Abstract
Immunocheckpoint inhibitors targeting the programmed cell death-1 (PD-1) and PD-1 ligand 1 (PD-L1) axis have shown promising results in patients with non-small-cell lung cancer (NSCLC). Recent research has shown that epidermal growth factor receptor (EGFR) signaling affects PD-L1 expression in NSCLC cells; however, the mechanism regulating PD-L1 expression in tumor cells remains unclear. Using immunohistochemistry, we evaluated the impact of expression of PD-L1 and EGF family receptors EGFR and human epidermal growth factor receptor 2 (HER2) in tumor cells from 91 patients with pathological Stage IA–IIIA NSCLC. Overexpression of PD-L1 was observed in 14% of the resected tumors, and associated with poor recurrence-free survival (p = 0.021) and overall survival (p = 0.033). PD-L1 expression is positively correlated with EGFR expression and inversely correlated with HER2. NSCLC cell lines were treated in vitro with the EGFR ligand EGF with or without inhibition of EGFR or HER2, after which PD-L1 expression was evaluated using flow cytometry. Consistent with previous reports, PD-L1 expression was clearly enhanced by EGF. EGFR-tyrosine kinase inhibitors or EGFR small interfering RNA (siRNA) blocked EGF-induced PD-L1 overexpression in NSCLC cell lines, but HER2 siRNA did not. Moreover, our findings suggest that PD-L1 expression could be partially regulated via the PI3K/AKT and JAK/STAT pathways. We conclude that PD-L1 overexpression is associated with poor prognosis and is positively correlated with EGFR expression but inversely correlated with HER2 expression in NSCLC. We also showed that EGFR and HER2 have different effects on EGF-induced PD-L1 expression in NSCLC cell lines.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-1986-y) contains supplementary material, which is available to authorized users.
Keywords: Non-small-cell lung cancer (NSCLC), Prognostic factor, PD-L1, EGFR, HER2
Introduction
The interaction between programmed cell death-1 (PD-1) and PD-1 ligand 1 (PD-L1) could inhibit T-cell activation and proliferation, which leads to the immune evasion of tumor cells [1–3]. Recent clinical studies with immunocheckpoint inhibitors targeting the PD-1/PD-L1 axis have shown promising results in patients with several types of cancer, including non-small-cell lung cancer (NSCLC) [4–7]. Some studies have reported that overexpression of PD-L1 was correlated with improved prognosis [8, 9], while others have described its expression as a poor prognostic factor for clinical outcome in patients with NSCLC [10–12]. The epidermal growth factor (EGF) family receptors EGFR and human epidermal growth factor receptor 2 (HER2) are transmembrane tyrosine kinase receptors and activate downstream pathways of these receptors [13]. Although the mechanism regulating PD-L1 expression in tumor cells remains unclear, recent research has revealed that EGFR affects PD-L1 expression in NSCLC cells [14–16]; however, no report to date has evaluated the relationship between PD-L1 and HER2.
In the present study, we evaluated the expression of PD-L1 in relation to the EGF family receptors EGFR and HER2 using immunohistochemistry (IHC) in resected pathological Stage I–IIIA NSCLC tissue samples, and determined the relationship between expression of these receptors, clinicopathological characteristics, and survival. In addition, in vitro assays were performed to assess the mechanism of regulation of PD-L1 expression via EGFR/HER2 signaling.
Our findings suggested that PD-L1 overexpression in tumor cells can be used as a predictive factor of poor clinical outcome. In addition, PD-L1 expression is positively correlated with EGFR overexpression in patients with NSCLC, while, interestingly, it is inversely correlated with HER2 overexpression in these patients. It is well known that PD-L1 expression is upregulated by interferon (IFN)-γ [1]. Moreover, other stimuli such as EGFR [14–16] and EML4-ALK [17] signaling are also known as inducers of PD-L1 in NSCLC. Our in vitro findings suggested that EGF-induced PD-L1 expression could be partially regulated via the PI3K/AKT and JAK/STAT pathways in NSCLC cells. These results suggest a promising area of focus for therapies targeting the EGFR signaling pathway to eliminate tumor cells via downregulation of PD-L1 in NSCLC. On the other hand, HER2 has different roles from that of EGFR in the regulation of PD-L1 expression.
Materials and methods
Patients and specimens
Our research was approved by the Kawasaki Medical School ethics committee (No. 1227-3), and written informed consent was obtained from all patients for the use of specimens. This retrospective study included 91 patients with primary invasive adenocarcinoma or squamous cell carcinoma who underwent evaluation with 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) scans (Discovery ST Elite; GE Healthcare, Fairfield, CT, USA) before undergoing lobectomy with lymph node dissection in our department between January 2007 and January 2011. Data regarding this patient population have previously been published [18]. Histologic diagnosis was based on hematoxylin and eosin staining according to the World Health Organization 2004 criteria [19] and the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) classification of lung adenocarcinoma [20]. Pathological stages were defined according to the 7th edition of the TNM classification [21]. The postoperative follow-up strategy employed in our institute was previously described [18].
Immunohistochemical staining
IHC was performed on the surgically resected tissues using the following antibodies: a mouse monoclonal anti-PD-L1 antibody (1:100, clone SP142, Spring Bioscience, Pleasanton, CA, USA), EGFR (1:50, clone A-10, Santa Cruz Biotechnology, Dallas, TX, USA), and HER2 (1:100, clone 3B5, Santa Cruz Biotechnology), according to a previously described protocol [18]. Two investigators (R. Okita and A. Maeda) who had no prior knowledge of the corresponding clinicopathological data jointly quantified the immunoreactivity of each molecule. The intensity scoring for staining was defined as follows: “0”: no staining, “1+”: weak staining that was visible only with high magnification, “2+”: moderate staining (between 1+ and 3+), and “3+”: strong staining that was visible with low magnification. The histoscore (H-score) was calculated according to the following formula: 1 × (%cells 1+) + 2 × (%cells 2+) + 3 × (%cells 3+) [22].
