correlation of CD163 expression, cytokine levels and early relapse. Int J Cancer. 2014;134:32–42. doi: 10.1002/ijc.28335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Vos van Steenwijk PJ, Ramwadhdoebe TH, Goedemans R, Doorduijn EM, van Ham JJ, Gorter A, van Hall T, Kuijjer ML, van Poelgeest MI, van der Burg SH, Jordanova ES. Tumor-infiltrating CD14-positive myeloid cells and CD8-positive T-cells prolong survival in patients with cervical carcinoma. Int J Cancer. 2013;133:2884–2894. doi: 10.1002/ijc.28309. [DOI] [PubMed] [Google Scholar]
- 18.Barros MH, Hassan R, Niedobitek G. Tumor-associated macrophages in pediatric classical Hodgkin lymphoma: association with Epstein–Barr virus, lymphocyte subsets, and prognostic impact. Clin Cancer Res. 2012;18:3762–3771. doi: 10.1158/1078-0432.CCR-12-0129. [DOI] [PubMed] [Google Scholar]
- 19.Obermajer N, Wong JL, Edwards RP, Chen K, Scott M, Khader S, Kolls JK, Odunsi K, Billiar TR, Kalinski P. Induction and stability of human Th17 cells require endogenous NOS2 and cGMP-dependent NO signaling. J Exp Med. 2013;210:1433–1445. doi: 10.1084/jem.20121277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pass HI, Levin SM, Harbut MR, Melamed J, Chiriboga L, Donington J, Huflejt M, Carbone M, Chia D, Goodglick L, Goodman GE, Thornquist MD, Liu G, de Perrot M, Tsao MS, Goparaju C. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N Engl J Med. 2012;367:1417–1427. doi: 10.1056/NEJMoa1115050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014;105:1–8. doi: 10.1111/cas.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirabe K, Mano Y, Muto J, Matono R, Motomura T, Toshima T, Takeishi K, Uchiyama H, Yoshizumi T, Taketomi A, Morita M, Tsujitani S, Sakaguchi Y, Maehara Y. Role of tumor-associated macrophages in the progression of hepatocellular carcinoma. Surg Today. 2012;42:1–7. doi: 10.1007/s00595-011-0058-8. [DOI] [PubMed] [Google Scholar]
- 23.Pettersen JS, Fuentes-Duculan J, Suarez-Farinas M, Pierson KC, Pitts-Kiefer A, Fan L, Belkin DA, Wang CQ, Bhuvanendran S, Johnson-Huang LM, Bluth MJ, Krueger JG, Lowes MA, Carucci JA. Tumor-associated macrophages in the cutaneous SCC microenvironment are heterogeneously activated. J Invest Dermatol. 2011;131:1322–1330. doi: 10.1038/jid.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, Zazzeroni F, Alesse E. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013:187204. doi: 10.1155/2013/187204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walter RB, Bachli EB, Schaer DJ, Ruegg R, Schoedon G. Expression of the hemoglobin scavenger receptor (CD163/HbSR) as immunophenotypic marker of monocytic lineage in acute myeloid leukemia. Blood. 2003;101:3755–3756. doi: 10.1182/blood-2002-11-3414. [DOI] [PubMed] [Google Scholar]
- 26.Garcia C, Gardner D, Reichard KK. CD163: a specific immunohistochemical marker for acute myeloid leukemia with monocytic differentiation. Appl Immunohistochem Mol Morphol. 2008;16:417–421. doi: 10.1097/PAI.0b013e31815db477. [DOI] [PubMed] [Google Scholar]
- 27.Lau SK, Chu PG, Weiss LM. CD163: a specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol. 2004;122:794–801. doi: 10.1309/QHD6YFN81KQXUUH6. [DOI] [PubMed] [Google Scholar]
- 28.Chung FT, Lee KY, Wang CW, Heh CC, Chan YF, Chen HW, Kuo CH, Feng PH, Lin TY, Wang CH, Chou CL, Chen HC, Lin SM, Kuo HP. Tumor-associated macrophages correlate with response to epidermal growth factor receptor-tyrosine kinase inhibitors in advanced non-small cell lung cancer. Int J Cancer. 2012;131:E227–E235. doi: 10.1002/ijc.27403. [DOI] [PubMed] [Google Scholar]
- 29.Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Behnes CL, Bremmer F, Hemmerlein B, Strauss A, Strobel P, Radzun HJ. Tumor-associated macrophages are involved in tumor progression in papillary renal cell carcinoma. Virchows Arch. 2014;464:191–196. doi: 10.1007/s00428-013-1523-0. [DOI] [PubMed] [Google Scholar]
