Abstract
The skeletal and immune systems have a complex relationship. Both systems are intimately coupled, with osteoclastogenesis and hematopoiesis occurring in the bone marrow. Bone and immune cells also share common hematopoietic precursors. Furthermore, the skeletal and immune systems share various cytokines, receptors, and transcription factors that regulate signal transduction pathways involved in osteoclastogenesis and immune system activation, including the receptor activator of nuclear factor-κΒ ligand/receptor activator of nuclear factor-κΒ/osteoprotegerin (RANKL–RANK–OPG) pathway. Cancer cells can disrupt both the skeletal and immune systems. Interaction between cancer and bone cells results in a vicious cycle of bone destruction and cancer growth. Bone remodeling generates a growth-factor-rich environment that attracts cancer cells and promotes their proliferation. In turn, cancer cells stimulate osteoclast formation and activity, resulting in additional bone resorption that further stimulates cancer cell growth. Currently available bone-targeted therapies may also modulate the immune system. Bisphosphonates such as zoledronic acid exert stimulating effects on the immune system, resulting in possible anticancer activity against malignant cells. Denosumab, an anti-RANKL monoclonal antibody with proven antiosteoclast activity, may suppress immune responses. This may result in the reported association with an increased risk of neoplasms, as well as serious skin and other infections as reported in some studies, mainly in the postmenopausal setting. When assessing bone-targeted therapies, it is important to consider the shared signaling pathways between bone and the immune system, as well as the clinical risk:benefit ratio.
Keywords: Anticancer, Bisphosphonates, Denosumab, Osteoimmunology, RANKL inhibition, Zoledronic acid
Introduction
The skeletal and immune systems are interconnected in normal (physiologic) and pathologic conditions. Both systems are intimately coupled, as osteoclastogenesis and hematopoiesis occur in the bone marrow. Osteoclasts, macrophages, and dendritic cells also share common precursors. Furthermore, the skeletal and immune systems share various cytokines, receptors, adaptor proteins, signaling molecules, and transcription factors, thereby allowing crosstalk to occur between the various cells and their respective signal transduction pathways involved in osteoclastogenesis and hematopoiesis.
Osteoclastogenesis and hematopoiesis
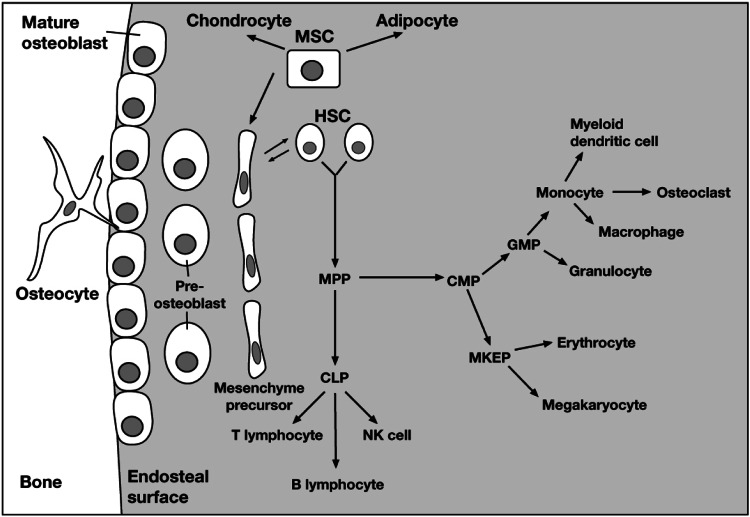
Hematopoietic stem cells are maintained in the bone marrow. Adjacent osteoblast precursors produce signals that control hematopoietic stem cell replication and differentiation. Hematopoietic stem cells may either maintain their pluripotency or differentiate into multipotential progenitor cells, which have the capacity to form common lymphoid progenitor or common myeloid precursor cells. Common lymphoid progenitor cells undergo additional differentiation to form T lymphocytes, B lymphocytes, or natural killer cells, whereas common myeloid precursor cells form all other myeloid lineages and preosteoclasts. Activated osteoclasts are formed from the fusion of preosteoclasts and multinucleated osteoclasts, the regulation of which is complex and affected by multiple factors. Multipotential stem cells differentiate into chondrocytes, adipocytes, and mesenchyme precursors; the latter undergo differentiation to form preosteoblasts and, eventually, mature matrix-producing osteoblasts. Osteoblasts may remain on the bone surface as lining cells or undergo terminal differentiation to form osteocytes, which become encased in the mineralized bone matrix (Fig. 1) [1]. The shared lineages and paracrine signaling between osteoclasts and hematopoietic cells highlight the potential for bone-targeted agents to influence the immune system.
Fig. 1.
Interaction between osteoblastic and hematopoietic cell lineages. Abbreviations: CLP, common lymphoid progenitor; CMP, common myeloid precursor; GMP, granulocyte–macrophage progenitor; HSC, hematopoietic stem cell; MKEP, megakaryocyte-erythroid progenitor; MPP, multipotential progenitor; MSC, mesenchymal stem cell; NK, natural killer. Reprinted from Lorenzo J, et al. (2008) Osteoimmunology: interactions of the bone and immune system. Endocr Rev 29:403–440. Copyright 2008 [1]
Crosstalk between skeletal and immune system components
The skeletal and immune systems share various signal transduction pathways, thereby allowing a complex interplay to occur between bone metabolism and immunology. Furthermore, immune system components, such as T cells, cytokines, and chemokines, can exert substantial effects on osteoclastogenesis.
Signal transduction in osteoclastogenesis
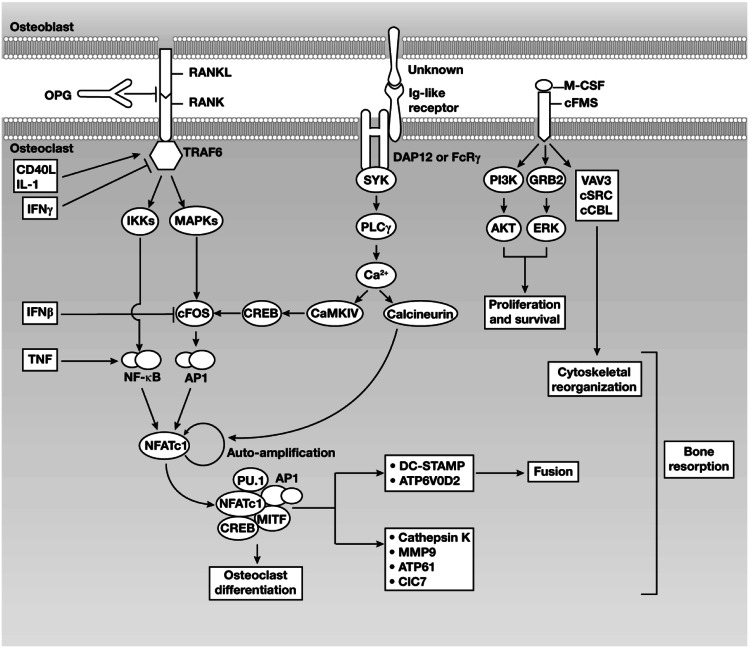
Osteoclastogenesis is primarily regulated via interactions between c-FMS and macrophage colony-stimulating factor, receptor activator of nuclear factor (NF)-κB (RANK) and RANK ligand (RANKL), and immunoglobulin (Ig)-like receptors and their ligands (Fig. 2) [2]. The role of RANK signaling in osteoclastogenesis has also been reviewed elsewhere [2–16]. Other key regulatory pathways are described below.
Fig. 2.
Osteoimmunologic interactions between osteoblasts and osteoclasts. Abbreviations: AP1, activator protein 1; CaMKIV, calcium-/calmodulin-dependent protein kinase type IV; CD40L, CD40 ligand; ClC, chloride channel; CREB, cyclic AMP responsive-element-binding protein; DAP, DNAX-activating protein; DC-STAMP, dendritic-cell-specific transmembrane protein; FcRγ, Fc-receptor common γ-subunit; GRB2-ERK, growth-factor-receptor-bound protein 2–extracellular-signal-regulated kinase; Ig, immunoglobulin; IFN, interferon; IL, interleukin; IKK, inhibitor of NF-κΒ kinase; MAPKs, mitogen-activated protein kinases; M-CSF, macrophage colony-stimulating factor; MITF, microphthalmia-associated transcription factor; MMP, matrix metalloproteinase; NFATc1, nuclear factor of activated T-cell cytoplasmic 1; NF-κΒ, nuclear factor-κΒ; OPG, osteoprotegerin; PI3K, phosphatidylinositol 3-kinase; PLC, phospholipase C; RANK, receptor activator of nuclear factor-κΒ; RANKL, receptor activator of nuclear factor-κΒ ligand; SYK, spleen tyrosine kinase; TNF, tumor necrosis factor; TRAF6, tumor necrosis factor receptor-associated factor 6. Reprinted by permission by Macmillan Publishers Ltd: Nature Reviews Immunology. Takayanagi H (2007) Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 7:292–304. Copyright 2007 [2]
RANK signaling
Receptor activator of NF-κΒ ligand is a member of the tumor necrosis factor (TNF) cytokine superfamily that is expressed by osteoblasts, monocytes, neutrophils, dendritic cells, B lymphocytes, and T lymphocytes [3]. Secretion of RANKL by osteoclastogenesis-supporting cells (osteoblasts and synovial fibroblasts) occurs in response to osteoclastogenic factors such as 1,25-dihydroxyvitamin D3, prostaglandin E2, and parathyroid hormone [2]. T cells express RANKL as a type-2 membrane-bound protein and also release it in soluble form, although the function of the soluble form remains unknown [16]. Inflammatory cytokines, such as interleukin (IL)-1, IL-6, and TNF-α, also potently induce RANKL expression on osteoblasts and synovial fibroblasts, thereby stimulating RANKL signaling [2].
