Abstract
The overexpression of B7-H1 in hepatocellular carcinoma (HCC) mediates HCC immune escape and obstructs the immunotherapy based on tumor-specific CD8+ T cells. Tumor-associated macrophages (TAM) are a major component of cancer-related inflammation and play a central role in tumor promotion. To classify the mechanism underlying the overexpression of B7-H1 in HCC, we examined B7-H1 expression and TAM infiltration in 63 cases of human HCC samples using immunohistochemistry method and found that B7-H1 overexpression was associated with TAM infiltration in HCC tissues. Furthermore, B7-H1 expression was upregulated at both mRNA level and protein level in HCC cells (BEL-7402 and SMMC-7721) cocultured with macrophages in a transwell system. The upregulation of B7-H1 expression induced by macrophage was inhibited by blocking NF-κB or STAT3 signal pathways. These results suggest that overexpression of B7-H1 in HCC may be induced by inflammatory microenvironment involving macrophages and imply that anti-inflammation therapy might be preventive for immune escape and assistant for immunotherapy of HCC.
Keywords: B7-H1, CD274, Hepatocellular carcinoma, PD-L1, Tumor-associated macrophages
Introduction
Hepatocellular carcinoma (HCC) represents the third most common cause of cancer-related death worldwide. Although resection and transplantation are possible therapeutic options, 5-year recurrence rates following resection can exceed 50% [1, 2]. Antitumor immunity mediated by CD8+ T cells plays an important role in controlling tumor progression or recurrence in HCC [3]. Immunotherapy based on CD8+ T cells is a potential therapeutic option for patients with HCC [4, 5]. However, tumors have also evolved numerous immune escape mechanisms, including the production of immunosuppressive cytokines such as IL-10 and TGF-β and generation of cells with immune suppressor functions, such as regulatory T cell (Treg) and myeloid-derived suppressor cell (MDSC) [5–7]. Expression of inhibitory costimulator on CD8+ T cells, such as B7-H1 (also termed PD-L1 and CD274), is also an important mechanism of tumor escape [8–11]. Better understanding of the mechanisms of tumor escape is essential for the design of effective immunotherapy-based clinical protocols to enhance tumor-specific CD8+ T-cell responses.
B7-H1 is a member of B7 family of cosignaling molecules, mainly expressed on immune cells such as B cells, dendritic cells, macrophages, mast cells and T cells. The binding of B7-H1 to its receptor programmed death 1 (PD-1) expressed on activated T cell delivers an inhibitory signal that regulates the balance among T-cell activation, tolerance and immune-mediated tissue damage [12]. In addition to immune cells, most human cancer cells also express high level of B7-H1 protein. The overexpression of B7-H1 in tumor cells leads to apoptosis or impaired activity of tumor-specific T cells infiltrated in tumor tissues, resulting in tumor immune escape [8–11]. Recent studies show that B7-H1 is also overexpressed in HCC cells, and its overexpression is associated with tumor aggressiveness, poor prognosis and postoperative recurrence [10, 11, 13]. However, the mechanisms underlying the overexpression of B7-H1 in HCC are not well understood.
Cancer-related inflammation is considered as the seventh hallmark of cancer, playing decisive role in tumor development [14]. Tumor-associated macrophage (TAM) is a pivotal component of cancer-related inflammation, promoting tumor growth, angiogenesis, invasion and metastasis. The major tumor-promoting mechanism of TAM is the production of cytokines that activate signaling pathways such as NF-κB and STAT3 in malignant cells to induce genes responsible for cell survival, proliferation and migration. In addition, TAM plays an import role in repression of antitumor immunity by producing immunosuppressive cytokines to inhibit the activity of CD8+ T cells, or secreting chemokines to preferentially attract Treg and MDSC [15–17].
The TAM in hepatocellular carcinoma also plays tumor-promoting roles. Macrophage activity facilitates tumor development in a murine model of chemically induced HCC [16]. Studies in human HCC demonstrate that TAM infiltration correlates to tumor size, metastasis, recurrence and angiogenesis, resulting in poor prognosis in HCC patients [18–21]. However, it is unknown whether the B7-H1 overexpression in HCC is associated with TAM. Therefore, we analyzed the relationship between B7-H1 expression and TAM infiltration in HCC tissues and investigated B7-H1 expression in HCC cell lines cocultured with macrophages. The results showed that the overexpression of B7-H1 in tumor cells was correlated with TAM infiltration in HCC tissues. Besides, the expression of B7-H1 in HCC cell lines was upregulated by macropahges through NF-κB and STAT3 signaling. These results suggested a close relation between B7-H1 overexpression in HCC cells and TAM.
Materials and methods
Cell lines and clinical specimens
Human hepatocellular carcinoma cell lines BEL-7402 and SMMC-7721 and human monocytic cell line THP-1 were obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Science (CBTCCCAS), Shanghai, China. BEL-7402, SMMC-7721 and THP-1 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum at 37°C with 5% CO2.
Sixty-three cases of tumor specimens (archived paraffin-embedded sections) included 34 cases from Qilu hospital and 29 cases from Shanghai Outdo Biotech Company. All the patients who the specimens derived from were untreated before receiving surgical excision of HCC from 2003 to 2009. Human peripheral blood was donated from 12 healthy volunteers. The medical ethical committee of Shandong University approved this study. Both the healthy volunteers and the patients with HCC gave their written informed consent. Conventional clinicopathologic variables, including age, gender, tumor size and differentiation, were obtained by reviewing the patients’ medical records.
