Abstract
Mistletoe extract (ME) is applied as an adjuvant treatment in cancer therapy in thousands of patients each year in Europe. The main immunostimulating component of mistletoe extract, mistletoe lectin, recently has been shown to be a pattern recognition receptor ligand and hence is binding to an important class of pathogen-sensing receptors. Pattern recognition receptor ligands are potent activators of dendritic cells. This activation is a prerequisite for a full-blown T-cell response against cancer cells. Pattern recognition receptor ligands are increasingly recognized as important players in cancer immunotherapy. We collect evidence from case studies on spontaneous regression, from epidemiology, from experiments in a mouse cancer model, and from protein structure comparisons to argue that a combination of mistletoe therapy with other pattern recognition receptor ligand substances leads to an increased immune stimulatory effect. We show that mistletoe lectin is a plant protein of bacterial origin with a 3D structure very similar to shiga toxin from Shigella dysenteriae, which explains the remarkable immunogenicity of mistletoe lectin. Secondly, we show that a combination of pattern recognition receptor ligands applied metronomically in a cancer mouse model leads to complete remission, while single pattern recognition receptor ligands slowed tumor growth. Taken together, we propose to combine mistletoe drugs with other pattern recognition receptor ligand drugs to increase its efficacy in adjuvant or even primary cancer therapy.
Keywords: Mistletoe extract, Mistletoe lectin, Pattern recognition receptor ligands, Shiga toxin, Cancer immunotherapy
Introduction
Spontaneous regressions and remissions
Spontaneous regressions and remissions in cancer are still regarded as a conundrum. However, by exhaustive scanning of case studies, we could show that many, if not a majority of spontaneous remissions, were preceded by an acute feverish infection [1]. In a syngeneic pancreatic carcinoma mouse model, we could confirm that a single intra-tumoral injection of live Streptococcus pyogenes can result in complete tumor regression [2].
Spontaneous regressions in cancer are regarded as a rare phenomenon in case of larger tumors; yet, they occur in up to 20 % of small breast cancer nodules [3]. If the link in time between acute infect and regressions was not only coincidental but causal, it should also precipitate as protection, such that a personal history of infections should reduce the likelihood to develop cancer later. Subsequently, indeed, we found more than 30 studies which, as an ensemble, appear to confirm this prediction (Table 1).
Table 1.
Anti-correlation between acute, cured infections, and the likelihood to develop cancer
| Observation | Effect | Year of publication | Pathogen | References |
|---|---|---|---|---|
| Lower risk of cancer in syphilitic prostitutes | Prophylactic | 1725 | Treponema pallidum | [4] |
| Collection of 302 cases of spontaneous regression (44 complete remissions), 27/302 cases accompanied by infection (9 %), 69 cases where “incomplete operation (was) often accompanied by postoperative fever” (28 %) | Therapeutic | 1918 | Diverse | [5] |
| Low risk of cancer in tuberculosis patients | Prophylactic | 1929 | Mycobacterium tuberculosis | [6] |
| Lower risk of cancer in malaria patients | Prophylactic | 1929 | Plasmodium falc., malariae, vivax | [7, 8] |
| Of 300 cancer patients, 113 had no febrile infectious childhood diseases (FICD), while in 300 controls, 16 lacked FICD | Prophylactic | 1934 | Diverse | [9] |
| Fewer childhood diseases, higher cancer risk in adults | Prophylactic | 1936 | Diverse | [10] |
| In a cohort of 300 cases of childhood leukemia, 26 spontaneous remissions were observed. 21/26 (80 %) were accompanied by infection | Therapeutic | 1951 | Diverse | [11] |
| Less benign ovary cysts in patients with childhood mumps | Prophylactic | 1960 | Paramyxovirus parotitis | [12] |
| “…according to the Cancer Centre in Sao Paulo (Brazil), among tens of thousands of cancer patients, only two gave a positive Machado reaction (indicating chronic or recovered trypanosoma infection), whereas among the remaining population, the number suffering from this infection varies from 10 to 20 percent.”, anecdotal remark | Prophylactic | 1963 | Trypanosoma cruzi | [13] |
| Lower cancer mortality in 5,460 survivors of typhoid fever | Prophylactic | 1970 | Salmonella typhi | Ref. 58 in [14] |
| Fewer physician visits, secondary illnesses, and hospital referrals in 150 controls versus 150 cancer patients | Prophylactic | 1970 | Diverse | [15] |
| In 62/224 cases of spontaneous regression (28 %), either an infection or a persistent temperature elevation was observed prior to regression | Therapeutic | 1971 | Diverse | [16] |
| Occasional remissions in Hodgkin’s lymphoma after measles attack | Therapeutic | 1971 | Morbillivirus | [17] |
| Patients developing empyema after lung cancer surgery have improved 5-year survival (50 % (n = 18) vs. 22 % (n = 411)) | Therapeutic | 1972 | Diverse | [18] |
| Lower incidence of mumps, measles, rubella in 300 patients with cancer of the ovary compared to control group | Prophylactic | 1977 | MMR viruses | [19] |
| Lower incidence of mumps in patients with cancer of the ovary compared to control group | Prophylactic | 1979 | Paramyxovirus parotitis | [20, 21] |
| Increased cancer risk with an odds ratio of 2.6 for missing history of infectious organ diseases, 5.7 for missing history of common colds, and 15.1 for missing history of fever | Prophylactic | 1983 | Diverse | [22] |
| Out of 353 individuals with a negative history of measles, 21 developed cancer versus 1 case in 230 controls with a positive history of measles (p 0.001) | Prophylactic | 1985 | Diverse | [23] |
| Much lower cancer rate in wool and hemp factories; wool or hemp dust can carry bacterial endotoxins. | Prophylactic | 1985 | Diverse | [24] |
| Lower frequency of infections in the first year of life for children with leukemia | Prophylactic | 1986 | Diverse | [25] |
| Lower cancer incidence after Herpes infections | Prophylactic | 1987 | Herpes simplex | [26] |
| Posttransfusional hepatitis in patients with acute myelogenous leukemia doubles survival rate | Therapeutic | 1982, 1992 | Hepatitis viruses | [27, 28] |