Receptor activator of NF-κB, the RANKL receptor, shares high homology with CD40, which is expressed on lymphocytes and, similar to RANKL, is reported to play a role in atherosclerosis and coronary artery disease [17–19]. Interaction of RANK with RANKL is inhibited by osteoprotegerin (OPG), a soluble competitor (decoy) receptor that binds to RANKL [12, 13]. Receptor activator of NF-κB lacks intrinsic enzymatic activity in its intracellular domain and transduces signals by recruiting adaptor molecules such as the TNF-receptor-associated factor (TRAF) family of proteins, especially TRAF6 [4, 5, 15]. By an unknown mechanism, RANKL binding to RANK induces trimerization of RANK and TRAF6, leading to activation of NF-κB and of mitogen-activated protein kinases such as Jun N-terminal kinase and p38 [6]. Activated RANK can also lead to stimulation of Ig-like receptor signaling.
Signaling through nuclear factor of activated T-cell cytoplasmic (NFATc)-1
Expression of NFATc-1, the master regulator of osteoclast differentiation, depends on induction of the TRAF6–NF-κB and c-FOS pathways, in addition to activation of calcium signaling [20]. Nuclear factor of activated T-cell cytoplasmic-1 is initially induced by TRAF6-activated NF-κB and NFATc-2. After translocation into the nucleus, NFATc-1 autoregulates its own expression by binding to the NFAT-binding site of its promoter, enabling robust induction of NFATc-1 expression [21]. Activator protein 1 and continuous activation of calcium signaling by calcineurin are crucial for NFATc-1 autoamplification [20]. Nuclear factor of activated T-cell cytoplasmic-1 cooperates with other transcription factors, such as AP1, PU.1, microphthalmia-associated transcription factor, and cyclic AMP responsive-element-binding protein, to regulate various osteoclast-specific genes, including tartrate-resistant acid phosphatase, cathepsin K, calcitonin receptor, osteoclast-associated receptor, and β3-integrin [2, 20, 22–24].
Effects of cytokines and chemokines on osteoclastogenesis
Immune cells produce a variety of proinflammatory cytokines that contribute to bone damage [25]. Tumor necrosis factor-alpha and IL-1, -3, -6, -7, -11, -15, and -17 potentiate bone loss by inducing RANKL expression on osteoblasts or by increasing osteoclast differentiation and activation. In contrast, IL-4, -5, -10, -12, -13, and -18 and interferon (IFN)-α, -β, and -γ inhibit osteoclastogenesis by directly or indirectly blocking RANKL signaling (Table 1). Interleukin-1 stimulates TRAF6 expression, thereby potentiating the RANKL–RANK signaling cascade and inducing mature osteoclasts to perform bone-resorbing activity. Interferon gamma down-regulates TRAF6 expression via proteosomal degradation, resulting in termination of osteoclast formation [26, 27]. Receptor activator of NF-κB induces expression of IFN-β in osteoclast precursor cells, and IFN-β functions as a negative feedback regulator of osteoclast differentiation by interfering with RANKL-induced c-FOS expression [28]. Tumor necrosis factor-alpha stimulates NF-κB activation primarily via interacting with TRAF2. Although TNF-α alone cannot induce osteoclastogenesis and TNF-α overexpression cannot rescue RANKL deficiency, TNF-α combined with transforming growth factor (TGF)-β induces osteoclastogenesis even in the absence of RANK or TRAF6 [29–31]. These results suggest that TNF-α plays a pivotal role in the pathologic activation of osteoclasts associated with inflammation (Fig. 2) [2]. Osteoblast-mediated bone formation is also affected by various soluble cytokines such as TNF-α, IL-1, and IL-4 [32]. The molecular mechanisms involved in osteoblast regulation by the immune system and the pathologic significance of such regulation are less understood than in osteoclasts.
Table 1.
Cytokines involved in osteoclastogenesis
| Cytokine | Main producer cells | Primary target in osteoclastogenesis | Effect on osteoclastogenesis | Role in osteoimmunology |
|---|---|---|---|---|
| RANKL | T-cells; Osteoblasts | Osteoclast precursor cells | Activation | Induction of osteoclast differentiation |
| TNF-α | Macrophages; Th1 cells | Osteoclast precursor cells; mesenchymal cells | Activation | RANKL induction on mesenchymal cells, RANKL synergy, inflammation |
| IL-6 | Th2 cells; dendritic cells | Mesenchymal cells; T cells | Activation | RANKL induction on mesenchymal cells, Th17-cell differentiation, inflammation |
| IL-17 | Th17 cells; memory T cells | Mesenchymal cells | Activation | RANKL induction on mesenchymal cells, inflammation |
| IFN-γ | Th1 cells; natural killer cells | Osteoclast precursor cells | Inhibition | RANKL signaling inhibition, cellular immunity |
| IL-4 | Th2 cells; natural killer T cells | Osteoclast precursor cells | Inhibition | RANKL signaling inhibition, humoral immunity |
| IL-10 | Th2 cells | Osteoclast precursor cells | Inhibition | RANKL signaling inhibition, antiinflammatory |
| IL-12 | Macrophages; dendritic cells | T cells | Inhibition | Th1-cell differentiation, IFN-γ and GM-CSF induction |
| IL-18 | Macrophages; dendritic cells | T cells | Inhibition | Th1-cell differentiation, IFN-γ induction |
| GM-CSF | Th1 cells | Osteoclast precursor cells | Inhibition | RANKL signaling inhibition, granulocyte differentiation |
GM-CSF granulocyte–macrophage colony-stimulating factor, IFN interferon, IL, interleukin, RANKL receptor activator of nuclear factor-κB ligand, Th T-helper, TNF tumor necrosis factor
Effects of T cells on osteoclastogenesis
In general, activated T cells exert an inhibitory effect on osteoclastogenesis. The CD4+ T-helper (Th) cells have traditionally been divided into 2 main subtypes—Th1 and Th2—based on their associated cytokine profiles. The Th1 cells mainly produce IFN-γ and IL-2 and mediate cellular immunity. In contrast, Th2 cells mainly produce IL-4, IL-5, and IL-10 and mediate humoral immunity. Although T cells express RANKL, most Th1 cytokines, as well as certain Th2 cytokines (e.g., IL-4 and IL-10), exert an inhibitory effect on osteoclastogenesis. However, the Th-cell subset involved in producing IL-17 (Th17 cells) is considered to be the typical osteoclastogenic Th subset. The Th17 cells express RANKL at higher levels than Th1 or Th2 cells and, as a result, may directly participate in osteoclastogenesis. In addition, Th17 cells do not produce large amounts of IFN-γ, an inhibitor of osteoclastogenesis. Furthermore, Th17 cells activate local inflammation, triggering release of proinflammatory cytokines that potentiate RANKL expression on osteoclastogenesis-supporting cells and RANKL–RANK signal transduction in osteoclast precursor cells [33]. Interleukin-17, produced by Th17 cells, induces the synthesis of matrix-degrading enzymes, such as matrix metalloproteinases, that mediate bone and cartilage degradation [34]. The effects of Th17 cells on osteoclastogenesis are balanced by regulatory T cells, which suppress osteoclast formation via a cytokine-dependent mechanism mediated by TGF-β, IFN-γ, IL-4, and IL-10 [35–37]. Therefore, the effects of T cells on osteoclastogenesis depend on the balance between positive and negative factors expressed by these cells under pathologic conditions.
