Abstract
We have recently shown that systemic administration of low molecular weight hyaluronan (LMW HA) significantly reduces colorectal carcinoma (CRC) growth in vivo. The elicited response is partially mediated by activated dendritic cells (DC). To potentiate the ability of DC loaded with whole tumor lysate (DC/TL) to induce immunity against CRC in mice, we aimed to study the effects of preconditioning DC with LMW HA for therapeutic vaccination. LMW HA improved maturation of ex vivo generated DC, increased IL-12, decreased IL-10 production, and enhanced a MLR activity in vitro. Although TNF-α showed a similar capacity to mature DC, preconditioning of DC/TL with LMW HA increased their ability to migrate in vitro toward CCL19 and CCL-21 in a CD44- and a TLR4-independent manner; this effect was superior to Poly(I:C), LPS, or TNF-α and partially associated with an increase in the expression of CCR7. Importantly, LMW HA dramatically enhanced the in vivo DC recruitment to tumor-regional lymph nodes. When these LMW HA-treated CRC tumor lysate-pulsed DC (DC/TL/LMW HA) were administered to tumor-bearing mice, a potent antitumor response was observed when compared to DC pulsed with tumor lysate alone and matured with TNF-α. Then, we showed that splenocytes isolated from animals treated with DC/TL/LMW HA presented a higher proliferative capacity, increased IFN-γ production, and secreted lower levels of the immunosuppressive IL-10. Besides, increased specific CTL response was observed in DC/TL/LMW HA-treated animals and induced long-term protection against tumor recurrence. Our data show that LMW HA is superior to other agents at inducing DC migration; therefore, LMW HA could be considered a new adjuvant candidate in the preparation of DC-based anticancer vaccines with potent immunostimulatory properties.
Keywords: Hyaluronan, Dendritic cells, Migration, CD44, TLR4, Colorectal carcinoma
Introduction
Colorectal carcinoma (CRC) is one of the leading causes for cancer death worldwide and, unfortunately, there is no curative treatment for patients not amenable to surgical resection [1]. Patients with metastatic disease have reported a median survival time close to 20 months as a result of the addition of bevacizumab (Avastin®) and cetuximab (Erbitux®) monoclonal antibodies to fluorouracil-based combination chemotherapy [2]. Therefore, new therapeutic options are urgently needed for the advanced disease. Recent evidences support that CRC could be considered an immunogenic tumor and may reasonably be thought as a target disease for immunotherapy [3, 4]. In search of new strategies, we have previously assessed the role of LMW HA as an immunotherapeutic adjuvant for the treatment of CRC in mice and found that systemic administration of LMW HA induced a potent antitumoral response partially mediated by DC activation [5].
Dendritic cells are the most potent specialized antigen-presenting cells (APCs) and the physiologic initiators of cellular immune responses [6]. Ex vivo generated, tumor antigen-loaded mature DC are currently exploited to immunity against cancer [7–9]. The ability of DC to elicit adaptive immune responses has been exploited not only in prophylactic vaccination but also in the induction of therapeutic immune responses against cancer including a large experience in phase-I clinical trials [7–10]. Issues regarding the optimal dose and route of administration for DC vaccination remain to be addressed; however, it is generally accepted that applying antigens as complex mixtures (e.g., tumor lysate) to DC is a reasonable strategy [11], because complex mixtures contain many antigenic sequences, making antigen-loss variants less likely to evade the immune response [11, 12]. In vitro maturation of DC for cancer immunotherapy may be generated by a not well-defined cytokine cocktails containing prostaglandin E2 (PGE2), poly (I:C), TNF-α, IFN-α [12].
Immature DC are located in the tissue wherein they capture antigens, while mature DC are capable of activating T cells in the lymph nodes; these functional state changes result from gene reprogramming in DC [13] and have a critical impact in terms of a tolerogenic or immunogenic response induction, respectively [14]. The ability of DC to migrate toward secondary lymphoid organs is one of their most important properties, because it is required for DC to prime T lymphocytes and to elicit an appropriate immune response [6]. During migration toward peripheral lymphoid organs, DC mature and upregulate CCR7 expression, the receptor for the CC chemokine ligands CCL19 and CCL21 expressed by stromal cells in the T cell zone of the lymph node [15, 16]. Nonetheless, in order to respond to CCL19 and CCL21 and to induce DC migration, CCR7 must be first sensitized [15]. Moreover, the ability of ex vivo generated DC to migrate in vivo to secondary lymphoid organs was shown to be rather inefficient; however, the incubation with pro-inflammatory cytokines and co-stimulatory factors, such as prostaglandin E2 and Poly (I:C) was found to improve this effect [17, 18].
Hyaluronan (HA) is a glycosaminoglycan with a simple chemical structure found in almost all tissues and particularly in those undergoing cell proliferation, regeneration, and repair such as embryonic, inflamed, and tumor stroma [19, 20]. An association between HA biosynthesis alteration and cancer progression has been demonstrated in different types of tumors including CRC [21]. HA functions are well known to be size-dependent [22], and the LMW HA form has been shown to induce the expression of inflammatory genes in many types of cells including DC and macrophages [22, 23]. There are different receptors for HA, being CD44 the major cellular HA receptor [24] and it is expressed at high levels on DC [25]. In addition, LMW HA or its small fragments were shown to stimulate T cell responses by activating and upregulating costimulatory molecules on DC [25, 26]. On the contrary, recent data suggest that high molecular weight (HMW) HA has an immunosuppressive function instead [27, 28]. We and others have observed that small HA fragments but not intermediate or HMW HA induce maturation of DC [5], in a CD44-independent and TLR4-dependent manner [29]. Moreover, Scheibner et al. [26] demonstrated that LMW HA can act as an adjuvant promoting antigen-specific T cell responses in vivo, in a TLR2-dependent manner. However, there is no data regarding the immunostimulatory effects of HA in cancer.
