Abstract
Reproductive malignancies are a major cause of cancer death in women worldwide. CD40 is a TNF receptor family member, which upon activation may mediate tumor regression. However, despite the great potential of CD40 agonists, their use as a therapeutic option for reproductive cancers has never been investigated. Because CD40 ligation is a potent pathway of macrophage activation, an in vitro model of pro-inflammatory type-1 (Mϕ-1) and anti-inflammatory type-2 (Mϕ-2) macrophages was developed to determine whether and how macrophage CD40 pathway activation might influence endometrial tumor cell behavior. Analysis of tumor growth kinetic in the endometrial cancer xenograft model indicates that, when injected once into the growing tumors, CD40-activated Mϕ-1 greatly reduced, while CD40-activated Mϕ-2 increased tumor size when compared to control isotype-activated Mϕ-1 and Mϕ-2, respectively. In vitro assays indicated that CD40-activated Mϕ-2 increased cell viability but failed to promote cell invasion. CD40-activated Mϕ-1, in contrast, decreased cell survival but greatly increased cell invasion in tumor cells less susceptible to cell death by apoptosis; they also induced the expression of some pro-inflammatory genes, such as IL-6, LIF, and TNF-α, known to be involved in tumor promotion and metastasis. The presence of IFN-γ is minimally required for CD40-activated Mϕ-1 to promote tumor cell invasion, a process that is mediated in part through the activation of the PI3K/Akt2 signaling pathway in tumor cells. From these results, we speculate that some functions of CD40 in tumor-associated Mϕs might limit the therapeutic development of CD40 agonists in endometrial cancer malignancies.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-012-1333-2) contains supplementary material, which is available to authorized users.
Keywords: CD40 receptor, Endometrial cancer, Immunotherapy, Polarized macrophages, Tumor–stroma interaction
Introduction
Endometrial cancer (EC) is the most common malignancy of the female genital tract in many countries with incidences increasing in these populations over the past two decades [1]. At initial diagnosis, the majority of these cancers (142,000 cases annually) are curable by means of hysterectomy and radiotherapy [1, 2]. However, because up to 26 % of patients still succumb of the disease (about 42,000 related deaths annually worldwide), major efforts must be invested to find alternative therapeutic approaches and identify new therapeutic targets.
The CD40 receptor is a member of the TNF receptor superfamily, which is expressed on many different normal cells, such as macrophages (Mϕ), dendritic cells (DC), B cells, epithelial cells, endothelial cells, and fibroblasts, as well as on transformed cells [3–6]. Consequently, via activation of multiple signal transduction pathways, CD40 ligation mediates a broad variety of immune and inflammatory responses [3–6]. CD40 activation plays an important role in anti-tumor immune responses [6–10]. Preliminary findings emerging from clinical trials indicate that agonist formulation as monoclonal antibodies (mAbs) to CD40 or soluble CD40 ligand (sCD40L) can enhance the anti-tumor immune response and induce clinical responses in cancer patients with solid and lymphoid malignancies [6–10]. As an alternative approach for novel tumor immunotherapy, CD40 agonists may mediate tumor regression mainly through the generation of cytotoxic T cell (CTL) and natural killer (NK) cells responses, or directly by inducing pro-apoptotic and anti-proliferative signaling on CD40-expressing malignant cells [6–10]. We have previously established that monocyte-derived macrophages may direct cytotoxic/cytostatic activities against tumor cells under the influence of interferon (IFN)-γ and lipopolysaccharide (LPS) stimulations [11]. Recent studies indicated that this action can be potently enhanced by ligation of CD40 on Mϕs in vitro [12, 13]. Furthermore, in vivo treatment with anti-CD40 mAbs resulted in Mϕ-mediated tumor cell death in immunocompetent and immunodeficient mice [13, 14].
Ongoing phase I clinical evaluation with CD40 agonists in advanced-stage cancer patients showed immune modulation and objective clinical responses [6–10]. The potential anti-tumor effect of CD40 agonists is thus a promising therapeutic approach in EC, but this remains to be established. However, because CD40 is present and functional in tumor cells and probably in tumor stromal cells such as Mϕs, fibroblasts, and endothelial cells as well, the implications of CD40 activation at the tumor–stroma interface are expected to be highly pleiotropic and should be evaluated carefully before targeting CD40 for anti-tumor strategies [6–10]. In this study, the main objective was to investigate whether and how pro-inflammatory type-1 (Mϕ-1) and anti-inflammatory type-2 (Mϕ-2) macrophages are able to influence EC cell behavior in response to CD40 agonists.
Materials and methods
Reagents, antibodies, and chemicals
All cell culture media, serum and cell culture reagents were from Wisent. The cytokines interleukin (IL)-4, IL-10, and interferon IFN-γ were purchased from Peprotech. The mouse anti-CD40 mAb G28.5 was purified from cell culture supernatants of hybridoma cells obtained from the American type culture collection (ATCC). The isotype-matched IgG1 mAb was from R&D Systems. Human IgG and goat anti-mouse fluorescein isothiocyanate (FITC)-conjugated IgG were from Jackson Immuno Research Laboratories. The anti-Akt3 was from Upstate Biotech. The HRP-conjugated goat anti-rabbit IgG was from Bio-Rad Laboratories. BD Matrigel™ basement membrane matrix was from BD Biosciences. Boyden chambers were from Corning. LPS, methylthiazolyldiphenyl-tetrazolium bromide (MTT) reagent, propidium iodide (PI), and all electrophoresis grade chemicals were from Sigma. Trizol reagent and PCR primers were from Invitrogen. Taq DNA polymerase and MMLV reverse transcriptase were from New England Biolabs.