Disruption of the skeletal and immune systems in cancer
Tumorigenesis can disrupt the skeletal and immune systems. Tumor growth and metastasis necessitate evasion of the immune system, especially phosphoantigen-targeted gamma delta T cells (γδ T cells), which can detect and destroy cancer cells. Immune system components also play other key roles in tumor development and progression. For example, tumor-associated macrophages (TAMs) are abundant in the bone microenvironment and influence multiple steps in tumor development, including growth, survival, invasion, and metastasis, as well as angiogenesis and lymphangiogenesis [38, 39]. During early metastasis of solid tumors, disseminated tumor cells (DTCs) survive in the bone marrow of patients with various tumor types. Cancers for which DTCs have been detected in patients who have not developed overt metastases include breast, colon, gastric, lung, and prostate cancers [40–46]. The hematopoietic niche in the bone marrow also provides a “harbor” for DTCs to survive despite anticancer therapies. Whether this niche also harbors cancer cells against anticancer immune defenses is unknown. However, the shared signal transduction pathways among the bone remodeling and immune system machineries in this common microenvironment suggest that activation of this vicious cycle of tumor growth and osteolytic bone destruction could also lead to localized immunosuppression or recruitment of metastasis-supporting TAMs, an unfortunate juxtaposition of osteoimmunology effects. Later in the disease course, interactions between malignant cells and bone may result in a vicious cycle of bone destruction and cancer growth (the “seed and soil theory”) [47]. The effects of cancer on bone can result in skeletal-related events (SREs) that include pathologic fracture, spinal cord compression, hypercalcemia of malignancy, and the need for radiotherapy. Furthermore, some cancers such as myeloma can exert additional deleterious effects on bone metabolism via inducing osteolysis, systemic bone loss, and suppression of new bone formation throughout the skeleton [48, 49].
Effects of osteoclastogenesis on cancer growth and metastases
Osteoclast-mediated osteolysis results in release of growth factors in the bone microenvironment that facilitate cancer growth and metastases. Bone-derived cytokines provide a chemotactic stimulus for directed tumor cell migration [50]. Recent studies established that RANKL is a chemoattractant that increases migration and invasion of RANK-positive cancer cells (bone tropism) [51, 52]. In preclinical models, bone resorption by bone cell cultures stimulated proliferation of various tumor cell types, including breast cancer that possessed bone-metastasizing properties [53]. In animal models, cancer cells located immediately adjacent to bone surfaces had significantly greater proliferation rates compared with those distant from bone, suggesting a mitogenic effect within the bone microenvironment [54]. Furthermore, in an animal model wherein bone resorption was stimulated by tumor cells, the proliferation rate of metastatic cancer cells was increased in bone but not in other tissues [55].
Effects of tumorigenesis on bone resorption
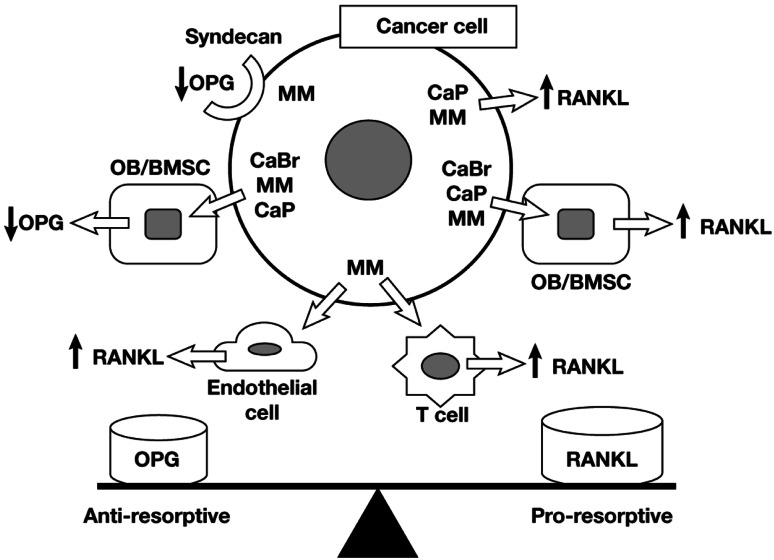
Cancer cells stimulate osteoclast-mediated osteolysis via several mechanisms. Cancer cells may express RANKL and RANK, up-regulate RANKL expression by other osteoimmune cell types, down-regulate OPG expression, and stimulate release of factors that activate RANKL–RANK signaling in osteoclasts (Fig. 3) [56]. Expression of RANKL has been detected in prostate cancer cells [57] and multiple myeloma (MM) cells [58, 59], and RANKL expression by MM cells correlated with the propensity to cause bone destruction [58]. Although breast cancer cells do not typically express RANKL [60, 61], they can up-regulate RANKL expression by osteoblasts [60, 61] and bone marrow stromal cells [61, 62]. Prostate cancer cells can up-regulate RANKL expression in osteoblasts [63], and MM cells up-regulate RANKL expression in bone marrow stromal cells [64], endothelial cells [65], and T cells [66]. Several studies also reported expression of functional RANK by breast cancer, prostate, and melanoma cell lines [51, 52]. Breast cancer cells and MM cells down-regulate OPG production by osteoblasts and bone marrow stromal cells [60, 64]. Multiple myeloma cells express the heparin sulfate proteoglycan, syndecan, on their surface, which sequesters and degrades heparin-binding proteins including OPG [67]. Notably, the RANKL–OPG balance is disturbed in severe osteolytic pathologies in favor of RANKL, with large quantities of OPG being released within the tumor microenvironment to counterbalance high RANKL concentrations [64, 68].
Fig. 3.
Mechanisms by which cancer cells may promote bone resorption via regulation of RANKL and/or OPG. Abbreviations: BMSC, bone marrow stromal cells; CaBr, breast cancer; CaP, prostate cancer; MM, multiple myeloma; OB, osteoblasts; OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor-κΒ ligand. Reprinted from Kearns AE, et al. Receptor activator of nuclear factor κB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 29:155–192. Copyright 2008 [56]
Effects of cancer treatment on the skeletal and immune systems
Bone-targeted therapies
Early generation bisphosphonates
In general, early generation bisphosphonates do not appear to activate the immune system against cancer cells. However, clodronate combined with IL-2 stimulated proliferation of γδ T cells in the absence of other cellular components in peripheral blood mononuclear cell (PBMC) cultures (wherein nitrogen-containing bisphosphonates have been tested), and clodronate-treated γδ T cells exhibited higher cytotoxic activity against neuroblastoma cells compared with untreated control cells [69]. There are currently no data on whether these effects can result in meaningful anticancer activities in in vivo models. Clodronate has shown efficacy in preventing SREs in patients with bone metastases from MM [70–73] and breast cancer [74] and was recently reported to significantly prolong survival in men with bone metastases from prostate cancer [75] (Table 2) [70, 72, 74–82]. Results from trials in the adjuvant breast cancer setting were inconsistent and provided some evidence to suggest that clodronate can delay not only metastasis to bone but also to visceral sites.
Table 2.
Efficacy of bone-targeted agents in patients with bone metastases
| Bisphosphonate | Cancer type | Patients, N | Reduction of SREs | Reduction of pain | Acute-phase reaction | Survival benefit |
|---|---|---|---|---|---|---|
| Clodronate [70] | Multiple myeloma | 350 | Yes | Yes | No | NE |
| Clodronate [72] | Multiple myeloma | 536 | Yes | Yes | No | ±a |
| Clodronate [74] | Breast cancer | 173 | Yes | Yes | No | No |
| Clodronate [75] | Prostate cancer | 819 | NR | NR | No | Yes |
| Pamidronate [76] | Multiple myeloma | 392 | Yes | Yes | Yes | ±b |
| Ibandronate [77] | Multiple myeloma | 198 | No | No | ± | No |
| Zoledronic acid [78] | Multiple myeloma or breast cancer | 1,648 | Yes | Yes | Yes | Yes |
| Zoledronic acid [79] | Breast cancer | 228 | Yes | Yes | Yes | NE |
| Zoledronic acid [80] | Lung cancer and other solid tumors | 773 | Yes | NE | Yes | No |
| Zoledronic acid [81] | Hormone-refractory prostate cancer | 122 | Yes | Yes | Yes | NE |
| Denosumab [82] | Breast cancer | 2,046 | Yes | NE | Yes | NE |
NE not evaluated, NR not reported, SREs skeletal-related events
aIn a post hoc analysis, patients without vertebral fracture at study entry survived significantly longer on clodronate therapy (median survival was 23 months longer compared with patients receiving placebo)
bSurvival of patients with more advanced disease was significantly increased in the pamidronate group (median survival of 21 vs. 14 months, P = .041)
Nitrogen-containing bisphosphonates
Nitrogen-containing bisphosphonates, such as zoledronic acid (ZOL) and pamidronate, cause immune system activation against cancer cells via activating γδ T cells [83, 84]. By blocking G-protein signaling, these agents prevent differentiation of monocytes into osteoclasts, inhibit osteoclast recruitment and maturation, induce osteoclast apoptosis, and inhibit adhesion of osteoclasts to bone [85].
Pamidronate—Pamidronate therapy is associated with SRE reductions in patients with bone metastases from MM [76]. Although there was no overall difference in survival between pamidronate- and placebo-treated patients, pamidronate prolonged survival among patients who had received more than 1 previous antimyeloma regimen (14 vs. 21 months; P = .041; N = 392) [86].
Although evidence is limited, pamidronate has demonstrated effects on the immune system that may result in anticancer activity. Treatment with pamidronate induced expansion of γδ T cells in PBMC cultures from healthy donors, and pamidronate-activated γδ T cells produced immunostimulatory cytokines and exhibited specific cytotoxicity against lymphoma and myeloma cell lines. Furthermore, pamidronate-treated bone marrow cultures from patients with MM exhibited reduced plasma cell survival compared with untreated cultures, especially in pamidronate-treated cultures, in which activation of bone marrow γδ T cells was evident (14 of 24 patients) [87].