Cell culture and reagents
Human lung adenocarcinoma cell lines A549, LC2/Ad, and RERF-LC-KJ were obtained from Riken BRC through the National BioResource Project of the Ministry of Education, Culture, Sports, Science and Technology (Tsukuba, Japan), while human adenocarcinoma cell line PC-9 was obtained from the Immuno-Biological Laboratories cell bank (Gunma, Japan). All cell lines were authenticated by genotyping with the PowerPlex 16 STR system (Promega, Madison, WI, USA) and maintained as previously described [23]. Gefitinib (Cayman Chemical, Ann Arbor, MI, USA), lapatinib (Selleckchem, Houston, TX, USA), LY294002 (Cayman Chemical), PD98059 (Cayman Chemical), and tofacitinib (Selleckchem) were dissolved in DMSO (Sigma-Aldrich, St. Louis, MO, USA), while EGF (R&D Systems, Minneapolis, MN, USA) was dissolved in PBS (−).
Flow cytometric analysis
Extracellular staining with fluorochrome-conjugated antibodies was performed according to standard flow cytometric staining protocols as previously described [24]. The following antibodies were used for flow cytometry: phycoerythrin (PE)-labeled PD-L1 (clone 29E.2A3), allophycocyanin (APC)-labeled EGFR (clone AY13), PE-labeled HER2 (clone 24D2), and the isotype control PE-labeled or APC-labeled anti-mouse IgG2aκ (clone MOPC-173) antibodies, which were all obtained from BioLegend (San Diego, CA, USA). Cells were acquired on a FACSCanto II flow cytometer with the FACSDiva software (BD Biosciences, San Diego, CA, USA) via FL2 filter (Dye 488 nm) for PE-labeled antibodies and FL4 filter (Dye: 633 nm) for APC-labeled antibodies, then analyzed with the FlowJo software 6.4.7 (Treestar, Ashland, OR, USA).
Small interfering RNA assay
NSCLC cell lines were grown at 30% confluence in 12-well plates, then transfected with EGFR-targeting small interfering RNA (siRNA) (#L-003114-00-0005, Dharmacon, Lafayette, CO, USA), HER2-targeting siRNA (#L-003126-00-0005, Dharmacon), or control siRNA (#L-001810-10-05, Dharmacon) with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) and Opti-MEM I medium (Invitrogen), as previously described [23]. After 24 h incubation, transfected cells were treated with EGF or left untreated for 24 h, then further analyzed.
Western blot analysis
Cell extracts were prepared and the protein concentrations were assessed using the bicinchoninic acid assay (Takara Bio, Kusatsu, Japan), after which immunoblotting was performed using the iBlot2 dry blotting system (Life Technologies, Carlsbad, CA, USA) and visualized with enhanced chemiluminescence prime detection reagent (GE Healthcare, Fairfield, CT, USA) and the LAS-4000 camera system (Fuji film, Tokyo, Japan), as previously described [23]. The following antibodies were used: EGFR (clone 1F4), HER2 (clone 44E7), phosphorylated (p)-EGFR (Tyr1068, clone D7A5), p-HER2 (Tyr1221/1222, clone 6B12), and β-actin (clone 8H10D10) (Cell Signaling Technology, Beverly, MA, USA). Membranes were stripped using stripping buffer (Takara Bio) for 30 min and reprobed up to two times.
Statistical analysis
Receiver operating characteristic (ROC) curves for EGFR, HER2, and PD-L1 to predict 2-year recurrence-free survival (RFS) were generated to determine the expected cut-off value that yielded optimal sensitivity and specificity. Differences in means were evaluated with the unpaired t test or Mann–Whitney test, while correlation analysis was evaluated with Spearman’s rank correlation test. Chi-square tests or Fisher’s exact tests were performed to compare the expression levels of PD-L1, EGFR, and HER2 among patients. Kaplan–Meier survival analysis was performed to determine the associations between PD-L1, EGFR, and HER2 expression and RFS or overall survival (OS). The significance of the differences in RFS or OS between groups was estimated using the log-rank test. The above statistical analyses were performed using GraphPad Prism 6.01 (GraphPad Software, La Jolla, CA, USA). Univariate and multivariate analyses were performed using the Cox-proportional hazards model to identify the independent prognostic factors with SPSS statistical package 17.0 (SPSS, Chicago, IL, USA). In all cases, a value of p < 0.05 was considered significant.
Results
Clinical characteristics
Patients with histories of other malignancy, limited resection, non-curative resection, or adenosquamous carcinoma, as well as those who had received radiotherapy or chemotherapy prior to surgery, were excluded from the present study. Therefore, a total of 91 patients with primary invasive adenocarcinoma or squamous cell carcinoma who had undergone lobectomy with node dissection were enrolled. The median follow-up time was 41.4 months (range 1–80 months) for RFS and 48.2 months (range 1–89 months) for OS.
Representative IHC patterns for EGFR, HER2, or PD-L1 are shown in Supplementary Fig. 1. The expected cut-off values for the H score of each molecule according to ROC curves (Supplementary Fig. 2) are as follows: EGFR: H-score ≦100, HER2: H-score ≦150, and PD-L1: H-score ≦100. Of the total 91 tumors, 12 cases (13%) showed EGFR overexpression, 35 cases (38%) showed HER2 overexpression, and 13 cases (14%) showed PD-L1 overexpression in tumor cells. EGFR overexpression was correlated with histology (p < 0.001), histologic grade (p = 0.030), lymphatic invasion (p = 0.010), vascular invasion (p = 0.049), and lymph node metastasis (p < 0.001), while HER2 overexpression was correlated with sex (p < 0.001), smoking status (p = 0.006), maximum standardized uptake value (SUVmax) on FDG-PET/CT scan (p < 0.001), tumor size (p = 0.038), histology (p < 0.001), histologic grade (p < 0.001), pleural invasion (p = 0.017), vascular invasion (p < 0.001), and pathological stage (p < 0.001). PD-L1 overexpression was correlated with sex (p = 0.004), smoking status (p = 0.002), SUVmax on FDG-PET/CT scan (p < 0.001), histology (p = 0.003), histologic grade (p = 0.003), lymph node metastasis (p = 0.015), and pathological stage (p = 0.014) (Table 1).
Table 1.