Monocyte-to-macrophage differentiation and activation
Human monocytic cell line THP-1 cells (ATCC number TIB-202) were cultured in RPMI-1640 supplement with 10 % FBS, 2 mM glutamine, 10 mM HEPES, 1 mM pyruvate, and 50 mg/L gentamycin (referred as 10 % FBS-RPMI). To induce Mϕ differentiation, THP-1 cells were gently washed and cultured for 48 h in serum-free culture media in the absence of cytokines to obtain control cells (Mϕ-O). Pro-inflammatory type-1 phenotype (Mϕ-1) was induced with 100 ng/ml LPS and 25 U/ml IFN-γ, and anti-inflammatory type-2 phenotype (Mϕ-2) was induced with 20 ng/ml IL-4 and 20 ng/ml IL-10. For CD40 activation, Mϕ-1 and Mϕ-2 were stimulated with 500 ng/ml of isotype-matched IgG1 antibody or anti-CD40 mAbs G28.5 (αCD40) as previously described [15–17]. To validate type-1 and type-2 polarization, THP-1 cells were collected after a 6-h stimulation period to evaluate specific gene expression by RT-PCR analysis and after a 48-h stimulation period to evaluate the expression level of CD40 by flow cytometry and Western blot analyses as described [11].
In vitro activation of tumor cells by polarized macrophages
Human EC cell lines Hec1A cells (ATCC number HTB-112), EN-1078D cells [18], KLE cells (ATCC number CRL-1622), and KLE cells stably expressing shRNA for Akt isoforms [19] were stimulated with control media or with conditioned media (CM) from isotype- or CD40-activated Mϕ-1 and Mϕ-2, as described [11]. EC cell viability was evaluated by MTT assays as described [11]. The inactive state of Akt isoforms, the activated state of Akt, and the cleaved form of the apoptosis marker caspase-3 were immunodetected by Western blot using EC cells exposed to CM from Mϕ1/isotype-CM and Mϕ1/αCD40-CM. To evaluate the number of dead cells, EC cells were washed and stained for 15 min with 2 μg/ml PI solution before analysis by flow cytometry. The transwell invasion assay was conducted in a modified Boyden chamber with membrane inserts (8 μm pore size) pre-coated with Matrigel at 2 mg/ml as described previously [11].
Total RNA extraction and RT-PCR analyses
Total RNA extraction from cultured EC cells, preparation of first strand cDNA by RT, and PCR amplifications were performed as described [11]. Primers for amplification were 5′–GTCAGTGGTGGACCTGACCT–3′ (sense, S) and 5′–TGAGCTTGACAAAGTGGTCG–3′ (antisense, AS) for GAPDH; 5′–ATGAACTCCTTCTCCACAAGC–3′ (S) and 5′–TGGACTGCAGGAACTCCTT–3′ (AS) for IL6; 5′–TGAGAACCAAGACCCAGACA–3′ (S) and 5′–TCATGGCTTTGTAGATGCCT–3′ (AS) for IL10; 5′–ATGTCGTAGAATTGGATTGGTATCCG–3′ (S) and 5′–GTACTGATTGTCGTCAGCCACCAGC–3′ (AS) for IL12p40; 5′–ACTGCACTCCTGGTTGTCCTCG–3′ (S) and 5′–GCCTCGGGCAGGAGTCTGAGGTCCAGTAG–3′ (AS) for CCL22; 5′–GGCCCGGACACCCATAGACG–3′(S) and 5′–CCACGCGCCATCCAGGTAAA–3′ (AS) for LIF; and 5′–CAGAGGGAAGAGTTCCCCAG–3′ (S) and 5′–CCTTGGTCTGGTAGGAGACG–3′ (AS) for TNFα.
In vivo activation of tumor cells by polarized macrophages
The EC xenograft model of tumor implants was developed in 6-week-old nude mice (Charles River Laboratories) as previously described [18]. Mice were injected at both flanks near the posterior legs. To monitor tumor growth, tumor volumes were calculated by caliper measurement twice each week using the formula 0.5 × length × (width)2. The proportion of tumor cells into the tumor nodules was also determined using RFP-expressing Hec1A cells and an in vivo real-time imaging system (IVIS Imaging System; Caliper Life Sciences). Our pilot studies have determined that all nude mice developed tumors by subcutaneous injections of 2 × 106 RFP-Hec1A cells in 100 μL of 2 mg/mL Matrigel and that tumor masses were palpable as soon as 7 days after injection. In order to investigate whether polarized Mϕs could influence tumor cell growth and survival in vivo, THP-1-derived Mϕ-1 and Mϕ-2 were mixed with RFP-Hec1A cells before subcutaneous injection on both flanks near the posterior legs of female nude mice. We have established that a minimal ratio of EC cells-to-Mϕs of 1:2 is compulsory to observe a significant influence of activated Mϕs on EC growth in vivo. To investigate whether CD40-activated Mϕs are able to regulate tumor cell proliferation in vivo, THP-1-derived Mϕ-1 and Mϕ-2 were treated for 24 h with 500 ng/ml isotype-matched IgG1 Ab (control groups) or anti-CD40 mAb G28.5 and then injected (4 × 106 cells/100 μl in Matrigel) into the growing (palpable) tumor nodules on day 21 after tumor implantation. All procedures with animals were conducted in accordance with the UQTR animal care committee guidelines.
Statistical analysis
For in vivo studies, differences between groups (n = 8 mice per group) were determined using two-way ANOVA and the Tukey’s and the Mann–Whitney tests (PRISM software version 2.0; GraphPad, San Diego, CA, USA). For in vitro studies, data were subjected to one-way ANOVA, and differences between experimental groups were determined by the Tukey’s test. Values were presented as mean ± SD from three independent experiments, and significance was accepted at p < 0.05.