Ibandronate—Administration of ibandronate to patients with advanced MM failed to reduce bone morbidity or prolong survival [77]. Ibandronate also produced a lesser reduction in markers of bone resorption and disease activity, including N-telopeptide of type I collagen (NTX), IL-6, and β2-microglobulin, compared with pamidronate [88]. However, ibandronate has demonstrated efficacy in the reduction of skeletal complications in other tumor types such as breast cancer [89].
Zoledronic acid—Numerous studies established that ZOL exhibits consistent efficacy in delaying and preventing SREs in patients with malignant bone disease from MM [78, 90, 91] and various solid tumors including breast [79], lung [80, 92], and prostate cancers [81]. In a 25-month randomized trial comparing ZOL with pamidronate in patients with bone lesions from MM or breast cancer (N = 1,648), a 15-minute infusion of 4 mg ZOL was at least as effective as a 2-hour infusion of 90 mg pamidronate at reducing the risk of SRE complications in the overall population [78]. Similarly, treating patients with lung cancer and other solid tumors with ZOL resulted in fewer patients developing SREs (ZOL 8 mg reduced to 4 mg = 36%, placebo = 46%; P = .023; N = 773) [80]. Administration of ZOL to men with hormone-refractory metastatic prostate cancer also reduced the proportion of patients with SREs (38% vs. 49%; P = .028 vs. placebo; N = 122) [81].
A recent study also demonstrated that ZOL may elicit anticancer effects associated with immune system stimulation. Zoledronic acid activated γδ T cells in vitro, and administration of ZOL to patients with prostate cancer resulted in the activation of γδ T cells in peripheral blood after the first infusion. Moreover, after the first ZOL infusion, serum prostate-specific antigen (PSA) levels were reduced in 3 of 11 evaluable patients, and PSA velocity was reduced in 5 of 10 evaluable patients [93]. These results suggest that ZOL-activated γδ T cells may be associated with the induction of an anticancer response in patients with prostate cancer.
Anticancer and antitumor activity of bisphosphonates
Numerous in vitro studies established that ZOL directly and indirectly inhibits multiple steps involved in the processes of cancer development and progression. In addition, ZOL stimulates cancer cell apoptosis and expansion of γδ T cells, which play an important role in immune surveillance against neoplasia [94]. Preclinical studies reported that ZOL elicits anticancer activity in various cancer types and exhibits synergy with cytotoxic agents [95–100]. Four separate studies reported that ZOL reduced the persistence of DTCs in the bone marrow of patients with breast cancer [101–104]. In the clinical setting, adding ZOL to standard anticancer therapy improved clinical outcomes in early breast cancer. Administration of ZOL combined with adjuvant endocrine therapy to premenopausal women improved disease-free survival (hazard ratio [HR] = 0.64; P = .01) compared with endocrine therapy alone in the ABCSG-12 trial (N = 1,803) [105]. Similarly, ZOL plus neoadjuvant chemotherapy reduced residual invasive tumor size by 44% compared with chemotherapy in an exploratory subgroup from the AZURE trial (P = .006; n = 205) [106]. A multivariate analysis adjusted for potential prognostic factors in addition to neoadjuvant treatment group demonstrated that patients treated with ZOL plus neoadjuvant chemotherapy had a twofold greater complete pathologic response rate (breast and axilla) compared with patients treated with chemotherapy alone (odds ratio = 2.2; P = .1457). In the ZO-FAST (N = 1,065; median follow-up = 48 months; HR = 0.59; P = .0176) and Z-FAST (N = 602; median follow-up = 61 months; P = .6283) studies in postmenopausal women receiving adjuvant letrozole, immediate addition of ZOL reduced disease recurrence [107, 108]. In contrast with ABCSG-12, which had disease-free survival as a primary endpoint, ZO-FAST and Z-FAST were not designed or powered to evaluate disease recurrence (primary endpoints were bone loss); however, these studies demonstrated that upfront administration of ZOL resulted in improved disease-free survival among women with breast cancer. Subset analyses of the phase III clinical studies revealed that ZOL significantly prolonged survival compared with placebo among patients with high baseline NTX levels. Benefits were independent of SRE prevention, and multiple anticancer mechanisms, some of which involved immune system activation, may have contributed [109, 110]. Additionally, ZOL elicited anticancer responses in patients with MM, bladder cancer, lung cancer, or advanced solid tumors [111–114]. The Medical Research Council (MRC) Myeloma IX trial demonstrated that, after median follow-up of 3.7 years, ZOL significantly improved overall survival (by 5.5 months; 16% reduction in risk of death; P = .0118) and progression-free survival (by 2 months; 12% reduction in risk of disease progression; P = .0179) versus clodronate in patients with newly diagnosed MM (N = 1,960 evaluable patients) [111]. The survival benefit associated with ZOL was maintained in analyses adjusting for the potential effects of SREs on survival (P = .0178 vs. clodronate), again supporting anticancer mechanisms for ZOL, which may involve positive effects on anticancer immune responses [111].
Denosumab
Denosumab is a fully human IgG2 monoclonal antibody that binds to RANKL with high affinity and specificity, thereby inhibiting osteoclastogenesis. The effects of denosumab on bone remodeling have been evaluated in patients with postmenopausal osteoporosis, rheumatoid arthritis, and various cancers [115–118]. Limited safety data from the advanced cancer setting have been released. However, results from phase III studies in bone-loss settings suggested that adverse immunologic effects might occur.
The FREEDOM trial, a phase III clinical study of 7,868 healthy postmenopausal women with osteoporosis, demonstrated that denosumab reduced the risk of new vertebral fractures by 68% compared with placebo (P < .001) [117]. A number of recent studies also demonstrated that denosumab can prevent SREs among patients with bone metastases from breast cancer, prostate cancer, other solid tumors, or MM. Denosumab was superior to ZOL in delaying time to first on-study SRE (HR = 0.82; P = .01 superiority), time to first and subsequent on-study SREs (rate ratio = 0.77; P = .001) in 2,046 patients with advanced breast cancer [82], and in delaying time to first on-study SRE in patients with advanced castration-resistant prostate cancer (CRPC) (HR = 0.82; P = .008 superiority; N = 1,901) [119]. Median time to first on-study SRE was 20.7 months for denosumab versus 17.1 months for ZOL [119]. However, a significantly greater proportion of denosumab-treated patients experienced increased PSA levels compared with ZOL-treated patients (3.8% vs. 2.0%, respectively; P < .05) [119]. Based on these results, it is possible that RANKL inhibition may impair immunosurveillance. Denosumab was noninferior to ZOL in delaying time to first SRE in 1,776 patients with other advanced solid tumors or MM (HR = 0.84; P = .0007) [115].
Anticancer and antitumor effects of denosumab therapy—Denosumab demonstrated antitumor activity in a phase II trial in 37 patients with benign giant-cell tumor (GCT) of bone, a tumor type that overexpresses RANKL and is associated with increased osteoclastic activity [120]. Given the low metastatic potential of GCT, the results observed in this patient population may not translate to patients with malignancies wherein the pathophysiology is distinct from that of GCT. Anticancer activity of blocking RANKL has been recently described in mouse models. RANKL inhibition was acting directly on hormone-induced mammary epithelium at early stages in tumorigenesis, and the permissive contribution of progesterone to increased mammary cancer incidence was due to RANKL-dependent proliferative changes in the mammary epithelium [121]. Based on these data, we assume that denosumab may have an anticancer activity; however, this has not yet been demonstrated in the clinical setting.
Risk of infections or new malignancies with denosumab therapy—Signaling via the RANKL–RANK pathway is involved in B-cell and T-cell differentiation and in survival of dendritic cells. As a result, concerns have been raised regarding possible immunosuppression with RANKL inhibitors. Recent clinical studies suggest that increased infection risk may be associated with denosumab therapy. The incidence of skin infections requiring hospitalization (cellulitis: 0.3 vs. <0.1% for placebo; P = .002) and endocarditis (3 patients vs. 0 for placebo) was increased among postmenopausal women with osteoporosis who received denosumab therapy (FREEDOM) [115, 117]. A meta-analysis of 10,329 patients with osteopenia or osteoporosis also reported an increased risk of serious infections (odds ratio = 4.54 for denosumab vs. placebo; P = .03) [122]. Serious infections were reported in 2.3% of denosumab-treated patients with early stage breast cancer compared with 0.8% of placebo-treated patients (P = not reported [NR]; N = 249; HALT-BC trial) [123]. Similarly, serious infections occurred at a higher incidence among denosumab-treated patients with androgen-dependent prostate cancer (5.9% vs. 4.6% for placebo; P = NR; N = 1,468; HALT-PC trial) [124]. Urinary tract infections also occurred more frequently among denosumab-treated patients with prostate cancer-related bone metastases (15% vs. 6% for bisphosphonates; P = NR; N = 49) [125].