| A history of common colds or gastroenteric influenza was found to be associated with a decreased cancer risk (odds ratio 0.18 and 0.23 vs. population and hospital controls, resp.) | Prophylactic | 1991 | Common cold viruses | [29] |
| Inverse correlation between number of infections and mortality from tumors in Italy in the period 1890–1960: each 2 % reduction in number of infectious diseases was followed by a 2 % increase in tumors about 10 years later | Prophylactic | 1998 | Diverse | [30] |
| Inverse association between number of carcinoma (but not breast cancer) and febrile infectious childhood diseases (FICD); association stronger for higher numbers of FICD and childhood in pre-antibiotic times; strongest protection by rubella (379 cancer cases vs. 379 office matched controls) | Prophylactic | 1998 | Diverse | [31] |
| Sixty-eight well-documented cases of spontaneous regression from melanoma, preceded in 21 (31 %) cases by a febrile infection | Therapeutic | 1998 | Streptococcus pyogenes | [32] |
| Statistically significant inverse association between a reported history of infections and glioma, meningioma (RR = 0.72, age- and gender-matched population control of 1,509 cases) | Prophylactic | 1999 | Diverse | [33] |
| Inverse correlation between melanoma risk and number of recorded infections on one hand and between melanoma risk and fever height on the other hand, leading to a combined reduction of melanoma risk of about 40 % for people with a history of three or more infections with high fever above 38.5 °C (age- and gender-matched population control) | Prophylactic | 1999 | Diverse | [34] |
| More than twofold higher incidence of cancer in Europe, GUS, and US compared to Africa and Asia of 381 versus 156 (ten most prominent cancer forms, age-standardized rate per 100,000 population; in Africa and Asia, a significant higher rate of infections is assumed here | Prophylactic | 2003 | Diverse | [35] |
| Prior immunization of melanoma patients with vaccinia or BCG is associated with better survival (age-matched controls) | Prophylactic | 2005 | Vaccinia, BCG vaccine | [36] |
| Dairy farmers, but not crop and orchard farmers, report one-third less cancers than the average population; protection diminishes over time after exposure is removed; dust in cattle houses can carry bacterial endotoxins which frequently lead to unspecific “day fever” | Prophylactic | 2005 | Diverse | [37] |
| The 10-year survival for patients with osteosarcoma with infection within 1 year after surgery (n = 41) was 84.5 % compared to 62.3 % in the non-infected group (n = 371) | Therapeutic | 2007 | Diverse | [38] |
| After allogeneic stem cell transplantation, patients who had a febrile infection (FI) before posttransplant day 21 (FI group) had a lower probability of leukemic relapse (p < 0.001) and a higher relapse-free survival rate (p = 0.012) than those patients who did not have a FI before posttransplant day 21 (non-FI group) | 2008 | [39] | ||
| Fourfold higher risk for Hodgkin lymphoma if tonsils are removed at age <15 years | Prophylactic | 2010 | Diverse | [40] |
| Reduced ALL (acute lymphoblastic leukemia) risk in kindergarten children (frequent mutual infectious contaminations presumed) (OR 0.8) or children with repeated common infections (OR 0.7) | Prophylactic | 2010 | Diverse | [41] |
| Reduced risk for ALL in children visiting kindergarten | Prophylactic | 2010, 2011 | Diverse | [42, 43] |
| Reduced HL risk (Hodgkin lymphoma, 128 cases aged 5–14) and NHL (non-Hodgkin lymphoma, 164 cases aged 2–15 years) versus 1,312 controls. HL + kindergarten: OR 0.5; HL + common infections + non-breast-feeding: OR 0.3; NHL + birth order 3: OR 0.7; NHL + prolonged breast-feeding: OR 0.5; NHL + frequent farm visits in early life: OR 0.5; NHL + asthma: OR 0.6 | Prophylactic | 2011 | Diverse | [44] |
Two publications were found, which could not confirm inverse association between infection and cancer [45, 46], one, in a low impact journal not listed in PubMed [47], reported an increased risk with mumps and whooping cough. All three publications are based on less than 200 cases. In one study, an inverse correlation between childhood mumps and ovary cancer could not be confirmed [48]
It should be emphasized that these were acute, fully cleared infections, in contrast to chronic viral and bacterial infections, which are causally related with up to 20 % of all cancers [49].
Coley’s cancer treatment using bacterial extracts
On a related line of evidence, it should be remembered that Coley and contemporaries, between 1895 and 1936, by injecting fever-inducing bacterial extracts prepared from S. pyogenes and Serratia marcescens (“Coley’s toxin”), “documented cases of the long-term survival of individuals with malignancies that remain a major challenge to treat now” [50] (see [51] for an impressive example reported 1928, to be downloaded from http://bioinfo.tg.fh-giessen.de/pamp-cancer/christian-1928-amjdsurg-coley.pdf). Coley used to increase the dosage of his extract on a per patient basis until a body temperature of 39 °C or above could be achieved [52]. He used a metronomic therapy regimen with 1–3 injections per week over month, thus stimulating the innate immune system again and again, with longer treatment resulting in better outcome.
As a biological mechanism underlying these three phenomena (spontaneous regression, epidemiological protection, and Coley’s experiments), we suggested the unspecific activation of dendritic cells (DC) by pattern recognition receptor ligands (PRRL), for instance Toll-like receptor (TLR) ligands [53]. In this sense, “Coley’s toxin” might be regarded as a PRRL mix.
PRRL drugs as potential substitutes for a proliferative infection
Pattern recognition receptor ligands act as mandatory co-stimulatory signals in DC activation and are the most potent activators of DC. DC activation, in turn, is needed for a full-blown T-cell response against cancer cells. Cancer tissues do not provide PRRL. DC activation by PRRL in infected cancer patients presumably extends to DC signalling both pathogen antigens and tumor antigens [53], leading to clonal expansion, maturation, and activation of both pathogen-specific and tumor cell-specific T-cell clones. We have outlined the immunological explanation in more detail in [54]. The repeated application of PRRL containing drugs, just like the repeated application of bacterial extracts, might serve as a substitute infection.