Clinicopathological characteristics by expressions of EGFR, HER2, and PD-L1 in 91 patients with NSCLC
| EGFR expression | HER2 expression | PD-L1 expression | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Number (n = 91) | Low | High | p | Low | High | p | Low | High | p |
| Age, 67.8 (37–83) | 67.3 ± 8.6 | 70.9 ± 6.4 | 0.166 | 68.3 ± 8.9 | 66.9 ± 7.5 | 0.439 | 67.7 ± 8.3 | 68.0 ± 9.4 | 0.919 | |
| Sex | ||||||||||
| Male | 59 (65%) | 49 | 10 | 0.150 | 44 | 15 | <0.001 | 46 | 13 | 0.004 |
| Female | 32 (35%) | 30 | 2 | 12 | 20 | 32 | 0 | |||
| Smokinga | ||||||||||
| Never | 33 (37%) | 31 | 2 | 0.123 | 14 | 19 | 0.006 | 32 | 1 | 0.002 |
| Current or former | 57 (63%) | 47 | 10 | 41 | 16 | 45 | 12 | |||
| CEA (ng/ml) | 9.4 ± 28.5 | 6.7 ± 6.5 | 0.747 | 12.4 ± 33.6 | 3.8 ± 3.3 | 0.135 | 9.5 ± 28.7 | 6.1 ± 4.4 | 0.669 | |
| SUVmax | 7.0 ± 5.5 | 10.1 ± 3.1 | 0.058 | 9.4 ± 5.4 | 4.2 ± 3.3 | <0.001 | 6.5 ± 5.0 | 12.4 ± 4.8 | <0.001 | |
| Tumor size (mm) | 30.6 ± 13.4 29.3 ± 12.2 | 0.767 | 32.7 ± 13.1 | 26.8 ± 12.8 | 0.038 | 29.7 ± 13.5 34.9 ± 10.6 | 0.190 | |||
| Histology | ||||||||||
| Ad | 71 (78%) | 67 | 4 | <0.001 | 37 | 34 | <0.001 | 65 | 6 | 0.003 |
| Sq | 20 (22%) | 12 | 8 | 19 | 1 | 13 | 7 | |||
| Histologic grade | ||||||||||
| G1 | 38 (42%) | 37 | 1 | 0.030 | 14 | 24 | <0.001 | 38 | 0 | 0.003 |
| G2 | 29 (32%) | 22 | 7 | 19 | 10 | 23 | 6 | |||
| G3 | 24 (26%) | 20 | 4 | 23 | 1 | 17 | 7 | |||
| Pleural invasion | ||||||||||
| Negative | 59 (65%) | 50 | 9 | 0.429 | 31 | 28 | 0.017 | 52 | 7 | 0.370 |
| Positive | 32 (35%) | 29 | 3 | 25 | 7 | 26 | 6 | |||
| Lymphatic invasion | ||||||||||
| Negative | 66 (73%) | 61 | 5 | 0.010 | 37 | 29 | 0.081 | 56 | 10 | 0.701 |
| Positive | 25 (27%) | 18 | 7 | 19 | 6 | 22 | 3 | |||
| Vascular invasion | ||||||||||
| Negative | 54 (59%) | 50 | 4 | 0.049 | 25 | 29 | <0.001 | 49 | 5 | 0.098 |
| Positive | 37 (41%) | 29 | 8 | 31 | 6 | 29 | 8 | |||
| Lymphnode metastasis | ||||||||||
| N0 | 72 (79%) | 67 | 5 | <0.001 | 41 | 31 | 0.080 | 65 | 7 | 0.015 |
| N1–2 | 19 (21%) | 12 | 7 | 15 | 4 | 13 | 6 | |||
| Pathological stage | ||||||||||
| IA | 35 (38%) | 33 | 2 | 0.096 | 13 | 22 | <0.001 | 34 | 1 | 0.014 |
| IB–IIIA | 56 (62%) | 46 | 10 | 43 | 13 | 44 | 12 | |||
CEA carcinoembryonic antigen, SUV max maximum standardized uptake value, G grade, Ad adenocarcinoma, Sq squamous cell carcinoma
Bold values indicate p < 0.05
aData not available for one patient
PD-L1 overexpression as an indicator of poor prognosis in resected NSCLC
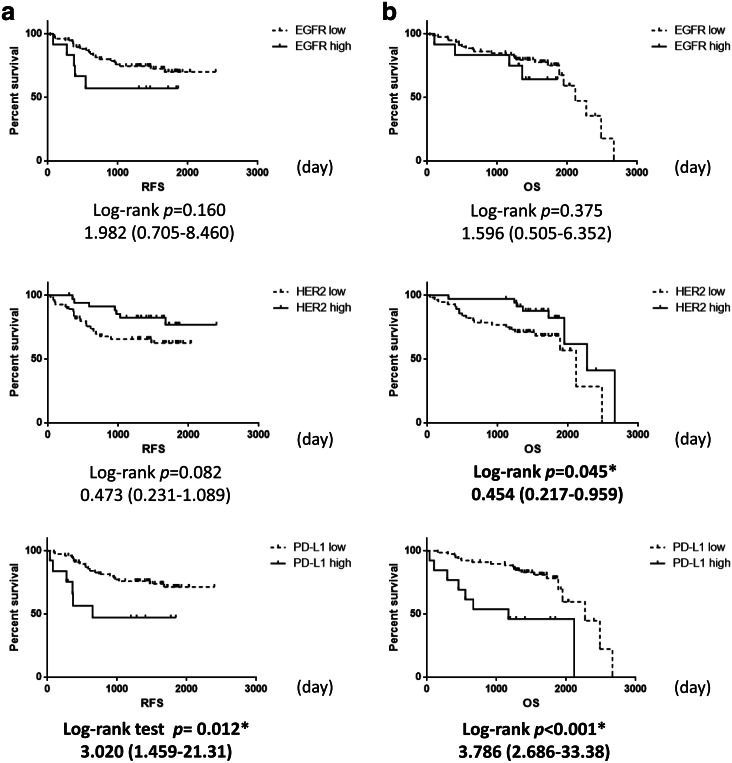
Survival analysis was performed in 91 patients who underwent curative resection for pathological Stage I–IIIA NSCLC. The results indicated that HER2 overexpression was associated with improved OS (p = 0.045), while PD-L1 overexpression was associated with poor RFS (p = 0.012) and OS (p < 0.001). EGFR overexpression had no impact on clinical outcome (Fig. 1).
Fig. 1.