Denosumab is specific for human and certain nonhuman primate RANKL and fails to inactivate rodent RANKL. Consequently, no carcinogenicity studies have been performed with denosumab because of the absence of an appropriate animal model. However, safety analyses from clinical trials of denosumab to prevent bone loss in patients receiving hormone ablation therapy (HALT) for early stage breast or prostate cancer suggest that the potential for cancer progression may be increased with denosumab therapy. Among 1,456 patients with androgen-dependent prostate cancer in HALT-PC, 8.2% (n = 60) of denosumab-treated patients and 5.5% (n = 40) of placebo-treated patients experienced metastatic events (P = NR) [115]. Similarly, metastatic events were reported in 7% (n = 9) of denosumab-treated patients compared with 4.2% (n = 5) of placebo-treated patients with breast cancer in HALT-BC (P = NR; N = 249) [115]. Indeed, given the significantly increased rates of PSA progression in patients with CRPC and the significantly reduced survival in patients with MM treated with denosumab versus ZOL in the phase III clinical trials program (HR = 2.26) [126], further investigations on the potential effects of RANKL inhibition on cancer immunosurveillance and response are warranted.
Conclusions
The skeletal and immune systems have a complex relationship under normal (physiologic) and pathologic conditions. The RANKL–RANK–OPG signal transduction pathway plays a key role in regulating osteoclastogenesis. However, the effects of RANKL signaling are not limited to the skeletal system; RANKL is also expressed in other regulatory systems including the immune, cardiovascular, endocrine, and nervous systems. Expression of RANKL in the immune system regulates antigen-specific T-cell and B-cell responses, as well as the ability of T cells to interact with dendritic cells. Furthermore, RANKL directly affects the survival of antigen-presenting dendritic cells, which help other cells in the immune system to recognize and destroy abnormal cells and foreign antigens. Because of the systemic nature of RANKL expression, RANKL inhibition to prevent bone destruction may result in unintended consequences outside of the bone, including immune suppression with resulting possible increases in risk of infection or new malignancies. The long-term safety profiles of agents targeting this pathway are not yet known.
Currently available therapies designed to reduce pathologic osteolysis may also result in modulation of the immune system. Nitrogen-containing bisphosphonates such as ZOL exert beneficial effects on the immune system, resulting in activation of anticancer responses, as demonstrated in several clinical studies in various malignancies. Careful consideration should be paid to the shared pathways in bone immunology to maximize beneficial and minimize potentially negative effects in the clinical setting.
Acknowledgments
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. I thank Ann Marie Fitzmaurice, PhD, ProEd Communications, Inc.®, for her medical editorial assistance with this manuscript.
Conflict of interest
Dr. Terpos has received honoraria from Amgen and Novartis; Dr. Dimopoulos has received honoraria from Novartis.
References
- 1.Lorenzo J, Horowitz M, Choi Y. Osteoimmunology: interactions of the bone and immune system. Endocr Rev. 2008;29:403–440. doi: 10.1210/er.2007-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 3.Caetano-Lopes J, Canhao H, Fonseca JE. Osteoimmunology—the hidden immune regulation of bone. Autoimmun Rev. 2009;8:250–255. doi: 10.1016/j.autrev.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 4.Gohda J, Akiyama T, Koga T, Takayanagi H, Tanaka S, Inoue J. RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J. 2005;24:790–799. doi: 10.1038/sj.emboj.7600564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadono Y, Okada F, Perchonock C, Jang HD, Lee SY, Kim N, Choi Y. Strength of TRAF6 signalling determines osteoclastogenesis. EMBO Rep. 2005;6:171–176. doi: 10.1038/sj.embor.7400345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi N, Kadono Y, Naito A, Matsumoto K, Yamamoto T, Tanaka S, Inoue J. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001;20:1271–1280. doi: 10.1093/emboj/20.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 8.Mao D, Epple H, Uthgenannt B, Novack DV, Faccio R. PLCgamma2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J Clin Invest. 2006;116:2869–2879. doi: 10.1172/JCI28775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mocsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, Majumdar S, Lanier LL, Lowell CA, Nakamura MC. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci USA. 2004;101:6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross FP, Teitelbaum SL. alphavbeta3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol Rev. 2005;208:88–105. doi: 10.1111/j.0105-2896.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 11.Sato K, Suematsu A, Nakashima T, Takemoto-Kimura S, Aoki K, Morishita Y, Asahara H, Ohya K, Yamaguchi A, Takai T, Kodama T, Chatila TA, Bito H, Takayanagi H. Regulation of osteoclast differentiation and function by the CaMK-CREB pathway. Nat Med. 2006;12:1410–1416. doi: 10.1038/nm1515. [DOI] [PubMed] [Google Scholar]
- 12.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 13.Tsuda E, Goto M, Mochizuki S, Yano K, Kobayashi F, Morinaga T, Higashio K. Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun. 1997;234:137–142. doi: 10.1006/bbrc.1997.6603. [DOI] [PubMed] [Google Scholar]
- 14.Wagner EF, Eferl R. Fos/AP-1 proteins in bone and the immune system. Immunol Rev. 2005;208:126–140. doi: 10.1111/j.0105-2896.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 15.Wong BR, Josien R, Lee SY, Vologodskaia M, Steinman RM, Choi Y. The TRAF family of signal transducers mediates NF-kappaB activation by the TRANCE receptor. J Biol Chem. 1998;273:28355–28359. doi: 10.1074/jbc.273.43.28355. [DOI] [PubMed] [Google Scholar]
- 16.Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS, 3rd, Frankel WN, Lee SY, Choi Y. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 17.Gururajan P, Gurumurthy P, Nayar P, Babu S, Sarasabharati A, Victor D, Cherian KM. Increased serum concentrations of soluble CD40 ligand as a prognostic marker in patients with acute coronary syndrome. Indian J Clin Biochem. 2009;24:229–233. doi: 10.1007/s12291-009-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montecucco F, Steffens S, Mach F. The immune response is involved in atherosclerotic plaque calcification: could the RANKL/RANK/OPG system be a marker of plaque instability? Clin Dev Immunol. 2007;2007:75805. doi: 10.1155/2007/75805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakraborty S, Cheek J, Sakthivel B, Aronow BJ, Yutzey KE. Shared gene expression profiles in developing heart valves and osteoblast progenitor cells. Phys Genomics. 2008;35:75–85. doi: 10.1152/physiolgenomics.90212.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/S1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 21.Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, Takayanagi H. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202:1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crotti TN, Flannery M, Walsh NC, Fleming JD, Goldring SR, McHugh KP. NFATc1 regulation of the human beta3 integrin promoter in osteoclast differentiation. Gene. 2006;372:92–102. doi: 10.1016/j.gene.2005.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y, Sato K, Asagiri M, Morita I, Soma K, Takayanagi H. Contribution of nuclear factor of activated T cells c1 to the transcriptional control of immunoreceptor osteoclast-associated receptor but not triggering receptor expressed by myeloid cells-2 during osteoclastogenesis. J Biol Chem. 2005;280:32905–32913. doi: 10.1074/jbc.M505820200. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto M, Kogawa M, Wada S, Takayanagi H, Tsujimoto M, Katayama S, Hisatake K, Nogi Y. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J Biol Chem. 2004;279:45969–45979. doi: 10.1074/jbc.M408795200. [DOI] [PubMed] [Google Scholar]
- 25.Herman S, Kronke G, Schett G. Molecular mechanisms of inflammatory bone damage: emerging targets for therapy. Trends Mol Med. 2008;14:245–253. doi: 10.1016/j.molmed.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Datta HK, Ng WF, Walker JA, Tuck SP, Varanasi SS. The cell biology of bone metabolism. J Clin Pathol. 2008;61:577–587. doi: 10.1136/jcp.2007.048868. [DOI] [PubMed] [Google Scholar]