Immunohistochemistry for B7-H1 and macrophages
Immunohistochemistry for B7-H1 was performed on a 4-mm-thick section using a two-step method with a polyclonal goat anti-B7-H1 antibody (1:75 dilution; Everest Biotech, Oxford, UK) as the primary antibody and a rabbit anti-goat IgG antibody conjugated to horseradish peroxidase (HRP) (ZSGB-BIO, Beijing, China) as the secondary antibody. Immunohistochemistry for macrophages was carried out on the consecutive section using a two-step protocol with a monoclonal mouse anti-CD68 antibody (1:100 dilution; ZSGB-BIO, Beijing, China) as the primary antibody and a goat anti-mouse IgG antibody conjugated to HRP (Maixin, Fuzhou, China) as the secondary antibody. Briefly, paraffin sections were dewaxed and hydrated before heat-mediated antigen retrieval. The endogenous peroxidase was blocked by incubation with 3% solution of hydrogen peroxide in methanol, and non-specific binding sites were blocked with blocking serum. After incubation with the primary antibodies at 4°C overnight, then the secondary antibodies at room temperature for 20 min, the sections were developed in diaminobenzidine solution and counterstained with hematoxylin. Negative controls were performed by omitting the primary antibodies.
B7-H1 expression was evaluated by a digital image system. Briefly, three images of representative fields were captured at a magnification of ×200 and saved as TIFF files. Images were analyzed with Image-Pro Plus version 6.0 software. In order to distinguish B7-H1 expression on HCC cells from those on some infiltrating immune cells, selection tool in the software was used to include cancer nest tissue and exclude peritumoral stroma with more immune cells. The area and the integrated optical density (IOD) of positive staining of B7-H1 were measured, and the mean density is calculated as IOD/area in each image. The average of mean densities of three images from each slide was used to represent an individual sample. TAM was identified as the cell positive of CD68 staining and counted manually at high-power field on the consecutive section. The number of TAM in each sample was determined by averaging the number of TAM in 3 high-power fields where the most CD68 staining was observed and there were not necrosis areas.
Macrophage and HCC cell coculture
THP-1 cells differentiate into macrophages when treated with phorbol-12-myristate-13-acetate (PMA) [22]. To generate macrophages, 1 × 106 THP-1 cells were seeded into the upper insert of a six-well transwell apparatus with 0.4-μm pore size (Corning, Lowell, MA, USA) and treated with PMA (Sigma, St. Louis, MO, USA) at a concentration of 320 nM for 24 h. After a thorough wash to remove all PMA, PMA-treated THP-1 macrophages were cocultured with BEL-7402 or SMMC-7721 cells (in a six-well plate, 2 × 105 cells per well), without direct contact. In the coculture system, BEL-7402 or SMMC-7721 cells were cultured with THP-1-differentiated macrophages for 6, 24 and 48 h and then harvested for use in subsequent experiments.
Human peripheral blood mononuclear cells (PBMC) were isolated by Ficoll Paque density centrifugation from peripheral blood donated by healthy volunteers. As previously described [23, 24], the monocytes were acquired by a plastic adsorptive process and seeded into six-well plates at a density of 5 × 105 cells/well. The monocytes were induced into macrophages by PMA at a concentration of 320 nM in DMEM supplemented with 10% heat-inactivated fetal bovine serum for 24 h at 37°C with 5% CO2. After a thorough wash to remove all PMA, the human macrophages were cocultured with BEL-7402 or SMMC-7721 cells (in the upper insert of a six-well transwell apparatus with 0.4-um pore size, 2 × 105 cells per well), without direct contact. In the coculture system, BEL-7402 or SMMC-7721 cells were cultured with PMA-induced macrophages for 12 and 24 h and then harvested for use in RT-PCR analysis.
Inhibition of NF-κB and STAT3 signaling pathways
For the inhibition of NF-κB pathway, BEL-7402 and SMMC-7721 cells were treated with pyrollidine dithiocarbamate (PDTC) (Sigma, St. Louis, MO, USA) at 10 ng/mL for 1 h. After thorough wash to remove all the PDTC, BEL-7402 or SMMC-7721 cell was cocultured with THP-1-differentiated macrophage for 6 h and then harvested for later use. For the inhibition of STAT3 pathway, BEL-7402 and SMMC-7721 cells were treated with tyrphostin AG490 (Sigma, St. Louis, MO, USA) at 20 ng/mL for 24 h. After thorough wash to remove all the AG490, BEL-7402 or SMMC-7721 cell was cocultured with THP-1-differentiated macrophage for 6 h and then harvested for later use.
RT-PCR analysis
Total RNA was extracted from HCC cells (BEL-7402 and SMMC-7721) with Trizol reagent (Invitrogen, USA) in accordance with the manufacturer’s protocol. After confirming RNA concentration and assessment of purity with Eppendorf Biophotometer (Eppendorf, Hamburg, Germany), equal amounts of total RNA (3 μg) from each sample were reversely transcribed into cDNAs using Reverse Transcription System (Promega, Madison, WI, USA) according to the manufacturer’s recommendation. Equal amounts of cDNA for each sample were used as template for PCR. The following sequence-specific primers were used for PCR amplification: (1) the internal control β-actin gene: forward, 5′-ATTGGCAATGAGCGGTTCCG-3′; reverse, 5′-AGGGCAGTGATCTCCTTCTG-3′ and (2) B7-H1 gene: forward, 5′-CTGGCACATCCTCCAAATGAAAG-3′; reverse, 5′-GAGGCATTGAGTGGAGGCAAAG-3′. RT-PCR of β-actin was performed with 26 cycles of 30 s at 94°C, 30 s at 60°C and 30 s at 72°C, followed by 5 min at 72°C. The product was 212 bp. RT-PCR of B7-H1 was performed with 30 cycles of 30 s at 94°C, 30 s at 56°C and 30 s at 72°C, followed by 5 min at 72°C. The product was 572 bp. The PCR products were electrophoretically separated on a 1.5% agarose gel and visualized by ethidium bromide staining. The gels were scanned on a digital imaging system, and the density of the bands was quantified by densitometry using GelPro 3.2 software. The amount of B7-H1 mRNA in each PCR was normalized on the basis of β-actin mRNA content. The B7-H1/β-actin density ratios represented the expression level of B7-H1 in each individual sample.