- 31.Santoni M, Massari F, Amantini C, Nabissi M, Maines F, Burattini L, Berardi R, Santoni G, Montironi R, Tortora G, Cascinu S. Emerging role of tumor-associated macrophages as therapeutic targets in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2013;62:1757–1768. doi: 10.1007/s00262-013-1487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helm O, Held-Feindt J, Grage-Griebenow E, Reiling N, Ungefroren H, Vogel I, Kruger U, Becker T, Ebsen M, Rocken C, Kabelitz D, Schafer H, Sebens S. Tumor-associated macrophages exhibit pro- and anti-inflammatory properties by which they impact on pancreatic tumorigenesis. Int J Cancer. 2014;135:843–861. doi: 10.1002/ijc.28736. [DOI] [PubMed] [Google Scholar]
- 33.Lim MH, Garrettc J, Mowlem L, Yap E. Diagnosing malignant pleural effusions: how do we compare? N Z Med J. 2013;126:42–48. [PubMed] [Google Scholar]
- 34.Gorgun D, Secik F, Midilli K, Akkaya V, Yildiz P. Diagnostic and prognostic significance of survivin levels in malignant pleural effusion. Respir Med. 2013;107:1260–1265. doi: 10.1016/j.rmed.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Neumann V, Loseke S, Nowak D, Herth FJ, Tannapfel A. Malignant pleural mesothelioma: incidence, etiology, diagnosis, treatment, and occupational health. Dtsch Arztebl Int. 2013;110:319–326. doi: 10.3238/arztebl.2013.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rieux C, Boisdron-Celle M, Morel A, Fey L, Urban T, Hureaux J. Biological diagnosis of resistance to erlotinib in a malignant pleural effusion. Rev Mal Respir. 2013;30:572–575. doi: 10.1016/j.rmr.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Johnston WW. The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer. 1985;56:905–909. doi: 10.1002/1097-0142(19850815)56:4<905::AID-CNCR2820560435>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 38.Sriram KB, Relan V, Clarke BE, Duhig EE, Windsor MN, Matar KS, Naidoo R, Passmore L, McCaul E, Courtney D, Yang IA, Bowman RV, Fong KM. Pleural fluid cell-free DNA integrity index to identify cytologically negative malignant pleural effusions including mesotheliomas. BMC Cancer. 2012;12:428. doi: 10.1186/1471-2407-12-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaczmarek M, Sikora J. Macrophages in malignant pleural effusions - alternatively activated tumor associated macrophages. Contemp Oncol (Pozn) 2012;16:279–284. doi: 10.5114/wo.2012.30054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mundt F, Heidari-Hamedani G, Nilsonne G, Metintas M, Hjerpe A, Dobra K. Diagnostic and prognostic value of soluble syndecan-1 in pleural malignancies. Biomed Res Int. 2014;2014:419853. doi: 10.1155/2014/419853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu C, Yu L, Zhan P, Zhang Y. Elevated pleural effusion IL-17 is a diagnostic marker and outcome predictor in lung cancer patients. Eur J Med Res. 2014;19:23. doi: 10.1186/2047-783X-19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong Y, Chen SX, Gao BA, Yao RC, Guan L. Cell origins and significance of IL-17 in malignant pleural effusion. Clin Transl Oncol. 2014;16:807–813. doi: 10.1007/s12094-013-1152-8. [DOI] [PubMed] [Google Scholar]
- 43.Gonda K, Shibata M, Shimura T, Abe N, Suzuki S, Yasuda M, Nakamura I, Ohtake T, Ohki S, Watanabe T, Fujimori K, Ohto H, Takenoshita S. Changes in myeloid-derived suppressor cells in malignant effusions of cancer patients following cancer chemotherapy. Gan To Kagaku Ryoho. 2012;39:2088–2091. [PubMed] [Google Scholar]
- 44.Romano A, Parrinello NL, Vetro C, Forte S, Chiarenza A, Figuera A, Motta G, Palumbo GA, Ippolito M, Consoli U, Raimondo FD. Circulating myeloid-derived suppressor cells correlate with clinical outcome in Hodgkin lymphoma patients treated up-front with a risk-adapted strategy. Br J Haematol. 2015;168:689–700. doi: 10.1111/bjh.13198. [DOI] [PubMed] [Google Scholar]
- 45.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.