- 27.Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, Nakamura K, Taniguchi T. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 28.Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, Yokochi T, Oda H, Nakamura K, Ida N, Wagner EF, Taniguchi T. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002;416:744–749. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- 29.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li P, Schwarz EM, O’Keefe RJ, Ma L, Boyce BF, Xing L. RANK signaling is not required for TNFalpha-mediated increase in CD11(hi) osteoclast precursors but is essential for mature osteoclast formation in TNFalpha-mediated inflammatory arthritis. J Bone Miner Res. 2004;19:207–213. doi: 10.1359/JBMR.0301233. [DOI] [PubMed] [Google Scholar]
- 31.Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, Kim JH, Kobayashi T, Odgren PR, Nakano H, Yeh WC, Lee SK, Lorenzo JA, Choi Y. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med. 2005;202:589–595. doi: 10.1084/jem.20050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, Choi Y. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 33.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.David JP. Osteoimmunology: a view from the bone. Adv Immunol. 2007;95:149–165. doi: 10.1016/S0065-2776(07)95005-1. [DOI] [PubMed] [Google Scholar]
- 35.Askenasy N, Kaminitz A, Yarkoni S. Mechanisms of T regulatory cell function. Autoimmun Rev. 2008;7:370–375. doi: 10.1016/j.autrev.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Kim YG, Lee CK, Nah SS, Mun SH, Yoo B, Moon HB. Human CD4+CD25+ regulatory T cells inhibit the differentiation of osteoclasts from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2007;357:1046–1052. doi: 10.1016/j.bbrc.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 37.Kelchtermans H, Geboes L, Mitera T, Huskens D, Leclercq G, Matthys P. Activated CD4+CD25+ regulatory T cells inhibit osteoclastogenesis and collagen-induced arthritis. Ann Rheum Dis. 2009;68:744–750. doi: 10.1136/ard.2007.086066. [DOI] [PubMed] [Google Scholar]
- 38.Biswas SK, Lewis CE. NF-κB as a central regulator of macrophage function in tumors. J Leukoc Biol. 2010;88:877–884. doi: 10.1189/jlb.0310153. [DOI] [PubMed] [Google Scholar]
- 39.Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE. Regulation of macrophage function in tumors: the multifaceted role of NF-kappaB. Blood. 2009;113:3139–3146. doi: 10.1182/blood-2008-12-172825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pantel K, Alix-Panabieres C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009;6:339–351. doi: 10.1038/nrclinonc.2009.44. [DOI] [PubMed] [Google Scholar]
- 41.Raimondi C, Gradilone A, Gandini O, Petracca A, Nicolazzo C, Palazzo A, Naso G, Cortesi E, Gazzaniga P (2010) Circulating tumor cells in breast cancer: are currently available detection methods enough? (abstract 170PD). In: Presented at the 35th ESMO Congress, 8–12 Oct 2010, Milan, Italy
- 42.De Giorgi U, Mego M, Scarpi E, Handy BC, Jackson SA, Reuben J, Valero V, Hortobagyi GN, Ueno N, Cristofanilli M (2010) Relationship between lymphopenia and circulating tumor cells as prognostic factors for overall survival in metastatic breast cancer (abstract 171PD). In: Presented at the 35th ESMO Congress, 8–12 Oct 2010, Milan, Italy
- 43.Sastre J, Maestro ML, Gomez MA, Rivera Herrero F, Valladares M, Massuti B, Gallen M, Benavides M, Diaz Rubio E, Aranda E (2010) Enumeration circulating tumor cells (CTCs) is a prognostic and predictive factor for progression-free survival (PFS) and overall survival (OS) in colon cancer patients receiving first-line chemotherapy plus bevacizumab. A TTD Spanish Group Cooperative Study (abstract 173PD). In: Presented at the 35th ESMO Congress, 8–12 Oct 2010, Milan, Italy
- 44.Seeliger H, Spatz H, Jauch KW. Minimal residual disease in gastric cancer. Recent Results Cancer Res. 2003;162:79–87. doi: 10.1007/978-3-642-59349-9_7. [DOI] [PubMed] [Google Scholar]
- 45.Passlick B. Micrometastases in non-small cell lung cancer (NSCLC) Lung Cancer. 2001;34(suppl 3):S25–S29. doi: 10.1016/S0169-5002(01)00375-0. [DOI] [PubMed] [Google Scholar]
- 46.Morgan TM, Lange PH, Porter MP, Lin DW, Ellis WJ, Gallaher IS, Vessella RL. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res. 2009;15:677–683. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roodman GD, Dougall WC. RANK ligand as a therapeutic target for bone metastases and multiple myeloma. Cancer Treat Rev. 2008;34:92–101. doi: 10.1016/j.ctrv.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson DR, Plevak ME, Therneau TM, Greipp PR. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 49.Hjorth-Hansen H, Seifert MF, Borset M, Aarset H, Ostlie A, Sundan A, Waage A. Marked osteoblastopenia and reduced bone formation in a model of multiple myeloma bone disease in severe combined immunodeficiency mice. J Bone Miner Res. 1999;14:256–263. doi: 10.1359/jbmr.1999.14.2.256. [DOI] [PubMed] [Google Scholar]
- 50.Orr W, Varani J, Gondex MK, Ward PA, Mundy GR. Chemotactic responses of tumor cells to products of resorbing bone. Science. 1979;203:176–179. doi: 10.1126/science.569363. [DOI] [PubMed] [Google Scholar]
- 51.Mori K, Le Goff B, Charrier C, Battaglia S, Heymann D, Redini F. DU145 human prostate cancer cells express functional receptor activator of NFkappaB: new insights in the prostate cancer bone metastasis process. Bone. 2007;40:981–990. doi: 10.1016/j.bone.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 52.Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R, Hojilla CV, Komnenovic V, Kong YY, Schreiber M, Dixon SJ, Sims SM, Khokha R, Wada T, Penninger JM. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 53.Manishen WJ, Sivananthan K, Orr FW. Resorbing bone stimulates tumor cell growth. A role for the host microenvironment in bone metastasis. Am J Pathol. 1986;123:39–45. [PMC free article] [PubMed] [Google Scholar]
- 54.Kostenuik PJ, Singh G, Suyama KL, Orr FW. A quantitative model for spontaneous bone metastasis: evidence for a mitogenic effect of bone on Walker 256 cancer cells. Clin Exp Metastasis. 1992;10:403–410. doi: 10.1007/BF00133469. [DOI] [PubMed] [Google Scholar]
- 55.Kostenuik PJ, Singh G, Suyama KL, Orr FW. Stimulation of bone resorption results in a selective increase in the growth rate of spontaneously metastatic Walker 256 cancer cells in bone. Clin Exp Metastasis. 1992;10:411–418. doi: 10.1007/BF00133470. [DOI] [PubMed] [Google Scholar]
- 56.Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29:155–192. doi: 10.1210/er.2007-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown JM, Corey E, Lee ZD, True LD, Yun TJ, Tondravi M, Vessella RL. Osteoprotegerin and rank ligand expression in prostate cancer. Urology. 2001;57:611–616. doi: 10.1016/S0090-4295(00)01122-5. [DOI] [PubMed] [Google Scholar]
- 58.Farrugia AN, Atkins GJ, To LB, Pan B, Horvath N, Kostakis P, Findlay DM, Bardy P, Zannettino AC. Receptor activator of nuclear factor-kappaB ligand expression by human myeloma cells mediates osteoclast formation in vitro and correlates with bone destruction in vivo. Cancer Res. 2003;63:5438–5445. [PubMed] [Google Scholar]
- 59.Sezer O, Heider U, Zavrski I, Kuhne CA, Hofbauer LC. RANK ligand and osteoprotegerin in myeloma bone disease. Blood. 2003;101:2094–2098. doi: 10.1182/blood-2002-09-2684. [DOI] [PubMed] [Google Scholar]
- 60.Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, Martin TJ, Gillespie MT. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology. 1999;140:4451–4458. doi: 10.1210/en.140.10.4451. [DOI] [PubMed] [Google Scholar]
- 61.Kitazawa S, Kitazawa R. RANK ligand is a prerequisite for cancer-associated osteolytic lesions. J Pathol. 2002;198:228–236. doi: 10.1002/path.1199. [DOI] [PubMed] [Google Scholar]
- 62.Mancino AT, Klimberg VS, Yamamoto M, Manolagas SC, Abe E. Breast cancer increases osteoclastogenesis by secreting M-CSF and upregulating RANKL in stromal cells. J Surg Res. 2001;100:18–24. doi: 10.1006/jsre.2001.6204. [DOI] [PubMed] [Google Scholar]
- 63.Fizazi K, Yang J, Peleg S, Sikes CR, Kreimann EL, Daliani D, Olive M, Raymond KA, Janus TJ, Logothetis CJ, Karsenty G, Navone NM. Prostate cancer cells-osteoblast interaction shifts expression of growth/survival-related genes in prostate cancer and reduces expression of osteoprotegerin in osteoblasts. Clin Cancer Res. 2003;9:2587–2597. [PubMed] [Google Scholar]
- 64.Giuliani N, Bataille R, Mancini C, Lazzaretti M, Barille S. Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood. 2001;98:3527–3533. doi: 10.1182/blood.V98.13.3527. [DOI] [PubMed] [Google Scholar]
- 65.Okada T, Akikusa S, Okuno H, Kodaka M. Bone marrow metastatic myeloma cells promote osteoclastogenesis through RANKL on endothelial cells. Clin Exp Metastasis. 2003;20:639–646. doi: 10.1023/A:1027362507683. [DOI] [PubMed] [Google Scholar]