Western blot assay
Cells were harvested and washed twice with ice-cold phosphate-buffered saline (PBS). The lysates were obtained with the lysis buffer (50 mM Tris–HCl pH 7.4, 150 mM NaCl, 0.5% deoxycholate 0.1% SDS, 1% Nonidet P-40, 0.02% sodium azide, 100 μg/ml PMSF, 1 μg/ml peptin and 1 μg/ml aprotinin) and then subjected to 10,000×g centrifugation at 4°C for 20 min. Protein levels were quantified with BCA Protein Assay Kit (Beyotime Biotechnology, Shanghai, China). Equal amount of protein from each lysate was subjected to electrophoresis on 12% SDS–polyacrylamide gels and subsequently transferred onto a polyvinylidene difluoride membrane. After blocking with blocking buffer (50 mM Tris–HCl pH 7.4, 0.5 M NaCl and 0.05% Tween-20) containing 5% (w/v) skim milk, the membrane was incubated with primary antibodies (goat anti-human B7-H1 antibody, 1:250 dilution, Everest Biotech, Oxford, UK; mouse anti-human β-actin antibody as an internal control, 1:1,000 dilution, ZSGB-BIO, Beijing, China) at 4°C overnight. After washing, the membrane was further incubated with HRP-conjugated secondary antibodies (rabbit anti-goat IgG and rabbit anti-mouse IgG antibody, 1:2,000 dilution, ZSGB-BIO, Beijing, China) at room temperature for 1 h and then developed using enhanced chemiluminescence (ECL) system (Amersham Biosciences, Buckinghamshire, UK). The density of the bands was quantified by densitometry using GelPro 3.2 software. The B7-H1/β-actin protein ratios were determined by densitometry and represented the normalized expression level of B7-H1 protein.
Statistical analysis
The statistical analysis was performed with the GraphPad Prism software, version 5.0. P < 0.05 was considered statistically significant. The statistical significance of differences between two experiment groups or among three experiment groups was determined by Mann–Whitney test, paired t test or One-way ANOVA, as appropriate.
Results
The overexpression of B7-H1 was associated with TAM infiltration in HCC tissues
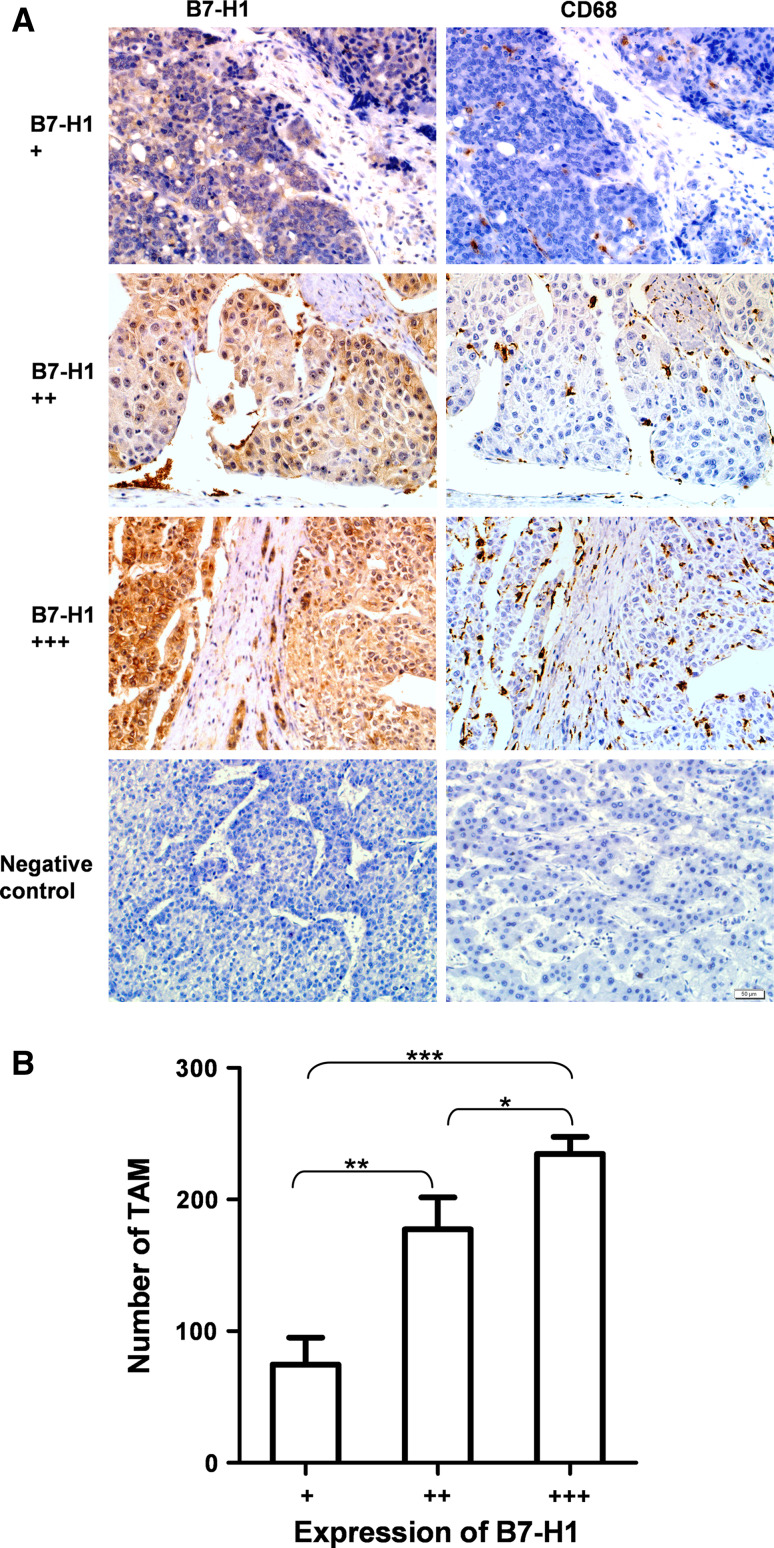
We analyzed B7-H1 expression and TAM infiltration in consecutive sections of 63 HCC tissues samples. Among all HCC specimens, the expression of B7-H1 was shown in cell membrane and cytoplasm and recorded as a value of optical density (average IOD/area). The range of the average IOD/area was 0–0.1 arbitrary unit. The strength of B7-H1 expression was classified into three grades based on the average IOD/area, that is grade +, 0–0.01; grade ++, 0.01–0.05; and grade +++, 0.05–0.1. Groups of grade +, grade ++ and grade +++ included 16, 18 and 29 cases of HCC patients, respectively. Among all HCC specimens, the number of TAM was in the range of 12–350 per high-power field. The averages of TAM number in the grade + group, grade ++ group and grade +++ group were 75, 177 and 235, respectively. TAM numbers in the three groups were significantly different (P < 0.0001, One-way ANOVA among the three groups; P = 0.0011, Mann–Whitney test between the grade + group and the grade ++ group; P < 0.0001, Mann–Whitney test between the grade + group and the grade +++ group; P = 0.022, Mann–Whitney test between the grade ++ group and the grade +++ group). These results clearly revealed a significant correlation between B7-H1 expression and TAM infiltration in HCC (Fig. 1).