Recurrence-free survival and overall survival in patients with NSCLC. Kaplan–Meier (a): recurrence-free survival (RFS) or (b): overall survival (OS) curves in patients with expression status of EGFR, HER2, or PD-L1. Significantly better overall survival was noted for the group with high HER2 expression than that with low HER2 expression. Significantly worse RFS and OS were noted for the group with high PD-L1 expression than that with low PD-L1 expression. Data were analyzed using log-rank test
Next, Cox regression analyses were performed to determine the predictive value of clinical variables for RFS or OS. Univariate analysis showed that lymphatic invasion, vascular invasion, lymph node metastasis, and PD-L1 expression status were potential predictors of RFS. Consistent with past reports [25, 26], multivariate analysis revealed lymphatic invasion to be a poor prognostic factor, and PD-L1 overexpression was also identified as an independent poor prognostic factor for RFS (Table 2). The association between clinical variables and OS was further investigated. Univariate analysis showed that pleural invasion, vascular invasion, lymph node metastasis, low expression of HER2, and overexpression of PD-L1 were potential predictors of OS. Multivariate analysis revealed only PD-L1 overexpression to be a poor prognostic factor for OS (Table 2).
Table 2.
Cox-proportional hazard model for RFS and OS (n = 91)
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |||
| RFS | ||||||
| Sex (male vs female) | 0.818 (0.364–1.837) | 0.627 | 1.551 (0.272–8.836) | 0.621 | ||
| Smokinga (non- vs smoker) | 1.066 (0.483–2.351) | 0.874 | 1.249 (0.220–7.077) | 0.802 | ||
| Histology (Ad vs Sq) | 1.070 (0.403–2.842) | 0.892 | 2.392 (0.732–7.816) | 0.149 | ||
| Pleural invasion (neg vs pos) | 1.553 (0.713–3.383) | 0.268 | 0.985 (0.336–2.885) | 0.978 | ||
| Lymphatic invasion (neg vs pos) | 5.244 (2.391–11.498) | <0.001 | 3.151 (1.024–9.694) | 0.045 | ||
| Vascular invasion (neg vs pos) | 3.749 (1.667–8.432) | 0.001 | 1.300 (0.550–3.073) | 0.549 | ||
| Lymphnode metastasis (neg vs pos) 8.087 (3.673–17.806) | <0.001 | 2.772 (0.873–8.799) | 0.084 | |||
| EGFR expression (low vs high) | 1.989 (0.748–5.289) | 0.168 | 1.184 (0.367–3.824) | 0.777 | ||
| HER2 expression (low vs high) | 0.471 (0.198–1.123) | 0.089 | 0.567 (0.183–1.759) | 0.326 | ||
| PD-L1 expression (low vs high) | 3.045 (1.217–7.619) | 0.017 | 4.103 (1.235–13.629) | 0.021 | ||
| OS | ||||||
| Sex (male vs female) | 0.554 (0.218–1.405) | 0.213 | 1.365 (0.196–9.485) | 0.753 | ||
| Smokinga (non- vs smoker) | 1.835 (0.721–4.670) | 0.203 | 1.304 (0.216–7.884) | 0.772 | ||
| Histology (Ad vs Sq) | 0.823 (0.302–2.238) | 0.702 | 1.732 (0.557–5.383) | 0.343 | ||
| Pleural invasion (neg vs pos) | 2.406 (1.037–5.585) | 0.041 | 1.952 (0.697–5.470) | 0.203 | ||
| Lymphatic invasion (neg vs pos) | 2.061 (0.900–4.720) | 0.087 | 1.066 (0.335–3.390) | 0.914 | ||
| Vascular invasion (neg vs pos) | 2.247 (1.302–3.877) | 0.004 | 1.309 (0.589–2.911) | 0.509 | ||
| Lymphnode metastasis (neg vs pos) | 3.727 (1.611–8.623) | 0.002 | 2.030 (0.627–6.575) | 0.238 | ||
| EGFR expression (low vs high) | 1.729 (0.580–5.154) | 0.326 | 1.505 (0.440–5.145) | 0.514 | ||
| HER2 expression (low vs high) | 0.340 (0.126–0.913) | 0.032 | 0.549 (0.150–2.002) | 0.363 | ||
| PD-L1 expression (low vs high) | 4.504 (1.894–10.707) | 0.001 | 3.315 (1.102–9.972) | 0.033 | ||
RFS recurrence free survival, OS overall survival, CI confidence interval, Ad adenocarcinoma, Sq squamous cell carcinoma, PD-L1 programed death ligand 1
Bold values indicate p < 0.05
aData not available for one patient
PD-L1 overexpression is positively correlated with EGFR expression but inversely correlated with HER2 expression in resected NSCLC tissue samples
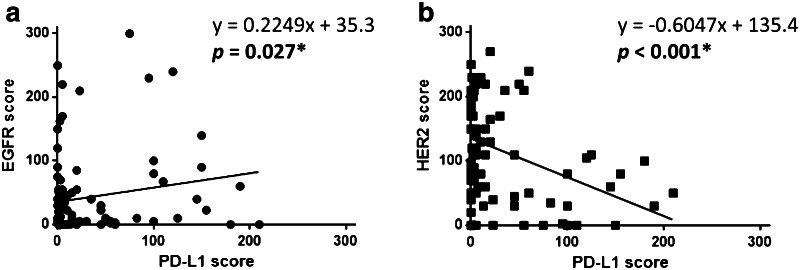
It was recently shown that EGFR driver mutation-positive tumors overexpressed PD-L1 in NSCLC tissues [14, 15], but there have been no published reports assessing the correlation between the expression of PD-L1 and EGFR or HER2. To further evaluate this finding, correlations between the H scores of PD-L1 and EGFR or HER2 were evaluated. Linear regression with Spearman’s rank correlation analysis showed that the H score of PD-L1 positively correlated with the H score of EGFR (r = 0.225, p = 0.027), while the H score of PD-L1 inversely correlated with the H score of HER2 (r = −0.605, p < 0.001) in resected NSCLC tissue samples (Fig. 2).
Fig. 2.