- 66.Giuliani N, Colla S, Sala R, Moroni M, Lazzaretti M, La Monica S, Bonomini S, Hojden M, Sammarelli G, Barille S, Bataille R, Rizzoli V. Human myeloma cells stimulate the receptor activator of nuclear factor-kappa B ligand (RANKL) in T lymphocytes: a potential role in multiple myeloma bone disease. Blood. 2002;100:4615–4621. doi: 10.1182/blood-2002-04-1121. [DOI] [PubMed] [Google Scholar]
- 67.Standal T, Seidel C, Hjertner O, Plesner T, Sanderson RD, Waage A, Borset M, Sundan A. Osteoprotegerin is bound, internalized, and degraded by multiple myeloma cells. Blood. 2002;100:3002–3007. doi: 10.1182/blood-2002-04-1190. [DOI] [PubMed] [Google Scholar]
- 68.Grimaud E, Soubigou L, Couillaud S, Coipeau P, Moreau A, Passuti N, Gouin F, Redini F, Heymann D. Receptor activator of nuclear factor kappaB ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. Am J Pathol. 2003;163:2021–2031. doi: 10.1016/S0002-9440(10)63560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schilbach K, Geiselhart A, Handgretinger R. Induction of proliferation and augmented cytotoxicity of gammadelta T lymphocytes by bisphosphonate clodronate. Blood. 2001;97:2917–2918. doi: 10.1182/blood.V97.9.2917. [DOI] [PubMed] [Google Scholar]
- 70.Lahtinen R, Laakso M, Palva I, Virkkunen P, Elomaa I. Randomised, placebo-controlled multicentre trial of clodronate in multiple myeloma. Finnish Leukaemia Group. Lancet. 1992;340:1049–1052. doi: 10.1016/0140-6736(92)93075-X. [DOI] [PubMed] [Google Scholar]
- 71.Laakso M, Lahtinen R, Virkkunen P, Elomaa I. Subgroup and cost-benefit analysis of the Finnish multicentre trial of clodronate in multiple myeloma. Finnish Leukaemia Group. Br J Haematol. 1994;87:725–729. doi: 10.1111/j.1365-2141.1994.tb06730.x. [DOI] [PubMed] [Google Scholar]
- 72.McCloskey EV, MacLennan IC, Drayson MT, Chapman C, Dunn J, Kanis JA. A randomized trial of the effect of clodronate on skeletal morbidity in multiple myeloma. MRC Working Party on Leukaemia in Adults. Br J Haematol. 1998;100:317–325. doi: 10.1046/j.1365-2141.1998.00567.x. [DOI] [PubMed] [Google Scholar]
- 73.McCloskey EV, Dunn JA, Kanis JA, MacLennan IC, Drayson MT. Long-term follow-up of a prospective, double-blind, placebo-controlled randomized trial of clodronate in multiple myeloma. Br J Haematol. 2001;113:1035–1043. doi: 10.1046/j.1365-2141.2001.02851.x. [DOI] [PubMed] [Google Scholar]
- 74.Paterson AHG, Powles TJ, Kanis JA, McCloskey E, Hanson J, Ashley S. Double-blind controlled trial of oral clodronate in patients with bone metastases from breast cancer. J Clin Oncol. 1993;11:59–65. doi: 10.1200/JCO.1993.11.1.59. [DOI] [PubMed] [Google Scholar]
- 75.Dearnaley DP, Mason MD, Parmar MKB, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10:872–876. doi: 10.1016/S1470-2045(09)70201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, Lipton A, Keller A, Ballester O, Kovacs MJ, Blacklock HA, Bell R, Simeone J, Reitsma DJ, Heffernan M, Seaman J, Knight RD. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334:488–493. doi: 10.1056/NEJM199602223340802. [DOI] [PubMed] [Google Scholar]
- 77.Menssen HD, Sakalova A, Fontana A, Herrmann Z, Boewer C, Facon T, Lichinitser MR, Singer CR, Euller-Ziegler L, Wetterwald M, Fiere D, Hrubisko M, Thiel E, Delmas PD. Effects of long-term intravenous ibandronate therapy on skeletal-related events, survival, and bone resorption markers in patients with advanced multiple myeloma. J Clin Oncol. 2002;20:2353–2359. doi: 10.1200/JCO.2002.02.032. [DOI] [PubMed] [Google Scholar]
- 78.Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, Apffelstaedt J, Hussein M, Coleman RE, Reitsma DJ, Seaman JJ, Chen BL, Ambros Y. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 2001;7:377–387. [PubMed] [Google Scholar]
- 79.Kohno N, Aogi K, Minami H, Nakamura S, Asaga T, Iino Y, Watanabe T, Goessl C, Ohashi Y, Takashima S. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2005;23:3314–3321. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- 80.Rosen LS, Gordon D, Tchekmedyian NS, Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, De Souza P, Zheng M, Urbanowitz G, Reitsma D, Seaman J. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, phase III, double-blind, placebo-controlled trial. Cancer. 2004;100:2613–2621. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 81.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Zheng M. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 82.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, Boer RH, Lichinitser M, Fujiwara Y, Yardley DA, Viniegra M, Fan M, Jiang Q, Dansey R, Jun S, Braun A. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 83.Coxon FP, Thompson K, Rogers MJ. Recent advances in understanding the mechanism of action of bisphosphonates. Curr Opin Pharmacol. 2006;6:307–312. doi: 10.1016/j.coph.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 84.Thompson K, Roelofs AJ, Jauhiainen M, Monkkonen H, Monkkonen J, Rogers MJ. Activation of gammadelta T cells by bisphosphonates. Adv Exp Med Biol. 2010;658:11–20. doi: 10.1007/978-1-4419-1050-9_2. [DOI] [PubMed] [Google Scholar]
- 85.Terpos E, Dimopoulos MA. Myeloma bone disease: pathophysiology and management. Ann Oncol. 2005;16:1223–1231. doi: 10.1093/annonc/mdi235. [DOI] [PubMed] [Google Scholar]
- 86.Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, Lipton A, Keller A, Ballester O, Kovacs M, Blacklock H, Bell R, Simeone JF, Reitsma DJ, Heffernan M, Seaman J, Knight RD. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J Clin Oncol. 1998;16:593–602. doi: 10.1200/JCO.1998.16.2.593. [DOI] [PubMed] [Google Scholar]
- 87.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–392. [PubMed] [Google Scholar]
- 88.Terpos E, Viniou N, de la Fuente J, Meletis J, Voskaridou E, Karkantaris C, Vaiopoulos G, Palermos J, Yataganas X, Goldman JM, Rahemtulla A. Pamidronate is superior to ibandronate in decreasing bone resorption, interleukin-6 and beta 2-microglobulin in multiple myeloma. Eur J Haematol. 2003;70:34–42. doi: 10.1034/j.1600-0609.2003.02823.x. [DOI] [PubMed] [Google Scholar]
- 89.Devitt B, McLachlan SA. Use of ibandronate in the prevention of skeletal events in metastatic breast cancer. Ther Clin Risk Manag. 2008;4:453–458. doi: 10.2147/tcrm.s1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berenson JR, Rosen LS, Howell A, Porter L, Coleman RE, Morley W, Dreicer R, Kuross SA, Lipton A, Seaman JJ. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. 2001;91:1191–1200. doi: 10.1002/1097-0142(20010401)91:7<1191::AID-CNCR1119>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 91.Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, Apffelstaedt J, Hussein MA, Coleman RE, Reitsma DJ, Chen BL, Seaman JJ. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735–1744. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 92.Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, de Souza P, Zheng M, Urbanowitz G, Reitsma D, Seaman JJ. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial—the Zoledronic acid lung cancer and other solid tumors study group. J Clin Oncol. 2003;21:3150–3157. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 93.Naoe M, Ogawa Y, Takeshita K, Morita J, Shichijo T, Fuji K, Fukagai T, Iwamoto S, Terao S. Zoledronate stimulates gamma delta T cells in prostate cancer patients. Oncol Res. 2010;18:493–501. doi: 10.3727/096504010X12671222663638. [DOI] [PubMed] [Google Scholar]
- 94.Lipton A. Emerging role of bisphosphonates in the clinic—antitumor activity and prevention of metastasis to bone. Cancer Treat Rev. 2008;34(suppl 1):S25–S30. doi: 10.1016/j.ctrv.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 95.Senaratne SG, Pirianov G, Mansi JL, Arnett TR, Colston KW. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer. 2000;82:1459–1468. doi: 10.1054/bjoc.1999.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Santini D, Martini F, Fratto ME, Galluzzo S, Vincenzi B, Agrati C, Turchi F, Piacentini P, Rocci L, Manavalan JS, Tonini G, Poccia F. In vivo effects of zoledronic acid on peripheral gammadelta T lymphocytes in early breast cancer patients. Cancer Immunol Immunother. 2009;58:31–38. doi: 10.1007/s00262-008-0521-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferretti G, Fabi A, Carlini P, Papaldo P, Cordiali Fei P, Di Cosimo S, Salesi N, Giannarelli D, Alimonti A, Di Cocco B, D’Agosto G, Bordignon V, Trento E, Cognetti F. Zoledronic-acid-induced circulating level modifications of angiogenic factors, metalloproteinases and proinflammatory cytokines in metastatic breast cancer patients. Oncology. 2005;69:35–43. doi: 10.1159/000087286. [DOI] [PubMed] [Google Scholar]