Fig. 1.
B7-H1 overexpression was associated with TAM infiltration in HCC tissues. a Expression of B7-H1 and CD68 was tested in consecutive sections from 63 HCC patients using immunohistochemitry method. CD68-positive staining indicated the TAM infiltration. The strength of B7-H1 expression was classified into grade +, grade ++ and grade +++ using Image-Pro Plus 6.0 software. Three representative cases showed B7-H1 expression and TAM infiltration. Positive cells were stained brown (×200). Negative controls were performed by omitting the primary antibodies. b Statistical analysis of the relationship between B7-H1 expression and TAM infiltration. TAM number in HCC tissues with high expression of B7-H1 was significantly higher than that in HCC tissues with low expression of B7-H1. Data shown are expressed as means ± SE. Asterisk indicates P < 0.05. Double asterisk indicates P < 0.01. Triple asterisk indicates P < 0.001
B7-H1 expression was upregulated in HCC cells by PMA-induced macrophages
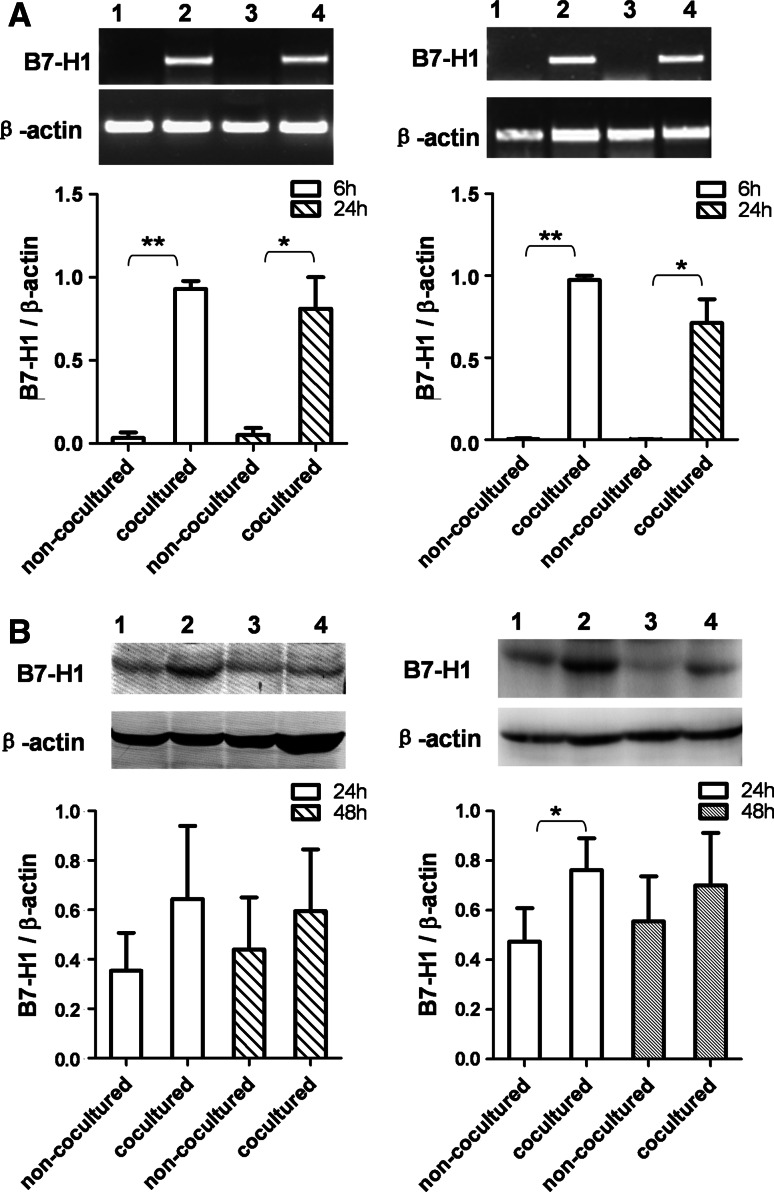
To determine whether macrophage induces the expression of B7-H1 on human HCC cells, we evaluated B7-H1 expression at mRNA and protein levels in BEL-7402 and SMMC-7721 cell lines cocultured with PMA-treated THP-1 cells by RT-PCR and Western blot assays. B7-H1 mRNA expression was significantly upregulated in BEL-7402 and SMMC-7721 cell lines cocultured with PMA-treated THP-1 cells for 6 and 24 h. B7-H1 protein expression was also increased in both cell lines after 24 and 48 h of coculture with PMA-treated THP-1 cells (Fig. 2).
Fig. 2.