PD-L1 expression in NSCLC tissue positively correlates with EGFR expression and inversely correlates with HER2 expression. a H score of PD-L1 in 91 NSCLC tissues positively correlates with the H score of EGFR. b H score of PD-L1 inversely correlates with the H score of HER2 in resected NSCLC tissues. Data were analyzed using Spearman’s rank correlation test. *p < 0.05
PD-L1 expression is regulated via EGFR signaling in NSCLC cells
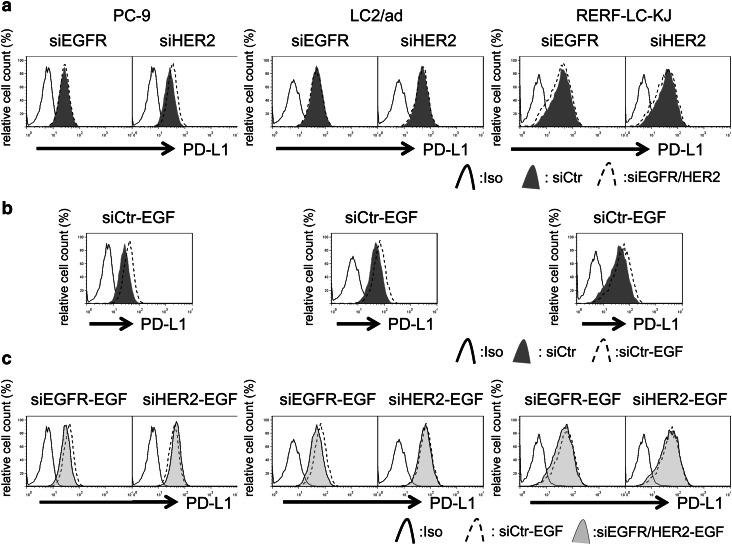
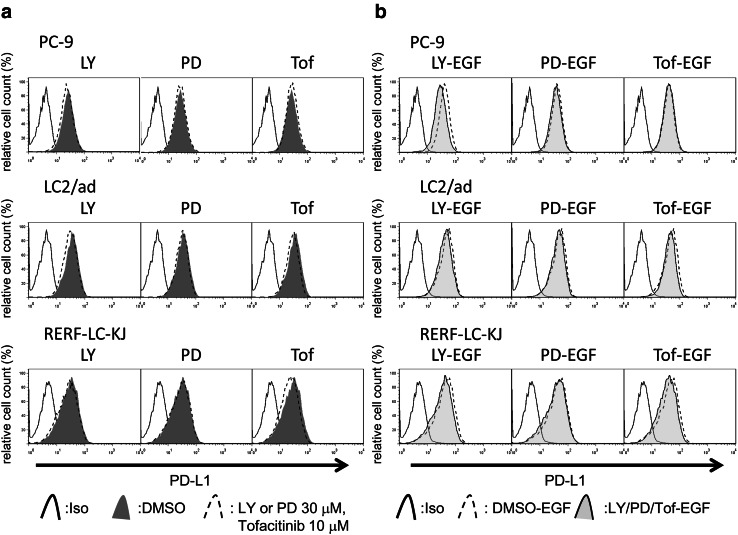
Basal expression levels of EGFR, HER2, and PD-L1 in four NSCLC cell lines were evaluated with flow cytometry and are shown in Supplementary Fig. 3. To analyze the ability of EGFR or HER2 signaling to influence PD-L1 expression, NSCLC cell lines were cultured in the presence or absence of the EGFR ligand EGF for 24 h. Consistent with the findings of a previous report [14–16], EGF upregulated PD-L1 expression in NSCLC cell lines (Supplementary Fig. 4a). To assess the influence of EGF on PD-L1 expression via EGFR or HER2 signaling, cancer cells were pretreated with the EGFR-tyrosine kinase inhibitor (TKI) gefitinib or the EGFR/HER2 dual TKI lapatinib followed by EGF, after which PD-L1 expression was evaluated using flow cytometry. Interestingly, neither gefitinib (Supplementary Fig. 4b) nor lapatinib (Supplementary Fig. 5) was able to downregulate the basal expression of PD-L1 in three of the four cell lines, but gefitinib clearly blocked EGF-induced upregulation of PD-L1 expression in all four cell lines (Supplementary Fig. 4c), suggesting that basal expression of PD-L1 and EGF-induced PD-L1 is regulated via different mechanisms. To assess these mechanisms more specifically, EGFR or HER2 expression was silenced by siRNA in three PD-L1 high-expressing cell lines (PC-9, RERF-LC-KJ, and LC2/ad cells). Western blot analysis showed that the addition of EGFR/HER2-targeting siRNA led to lower EGFR/HER2 expression levels in these cell lines (Supplementary Fig. 6). To determine whether the EGFR/HER2 pathway regulates EGF-induced PD-L1 expression, expression of PD-L1 was analyzed in cell lines pre-treated with EGFR- or HER2-targeting siRNA followed by EGF exposure. Consistent with the results obtained with EGFR-TKIs, neither EGFR- nor HER2-targeting siRNA decreased the basal expression of PD-L1 in these cell lines, although HER2 siRNA could attenuate basal expression of PD-L1 in PC-9 cells. Interestingly, EGFR-targeting siRNA clearly attenuated EGF-induced enhancement of PD-L1 expression, although HER2-targeting siRNA could not block EGF-induced PD-L1 expression in the cell lines (Fig. 3). It is well known that the main downstream pathways of EGF family receptor are PI3K/AKT, RAS/RAF/MAPK, and JAK/STAT [27]. We then assessed which downstream signaling pathway of EGFR/HER2 regulated the expression of PD-L1 in NSCLC cell lines. Interestingly, inhibitors of PI3K or JAK marginally downregulated basal expression of PD-L1, while MEK1 inhibitor did not. EGF-induced enhancement of PD-L1 expression was also marginally blocked by PI3K inhibitor or JAK inhibitor, but not by MEK1 inhibitor (Fig. 4).
Fig. 3.
Silencing EGFR blocks EGF-induced PD-L1 but silencing HER2 does not. a PD-L1 expression in cells treated with small interfering RNA (siRNA) targeting each EGF family receptor (siEGFR or siHER2) or control siRNA (siCtr) for 48 h, after which expression of PD-L1 was assessed by flow cytometry. b PD-L1 expression in cells treated with control siRNA for 24 h followed by EGF for 24 h, after which PD-L1 expression was evaluated. c PD-L1 expression in cells treated with siRNA targeting EGFR or HER2 or control for 24 h followed by 100 ng/mL of EGF for 24 h, after which expression of PD-L1 was evaluated. Representative data from at least three independent experiments are shown
Fig. 4.