Results
Polarized THP-1 cell is a valuable model of both type-1 and type-2 Mϕs
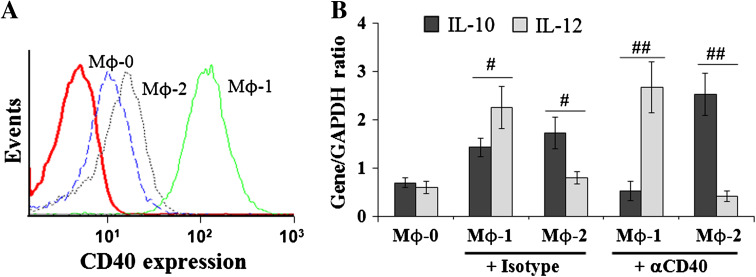
Phenotypic analysis by flow cytometry using THP-1-derived Mϕ-0 as control cells indicates that combined stimulation with LPS/IFN-γ increased the expression of CD40 in THP-1-derived Mϕ-1 (Fig. 1a). In contrast, combined stimulation with IL-4/IL-10 slightly increased CD40 expression in THP-1-derived Mϕ-2 (Fig. 1a). In accordance with previous studies [20–23], we found that gene expression of pro-inflammatory cytokines IL-12 and tumor necrosis factor (TNF)-α were preferentially high in Mϕ-1, whereas gene expression of anti-inflammatory cytokines IL-10 and CCL22 was especially high in Mϕ-2 (Online Resource 1). Functionally, CD40 receptor activation in THP-1-derived Mϕs leads to the generation of Mϕ-1 with an IL-10negativeIL-12high profile and Mϕ-2 with an IL-10highIL-12negative profile (Fig. 1b). Together, these results confirm that polarized THP-1 cell is a valuable cell model to study the regulatory role of CD40-activated type-1 and type-2 Mϕs in EC behavior changes in vivo and in vitro.
Fig. 1.
Functional features of polarized Mϕs. a Flow cytometry analysis. CD40 expression is higher in Mϕ-1 than in Mϕ-2 and in control cells. b RT-PCR analysis. CD40 activation leads to the generation of Mϕ-1 with negative-IL-10 and high-IL-12 expression profile and Mϕ-2 with high-IL-10 and negative-IL-12 expression profile. The relative mRNA expression of gene of interest was expressed as gene/GAPDH ratio; # p < 0.05 and ## p < 0.01 denote significant difference between groups
Tumor cell growth is differentially influenced by polarized Mϕs
Analysis of tumor growth kinetic indicates that CD40-activated Mϕ-1 was more efficient than isotype-activated Mϕ-1 to decrease the size of xenografted tumors developing from RFP-Hec1A cells (Fig. 2a). Injection of CD40-activated Mϕ-2 at day 21, however, modestly increased tumor size when compared with isotype-activated Mϕ-2 (Fig. 2a). Analysis of tumor cell fluorescence intensity by in vivo real-time imaging system at day 45 confirms that CD40-activated Mϕ-2 discreetly enhanced, while CD40-activated Mϕ-1 significantly decreased tumor volume when compared to respective control isotype-activated cells (Fig. 2b).
Fig. 2.
Influence of polarized Mϕs on the growth of xenografted endometrial tumors. a Tumor growth kinetic analyses. Control (Mϕ-0) and polarized Mϕs were injected into the tumor mass on day 21 after implantation of RFP-expressing Hec1A cells. Injection of CD40-activated Mϕ-1 decreases tumor size more efficiently than isotype-activated Mϕ-1, and CD40-activated Mϕ-2 significantly increases tumor size when compared to isotype-activated Mϕ-2; # p < 0.05 and ## p < 0.01 denote significant difference between groups. b Visualization of tumors cells at the end of the experiment confirms that CD40-activated Mϕ-2 enhance, while CD40-activated Mϕ-1 decreases tumor size compared to respective control isotype-activated cells; representative images of tumor fluorescence intensity are shown
CD40-activated Mϕ-1 enhances invasiveness and gene expression in tumor cells
As assessed by MTT assays (Fig. 3a), the number of viable Hec1A cells was reduced when incubated in conditioned media (CM) from isotype-stimulated Mϕ-1 (Mϕ1/isotype-CM) and even more when stimulated with Mϕ1/αCD40-CM. The number of viable Hec1A cells increased when incubated in Mϕ2-CM, while significantly increased in Mϕ2/αCD40-CM (Fig. 3a). Data from micro-invasion assays (Fig. 3b) indicates that Mϕ-1 but not Mϕ-2 significantly increased tumor cell invasion. Co-incubation of Hec1A cells with THP-1-derived Mϕ-2 does not result in enhanced tumor cell invasion, even if Mϕ-2 was activated via CD40 ligation (Fig. 3b). In order to explore the possibility that tumor cells contribute to the inflammatory process in response to paracrine signals from polarized Mϕs, we next investigated whether Mϕ-1 and Mϕ-2 have the ability to induce cytokine gene expression in Hec1A cells. Semi-quantitative RT-PCR analysis indicates that gene expression of IL-6, leukemia inhibitory factor (LIF), and TNF-α was significantly enhanced in Hec1A cells in response to soluble factors from CD40-activated Mϕ-1 when compared with isotype-stimulated Mϕ-1 (Fig. 3c). Inconsistent results were obtained with CD40-activated Mϕ-2: the transcription level of IL-6 is increased, while that of LIF is decreased and that of TNF-α is unchanged in response to CD40 ligation (Fig. 3c).
Fig. 3.