Immune-stimulating effects of mistletoe lectin and PRRL are similar
Mistletoe extract (ME) is manufactured from the European mistletoe Viscum album and available as pharmaceutical preparation under various brands including Helixor, Iscador, and Abnoba. ME therapy is an adjuvant treatment applied to thousands of cancer patients each year in Europe [55]. Immune-stimulating effects of ME depend largely on mistletoe lectin-1 (ML) [56]. Physicians often report better well-being and occasional remissions or disease stabilisations. Meta-analyses show small benefits of ME regarding survival [57] and clear benefits with respect to quality of life [58, 59]. Occasional cures have been reported after very long repeated application in otherwise therapy naive patients [60, 61].
Several immune-stimulating effects of ME or ML have been observed. In vitro effects include increased cytokine expression (IL-1b, IL-5, IL-6, TNF-alpha, and GM-CSF), higher phagocytic uptake by macrophages, increased NK-cell and cytotoxic T-lymphocyte (CTL) activity [62]. In vivo effects include increased counts of CD4 cells [63], of DC infiltrating the primary tumor [64], of tumor antigen-specific CD8+-T cells, of NK- and gamma-delta-T cells [65].
Recently, it has been shown that the Korean variant of mistletoe lectin (KMLC) is a TLR-4 ligand [66]. KMLC and European ML have a sequence identity of 84 % and thus the same 3D structures [67], so European ML presumably is a TLR-4 ligand as well.
The most prominent TLR-4-binding molecule is lipopolysaccharide (LPS). At first sight, it seems puzzling that KMLC shares binding to TLR-4 with LPS, which has a completely different molecular weight and 3D structure. However, LPS binds not directly to TLR-4 but its binding is mediated through scaffold proteins, including LPS-binding protein (LBP), CD14 and MD-2 [68]. Thus, mistletoe lectin binding to TLR-4 might as well be mediated by respective scaffold proteins. Several cytokines, including IL-1, IL-6, IL-12, TNF-alpha, and GM-CSF, show elevated levels both upon PRRL application [69] and ML application [68]; both KMLC [62] and LPS [70] increase the production of IL-1 and IL-6 in antigen-presenting cells.
In this study, we elucidated the remarkable immunogenicity of ML and set out to substantiate our hypothesis that the application of a combination of PRRL has unleveraged therapeutic potential.
Materials and methods
Bioinformatics
Sequence database searches were performed using Blast. Alignment, 3D overlay and rendering of mistletoe lectin-1 (PDB-ID 1SZ6-A) and shiga toxin (PDB-ID 1R4P-A) were done using PyMol.
Mouse experiments
Panc02 pancreatic tumor cells were injected s.c. (1 × 106) in C57BL/6J mice, leading to surface lesions of about 50 mm2 after 7 days. PRRL (n = 5) or saline solution (control, n = 8) were injected s.c. (in 50 μl) 10 times over 21 days in regular intervals. Tumor size was measured at each injection day. PRRL applied were flagellin (Sigma, 25 μg/kg BW) targeting TLR-5, LPS (2 mg/kg BW) targeting TLR-4, MDP (100 μg/kg BW) targeting NOD-2, Resiquimod (60 μg/kg BW) targeting TLR-7/8 or a combination of LPS, Resiquimod and MDP. All chemicals were purchased from Enzo, Lörrach, Germany. Mice were killed at day 31 and residual tumor volume compared. Content of myeloid suppressor cells (MDSC) in spleen were analyzed by flow cytometry.
Body temperature was monitored wireless by subcutaneously implanted sensors (Respironics, PDT-4000 E-mitter).
Results
Mistletoe lectin chain A shares a striking structural similarity with shiga toxin chain A
The remarkable immunogenicity of ML, a protein produced by a plant, so far is unexplained. ML is classified as a protein with rRNA N-glycosylase activity, thus inhibiting ribosomes leading to mild ML cytotoxic activity in vitro. ML has two domains, the A-chain carrying the enzymatic site and the B-chain acting as cellular-binding domain. The closest related structure is ricin, with 49 % sequence identity, the same EAAR motif at the active site (catalytic residues E, R), similar length of both A- and B-chains, and a very similar 3D structure extending over both chains. Ricin is produced by the castor bean and exhibits a much stronger cytotoxicity than ML. While ricin does not bind to CD75s-ganglioside-linked cell surface receptors, a recombinant ML does [71]. Hence, the different toxicities might be attributed to different cell-type binding preferences or affinities of the B-chains.
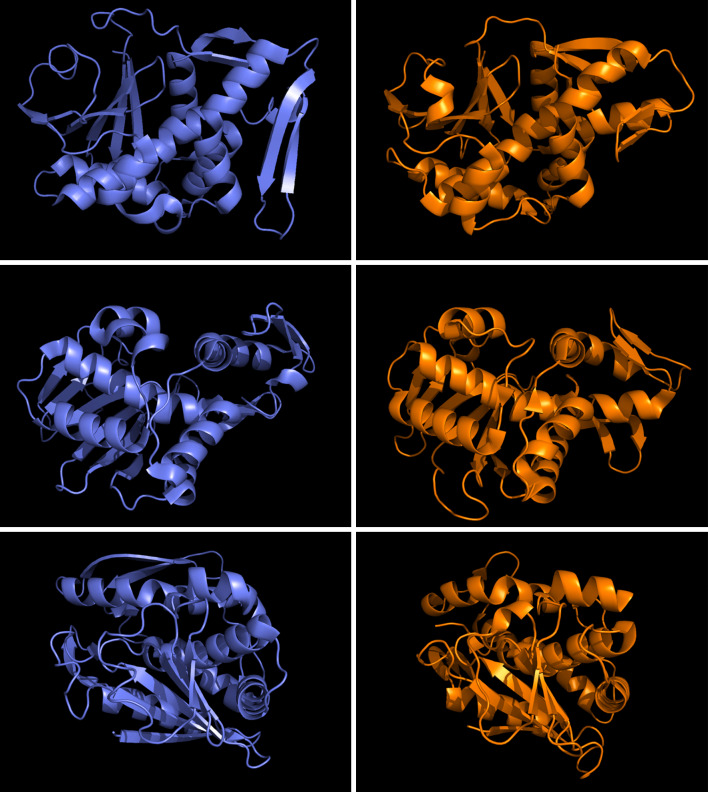
Apart from the obvious relationship with ricin, we noticed that shiga toxin, a bacterial protein, has a sequence identity with ML at the borderline of significance (25 % sequence identity over 263 residues alignment length and Blast e value 0.00026). At this low sequence identity, indicating considerable evolutionary divergence, one would expect quite dissimilar functions and 3D structures.