B7-H1 expression was upregulated in HCC cells by PMA-treated THP-1 cells. a B7-H1 mRNA expression was upregulated in HCC cells by PMA-treated THP-1 cells. After coculture for 6 and 24 h with PMA-treated THP-1 cells in a transwell system, BEL-7402 (left figure) and SMMC-7721 (right figure) cells were harvested, and B7-H1 mRNA expression was determined by RT-PCR. Lane 1, non-cocultured 6 h; lane 2, cocultured 6 h; lane 3, non-cocultured 24 h; lane 4, cocultured 24 h. Densitometric data shown are means ± SE of the results for three independent experiments. b B7-H1 protein expression was upregulated in HCC cells by PMA-treated THP-1 cells. HCC cells were cocultured with PMA-treated THP-1 cells in a transwell system. After 24 and 48 h of coculture, BEL-7402 (left figure) and SMMC-7721 (right figure) cells were harvested, and B7-H1 protein expression was determined by Western blot. Lane 1, non-cocultured 24 h; lane 2, cocultured 24 h; lane 3, non-cocultured 48 h; lane 4, cocultured 48 h. Densitometric data shown are means ± S.E of the results for three independent experiments. Asterisk indicates P < 0.05. Double asterisk indicates P < 0.01
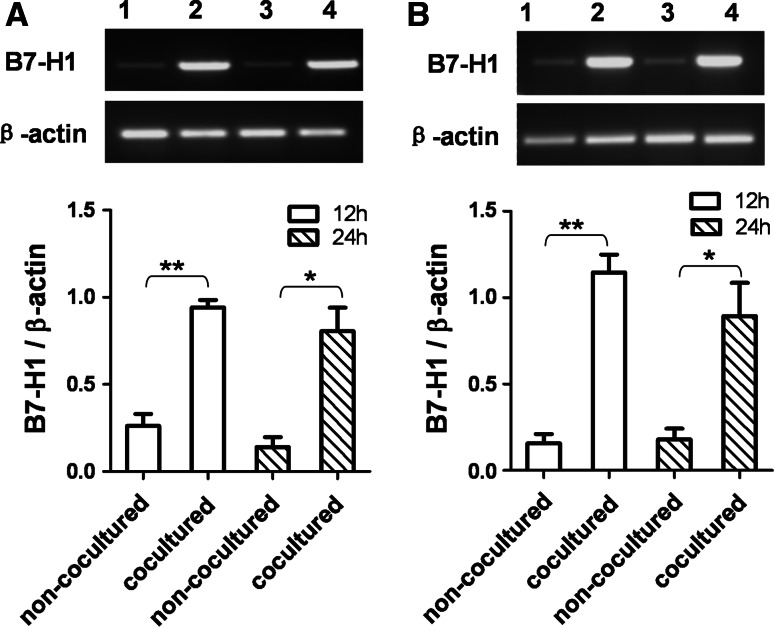
To generalize our findings, monocytes were isolated from human peripheral blood and induced to macrophages by PMA. The B7-H1 mRNA expression in BEL-7402 or SMMC-7721 cell line cocultured with the PMA-induced human macrophages was significantly upregulated (Fig. 3).
Fig. 3.
B7-H1 expression was upregulated in HCC cells by PMA-induced human macrophages After coculture for 12 and 24 h with PMA-induced human macrophages in a transwell system, BEL-7402 (a) and SMMC-7721 (b) cells were harvested, and B7-H1 mRNA expression was determined by RT-PCR. Lane 1, non-cocultured 12 h; lane 2, cocultured 12 h; lane 3, non-cocultured 24 h; lane 4, cocultured 24 h. Densitometric data shown are means ± S.E of the results for three independent experiments. Asterisk indicates P < 0.05. Double asterisk indicates P < 0.01
The upregulation of B7-H1 expression was inhibited by blocking NF-κB and STAT3 signal pathways
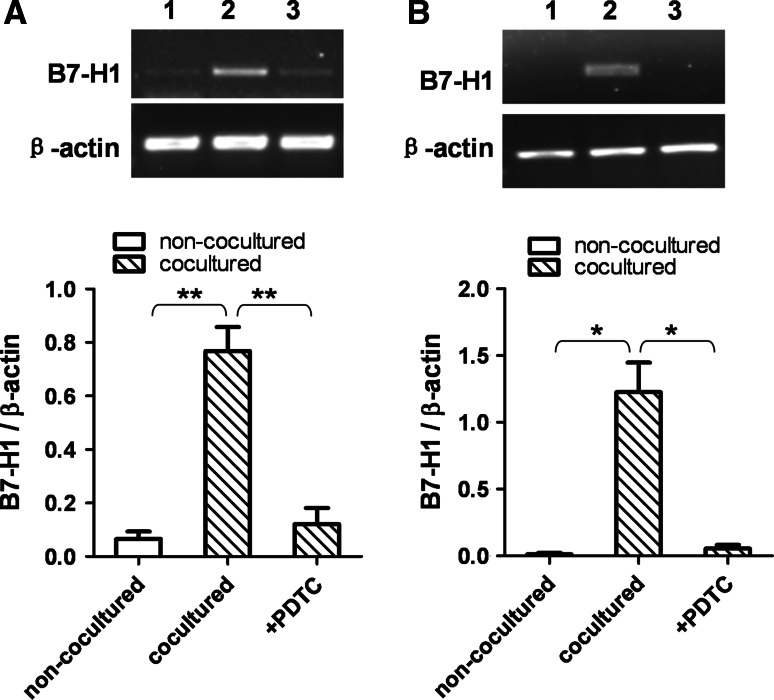
To further clarify the mechanism of upregulation of B7H1 expression in HCC cells cocultured with THP-1-differentiated macrophages, experiments of blocking NF-κB and STAT3 signal pathway were performed. PDTC is a specific inhibitor of NF-κB pathway, while AG490 can selectively inhibit STAT3 pathway. PDTC abrogated the upregulation of B7H1 mRNA expression in both BEL-7402 and SMMC-7721 cells cocultured with PMA-treated THP-1 cells for 6 h (Fig. 4). AG490 partially inhibited the increase in B7-H1 mRNA expression in BEL-7402 and SMMC-7721 cells cocultured with PMA-treated THP-1 cells for 6 h (Fig. 5). These results suggested that PMA-treated THP-1 cells might induce upregulation of B7-H1 expression in BEL-7402 and SMMC-7721 cells through NF-κB and STAT3 signal pathways.
Fig. 4.