In vitro activation of Hec1A cells by polarized Mϕs. a MTT assays. Result shows the relative number of viable Hec1A cells, which were stimulated for 48 h with CM from polarized Mϕs (Mϕ1/isotype-CM, Mϕ1/αCD40-CM, Mϕ2/isotype-CM or Mϕ2/αCD40-CM). Different superscripts denote significant differences between groups; * p < 0.05, significantly decreased when compared with control mean; ** p < 0.05, significantly increased when compared with control mean. b Invasion assays. Result shows the number of invasive cells/field after a 48 h co-culture period of Hec1A cells with isotype- and CD40-activated Mϕ-1 or Mϕ-2. * p < 0.05, ** p < 0.01, significantly increased when compared to basal level. c RT-PCR analysis. Results show regulation of mRNA expression levels of IL6, LIF, and TNFα in Hec1A cells by soluble factors present in Mϕ1/isotype-CM, Mϕ1/αCD40-CM, Mϕ2/isotype-CM, and Mϕ2/αCD40-CM. The relative mRNA expression of gene of interest (gene/GAPDH ratio) was expressed as fold induction of basal level; # p < 0.05, denote significant difference between groups
CD40-activated Mϕ-1 increases invasiveness of tumor cells less susceptible to caspase-3-mediated apoptosis
To explore the possibility that the dual influence of CD40-activated Mϕ-1 on tumor cell survival and invasion is not restricted to Hec1A cells; two other human EC cell lines, KLE and EN-1078D cells, were used as experimental models. As shown in Fig. 4a, the numbers of viable KLE cells and EN-1078D were significantly reduced in Mϕ1/αCD40-CM when compared with Mϕ1/isotype-CM. In regard to Matrigel invasion of KLE cells, the basal level of invasive cells per field was significantly increased when co-cultured with CD40-activated Mϕ-1 (Fig. 4b). However, in contrast to Hec1A and KLE cells, the basal level of invasive EN-1078D cells was increased when co-cultured with isotype-stimulated Mϕ-1, but the level does not increase and remains stable in the presence of CD40-activated Mϕ-1 (Fig. 4b). Having that CD40-activated Mϕ-1 affects EC cell survival, we next investigate whether Hec1A, KLE, and EN-1078D cells are susceptible to apoptosis-inducing factors, which are released from pro-inflammatory Mϕ-1 [11]. Cells were thus exposed to Mϕ1/isotype-CM and Mϕ1/αCD40-CM during 6, 24, and 48 h; after that, the cells were processed for immunodetection of cleaved caspase-3, an apoptosis marker. Maximal effects were observed at 48 h, and the results are presented in Fig. 4c. Western blot analysis reveals that cytotoxic factors in both types of CM do not induce caspase-3-dependent apoptosis in Hec1A cells. However, the basal level of cleaved caspase-3 in KLE cells (expressed as cleaved caspase-3/β-actin ratio) was significantly increased when exposed to Mϕ1/αCD40-CM when compared with Mϕ1/isotype-CM. EN-1078D cells were more sensitive to caspase-3-mediated apoptosis (Fig. 4c).
Fig. 4.
Enhancement of cell invasion by CD40-activated Mϕ-1 depends on tumor cell susceptibility to apoptosis. a MTT assays. KLE cells and EN-1078D cells were stimulated for 48 h in control media or with soluble factors from Mϕ1/isotype-CM or Mϕ1/αCD40-CM. Result shows viable cell number in percent of control mean. Different superscripts denote significant differences between groups; * p < 0.05, significantly decreased when compared with control mean. b Invasion assays. Result shows the number of invasive cells/field alone (basal) or after a 48 h co-culture period of KLE cells or EN-1078D cells with isotype- and CD40-activated Mϕ-1. Compared with KLE cells, EN-1078D cells are no responsive to the invasiveness induced by Mϕ-1/αCD40 macrophages. Different superscripts denote significant differences between groups; * p < 0.01, significantly increased when compared with the basal level of invasive cells. c Apoptosis assays. Hec1A, KLE, and EN-1078D cells were stimulated with CM from Mϕ1/isotype or Mϕ1/αCD40 macrophages for 48 h. Results from Western blot analysis show expression of cleaved caspase-3 (left panel). The protein β-actin was used as control to correct for loading, and blots are representative of three independent experiments. Relative level of cell apoptosis was expressed as cleaved caspase-3/β-actin ratio (right panel). Compared with KLE cells, EN-1078D cells are highly sensitive to the apoptosis induced by Mϕ1/isotype and even more by Mϕ1/αCD40 macrophage; * p < 0.01 denotes significant difference between groups
IFN-γ is minimally required for CD40-activated Mϕ-1 to promote tumor cell invasion
We have previously established that anti-tumor response of Mϕs can be induced by IFN-γ and/or LPS stimulation [11]. Other independent studies indicate that CD40 ligation increased Mϕ-mediated tumor cell cytotoxicity in vitro and in vivo through an IFN-γ-dependent mechanism [12–14]. To investigate the minimal requirement responsible for the pro-invasive effect of Mϕ-1 on EC tumor cells, we further examined the priming effects of IFN-γ and LPS toward a type-1 phenotype that causes EC tumor cell death and cell invasion upon CD40 stimulation on Mϕs. Flow cytometry analysis showed that the basal level of CD40 expression in THP-1 cells gradually increases when stimulated as follows: vehicle (PBS) < LPS < IFN-γ < LPS/IFN-γ (Fig. 5a). In respect of the cytotoxic effects on EN-1078D cells, we found that the percent of dead cells gradually increased with CM from IFN-γ-stimulated THP-1 cells, from LPS-stimulated THP-1 cells, and even more with CM from IFN-γ/LPS-stimulated THP-1 cells (Fig. 5b). In the presence of αCD40 mAbs, the number of dead cells was higher and progressively increased with CM from THP-1 cells stimulated with IFN-γ, LPS, and combined IFN-γ/LPS (Fig. 5b). In regard to Matrigel invasion of Hec1A cells, no pro-invasive effects were observed when they were co-cultured with THP-1 cells, which were primed with either PBS + isotype, PBS + αCD40, LPS + isotype, LPS + αCD40, and IFN-γ + isotype (Fig. 5c). In contrast, there is a significant increase in the number of invasive cells per field when THP-1 cells were activated with IFN-γ + αCD40. Activation of THP-1 cells with IFN-γ and LPS results in an enhanced number of invasive cells, while together they exert a synergistic effect in EC tumor cell invasion after CD40 ligation (Fig. 5c).