To our surprise, both ML (PDB-Id 1SZ6 chain A) and ricin A-chains turned out to be structurally related to shiga toxin type-2 (PDB-Id 1R4P chain A). Shiga toxin is produced by Shigella dysenteriae and enterohemorrhagic E. coli (EHEC) and is, like ricin, a strong cytotoxin. Structural overlay of ML and shiga toxin showed that secondary structure elements are in striking spacial match (see Fig. 1): root mean square deviation (RSMD) is 2.1 over 240 residues. This almost perfect structural identity therefore implies a common evolutionary origin [72]. Both proteins share the same enzymatic activity and a conserved 4-amino acid active site motif (ML: EAAR, shiga toxin: EALR). Thus, ML might be considered in vaccination strategies against EHEC infections.
Fig. 1.
Structural identity of mistletoe lectin-1 (blue) and shiga toxin (orange) shown in 3 orientations. RMSD = 2.1 along residues 1–240
A lookup in the CATH database of related structures [73] revealed that structural motifs similar to parts of ML chain A (CATH IDs 3.40.420.10 and 4.10.470.10) can be found in some other bacteria including particular strains of E. coli and Streptomyces, but also in enterobacterial phages, and in a range of plants besides mistletoe.
Presumably, mistletoe, castor bean, and some other plants have captured bacterial ribosome inhibitors by horizontal gene transfer. The function of a toxin such as ML in plants might be the rebuff of herbivores. The evolutionary provenance of ML from bacteria can explain why human Toll-like receptors, otherwise preferentially binding pathogenic substances, could be targeted by ME. Mistletoe plant has strong immune stimulatory capacity because it produces a bacterial toxin.
PRRL should be applied in combination
To resemble a proliferative infection and according to the lessons to be learned from Coley’s experiments, from case observations on spontaneous regressions [1] and epidemiological findings (Table 1), we have suggested to apply PRRL (1) in immuno-competent organisms, (2) in combination to address more than one PRR, (3) over a longer fixed period (metronomic treatment regimen), and (4) under appreciation of fever [53, 74]. To the best of our knowledge, this paradigm has not been tested before.
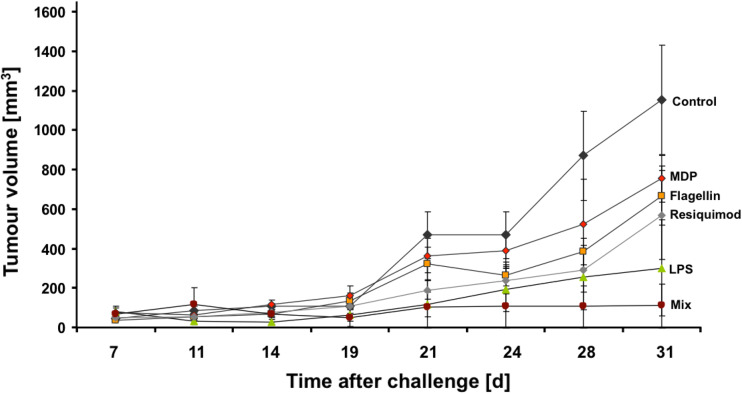
Hence, we challenged C57BL6J mice with Panc02 autologous pancreatic tumor cells and applied single and multiple PRRL 10 times over 3 weeks. While individual PRRL slowed tumor growth, only the combination induced complete remission in 4/5 mice. The tumor of the fifth mouse in the combi group, which was the largest in the mix group at treatment start, stopped tumor growth at day 14. The tumor size difference between control and mix at day 31 is significant at the 1 % level (t-Test, p = 0.0068) (see Fig. 2). The content of myeloid-derived suppressor cells (MDSC) in spleen, a heterogeneous population of cells expanding during cancer development with the ability to suppress T-cell responses [75], was reduced by all PRRL between twofold (MDP) to sixfold (Resiquimod, LPS), indicating counterbalanced immune suppression upon PRRL application.
Fig. 2.
C57BL/6J mice (n = 5) were challenged with autologous Panc02 tumor cells at day 0. PRRL treatment started at day 7 and was repeated 10× until day 28. PRR targeted were TLR-4 (LPS), TLR-5 (Flagellin), TLR-7/8 (Resiquimod), NOD2 (MDP). Mix contained LPS, Resiquimod, and MDP. Error bars are SEM. Tumor size difference between mix and control at day 31 is significant (t-Test, p = 0.0068)
Mice have limited capabilities to raise their body temperature. Still, a PRRL such as LPS can raise the body temperature of mice by about 1 °C (measured by subcutaneous wireless sensor, averaged over 24 h, data not shown).