Inhibition of PI3K/AKT or JAK/STAT pathways attenuates EGF-induced PD-L1 expression in NSCLC cells. a NSCLC cell lines were cultured in the presence or absence of LY294002 (LY), PD98059 (PD), or tofacitinib (Tof) for 24 h and then the expression of PD-L1 was assessed by flow cytometry. b NSCLC cell lines were cultured in the presence or absence of LY294002, PD98059, or tofacitinib for 24 h followed by 100 ng/mL of EGF for 24 h, and then the expression of PD-L1 was assessed by flow cytometry. Representative data from three independent experiments are shown
Discussion
In the present study, we have shown that overexpression of PD-L1 in NSCLC tissue is independently associated with poor prognosis in terms of both RFS and OS. This finding was consistent with the results of previous studies [10–12]. One reasonable hypothesis to explain why PD-L1 overexpressing tumors have poor clinical outcomes is that the upregulation of PD-L1 in tumor cells inactivates cytotoxic T lymphocytes (CTLs), leading to evasion from host immunity.
Interestingly, we also found that PD-L1 overexpression was significantly associated with male sex, smoking history, higher SUVmax, squamous cell carcinoma histology, higher histological grade, node metastasis-positive status, and advanced pathological stage in resected NSCLC tissue samples. To the best of our knowledge, there have been no previous studies regarding the relationship between PD-L1 expression and SUVmax or histological grade in NSCLC. It is interesting that tumors with high SUVmax values or dedifferentiated tumors had high levels of PD-L1 expression. One possible reason for these findings is that these tumors have been immunoselected based on the overexpression of PD-L1 and have enlarged, resulting in larger size with dedifferentiation, as PD-L1 overexpression in tumors attenuates T-cell anti-tumor immunity [28, 29].
We additionally showed that PD-L1 overexpression positively correlates with the expression of EGFR, while it is inversely correlated with the expression of HER2 in resected NSCLC tissue samples. It may be easy to understand why EGFR expression positively correlates with PD-L1 expression, as EGFR regulates PD-L1 expression and EGFR driver mutation positive cell lines express high PD-L1 compared with cell lines with wild-type EGFR oncogene [14, 15]. It is known that A549 and RERF-LC-KJ are wild-type EGFR, while PC-9 and LC2/ad cell lines harbor EGFR driver mutation [15, 30]. In line with previous studies, we showed that A549 cell expresses low PD-L1, while both PC-9 and LC2/ad cells express high PD-L1. Interestingly, EGFR wild-type RERF-LC-KJ also expresses high PD-L1 and suggested that other oncogene mutation also affects the expression of PD-L1 in NSCLC cells. Indeed, our in vitro data also suggested that EGF enhanced PD-L1 expression and that either EGFR-TKIs or EGFR siRNA could block EGF-induced PD-L1 overexpression in cell lines. However, the interesting finding is that neither EGFR-TKIs nor EGFR siRNA could downregulate basal expression of PD-L1 in three of four NSCLC cell lines. After EGF binds to EGFR, EGFR undergoes homo- or heterodimerization with other EGF family receptors such as HER2. In the field of anticancer therapy, EGFR is a therapeutic target in NSCLC with EGFR driver mutations, while HER2 is a therapeutic target in breast cancer with HER2 overexpression. Thus, our next interesting finding regards the role of HER2 in the expression of PD-L1. The dual EGFR/HER2 TKI lapatinib shows similar effects to those of gefitinib on basal expression of PD-L1 in NSCLC cell lines, suggesting that additional inhibition of HER2 with EGFR has no additional effect on the expression of PD-L1. In addition, HER2 siRNA has no effect on basal expression of PD-L1 or EGF-induced enhancement of PD-L1 in LC2/ad and RERF-LC-KJ cell lines, suggesting that EGF-induced upregulation of PD-L1 depends mainly on EGFR, but does not depend on HER2 in these two cell lines. The main pathways involved in the downstream signaling of EGFR/HER2 are PI3K/AKT, RAS/RAF/MAPK, and JAK/STAT [27]; therefore, we attempted to block each pathway with specific signal inhibitors, and found that both PI3K/AKT and JAK/STAT inhibitors marginally downregulated basal PD-L1 expression as well as EGF-induced PD-L1 expression in NSCLC cell lines, but MEK1 inhibitor did not. These findings suggested that PD-L1 expression is not regulated by one specific downstream pathway but by both the PI3K/AKT and JAK/STAT pathways, although other unknown pathways may also regulate the expression of this molecule. The mystery is that HER2 expression was inversely correlated with the expression of PD-L1. We are presently unable to explain this finding, but one possible hypothesis is that PD-L1 high-expressing tumors are residual tumors following attack by IFN-γ from CTLs. In addition to our group, several others have reported that the HER2 oncogene downregulates MHC class I molecules [31, 32], which are required for tumor recognition by CTLs. Theoretically, HER2 low-expressing tumors have high expression of MHC class I molecules and are attacked by CTLs with IFN−γ, which induce PD-L1 overexpression [33, 34]. On the other hand, HER2 high-expressing tumors have low expression of MHC class I molecules, resulting in escape from CTLs; therefore, PD-L1 expression is not induced. Another possibility is that EGFR and HER2 have different roles in the regulation of PD-L1, as silencing EGFR or HER2 demonstrated different effects on the expression of PD-L1 in NSCLC cells. If EGFR is silenced, EGF family receptor signaling will depend mainly on HER2/HER3 heterodimers, as HER2 lacks its own ligand and HER3 has no kinase activity [35]; therefore, EGF, a ligand of EGFR, has no effect on this signaling. On the other hand, if HER2 is silenced, EGF family signaling will depend on both EGFR/EGFR homodimers and EGFR/HER3 heterodimers, and EGF could still activate signaling pathways of EGFR. We found that HER2 siRNA enhanced basal expression of PD-L1, and moreover, HER2 siRNA did not block but enhanced the EGF-induced PD-L1 expression in PC-9 cells, suggesting that HER2 is a negative regulator of PD-L1 expression in this cell line. The possibility that it will be easier for PC-9 cells to demonstrate attenuated HER2 expression by HER2 siRNA than LC2/ad and RERF-LC-KJ cells, as PC-9 cells have lower levels of HER2 expression than the other two cell lines, might be another possible reason why HER2 siRNA showed a different effect on PD-L1 expression compared with HER2 high-expressing LC2/ad and RERF-LC-KJ cells.