Fig. 5.
IFN-γ is minimally required for CD40-activated Mϕ-1 to promote tumor cell invasion. a Result from flow cytometry analysis shows the expression level of CD40 in THP-1 cells treated for 48 h with vehicle (Ve), LPS, IFN-γ, and IFN-γ + LPS, in the presence of G8.5 (αCD40) or isotype-matched antibody. Compared with the effects of LPS and IFN-γ alone, combined IFN-γ/LPS induced higher expression levels of CD40. b Result from flow cytometry analysis shows the level of cell death (percent of PI positive cells). EN-1078D cells were stimulated for 48 h with control media or CM from THP-1 cells treated with PBS (Ve), LPS, IFN-γ, and IFN-γ + LPS, in the presence of αCD40 or isotype-matched antibody, and then stained with PI solution. c Result from invasion assays shows the number of invasive cells/field after 48 h of co-culture of Hec1A cells with THP-1 cells treated with PBS (Ve), LPS, IFN-γ, and IFN-γ + LPS, in the presence of αCD40 or isotype-matched antibody. * p < 0.05 and ** p < 0.01 denote significant difference between groups
Macrophage-mediated tumor cell invasion involves PI3K/Akt2 signaling pathway
We have previously reported that increased invasiveness of bladder cancer T24 cells in response to Mϕ-1-derived factors is in part dependent on tumor cell PI3K/Akt signaling pathway activation [11]. Western blot analysis indicates that paracrine activation of this signaling pathway is also induced by isotype-activated Mϕ-1 and even more by CD40-activated Mϕ-1 in KLE cells, but not in Hec1A and EN-1078D cells (Fig. 6a). Here, we used a well-established model of KLE cells stably expressing shRNA plasmids for Akt1, Akt2, and Akt3 isoforms [19] to further investigate the mechanism by which Mϕ-1 enhances tumor cell invasion via paracrine cell interactions. Micro-invasion assays (Fig. 6b) indicate that there is no difference in the number of invasive cells using Akt1-, Akt2-, and Akt3-deficient KLE cells in response to isotype-activated Mϕ-1. However, the increased invasiveness of tumor cells induced in response to CD40-activated Mϕ-1 was completely impeded by Akt2 knockdown, but not by either Akt1 or Akt3 knockdown, in KLE cells (Fig. 6b). Cell signaling studies indicate that Akt2 expression and activation is in part required for CD40-activated Mϕ-1 to increase cell invasiveness in KLE cells (Fig. 6c).
Fig. 6.
Mϕ-1 induced tumor cell invasion via tumor cell PI3 K/Akt2 signaling pathway activation. a KLE, Hec1A, and EN-1078D cells were stimulated with control media (basal) or CM from Mϕ1/isotype or Mϕ1/αCD40 macrophages for 15 min. Result from Western blot analysis shows representative expression of phosphorylated/active form of Akt (pAkt) and total Akt, from three independent experiments. b KLE cells were transfected with plasmids expressing scramble shRNA (empty) or shRNA against Akt1, Akt2, and Akt3 mRNA. Result shows the number of invasive cells/field after a 48-h co-culture period. * p < 0.01 denotes significant difference between groups. c Control KLE cells (shEmpty) and KLE deficient for Akt1 (shAKt1), Akt2 (shAKt2), and Akt3 (shAkt3) were stimulated with control media (basal) or CM from Mϕ1/isotype or Mϕ1/αCD40 macrophages for 15 min. Result from Western blot analysis shows representative expression of phosphorylated/active form of Akt (pAkt) and total Akt from three independent experiments
Discussion
Compelling clinical and experimental data suggest that CD40 agonists can be exploited to offer, in one therapy, different anti-cancer approaches: inhibition of tumor cell proliferation, sensitization to other cytotoxic drugs, improving immunogenicity of tumors, and stimulation of anti-tumor immune responses via activation of DC, CTL, NK cells, and Mϕs [6–10, 12–14]. Engagement of CD40 on pro-inflammatory Mϕ-1 induces the expression of several factors, such as nitric oxide and TNF-α, which are involved in the cytotoxic effector mechanisms of Mϕs [3, 4]. As expected, we found that injection of polarized Mϕs have opposing effects on the growth of tumor xenografts. These results are consistent with previous observations that injection of type-1 cytokine-activated human Mϕs inhibits while of type-2 cytokine-activated human Mϕs increases the growth of human tumors implanted subcutaneously in immunodeficient mice [24, 25]. We show for the first time that CD40 activation in Mϕ-1 results in efficient tumor growth inhibition, but fails to reverse the proliferative effects of Mϕ-2. Indeed, tumor size and fluorescence in the presence of CD40-activated Mϕs were modestly decreased with Mϕ-1 or increased with Mϕ-2 pointing out on the necessity to sustain their effects by repetitive injections into the tumor mass. Of note, repetitive injections of polarized Mϕs once a week are expected to imitate a chronic Mϕ-mediated inflammatory response. Thus, although the functional role in cancer development still remains unclear and controversial, CD40 pathway activation may provide an attractive option for future clinical trials in gynecological malignancies [7–10]. However, for several considerations, further investigations are needed in regard to host-tumor interactions prior to recommending clinical trials with CD40 agonists.