The induction of B7-H1 expression was inhibited by blocking NF-κB pathway. BEL-7402 and SMMC-7721 cells were treated with PDTC (10 ng/mL) for one h before coculture with PMA-treated THP-1 cells. After 6 h of coculture, BEL-7402 (a) and SMMC-7721 (b) cells were harvested, and B7-H1 mRNA expression was determined by RT-PCR. Lane 1, non-cocultured; lane 2, cocultured; lane 3, PDTC-treated and cocultured. Densitometric data shown are the means ± SE of triplicate experiments. Asterisk indicates P < 0.05. Double asterisk indicates P < 0.01
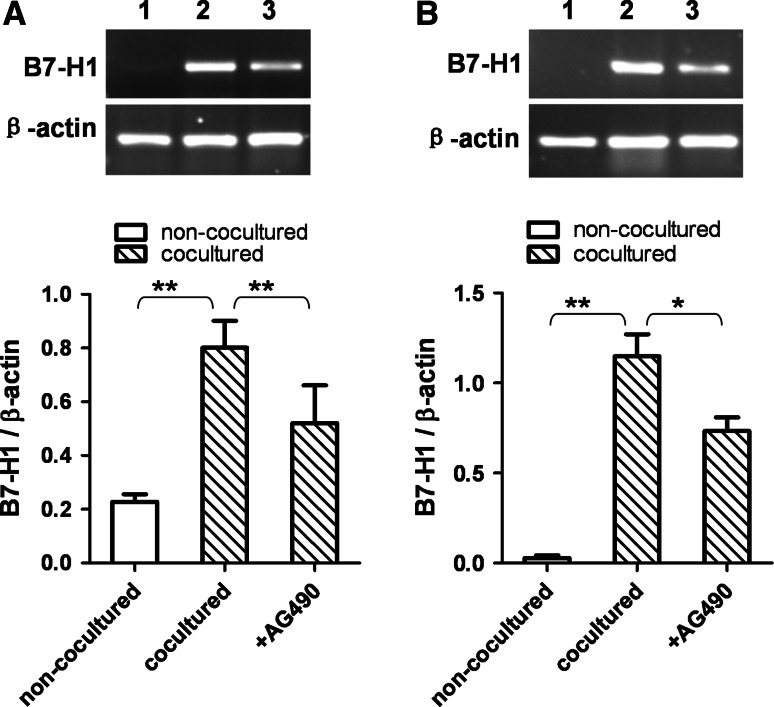
Fig. 5.
The induction of B7-H1 expression was inhibited by blocking STAT3 pathway. BEL-7402 and SMMC-7721 cells were treated with AG490 (20 ng/mL) for 24 h before coculture with PMA-treated THP-1 cells. After 6 h of coculture, BEL-7402 (a) and SMMC-7721 (b) cells were harvested, and B7-H1 mRNA expression was determined by RT-PCR. Lane 1, non-cocultured; lane 2, cocultured; lane 3, AG490-treated and cocultured. Densitometric data shown are the means ± SE of triplicate experiments. Asterisk indicates P < 0.05. Double asterisk indicates P < 0.01
Discussion
B7-H1 is overexpressed in HCC and leads to apoptosis of antitumor CD8+ T cells, contributing to immune escape [10, 11]. In order to explore the mechanisms underlying the overexpresion of B7-H1, we analyzed the relationship between the B7-H1 expression and TAM in HCC. The results showed that the B7-H1 expression in tumor cells was correlated with the infiltration of TAM in HCC tissues. Furthermore, THP-1-differentiated macrophages induced the upregulation of B7-H1 expression in HCC cells lines, depending on the activity of NF-κB and STAT3 signal pathways. These findings suggest that B7-H1 overexpression in tumor cells may result from the cancer-related inflammation involving macrophages in HCC.
Macrophages may induce the B7-H1 expression in HCC through the production of multiple inflammatory cytokines. B7-H1 is inducible in both immune cells and non-immune cells. For instance, cytokines IL-2, IL-7 and IL-15 increase B7-H1 expression on human T cells [25]. Cytokine IL-27 leads to specific upregulation of B7-H1 in DC cells [26]. Autocrine TNF-α and IL-10 released from activated monocytes stimulated monocyte to express B7-H1 in the HCC environment [8, 9]. As for non-immune cells, B7-H1 can be induced by IFN-γ and TNF-α on endothelial cells [27]. And B7-H1 experession on renal tubular epithelial cells (TECs) was increased after TECs were stimulated with IL-1α, TNF-α and IFN-γ [28]. In addition, IFN-α and -γ also can induce B7-H1 expression in hepatocytes and cholangiocytes [29, 30]. Inflammation-associated cytokines, such as TNF-α, IL-10, IL-1α, IL-6, IL-8, IL-12p40 and GM-CSF, are abundant in HCC microenvironment, and the abundance of cytokines can be reflected by their elevated levels in serum of HCC patients [31, 32]. TAM is the major source of the inflammatory cytokines in tumor microenvironment [15–17]. Our results showed that the overexpression of B7-H1 in tumor cells was correlated with TAM infiltration in HCC tissues. Coculture experiment revealed that B7-H1 expression was increased by PMA-treated THP-1 cells or PMA-treated human monocytes. Therefore, the overexpression of B7-H1 in HCC might be induced by cytokines released from TAM.
The overexpression of B7-H1 induced by activated macrophages was dependent on NF-κB and STAT3 signal pathways in HCC cells. Recent studies demonstrate that the expression of B7-H1 gene is involved in activation of STAT3 and NF-κB pathways. Moreover, STAT3 and NF-κB binding sites are identified on the promoter region of B7-H1 gene [33, 34]. In tumor microenviroment, the inflammatory cytokines secreted from TAM mainly activate STAT3 and NF-κB pathways in tumor cells to induce expression of genes responsible for promotion and progression of tumor [15–17]. In this study, the upregulation of B7-H1 expression in HCC cells cocultured with THP-1-differentiated macrophages was abrogated by inhibitors specific for NF-κB or STAT3 signaling. Therefore, the overexpression of B7-H1 may be induced by the inflammatory cytokines released from TAM infiltrated in HCC tissues through activating NF-κB or STAT3 signal pathway.