Discussion
Mistletoe therapy might be augmented by PRRL
Besides Coley’s seminal treatments for cancer patients with bacterial extracts of S. pyogenes and S. marcescens about a century ago, the application of pathogens in cancer therapy has a long history [76]. A clinical success is the administration of Bacillus Calmette–Guérin (BCG) immunotherapy as treatment standard for non-muscle invasive bladder cancer [77]. Repeated instillations with live BCG are necessary for a robust immune response. Some facultative anaerobic bacteria like Bifidobacterium bifidum [78], Clostridium novyi, and Salmonella typhimurium are known to home preferentially into solid tumors after systemic application, perhaps favouring hypoxic tumor environments. Hoffman et al. used a genetically modified strain of live S. typhimurium to treat prostate [79], breast [80], pancreatic [81], spinal chord [82], lung [83], and glioma [84] tumors in orthotopic or syngeneic mouse models and achieved some impressive remission rates. The authors suggest direct tumor killing effects; however, metronomic treatment was more effective and better tolerated compared to bolus or few applications [84], and complete cure can be observed despite a residual well-oxygenated tumor rim after treatment [85]. So innate immune stimulation of an existing T-cell response as suggested here could be an alternative explanation at least in the immunocompetent mouse models.
The approval of live or even killed bacterial drugs nowadays faces severe obstacles due to batch-to-batch variability during production and the general lack of structure–function relationship data, which are usually requested by the authorities. Thus, drugs resembling a proliferative infection, where clear structure–function relationships can be established, are preferable compared to bacterial extracts. We suggest that PRRL combinations could serve as substitute infection. Different combinations of PRRL could be compared and optimized for effectivity.
Several PRRL were tested in clinical trials, mainly as adjuvants, to this end with uncertain benefits [86–88]. In these trials, PRRL are applied as single substances, in immunocompromised patients with a record of chemotherapy or radiation, a few times, with fever treated as toxic side effect. Previously, we have suggested to change PRRL therapy regimen and rather apply PRRL in immunocompetent cancer patients, in a metronomic fashion over several weeks, under the appreciation of periods of short (1-day) fever and in combination [53, 74].
Our preliminary results show (1) that single PRRL applied metronomically can slow tumor progression, (2) PRRL in combination and applied multiple times over 3 weeks can induce complete remissions, and (3) the count of MDSC was reduced 2- to 6-fold after PRRL application.
While it is known that chronic CpG application in tumor-free hosts can lead to expansion of MDSC and T-cell suppression, the therapeutic application of CpG in tumor-bearing mice decreased MDSC and blocked suppressive activity on T-cell proliferation [89]. We confirmed a similar decrease on MDSC count after PRRL application, likely leading to a similar lift of T-cell suppression.
Protein PRRL like flagellin or ML are expected to induce neutralizing antibodies after repeated application. Yet, immune stimulatory effects of ME can be found in patients after repeated application despite ML serum antibody titers. Innate immune responses can occur within minutes, so innate responses are likely triggered before any neutralization takes place, in particular upon s.c. or intra-tumoral or peri-tumoral application. For i.v. application, nonprotein PRRL might be preferable.
Mistletoe extract, in particular at higher dosages and at the beginning of treatment, often leads to fever. Clinicians with long experience in mistletoe therapy try to adjust a fever-inducing dosage on a per patient basis. ME-induced fever usually rises and declines within 1 day and lacks potential severe side effects of fever induced by a proliferative infection. Mistletoe therapy often is applied over longer periods. Thus, our suggestions to apply PRRL in a metronomic fashion and not suppressing fever are already implemented by present-day ME therapy regimen, since the main actor in ME, mistletoe lectin, is a PRRL of bacterial origin. To improve the outcome of mistletoe therapy, we suggest to combine ML with other PRRL to extend the range of PRR addressed.
Conflict of interest
All authors declare that neither of us has a significant financial arrangement or affiliation with any product or services used or discussed in our paper, nor any potential bias against another product or service.
Animal experiment declaration
Animal experiments were performed in accordance with the German legislation on the protection of animals and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council; NIH Guide, vol. 25, No. 28, 1996). All animal experiments were approved by the local agency for animal welfare (Landesamt für Landwirtschaft, Lebensmittelsicherheit und Fischerei, Mecklenburg-Vorpommern).
References
- 1.Hobohm U. Fever and cancer in perspective. Cancer Immunol Immunother. 2001;50:391–396. doi: 10.1007/s002620100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maletzki C, Linnebacher M, Kreikemeyer B, Emmrich J. Pancreatic cancer regression by intratumoural injection of live Streptococcus pyogenes in a syngeneic mouse model. Gut. 2008;57:483–491. doi: 10.1136/gut.2007.125419. [DOI] [PubMed] [Google Scholar]
- 3.Zahl PH, Maehlen J, Welch HG. The natural history of invasive breast cancers detected by screening mammography. Arch Intern Med. 2008;168:2311–2316. doi: 10.1001/archinte.168.21.2311. [DOI] [PubMed] [Google Scholar]