First, in advanced-stage EC patients, a subset of invasive tumors was found to be highly enriched with Mϕs, but the exact role that such inflammatory cells are playing in EC is poorly understood [26–28]. For instance, high number of tumor-infiltrating Mϕs was significantly associated with clinical manifestations, such as deep myometral invasion, angiogenesis, and the presence of lymph node metastasis [26–28]. Indeed, tumor-infiltrating Mϕs are able to exert pro- and anti-tumor effects in the tumor microenvironment depending on their activation state [20–23]. In this context, we have previously established that differentiation of peripheral blood monocytes with IFN-γ and LPS results in pro-inflammatory Mϕ-1 that decreased cell viability of urothelial bladder carcinoma cells, while significantly increased their invasiveness [11]. Conversely, after differentiation with combined IL-4 and IL-10, Mϕ-2 supports tumor cell survival/proliferation and suppresses the cytotoxic activities of Mϕ-1 via secretion of IL-10 and other soluble factors [11]. In line with our data, a previous study demonstrated that IFN-γ is required to shift CD40-induced cytokine profiles in human DC toward high IL-12 and low IL10 production [29]. In addition, CD40 expression and CD40-triggered antigen-presentation function in DC are down-regulated via tumor cell-derived IL-10 [30]. Moreover, it was established that CD40-induced IL-10 expression in DC can result in the inhibition of the anti-tumor immune response [31, 32]. From these results and our finding that CD40 receptor activation leads to the generation of Mϕ-2 with an IL-10highIL-12negative profile, it is tempting to presume that some functions of CD40 in tumor-associated Mϕs [20–23] might limit the therapeutic potential of CD40 agonists to inhibit human EC growth.
Second, CD40 ligation on Mϕs leads to the production of inflammatory mediators known to be involved in tumor cell motility and invasion, a phenomenon associated with metastatic spread of malignant cells. The listed factors include the cytokines IL-6, TNF-α, monocyte chemoattractant protein (MCP)-1, transforming growth factor (TGF)-β1, and the matrix-metalloproteinases MMP-2 and MMP-9 [3, 4, 6, 8]. This could explain why CD40 activation in Mϕ-1 can boost their cytotoxicity but does not reverse their pro-invasive effects on EC tumor cells. In fact, we have established that CD40-activated Mϕ-1 enhances cell invasion mainly in tumor cells highly resistant to caspase-3-mediated apoptosis. In addition, they enhance the expression of tumor cell pro-inflammatory genes known to be involved in tumor promotion, invasion, and metastasis, such as IL-6, LIF, and TNF-α [33–35]. Mechanistically, our study provides evidence that CD40-activated Mϕ-1 differentially modulates cell survival and invasion through a synergistic effect of IFN-γ and LPS. Of note, CD40, LPS, and IFN-γ are major ways to induce extracellular matrix degradation via the expression of MMPs by Mϕs [3, 36–38]. Moreover, it demonstrated that EC cell PI3K/Akt2 pathway activation is partially required during these processes. In one hand, KLE and EN-1078D cells, but not Hec1A cells, are susceptible to apoptosis-inducing factors, which are released from pro-inflammatory Mϕ-1; because KLE cells are induced to express phosphorylated Akt, but not EN-1078D and Hec1A cells, this effect is probably independent of the PI3K/Akt signaling pathway, but it is probably due to the balance of pro-apoptotic and anti-apoptotic factors that such cells express [18, 19]. On the other hand, this suggests that the pro-invasive function of CD40-activated Mϕ-1 is independent of the PI3K/Akt2 signaling pathway in Hec1A and EN-1078D cells and that other pathways could be involved. In this context, our preliminary data actually shown that soluble factors released from pro-inflammatory Mϕ-1 are able to induce other signaling pathways involved in cell motility and invasion such as the JAK/STAT (Janus-Activated Kinase/Signal Transducers and Activators of Transcription), as well as the MAP kinases p38, ERK, and JNK signaling pathways. Certainly, our study further supports our previously published data suggesting that paracrine activation of the PI3K/Akt signaling pathway in tumor cells by pro-inflammatory Mϕs is involved in the regulation of tumor cell invasion [11].
Third, CD40-activated Mϕs are known to be major producers of inflammatory mediators at sites of acute and chronic inflammation [3, 4]. In a pathological setting, the deregulation of CD40 signaling has been associated with Mϕ dysfunction and the pathogenesis of chronic inflammatory diseases, such as rheumatoid arthritis and atherosclerosis [39, 40]. Mature Mϕs are characterized by their plasticity [41], and as observed in chronic inflammatory diseases [39, 40], their normal function could be subverted during tumor development under the influence of activating factors from the tumor microenvironment [20–23].
Finally, normal human endometrium expresses functional CD40 receptor and CD40L, and CD40 activation via CD40L induces uterine fibroblasts and perivascular cells to produce large amounts of the inflammatory cytokines IL-6, IL-8, and MCP-1 [42, 43]. CD40 has been detected in cervical and ovarian carcinoma, with CD40L expressed by infiltrating T cells or the tumor itself [44, 45]. CD40 ligation induces the expression of MCP-1 and MMP-9 in cervical carcinomas and the expression of IL-6 and IL-8 in ovarian carcinomas [44, 45]. In line with our observation that CD40-activated Mϕ-1 enhances the expression of pro-inflammatory genes in EC cells, these findings further support the hypothesis that tumor cells contribute to the inflammatory process and that CD40 agonists might play a dual role in pathogenesis and treatment of EC [7, 8, 10].
In conclusion, our study emphasizes the duality of CD40-activated Mϕs in the immune response to tumors. In the EC xenograft model, CD40 activation in Mϕ-1 results in efficient tumor growth inhibition but fails to reverse the proliferative effects of Mϕ-2. The presence of IFN-γ is minimally required for CD40-activated Mϕ-1 to promote tumor cell invasion, and the PI3K/Akt2 pathway activation in EC cells may play an important role in this process. To the best of our knowledge, this is the first report showing the dual effects of CD40-activated Mϕs on the survival and the invasion of reproductive cancer cells. Subsequently, before recommending immunotherapy with CD40 agonists, further studies should be made in order to eliminate the putative pro-tumorigenic effects while preserving the capacity of stimulating anti-tumor immune responses.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was financially supported by grants from The Fonds de la Recherche en Santé du Québec (FRSQ) and the Natural Science and Engineering Research Council (NSERC) of Canada to C.R.M. G.D. and was supported by the Research Awards Program of the NSERC. J.G. holds a postdoctoral fellowship from the FRSQ. E.A. is holder of the Canada research chair in Molecular Gyneco–Oncology.