Our findings that B7-H1 overexprssion in HCC is due to the inflammatory environment provide supportive data for anti-inflammation therapy of HCC. B7-H1 expressed on malignant cells inhibits antitumor immunity by leading to CD8+ T-cell apoptosis, induction of Treg and secretion of IL-10 [12, 35, 36]. Anti-inflammatoy therapies targeting on TAM or signaling pathways like NF-κB and STAT3 may downregulate the B7-H1 expression on malignant cells and enhance the efficacy of immunotherapy based on tumor-specific CD8+ T cells.
PMA-treated THP-1 cells present stable M2 phenotye similar to tumor-associated macrophages. Most TAMs in tumor microenvironment resemble M2 macrophages [15, 17]. We used PMA-treated THP-1 cells as a cell coculture model to provide inflammatory microenvironment for HCC cell lines in vitro because the PMA-treated THP-1 exhibits the functional profile of M2 macrophages. In a previous study [22], M1-polarized THP-1 macrophages were generated by treating THP-1 cells with PMA, IFN-γ and LPS for 24 h. M2-polarized THP-1 macrophages were obtained from THP-1 cells treated with PMA, IL-4 and IL-13 for 24 h. Then, the cytokine profiles of the PMA-treated, M1-polarized and M2-polarized THP-1 macrophages were compared. The PMA-treated and M2-polarized THP-1 macrophages shared the same profile, that is low TNF-α, IL-1b, IL-6 and high TGF-β, which was exact opposite to that of M1-polarized THP-1 macrophages. The PMA-treated THP-1 macrophages also exhibited significant expression of M2 macrophage surface markers CD206 and CD204. Therefore, the phenotype of the PMA-treated THP-1 cells is M2 type.
Our results must be carefully explained with great caution. There are some limitations in the present study. First, it is difficult to completely distinguish B7-H1 molecules on HCC cells from those on some infiltrating immune cells when presenting B7-H1 expression as integrated optical density/area in immunohistochemistry assay, although the immune cells are a minor fraction of the tumor, accounting for 0.5–5% of total cells [37]. In our present study, we therefore used selection tool to include the cancer nest and exclude peritumoral stroma in the Image-Pro Plus version 6.0 software. This is the way we attempted to make the cells expressing B7-H1 molecule be mostly HCC cells. However, we should still not ignore the B7-H1 expression of the tumor-infiltrating immune cells even if they are a minor population. The phenotype of those cells may be different from immune cells in the peri-tumor tissue. Second, the macrophages used in this study were derived from THP-1 cell line and human PBMC. They may be different from TAM in HCC tissues, although PMA-treated THP-1 cells are able to present stable M2 phenotype and mimic the activity of tumor-associated macrophages to a certain extent [22]. Since there is no evidence that tumor-associated macrophages directly enhance the B7-H1 molecules on HCC cells, our findings presented here might be observed only under a specific condition. To generalizing our findings further, macrophages derived from HCC tissues will be performed as an emphasis in our next work. Third, cytokines responsible for the increased B7-H1 expression in HCC cells are not identified. It would be highly relevant to know which soluble factors, released by TAM, can upregulate B7-H1 expression in HCC cells. In our future work, we will perform protein array to identify the cytokines and use immunohistochemistry method to analyze the relation between the cytokine expression and B7-H1 expression in HCC tissues to improve the relevance of our findings.
In summary, the present study analyzed the relationship between B7-H1 expression and TAM in HCC. We found that the overexpression of B7-H1 in malignant cells was associated with TAM infiltration in HCC tissues. The B7-H1 expression in HCC cell lines was upregulated by PMA-induced macrophages, and the upregulation of B7-H1 expression depended on NF-κB and STAT3 signaling pathways. These results suggested that inflammatory microenvironment may be responsible for B7-H1 overexpression in tumor cells and involved in immune escape of HCC. Therefore, anti-inflammation therapy may be preventive for immune escape and assistant in immunotherapy of HCC.
Acknowledgments
This work was supported by grants from the National Nature Science Foundation of China (No. 81071705 and No. 30500591), the Award Funds for Excellent Young and Middle-aged Scientists of Shandong Province (BS2009YY002) and the National “973” Program of China (No. 2011CB503906).
Footnotes
Jie Chen and Guosheng Li contributed equally to this work.