- 4.Deidier A (1725) Dissertation Medecinal et Chirurgical sur les Tumeurs, Paris
- 5.Rohdenburg G. Fluctuations in the growth energy of malignant tumors in man, with especial reference to spontaneous recession. J Cancer Res. 1918;3:193–225. [Google Scholar]
- 6.Pearl R. Cancer and tuberculosis. Am J Hyg. 1929;9:97–162. [Google Scholar]
- 7.Braunstein A. Krebs und malaria. Z Krebsforsch. 1929;29:330–333. doi: 10.1007/BF01634496. [DOI] [Google Scholar]
- 8.Braunstein A. Experimentelle und klinische Grundlagen fuer Malariabehandlung des Krebses. Z Krebsforsch. 1929;29:468–490. [Google Scholar]
- 9.Engel P. Ueber den Infektionsindex der Krebskranken. Wien Klin Wochenschr. 1934;47:1118–1119. [Google Scholar]
- 10.Sinek F. Versuch einer statistischen Erfassung endogener Faktoren bei Carcinomerkrankungen. Z Krebsforsch. 1936;44:492–527. doi: 10.1007/BF01668080. [DOI] [Google Scholar]
- 11.Diamond LK, Luhby LA. Pattern of ‘spontaneous’ remissions in leukemia of the childhood, observed in 26 of 300 cases. Am J Med. 1951;10:238ff. doi: 10.1016/0002-9343(51)90263-X. [DOI] [Google Scholar]
- 12.West RO. Epidemiologic study of malignancies of the ovaries. Cancer. 1966;19(7):1001–1007. doi: 10.1002/1097-0142(196607)19:7<1001::AID-CNCR2820190714>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 13.Klyuyeva NG, Roskin GI. Biotherapy of malignant tumours. Oxford: Pergamon Press; 1963. [Google Scholar]
- 14.Kienle GS. Fever in cancer treatment: Coley’s therapy and epidemiological observations. Glob Adv Health Med. 2012;1(1):90–98. doi: 10.7453/gahmj.2012.1.1.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witzel L. History and other diseases in patients with malignant neoplasms. Med Klin. 1970;65(18):876–879. [PubMed] [Google Scholar]
- 16.Stephenson HE, Jr, et al. Host immunity and spontaneous regression of cancer evaluated by computerized data reduction study. Surg Gynecol Obstet. 1971;133(4):649–655. [PubMed] [Google Scholar]
- 17.Zygiert Z. Hodgkin’s disease: remissions after measles. Lancet. 1971;1(7699):593. doi: 10.1016/S0140-6736(71)91186-X. [DOI] [PubMed] [Google Scholar]
- 18.Ruckdeschel JC, et al. Postoperative empyema improves survival in lung cancer. documentation and analysis of a natural experiment. N Engl J Med. 1972;287(20):1013–1017. doi: 10.1056/NEJM197211162872004. [DOI] [PubMed] [Google Scholar]
- 19.Newhouse ML, et al. A case control study of carcinoma of the ovary. Br J Prev Soc Med. 1977;31(3):148–153. doi: 10.1136/jech.31.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menczer J, et al. Possible role of mumps virus in the etiology of ovarian cancer. Cancer. 1979;43(4):1375–1379. doi: 10.1002/1097-0142(197904)43:4<1375::AID-CNCR2820430427>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 21.Cramer DW, et al. Mumps, menarche, menopause, and ovarian cancer. Am J Obstet Gynecol. 1983;147(1):1–6. doi: 10.1016/0002-9378(83)90073-x. [DOI] [PubMed] [Google Scholar]
- 22.Remy W, et al. Tumorträger haben selten Infekte in der Anamnese. Med Klin. 1983;78:95–98. [Google Scholar]
- 23.Ronne T. Measles virus infection without rash in childhood is related to disease in adult life. Lancet. 1985;1(8419):1–5. doi: 10.1016/S0140-6736(85)90961-4. [DOI] [PubMed] [Google Scholar]
- 24.Enterline PE, et al. Endotoxins, cotton dust, and cancer. Lancet. 1985;2(8461):934–935. doi: 10.1016/S0140-6736(85)90861-X. [DOI] [PubMed] [Google Scholar]
- 25.van Steensel-Moll HA, Valkenburg HA, van Zanen GE. Childhood leukemia and infectious diseases in the first year of life: a register-based case–control study. Am J Epidemiol. 1986;124(4):590–594. doi: 10.1093/oxfordjournals.aje.a114431. [DOI] [PubMed] [Google Scholar]
- 26.Grossarth-Maticek R, et al. Reported herpes-virus-infection, fever and cancer incidence in a prospective study. J Chronic Dis. 1987;40(10):967–976. doi: 10.1016/0021-9681(87)90147-0. [DOI] [PubMed] [Google Scholar]
- 27.Rotoli B, et al. Long-term survival in acute myelogenous leukemia complicated by chronic active hepatitis. N Engl J Med. 1982;307(27):1712–1713. doi: 10.1056/NEJM198212303072721. [DOI] [PubMed] [Google Scholar]
- 28.Treon SP, Broitman SA. Beneficial effects of post-transfusional hepatitis in acute myelogenous leukemia may be mediated by lipopolysaccharides, tumor necrosis factor alpha and interferon gamma. Leukemia. 1992;6(10):1036–1042. [PubMed] [Google Scholar]
- 29.Abel U, et al. Common infections in the history of cancer patients and controls. J Cancer Res Clin Oncol. 1991;117(4):339–344. doi: 10.1007/BF01630717. [DOI] [PubMed] [Google Scholar]
- 30.Mastrangelo G, Fadda E, Milan G. Cancer increased after a reduction of infections in the first half of this century in Italy: etiologic and preventive implications. Eur J Epidemiol. 1998;14(8):749–754. doi: 10.1023/A:1007560709013. [DOI] [PubMed] [Google Scholar]
- 31.Albonico HU, Braker HU, Husler J. Febrile infectious childhood diseases in the history of cancer patients and matched controls. Med Hypotheses. 1998;51(4):315–320. doi: 10.1016/S0306-9877(98)90055-X. [DOI] [PubMed] [Google Scholar]
- 32.Maurer S, Koelmel KF. Spontaneous regression of advanced malignant melanoma. Onkologie. 1998;21:14–18. doi: 10.1159/000026785. [DOI] [Google Scholar]
- 33.Schlehofer B, et al. Role of medical history in brain tumour development. Results from the international adult brain tumour study. Int J Cancer. 1999;82(2):155–160. doi: 10.1002/(SICI)1097-0215(19990719)82:2<155::AID-IJC1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 34.Koelmel KF, et al. Infections and melanoma risk: results of a multicenter EORTC case study. Melanoma Res. 1999;9:511–519. doi: 10.1097/00008390-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Stewart BW, Kleihues P. World Cancer report. Lyon: World Health Organization, IARC Press; 2003. [Google Scholar]