Conflict of interest
The authors declare no financial conflict of interest.
Footnotes
Geneviève Dumas and Mathieu Dufresne contributed equally to this work.
References
- 1.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 2.Sohaib SA, Houghton SL, Meroni R, Rockall AG, Blake P, Reznek RH (2007) Recurrent endometrial cancer: patterns of recurrent disease and assessment of prognosis. Clin Radiol 62: 28–34; discussion 35–26. doi:10.1016/j.crad.2006.06.015 [DOI] [PubMed]
- 3.Stout RD, Suttles J. The many roles of CD40 in cell-mediated inflammatory responses. Immunol Today. 1996;17:487–492. doi: 10.1016/0167-5699(96)10060-I. [DOI] [PubMed] [Google Scholar]
- 4.Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58:4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Semin Immunol. 2009;21:293–300. doi: 10.1016/j.smim.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costello RT, Gastaut JA, Olive D. What is the real role of CD40 in cancer immunotherapy? Immunol Today. 1999;20:488–493. doi: 10.1016/S0167-5699(99)01507-8. [DOI] [PubMed] [Google Scholar]
- 8.Eliopoulos AG, Young LS. The role of the CD40 pathway in the pathogenesis and treatment of cancer. Curr Opin Pharmacol. 2004;4:360–367. doi: 10.1016/j.coph.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Vonderheide RH. Prospect of targeting the CD40 pathway for cancer therapy. Clin Cancer Res. 2007;13:1083–1088. doi: 10.1158/1078-0432.CCR-06-1893. [DOI] [PubMed] [Google Scholar]
- 10.Bereznaya NM, Chekhun VF. Expression of CD40 and CD40L on tumor cells: the role of their interaction and new approach to immunotherapy. Exp Oncol. 2007;29:2–12. [PubMed] [Google Scholar]
- 11.Dufresne M, Dumas G, Asselin E, Carrier C, Pouliot M, Reyes-Moreno C. Pro-inflammatory type-1 and anti-inflammatory type-2 macrophages differentially modulate cell survival and invasion of human bladder carcinoma T24 cells. Mol Immunol. 2011;48:1556–1567. doi: 10.1016/j.molimm.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Buhtoiarov IN, Lum H, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL. CD40 ligation activates murine macrophages via an IFN-gamma-dependent mechanism resulting in tumor cell destruction in vitro. J Immunol. 2005;174:6013–6022. doi: 10.4049/jimmunol.174.10.6013. [DOI] [PubMed] [Google Scholar]
- 13.Rakhmilevich AL, Buhtoiarov IN, Malkovsky M, Sondel PM. CD40 ligation in vivo can induce T cell independent antitumor effects even against immunogenic tumors. Cancer Immunol Immunother. 2008;57:1151–1160. doi: 10.1007/s00262-007-0447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lum HD, Buhtoiarov IN, Schmidt BE, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL. In vivo CD40 ligation can induce T-cell-independent antitumor effects that involve macrophages. J Leukoc Biol. 2006;79:1181–1192. doi: 10.1189/jlb.0405191. [DOI] [PubMed] [Google Scholar]
- 15.Reyes-Moreno C, Girouard J, Lapointe R, Darveau A, Mourad W. CD40/CD40 homodimers are required for CD40-induced phosphatidylinositol 3-kinase-dependent expression of B7.2 by human B lymphocytes. J Biol Chem. 2004;279:7799–7806. doi: 10.1074/jbc.M313168200. [DOI] [PubMed] [Google Scholar]
- 16.Girouard J, Reyes-Moreno C, Darveau A, Akoum A, Mourad W. Requirement of the extracellular cysteine at position six for CD40/CD40 dimer formation and CD40-induced IL-8 expression. Mol Immunol. 2005;42:773–780. doi: 10.1016/j.molimm.2004.07.048. [DOI] [PubMed] [Google Scholar]
- 17.Reyes-Moreno C, Sharif-Askari E, Girouard J, Leveille C, Jundi M, Akoum A, Lapointe R, Darveau A, Mourad W. Requirement of oxidation-dependent CD40 homodimers for CD154/CD40 bidirectional signaling. J Biol Chem. 2007;282:19473–19480. doi: 10.1074/jbc.M701076200. [DOI] [PubMed] [Google Scholar]
- 18.Dery MC, Van Themsche C, Provencher D, Mes-Masson AM, Asselin E. Characterization of EN-1078D, a poorly differentiated human endometrial carcinoma cell line: a novel tool to study endometrial invasion in vitro. Reprod Biol Endocrinol. 2007;5:38. doi: 10.1186/1477-7827-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouette A, Parent S, Girouard J, Leblanc V, Asselin E. Cisplatin increases B-cell-lymphoma-2 expression via activation of protein kinase C and Akt2 in endometrial cancer cells. Int J Cancer. 2012;130:1755–1767. doi: 10.1002/ijc.26183. [DOI] [PubMed] [Google Scholar]
- 20.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 21.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 22.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 23.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Chakraborty NG, Okino T, Stabach P, Padula SJ, Yamase H, Morse E, Sha’afi RI, Twardzik DR, Shultz LJ, Mukherji B. Adoptive transfer of activated human autologous macrophages results in regression of transplanted human melanoma cells in SCID mice. In Vivo. 1991;5:609–614. [PubMed] [Google Scholar]