Theoretically, EGFR mutation-positive tumor cells that express higher PD-L1 would provide a good predictive biomarker for PD-1/PD-L1 targeting therapy. However, recent studies have shown that second line PD-1/PD-L1 targeting therapy could not improve OS over that with docetaxel in patients with EGFR-mutated NSCLC [36, 37]. The mutation burden is considered a predictive factor for treatment with immunocheckpoint inhibitors including PD-1/PD-L1 targeting drugs [38], and EGFR-mutated lung adenocarcinomas have a significantly lower mutation burden than EGFR wild-type tumors [39]. These findings provide a biological explanation for the results of clinical trials.
Taken together, PD-L1 overexpression in tumor cells is correlated with worse prognosis in NSCLC patients, and is correlated positively with EGFR overexpression but inversely with HER2 expression. Our findings suggest that tumors expressing higher levels of PD-L1 are more aggressive and that administration of adjuvant chemotherapy should be considered for patients with these tumors.
Our in vitro findings suggested that EGFR and HER2 have different effects on the expression of PD-L1 and that both the PI3K/AKT and JAK/STAT pathways partially regulate PD-L1 expression in NSCLC cells. Attenuation of PD-L1 expression by specific signal inhibitors is a promising strategy for the treatment of NSCLC and further investigation to understand the mechanisms of PD-L1 regulation in cancer cells, particularly via downstream signaling of EGFR and HER2, should be considered.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Ms. Maitani and the staff of the Tissue Culture & Immunology and the Tissue Biology & Electron Microscopy Research Centers (Kawasaki Medical School) for providing technical assistance. This work was supported by grants from the Japan Society for the Promotion of Science (JSPS) Kakenhi Grant (25462189 and 16K10696), Kawasaki Medical School Project Grants (27–82 and 28–105), The Okayama Medical Foundation (no Grant Number) (to R. Okita), and Strategic Research Foundation Grant-aided Project for Private Universities from the Ministry of Education, Culture, Sport, Science, and Technology (S1291010) (to M. Nakata). The authors thank Editage for the language editing.
Author contributions
Conceived and designed the experiments: R. Okita. Performed the experiments: R. Okita. Analyzed the data: R. Okita and A. Maeda. Collected clinical data and samples: R. Okita, K. Shimizu, Y. Nojima, A. Maeda, S. Saisho, and M. Nakata. Contributed reagents/materials/analysis tools: R. Okita and M. Nakata. Wrote the manuscript: R. Okita and M. Nakata. All authors read and approved the final manuscript.
Abbreviations
- Ad
Adenocarcinoma
- APC
Allophycocyanin
- CEA
Carcinoembryonic antigen
- CI
Confidence interval
- CTL
Cytotoxic T lymphocyte
- EGFR
Epidermal growth factor receptor
- FDG-PET/CT
18F-fluorodeoxyglucose positron emission tomography/computed tomography
- HER2
Human epidermal growth factor receptor 2
- H-score
Histoscore
- IHC
Immunohistochemical staining
- IASLC/ATS/ERS
International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society
- NSCLC
Non-small-cell lung cancer
- OS
Overall survival
- p-
Phosphorylated-
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death-1 ligand 1
- PE
Phycoerythrin
- RFS
Recurrence-free survival
- ROC
Receiver operating characteristic
- siRNA
Small interfering RNA
- Sq
Squamous cell carcinoma
- SUVmax
Maximum standard uptake value
- TKI
Tyrosine kinase inhibitor
Compliance with ethical standards
Our research was approved by the Kawasaki Medical School ethics committee (No. 1227-3).
Conflict of interest
Dr. M. Nakata received research funding from Kyowa Kirin and Taiho Pharma for this study. The sponsors had no control over the interpretation, writing, or publication of this work. Dr. M. Nakata also received research funding from CSL Behring and Eli Lilly for research outside the scope of the submitted work. All other authors declare no conflicts of interest.
References
- 1.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307–314. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Jr, Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 5.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. New Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR, Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. New Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 8.Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L, Rimm DL. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94:107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang CY, Lin MW, Chang YL, Wu CT, Yang PC. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer. 2014;50:1361–1369. doi: 10.1016/j.ejca.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 11.Sun JM, Zhou W, Choi YL, Choi SJ, Kim SE, Wang Z, Dolled-Filhart M, Emancipator K, Wu D, Weiner R, Frisman D, Kim HK, Choi YS, Shim YM, Kim J. Prognostic significance of PD-L1 in patients with non-small cell lung cancer: a large cohort study of surgically resected cases. J Thorac Oncol. 2016;11:1003–1011. doi: 10.1016/j.jtho.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Shimoji M, Shimizu S, Sato K, Suda K, Kobayashi Y, Tomizawa K, Takemoto T, Mitsudomi T. Clinical and pathologic features of lung cancer expressing programmed cell death ligand 1 (PD-L1) Lung Cancer. 2016;98:69–75. doi: 10.1016/j.lungcan.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- 14.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, Wilkerson MD, Fecci PE, Butaney M, Reibel JB, Soucheray M, Cohoon TJ, Janne PA, Meyerson M, Hayes DN, Shapiro GI, Shimamura T, Sholl LM, Rodig SJ, Freeman GJ, Hammerman PS, Dranoff G, Wong KK. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, Matsumoto K, Takayama K, Takamori S, Kage M, Hoshino T, Nakanishi Y, Okamoto I. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25:1935–1940. doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- 16.Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, Zhang Y, He X, Zhou T, Qin T, Huang Y, Yi X, Zhang L. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10:910–923. doi: 10.1097/JTO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 17.Ota K, Azuma K, Kawahara A, Hattori S, Iwama E, Tanizaki J, Harada T, Matsumoto K, Takayama K, Takamori S, Kage M, Hoshino T, Nakanishi Y, Okamoto I. Induction of PD-L1 expression by the EML4-ALK oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin Cancer Res. 2015;21:4014–4021. doi: 10.1158/1078-0432.CCR-15-0016. [DOI] [PubMed] [Google Scholar]