- 36.Koelmel KF, et al. Prior immunisation of patients with malignant melanoma with vaccinia or BCG is associated with better survival. An European Organization for Research and Treatment of Cancer cohort study on 542 patients. Eur J Cancer. 2005;41(1):118–125. doi: 10.1016/j.ejca.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 37.Mastrangelo G, et al. Lung cancer risk: effect of dairy farming and the consequence of removing that occupational exposure. Am J Epidemiol. 2005;161(11):1037–1046. doi: 10.1093/aje/kwi138. [DOI] [PubMed] [Google Scholar]
- 38.Jeys LM, et al. Post operative infection and increased survival in osteosarcoma patients: are they associated? Ann Surg Oncol. 2007;14(10):2887–2895. doi: 10.1245/s10434-007-9483-8. [DOI] [PubMed] [Google Scholar]
- 39.Kim SY, et al. The influence of infection early after allogeneic stem cell transplantation on the risk of leukemic relapse and graft-versus-host disease. Am J Hematol. 2008;83(10):784–788. doi: 10.1002/ajh.21227. [DOI] [PubMed] [Google Scholar]
- 40.Vestergaard H, et al. Tonsillitis, tonsillectomy and Hodgkin’s lymphoma. Int J Cancer. 2010;127(3):633–637. doi: 10.1002/ijc.24973. [DOI] [PubMed] [Google Scholar]
- 41.Rudant J, et al. Childhood acute leukemia, early common infections, and allergy: the ESCALE Study. Am J Epidemiol. 2010;172(9):1015–1027. doi: 10.1093/aje/kwq233. [DOI] [PubMed] [Google Scholar]
- 42.Urayama KY, et al. A meta-analysis of the association between day-care attendance and childhood acute lymphoblastic leukaemia. Int J Epidemiol. 2010;39(3):718–732. doi: 10.1093/ije/dyp378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urayama KY, et al. Early life exposure to infections and risk of childhood acute lymphoblastic leukemia. Int J Cancer. 2011;128(7):1632–1643. doi: 10.1002/ijc.25752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudant J, et al. Childhood Hodgkin’s lymphoma, non-Hodgkin’s lymphoma and factors related to the immune system: the Escale Study (SFCE) Int J Cancer. 2011;129(9):2236–2247. doi: 10.1002/ijc.25862. [DOI] [PubMed] [Google Scholar]
- 45.Chilvers C, et al. The common cold, allergy, and cancer. Br J Cancer. 1986;54(1):123–126. doi: 10.1038/bjc.1986.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cardwell CR, et al. Infections in early life and childhood leukaemia risk: a UK case–control study of general practitioner records. Br J Cancer. 2008;99(9):1529–1533. doi: 10.1038/sj.bjc.6604696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoffmann C, et al. Childhood diseases, infectious diseases, and fever as potential risk factors for cancer? Forsch Komplementarmed Klass Naturheilkd. 2002;9:324–330. doi: 10.1159/000069231. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y, et al. Risk factors for epithelial ovarian cancer in Beijing, China. Int J Epidemiol. 1992;21(1):23–29. doi: 10.1093/ije/21.1.23. [DOI] [PubMed] [Google Scholar]
- 49.Mantovani A, Pierotti MA. Cancer and inflammation: a complex relationship. Cancer Lett. 2008;267:180–181. doi: 10.1016/j.canlet.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 51.Christian S, Palmer L. An apparent recovery from multiple sarcoma with involvement of both bone and soft parts treated by toxin of erysipelas and bacillus prodigiosus. Am J Surg. 1928;43:188–197. doi: 10.1016/S0002-9610(28)90373-6. [DOI] [Google Scholar]
- 52.Coley-Nauts HC, Fowler GAA, Bogatko FH. A review of the influence of bacterial infection and of bacterial products (Coley’s toxins) on malignant tumors in man. Acta Med Scand. 1953;145:5–102. [PubMed] [Google Scholar]
- 53.Hobohm U, Stanford JL, Grange JM. Pathogen-associated molecular pattern in cancer immunotherapy. Crit Rev Immunol. 2008;28:95–107. doi: 10.1615/CritRevImmunol.v28.i2.10. [DOI] [PubMed] [Google Scholar]
- 54.Hobohm U. Toward general prophylactic cancer vaccination. BioEssays. 2009;31:1071–1079. doi: 10.1002/bies.200900025. [DOI] [PubMed] [Google Scholar]
- 55.Molassiotis A, Fernadez-Ortega P, Pud D, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 2005;16:655–663. doi: 10.1093/annonc/mdi110. [DOI] [PubMed] [Google Scholar]
- 56.Kirsch A, Hajto T. Case reports of sarcoma patients with optimized lectin-oriented mistletoe extract therapy. J Altern Complement Med. 2011;17:973–979. doi: 10.1089/acm.2010.0596. [DOI] [PubMed] [Google Scholar]
- 57.Ostermann T, Bussing A. Retrolective studies on the survival of cancer patients treated with mistletoe extracts: a meta-analysis. Explore (NY) 2012;8:277–281. doi: 10.1016/j.explore.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Bussing A, Raak C, Ostermann T. Quality of life and related dimensions in cancer patients treated with mistletoe extract (iscador): a meta-analysis. Evid Based Complement Alternat Med. 2012;2012:219402. doi: 10.1155/2012/219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kienle GS, Kiene H. Review article: influence of Viscum album L. (European mistletoe) extracts on quality of life in cancer patients: a systematic review of controlled clinical studies. Integr Cancer Ther. 2010;9:142–157. doi: 10.1177/1534735410369673. [DOI] [PubMed] [Google Scholar]
- 60.Orange M, Fonseca M, Lace A, Laue HB, Geider S. Durable tumour responses following primary high dose induction with mistletoe extracts: two case reports. Eur J Integr Med. 2010;2:63–69. doi: 10.1016/j.eujim.2010.04.001. [DOI] [Google Scholar]