- 25.Craig M, Ying C, Loberg RD. Co-inoculation of prostate cancer cells with U937 enhances tumor growth and angiogenesis in vivo. J Cell Biochem. 2007;103:1–8. doi: 10.1002/jcb.21379. [DOI] [PubMed] [Google Scholar]
- 26.Salvesen HB, Akslen LA. Significance of tumour-associated macrophages, vascular endothelial growth factor and thrombospondin-1 expression for tumour angiogenesis and prognosis in endometrial carcinomas. Int J Cancer. 1999;84:538–543. doi: 10.1002/(SICI)1097-0215(19991022)84:5<538::AID-IJC17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto J, Aoki I, Khatun S, Toyoki H, Tamaya T. Clinical implications of expression of interleukin-8 related to myometrial invasion with angiogenesis in uterine endometrial cancers. Ann Oncol. 2002;13:430–434. doi: 10.1093/annonc/mdf078. [DOI] [PubMed] [Google Scholar]
- 28.Ohno S, Ohno Y, Suzuki N, Kamei T, Koike K, Inagawa H, Kohchi C, Soma G, Inoue M. Correlation of histological localization of tumor-associated macrophages with clinicopathological features in endometrial cancer. Anticancer Res. 2004;24:3335–3342. [PubMed] [Google Scholar]
- 29.Conzelmann M, Wagner AH, Hildebrandt A, Rodionova E, Hess M, Zota A, Giese T, Falk CS, Ho AD, Dreger P, Hecker M, Luft T. IFN-gamma activated JAK1 shifts CD40-induced cytokine profiles in human antigen-presenting cells toward high IL-12p70 and low IL-10 production. Biochem Pharmacol. 2010;80:2074–2086. doi: 10.1016/j.bcp.2010.07.040. [DOI] [PubMed] [Google Scholar]
- 30.Shurin MR, Yurkovetsky ZR, Tourkova IL, Balkir L, Shurin GV. Inhibition of CD40 expression and CD40-mediated dendritic cell function by tumor-derived IL-10. Int J Cancer. 2002;101:61–68. doi: 10.1002/ijc.10576. [DOI] [PubMed] [Google Scholar]
- 31.Murugaiyan G, Agrawal R, Mishra GC, Mitra D, Saha B. Functional dichotomy in CD40 reciprocally regulates effector T cell functions. J Immunol. 2006;177:6642–6649. doi: 10.4049/jimmunol.177.10.6642. [DOI] [PubMed] [Google Scholar]
- 32.Murugaiyan G, Martin S, Saha B. Levels of CD40 expression on dendritic cells dictate tumour growth or regression. Clin Exp Immunol. 2007;149:194–202. doi: 10.1111/j.1365-2249.2007.03407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 34.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Mylonas I, Makovitzky J, Shabani N, Richter DU, Kuhn C, Jeschke U, Briese V, Friese K. Leukaemia inhibitory factor (LIF) is immunohistochemically expressed in normal, hyperplastic and malignant endometrial tissue. Eur J Obstet Gynecol Reprod Biol. 2005;118:101–108. doi: 10.1016/j.ejogrb.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 36.Lu Y, Wahl LM. Production of matrix metalloproteinase-9 by activated human monocytes involves a phosphatidylinositol-3 kinase/Akt/IKKalpha/NF-kappaB pathway. J Leukoc Biol. 2005;78:259–265. doi: 10.1189/jlb.0904498. [DOI] [PubMed] [Google Scholar]
- 37.Malik N, Greenfield BW, Wahl AF, Kiener PA. Activation of human monocytes through CD40 induces matrix metalloproteinases. J Immunol. 1996;156:3952–3960. [PubMed] [Google Scholar]
- 38.Zhou M, Zhang Y, Ardans JA, Wahl LM. Interferon-gamma differentially regulates monocyte matrix metalloproteinase-1 and -9 through tumor necrosis factor-alpha and caspase 8. J Biol Chem. 2003;278:45406–45413. doi: 10.1074/jbc.M309075200. [DOI] [PubMed] [Google Scholar]
- 39.Phipps RP. Atherosclerosis: the emerging role of inflammation and the CD40-CD40 ligand system. Proc Natl Acad Sci USA. 2000;97:6930–6932. doi: 10.1073/pnas.97.13.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monaco C, Andreakos E, Kiriakidis S, Feldmann M, Paleolog E. T-cell-mediated signalling in immune, inflammatory and angiogenic processes: the cascade of events leading to inflammatory diseases. Curr Drug Targets. 2004;3:35–42. doi: 10.2174/1568010043483881. [DOI] [PubMed] [Google Scholar]
- 41.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 42.Kelly RW, King AE, Critchley HO. Inflammatory mediators and endometrial function–focus on the perivascular cell. J Reprod Immunol. 2002;57:81–93. doi: 10.1016/S0165-0378(02)00008-6. [DOI] [PubMed] [Google Scholar]
- 43.King AE, Kelly RW, Critchley HO, Malmstrom A, Sennstrom M, Phipps RP. Cd40 expression in uterine tissues: a key regulator of cytokine expression by fibroblasts. J Clin Endocrinol Metab. 2001;86:405–412. doi: 10.1210/jc.86.1.405. [DOI] [PubMed] [Google Scholar]
- 44.Altenburg A, Baldus SE, Smola H, Pfister H, Hess S. CD40 ligand-CD40 interaction induces chemokines in cervical carcinoma cells in synergism with IFN-gamma. J Immunol. 1999;162:4140–4147. [PubMed] [Google Scholar]
- 45.Gallagher NJ, Eliopoulos AG, Agathangelo A, Oates J, Crocker J, Young LS. CD40 activation in epithelial ovarian carcinoma cells modulates growth, apoptosis, and cytokine secretion. Mol Pathol. 2002;55:110–120. doi: 10.1136/mp.55.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.