References
- 1.Andreana L, Burroughs AK. Treatment of early hepatocellular carcinoma: how to predict and prevent recurrence. Dig Liver Dis. 2010;42(Suppl 3):S249–S257. doi: 10.1016/S1590-8658(10)60513-0. [DOI] [PubMed] [Google Scholar]
- 2.Nowak AK, Chow PK, Findlay M. Systemic therapy for advanced hepatocellular carcinoma: a review. Eur J Cancer. 2004;40(10):1474–1484. doi: 10.1016/j.ejca.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 3.Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, Hiraide A, Sakaki M, Doi H, Uozumi S, Omori R, Matsumura T, Yanagawa T, Ito T, Imawari M. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2010;45(4):451–458. doi: 10.1007/s00535-009-0155-2. [DOI] [PubMed] [Google Scholar]
- 4.Sun JC, Pan K, Chen MS, Wang QJ, Wang H, Ma HQ, Li YQ, Liang XT, Li JJ, Zhao JJ, Chen YB, Pang XH, Liu WL, Cao Y, Guan XY, Lian QZ, Xia JC. Dendritic cells-mediated CTLs targeting hepatocellular carcinoma stem cells. Cancer Biol Ther. 2010;10(4):368–375. doi: 10.4161/cbt.10.4.12440. [DOI] [PubMed] [Google Scholar]
- 5.Zhang HH, Mei MH, Fei R, Liao WJ, Wang XY, Qin LL, Wang JH, Wei L, Chen HS. Regulatory T cell depletion enhances tumor specific CD8 T-cell responses, elicited by tumor antigen NY-ESO-1b in hepatocellular carcinoma patients, in vitro. Int J Oncol. 2010;36(4):841–848. doi: 10.3892/ijo_00000561. [DOI] [PubMed] [Google Scholar]
- 6.Kim R, Emi M, Tanabe K. Cancer cell immune escape and tumor progression by exploitation of anti-inflammatory and pro-inflammatory responses. Cancer Biol Ther. 2005;4(9):924–933. doi: 10.4161/cbt.4.9.2101. [DOI] [PubMed] [Google Scholar]
- 7.Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50(3):799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7–H1/programmed death-1 interactions. Cancer Res. 2009;69(20):8067–8075. doi: 10.1158/0008-5472.CAN-09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206(6):1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, Yang YP, Tien P, Wang FS. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128(4):887–896. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 11.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15(3):971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 12.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gehring AJ, Ho ZZ, Tan AT, Aung MO, Lee KH, Tan KC, Lim SG, Bertoletti A. Profile of tumor antigen-specific CD8 T cells in patients with hepatitis B virus-related hepatocellular carcinoma. Gastroenterology. 2009;137(2):682–690. doi: 10.1053/j.gastro.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 14.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 15.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Karin M. The IkappaB kinase—a bridge between inflammation and cancer. Cell Res. 2008;18(3):334–342. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- 17.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding T, Xu J, Wang F, Shi M, Zhang Y, Li SP, Zheng L. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum Pathol. 2009;40(3):381–389. doi: 10.1016/j.humpath.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26(16):2707–2716. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 20.Peng SH, Deng H, Yang JF, Xie PP, Li C, Li H, Feng DY. Significance and relationship between infiltrating inflammatory cell and tumor angiogenesis in hepatocellular carcinoma tissues. World J Gastroenterol. 2005;11(41):6521–6524. doi: 10.3748/wjg.v11.i41.6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J, Ding T, Pan W, Zhu LY, Li L, Zheng L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer. 2009;125(7):1640–1648. doi: 10.1002/ijc.24556. [DOI] [PubMed] [Google Scholar]
- 22.Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao YH, Chu CY, Tsai TF, Chiu HC, Dai YS, Inoue H, Yang PC, Kuo ML, Jee SH. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol. 2009;129(4):1016–1025. doi: 10.1038/jid.2008.310. [DOI] [PubMed] [Google Scholar]
- 23.Wu P, Liang DH, Liu YF, Liu YY, Zhang XL, Fu Q, Miao F. Effects of high-density lipoprotein 1 on the formation of foam cells from human monocyte-derived macrophages. Zhonghua Xin Xue Guan Bing Za Zhi. 2009;37(2):156–160. [PubMed] [Google Scholar]
- 24.Bever CT, Jr, Morgan KD, Whitaker JN. Cathepsin D activity in human peripheral blood mononuclear leukocytes. Inflammation. 1989;13(3):309–316. doi: 10.1007/BF00914397. [DOI] [PubMed] [Google Scholar]
- 25.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, Fauci AS. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181(10):6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 26.Karakhanova S, Bedke T, Enk AH, Mahnke K. IL-27 renders DC immunosuppressive by induction of B7–H1. J Leukoc Biol. 2011;89(6):837–845. doi: 10.1189/jlb.1209788. [DOI] [PubMed] [Google Scholar]
- 27.Mazanet MM, Hughes CC. B7–H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002;169(7):3581–3588. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Zhang J, Li J, Zou L, Zhao T, Tang Y, Wu Y. Expression of B7–H1 in inflammatory renal tubular epithelial cells. Nephron Exp Nephrol. 2006;102(3–4):e81–e92. doi: 10.1159/000089686. [DOI] [PubMed] [Google Scholar]
- 29.Gong AY, Zhou R, Hu G, Li X, Splinter PL, O’Hara SP, LaRusso NF, Soukup GA, Dong H, Chen XM. MicroRNA-513 regulates B7–H1 translation and is involved in IFN-gamma-induced B7–H1 expression in cholangiocytes. J Immunol. 2009;182(3):1325–1333. doi: 10.4049/jimmunol.182.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mühlbauer M, Fleck M, Schütz C, Weiss T, Froh M, Blank C, Schölmerich J, Hellerbrand C. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006;45(4):520–528. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Castello G, Scala S, Palmieri G, Curley SA, Izzo F. HCV-related hepatocellular carcinoma: from chronic inflammation to cancer. Clin Immunol. 2010;134(3):237–250. doi: 10.1016/j.clim.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Capone F, Costantini S, Guerriero E, Calemma R, Napolitano M, Scala S, Izzo F, Castello G. Serum cytokine levels in patients with hepatocellular carcinoma. Eur Cytokine Netw. 2010;21(2):99–104. doi: 10.1684/ecn.2010.0192. [DOI] [PubMed] [Google Scholar]
- 33.Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA, Wasik MA. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7–H1) Proc Natl Acad Sci USA. 2008;105(52):20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Zhang J, Guo G, Ruan Z, Jiang M, Wu S, Guo S, Fei L, Tang Y, Yang C, Jia Z, Wu Y. Induced B7–H1 expression on human renal tubular epithelial cells by the sublytic terminal complement complex C5b–9. Mol Immunol. 2009;46(3):375–383. doi: 10.1016/j.molimm.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 35.Dong H, Zhu G, Tamada K, Chen L. B7–H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 36.Ge W, Ma X, Li X, Wang Y, Li C, Meng H, Liu X, Yu Z, You S, Qiu L. B7–H1 up-regulation on dendritic-like leukemia cells suppresses T cell immune function through modulation of IL-10/IL-12 production and generation of Treg cells. Leuk Res. 2009;33(7):948–957. doi: 10.1016/j.leukres.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Chew V, Tow C, Teo M, Wong HL, Chan J, Gehring A, Loh M, Bolze A, Quek R, Lee VK, Lee KH, Abastado JP, Toh HC, Nardin A. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J Hepatol. 2010;52(3):370–379. doi: 10.1016/j.jhep.2009.07.013. [DOI] [PubMed] [Google Scholar]