- 98.Santini D, Vincenzi B, Galluzzo S, Battistoni F, Rocci L, Venditti O, Schiavon G, Angeletti S, Uzzalli F, Caraglia M, Dicuonzo G, Tonini G. Repeated intermittent low-dose therapy with zoledronic acid induces an early, sustained, and long-lasting decrease of peripheral vascular endothelial growth factor levels in cancer patients. Clin Cancer Res. 2007;13:4482–4486. doi: 10.1158/1078-0432.CCR-07-0551. [DOI] [PubMed] [Google Scholar]
- 99.Ottewell PD, Monkkonen H, Jones M, Lefley DV, Coleman RE, Holen I. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J Natl Cancer Inst. 2008;100:1167–1178. doi: 10.1093/jnci/djn240. [DOI] [PubMed] [Google Scholar]
- 100.Neville-Webbe HL, Rostami-Hodjegan A, Evans CA, Coleman RE, Holen I. Sequence- and schedule-dependent enhancement of zoledronic acid induced apoptosis by doxorubicin in breast and prostate cancer cells. Int J Cancer. 2005;113:364–371. doi: 10.1002/ijc.20602. [DOI] [PubMed] [Google Scholar]
- 101.Rack B, Schindlbeck C, Strobl B, Sommer H, Friese K, Janni W. Efficacy of zoledronate in treating persisting isolated tumor cells in bone marrow in patients with breast cancer. A phase II pilot study. Dtsch Med Wochenschr. 2008;133:285–289. doi: 10.1055/s-2008-1046707. [DOI] [PubMed] [Google Scholar]
- 102.Solomayer E, Gebauer G, Hirnle P, Janni W, Lück H-J, Becker S, Huober J, Kraemer B, Fehm T. Influence of zoledronic acid on disseminated tumor cells (DTC) in primary breast cancer patients (abstract 2048) Cancer Res. 2009;69(suppl):170s–171s. doi: 10.1158/0008-5472.SABCS-2048. [DOI] [PubMed] [Google Scholar]
- 103.Aft R, Naughton M, Ylagen L, Watson M, Chavez-MacGregor M, Trinkaus K, Zhai J, Weilbaecher K (2008) Effect of zoledronic acid on bone marrow micrometastases in women undergoing neoadjuvant chemotherapy for breast cancer (abstract 1021). Presented at the 44th annual meeting of the American society of clinical oncology, 30 May to 3 June 2008, Chicago, IL, USA
- 104.Lin AY, Park JW, Scott J, Melisko M, Goga A, Moasser MM, Moore DH, Rugo HS (2008) Zoledronic acid as adjuvant therapy for women with early-stage breast cancer and disseminated tumor cells in bone marrow (abstract 559). In: Presented at the 44th annual meeting of the American society of clinical oncology, 30 May to 3 June 2008, Chicago, IL, USA
- 105.Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C, Jakesz R, Seifert M, Hubalek M, Bjelic-Radisic V, Samonigg H, Tausch C, Eidtmann H, Steger G, Kwasny W, Dubsky P, Fridrik M, Fitzal F, Stierer M, Rucklinger E, Greil R, Marth C. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 106.Coleman RE, Winter MC, Cameron D, Bell R, Dodwell D, Keane MM, Gil M, Ritchie D, Passos-Coelho JL, Wheatley D, Burkinshaw R, Marshall SJ, Thorpe H. The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: exploratory evidence for direct anti-tumour activity in breast cancer. Br J Cancer. 2010;102:1099–1105. doi: 10.1038/sj.bjc.6605604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Coleman R, Bundred N, De Boer R, Llombarto A, Campbell I, Neven P, Barrios C, Dias R, Miller J, Brufsky A. Impact of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: Z-FAST, ZO-FAST, E-ZO-FAST (abstract 4082) Cancer Res. 2009;69(suppl):733s. [Google Scholar]
- 108.Brufsky A, Graydon Harker W, Beck JT, Carroll R, Jin L, Warsi G, Argonza-Aviles E, Ericson S, Perez EA (2009) The effect of zoledronic acid on aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: the Z-FAST study 5-year final follow-up (abstract 4083). In: Presented at the 32nd annual San Antonio breast cancer symposium, 9 to 13 Dec 2009, San Antonio, TX, USA
- 109.Costa L, Cook R, Body J-J, Brown J, Terpos E, Saad F, Lipton A, Coleman R (2009) Zoledronic acid treatment delays disease progression and improves survival in patients with bone metastases from solid tumors and elevated levels of bone resorption (abstract 50) In: Presented at the IX International meeting on cancer induced bone disease, 28–31 Oct 2009, Arlington, VA, USA
- 110.Body J-J, Cook R, Costa L, Brown J, Terpos E, Saad F, Lipton A, Coleman R. Possible survival benefits from zoledronic acid treatment in patients with bone metastases from solid tumors and poor prognostic features (abstract 71). In: Presented at the IX International Meeting on Cancer Induced Bone Disease, 28–31 October 2009. VA, USA: Arlington; 2009. [Google Scholar]
- 111.Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, Navarro-Coy N, Drayson MT, Owen RG, Feyler S, Ashcroft AJ, Ross F, Byrne J, Roddie H, Rudin C, Cook G, Jackson GH, Child JA, National Cancer Research Institute Haematological Oncology Clinical Study Group First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376:1989–1999. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zaghloul MS, Boutrus R, El-Hosieny H, A-Kader Y, El-Attar I, Nazmy M. A controlled prospective randomized placebo-controlled trial of zoledronic acid in bony metastatic bladder cancer patients (abstract 5033) J Clin Oncol. 2008;26(suppl):257s. doi: 10.1007/s10147-010-0074-5. [DOI] [PubMed] [Google Scholar]
- 113.Zarogoulidis K, Boutsikou E, Zarogoulidis P, Eleftheriadou E, Kontakiotis T, Lithoxopoulou H, Tzanakakis G, Kanakis I, Karamanos NK. The impact of zoledronic acid therapy in survival of lung cancer patients with bone metastasis. Int J Cancer. 2009;125:1705–1709. doi: 10.1002/ijc.24470. [DOI] [PubMed] [Google Scholar]
- 114.Mystakidou K, Katsouda E, Parpa E, Kelekis A, Galanos A, Vlahos L. Randomized, open label, prospective study on the effect of zoledronic acid on the prevention of bone metastases in patients with recurrent solid tumors that did not present with bone metastases at baseline. Med Oncol. 2005;22:195–201. doi: 10.1385/MO:22:2:195. [DOI] [PubMed] [Google Scholar]
- 115.United States Food and Drug Administration (2009) Background document for meeting of Advisory Committee for Reproductive Health Drugs (13 August). Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ReproductiveHealthDrugsAdvisoryCommittee/UCM176605.pdf (Accessed 21 April 2010)
- 116.Anastasilakis AD, Toulis KA, Polyzos SA, Terpos E. RANKL inhibition for the management of patients with benign metabolic bone disorders. Expert Opin Investig Drugs. 2009;18:1085–1102. doi: 10.1517/13543780903048929. [DOI] [PubMed] [Google Scholar]
- 117.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 118.Stopeck A, Body JJ, Fujiwara Y, Lipton A, Steger GG, Viniegra M, Fan M, Braun A, Dansey R, Jun S. Denosumab versus zoledronic acid for the treatment of breast cancer patients with bone metastases: results of a randomized phase 3 study (abstract 2LBA) Eur J Cancer Suppl. 2009;7:2. doi: 10.1016/S1359-6349(09)72028-2. [DOI] [Google Scholar]
- 119.Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, Milecki P, Rader M, Shore N, Tadros S, Wang H, Jiang Q, Dansey R, Goessl C. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with castration-resistant prostate cancer (abstract LBA4507). In: Presented at the 46th annual meeting of the American society of clinical oncology, 4–8 June 2010. IL, USA: Chicago; 2010. [Google Scholar]
- 120.Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY, Roudier M, Smith J, Ye Z, Sohn W, Dansey R, Jun S. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11:275–280. doi: 10.1016/S1470-2045(10)70010-3. [DOI] [PubMed] [Google Scholar]
- 121.Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D, Dougall WC. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468:103–107. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- 122.Anastasilakis AD, Toulis KA, Goulis DG, Polyzos SA, Delaroudis S, Giomisi A, Terpos E. Efficacy and safety of denosumab in postmenopausal women with osteopenia or osteoporosis: a systematic review and a meta-analysis. Horm Metab Res. 2009;41:721–729. doi: 10.1055/s-0029-1224109. [DOI] [PubMed] [Google Scholar]
- 123.Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Smith J, Fan M, Jun S. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26:4875–4882. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 124.Smith MR, Egerdie B, Hernandez Toriz N, Feldman R, Tammela TL, Saad F, Heracek J, Szwedowski M, Ke C, Kupic A, Leder BZ, Goessl C. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fizazi K, Bosserman L, Gao G, Skacel T, Markus R. Denosumab treatment of prostate cancer with bone metastases and increased urine N-telopeptide levels after therapy with intravenous bisphosphonates: results of a randomized phase II trial. J Urol. 2009;182:509–515. doi: 10.1016/j.juro.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 126.Xgeva (denosumab) injection [prescribing information]. Amgen, Thousand Oaks, CA, USA, 2010