- 18.Okita R, Yukawa T, Nojima Y, Maeda A, Saisho S, Shimizu K, Nakata M. MHC class I chain-related molecule A and B expression is upregulated by cisplatin and associated with good prognosis in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2016;65:499–509. doi: 10.1007/s00262-016-1814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC. Tumours of the lung, pleura, thymus and heart. Pathology & genetics. Lyon: IARC; 2004. [Google Scholar]
- 20.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, Garg K, Austin JH, Asamura H, Rusch VW, Hirsch FR, Scagliotti G, Mitsudomi T, Huber RM, Ishikawa Y, Jett J, Sanchez-Cespedes M, Sculier JP, Takahashi T, Tsuboi M, Vansteenkiste J, Wistuba I, Yang PC, Aberle D, Brambilla C, Flieder D, Franklin W, Gazdar A, Gould M, Hasleton P, Henderson D, Johnson B, Johnson D, Kerr K, Kuriyama K, Lee JS, Miller VA, Petersen I, Roggli V, Rosell R, Saijo N, Thunnissen E, Tsao M, Yankelewitz D. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L, International Association for the Study of Lung Cancer International Staging C, Participating I The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 22.Nunez MI, Behrens C, Woods DM, Lin H, Suraokar M, Kadara H, Hofstetter W, Kalhor N, Lee JJ, Franklin W, Stewart DJ, Wistuba II. High expression of folate receptor alpha in lung cancer correlates with adenocarcinoma histology and EGFR mutation. J Thorac Oncol. 2012;7:833–840. doi: 10.1097/JTO.0b013e31824de09c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okita R, Wolf D, Yasuda K, Maeda A, Yukawa T, Saisho S, Shimizu K, Yamaguchi Y, Oka M, Nakayama E, Lundqvist A, Kiessling R, Seliger B, Nakata M. Contrasting effects of the cytotoxic anticancer drug gemcitabine and the EGFR tyrosine kinase inhibitor gefitinib on NK cell-mediated cytotoxicity via regulation of NKG2D ligand in non-small-cell lung cancer cells. PLoS One. 2015;10:e0139809. doi: 10.1371/journal.pone.0139809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okita R, Mougiakakos D, Ando T, Mao Y, Sarhan D, Wennerberg E, Seliger B, Lundqvist A, Mimura K, Kiessling R. HER2/HER3 signaling regulates NK cell-mediated cytotoxicity via MHC class I chain-related molecule A and B expression in human breast cancer cell lines. J Immunol. 2012;188:2136–2145. doi: 10.4049/jimmunol.1102237. [DOI] [PubMed] [Google Scholar]
- 25.Rigau V, Molina TJ, Chaffaud C, Huchon G, Audouin J, Chevret S, Brechot JM. Blood vessel invasion in resected non small cell lung carcinomas is predictive of metastatic occurrence. Lung Cancer. 2002;38:169–176. doi: 10.1016/S0169-5002(02)00213-1. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchiya T, Akamine S, Muraoka M, Kamohara R, Tsuji K, Urabe S, Honda S, Yamasaki N. Stage IA non-small cell lung cancer: vessel invasion is a poor prognostic factor and a new target of adjuvant chemotherapy. Lung Cancer. 2007;56:341–348. doi: 10.1016/j.lungcan.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Avraham R, Yarden Y. Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat Rev Mol Cell Biol. 2011;12:104–117. doi: 10.1038/nrm3048. [DOI] [PubMed] [Google Scholar]
- 28.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm0902-1039c. [DOI] [PubMed] [Google Scholar]
- 29.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Nagai Y, Miyazawa H, Huqun, Tanaka T, Udagawa K, Kato M, Fukuyama S, Yokote A, Kobayashi K, Kanazawa M, Hagiwara K. Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clamp. Cancer Res. 2005;65(16):7276–7282. doi: 10.1158/0008-5472.CAN-05-0331. [DOI] [PubMed] [Google Scholar]
- 31.Maruyama T, Mimura K, Sato E, Watanabe M, Mizukami Y, Kawaguchi Y, Ando T, Kinouchi H, Fujii H, Kono K. Inverse correlation of HER2 with MHC class I expression on oesophageal squamous cell carcinoma. Br J Cancer. 2010;103:552–559. doi: 10.1038/sj.bjc.6605772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mimura K, Ando T, Poschke I, Mougiakakos D, Johansson CC, Ichikawa J, Okita R, Nishimura MI, Handke D, Krug N, Choudhury A, Seliger B, Kiessling R. T cell recognition of HLA-A2 restricted tumor antigens is impaired by the oncogene HER2. Int J Cancer. 2011;128:390–401. doi: 10.1002/ijc.25613. [DOI] [PubMed] [Google Scholar]
- 33.Wei S, Shreiner AB, Takeshita N, Chen L, Zou W, Chang AE. Tumor-induced immune suppression of in vivo effector T-cell priming is mediated by the B7-H1/PD-1 axis and transforming growth factor beta. Cancer Res. 2008;68:5432–5438. doi: 10.1158/0008-5472.CAN-07-6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 35.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 36.Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, Huynh TG, Zhao L, Fulton L, Schultz KR, Howe E, Farago AF, Sullivan RJ, Stone JR, Digumarthy S, Moran T, Hata AN, Yagi Y, Yeap BY, Engelman JA, Mino-Kenudson M. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22:4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, Yang JC. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol. 2017;12:403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadara H, Choi M, Zhang J, Parra ER, Rodriguez-Canales J, Gaffney SG, Zhao Z, Behrens C, Fujimoto J, Chow C, Yoo Y, Kalhor N, Moran C, Rimm D, Swisher S, Gibbons DL, Heymach J, Kaftan E, Townsend JP, Lynch TJ, Schlessinger J, Lee J, Lifton RP, Wistuba II, Herbst RS. Whole-exome sequencing and immune profiling of early-stage lung adenocarcinoma with fully annotated clinical follow-up. Ann Oncol. 2016 doi: 10.1093/annonc/mdw436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.