- 61.Orange M, Lace A, Fonseca MP, Laue BH, Geider S, Kienle GS. Durable regression of primary cutaneous B-cell lymphoma following fever-inducing mistletoe treatment: two case reports. Global Adv Health Med. 2012;1:18–25. doi: 10.7453/gahmj.2012.1.1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee CH, Kim JK, Kim HY, Park SM, Lee SM. Immunomodulating effects of Korean mistletoe lectin in vitro and in vivo. Int Immunopharmacol. 2009;9:1555–1561. doi: 10.1016/j.intimp.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 63.Huber R, Ludtke H, Wieber J, Beckmann C. Safety and effects of two mistletoe preparations on production of Interleukin-6 and other immune parameters: a placebo controlled clinical trial in healthy subjects. BMC Complement Altern Med. 2011;11:116. doi: 10.1186/1472-6882-11-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thies A, Dautel P, Meyer A, Pfuller U, Schumacher U. Low-dose mistletoe lectin-I reduces melanoma growth and spread in a scid mouse xenograft model. Br J Cancer. 2008;98:106–112. doi: 10.1038/sj.bjc.6604106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma YH, Cheng WZ, Gong F, Ma AL, Yu QW, Zhang JY, Hu CY, Chen XH, Zhang DQ. Active Chinese mistletoe lectin-55 enhances colon cancer surveillance through regulating innate and adaptive immune responses. World J Gastroenterol. 2008;14:5274–5281. doi: 10.3748/wjg.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park HJ, Hong JH, Kwon HJ, Kim Y, Lee KH, Kim JB, Song SK. TLR4-mediated activation of mouse macrophages by Korean mistletoe lectin-C (KML-C) Biochem Biophys Res Commun. 2010;396:721–725. doi: 10.1016/j.bbrc.2010.04.169. [DOI] [PubMed] [Google Scholar]
- 67.Abagyan RA, Batalov S. Do aligned sequences share the same fold? J Mol Biol. 1997;273:355–368. doi: 10.1006/jmbi.1997.1287. [DOI] [PubMed] [Google Scholar]
- 68.Peri F, Piazza M. Therapeutic targeting of innate immunity with Toll-like receptor 4 (TLR4) antagonists. Biotechnol Adv. 2012;30:251–260. doi: 10.1016/j.biotechadv.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 69.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 70.Benwell RK, Lee DR. Essential and synergistic roles of IL1 and IL6 in human Th17 differentiation directed by TLR ligand-activated dendritic cells. Clin Immunol. 2010;134:178–187. doi: 10.1016/j.clim.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 71.Muthing J, Meisen I, Kniep B, et al. Tumor-associated CD75s gangliosides and CD75s-bearing glycoproteins with Neu5Acalpha2-6Galbeta1-4GlcNAc-residues are receptors for the anticancer drug rViscumin. FASEB J. 2005;19:103–105. doi: 10.1096/fj.04-2494fje. [DOI] [PubMed] [Google Scholar]
- 72.Branden C, Tooze J. Introduction to protein structure. New York: Garland Science; 1999. [Google Scholar]
- 73.Greene LH, Lewis TE, Addou S, et al. The CATH domain structure database: new protocols and classification levels give a more comprehensive resource for exploring evolution. Nucleic Acids Res. 2007;35:D291–D297. doi: 10.1093/nar/gkl959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hobohm U. Fever therapy revisited. Br J Cancer. 2005;92:421–425. doi: 10.1038/sj.bjc.6602386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pawelek JM, Low KB, Bermudes D. Bacteria as tumour-targeting vectors. Lancet Oncol. 2003;4:548–556. doi: 10.1016/S1470-2045(03)01194-X. [DOI] [PubMed] [Google Scholar]
- 77.Gandhi NM, Morales A, Lamm DL (2013) Bacillus Calmette–Guerin immunotherapy for genitourinary cancer. BJU Int. doi:10.1111/j.1464-410X.2012.11754.x [DOI] [PubMed]
- 78.Kimura NT, Taniguchi S, Aoki K, Baba T. Selective localization and growth of Bifidobacterium bifidum in mouse tumors following intravenous administration. Cancer Res. 1980;40:2061–2068. [PubMed] [Google Scholar]
- 79.Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, Xu M, Penman S, Hoffman RM. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium . Proc Natl Acad Sci USA. 2005;102:755–760. doi: 10.1073/pnas.0408422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao M, Yang M, Ma H, Li X, Tan X, Li S, Yang Z, Hoffman RM. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006;66:7647–7652. doi: 10.1158/0008-5472.CAN-06-0716. [DOI] [PubMed] [Google Scholar]
- 81.Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Hoffman RM. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium . J Cell Biochem. 2009;106:992–998. doi: 10.1002/jcb.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kimura H, Zhang L, Zhao M, Hayashi K, Tsuchiya H, Tomita K, Bouvet M, Wessels J, Hoffman RM. Targeted therapy of spinal cord glioma with a genetically modified Salmonella typhimurium . Cell Prolif. 2010;43:41–48. doi: 10.1111/j.1365-2184.2009.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu F, Zhang L, Hoffman RM, Zhao M. Vessel destruction by tumor-targeting Salmonella typhimurium A1-R is enhanced by high tumor vascularity. Cell Cycle. 2010;9:4518–4524. doi: 10.4161/cc.9.22.13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Momiyama M, Zhao M, Kimura H, Tran B, Chishima T, Bouvet M, Endo I, Hoffman RM. Inhibition and eradication of human glioma with tumor-targeting Salmonella typhimurium in an orthotopic nude-mouse model. Cell Cycle. 2012;11:628–632. doi: 10.4161/cc.11.3.19116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Agrawal N, Bettegowda C, Cheong I, et al. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc Natl Acad Sci USA. 2004;101:15172–15177. doi: 10.1073/pnas.0406242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 87.Krishnan J, Lee G, Choi S. Drugs targeting Toll-like receptors. Arch Pharm Res. 2009;32:1485–1502. doi: 10.1007/s12272-009-2100-6. [DOI] [PubMed] [Google Scholar]
- 88.Goutagny N, Estornes Y, Hasan U, Lebecque S, Caux C. Targeting pattern recognition receptors in cancer immunotherapy. Target Oncol. 2012;7:29–54. doi: 10.1007/s11523-012-0213-1. [DOI] [PubMed] [Google Scholar]
- 89.Zoglmeier C, Bauer H, Norenberg D, et al. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin Cancer Res. 2011;17:1765–1775. doi: 10.1158/1078-0432.CCR-10-2672. [DOI] [PubMed] [Google Scholar]