In this study, we have analyzed the effect of LMW HA preconditioning tumor lysate-pulsed DC in a protocol scheme for vaccination against CRC in mouse. This treatment was found to induce efficient immune responses and long-term protection against established subcutaneous tumors through the enhancement of DC maturation and activation. In addition, LMW HA-treated DC/TL showed an increased CD44- and TLR4-independent migratory capacity toward the secondary lymphoid organs-derived chemokines CCL-19 and CCL-21, probably by modulating CCR7 expression. To our knowledge, this is the first work describing an effect of LMW HA in enhancing the migratory ability of DC toward antigen-presenting regions in a tumor vaccination protocol scheme against CRC in mice.
Materials and methods
Reagents
Pharmaceutical endotoxin-free LMW HA of definite size (1–3 × 105 Da) from CPN spol.s.r.o (Czech Republic) was kindly supplied by Farmatrade (Buenos Aires, Argentina). A stock solution of 5 mg/ml LMW HA was prepared, and the presence of endotoxins was determined by Limulus amebocyte lysate (LAL) assays with a sensitivity limit of 0.05–0.1 endotoxin units (EU) per ml (Sigma–Aldrich). GM-CSF, TNF-α, CCL21, and CCL19 were from PeproTech; Poly(I:C) was from Invivogen.
Animals and cell lines
Six-to-eight-week-old male BALB/c and C57BL/6 mice were purchased from Biofucal S.A. (Buenos Aires, Argentina). B6.B10ScN-Tlr4lps-del/JthJ (TLR4 null) mice were kindly provided by Dr. F. Polack (Infant Foundation, Buenos Aires, Argentina). CD44 (C57BL/6 background) knockout mice were purchased from Jackson laboratories. Animals were maintained at our Animal Resource Facilities (School of Biomedical Sciences, Austral University) in accordance with the experimental ethical committee and the NIH guidelines on the ethical use of animals. CT26 tumor cell line, an undifferentiated murine colorectal adenocarcinoma, established from a N-nitroso-N-methylurethan-induced transplantable tumor in BALB/c (H-2d) mice (kindly provided by Dr. J. Prieto, University of Navarra, Spain) was herein used. YAC-1 cells, a Moloney leukemia virus-transformed lymphoma cell line derived from A/Sn mice, were kindly provided by Dr. Mirta Giordano (National Academy of Medicine, Buenos Aires, Argentina). Cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM Lglutamine, 100 U/ml streptomycin, and 100 mg/ml penicillin and incubated at 37°C in a 5% CO2 humidified atmosphere.
Tumor lysates
Confluent cultures of CT26 cells (106 approximately) were detached with a cell scraper, washed twice in PBS, and resuspended in serum-free medium. Cell suspensions were frozen at −80°C and disrupted by 5 freeze–thaw cycles. To remove large debris, tumor lysates were centrifuged at 300 rpm for 10 min. The supernatant was collected and passed through a 0.2-μm filter. The protein concentration of the lysate was determined by Bradford assay. The resulting tumor lysates were aliquoted and stored at −40°C until use.
Bone marrow-derived DC generation
Dendritic cells generation from bone marrow (BM) of BALB/c, C57BL/6, TLR4−/−, and CD44−/− mice was carried out as previously described [5]. Briefly, BM cells (106 cells/ml) were placed into 6-well plates and cultured for up to 7 days with 20 ng/ml of GM-CSF (PreproTech) at 37°C with 5% CO2. Medium was replaced on days 3 and 5 of culture. In HA stimulation experiments, cells were treated from day 3 with LMW HA (50 μg/ml). At day 7, DC were pulsed with whole tumor lysates alone (200 μg/106 cells/ml) or with LMW HA (50 μg/ml), TNF-α (20 ng/ml), LPS (1 μg/ml), or Poly(I:C) (10 μg/ml) at 37°C for 18 h. Cells were then centrifuged, characterized by flow cytometry, and used for experiments.
Flow cytometric analyses
Staining and flow cytometric analyses of generated DC were carried out using standard procedures. Briefly, 106 cells were stained with different conjugated antibodies as it follows: anti-CD11c (HL3), anti-MHC-II (2G9), anti-CD40 (3/23), anti-CD80 (16-10A1), anti-CD86 (GL1), anti-CCR7 (4B12), and their respective isotypes (negative controls) (BD Biosciences, San Diego, CA, USA) on ice for 30 min, washed thoroughly with PBS-1% BSA. Then, cells were fixed with 1% paraformaldehyde and subjected to flow cytometry (FACSCalibur, BectonDickinson-BD, USA). Data were analyzed using WinMDI software.
ELISA assays
IL-12, IFN-γ, and IL-10 expression levels were determined by a sandwich ELISA assay (OptEIATM, BD Biosciences Pharmingen, San Diego, CA, USA) from free-cell culture supernatant of DC/TL/LMW HA, DC/TL/TNF-α, or DC/TL. IL-10 and IFN-γ expression was also determined from supernatant of splenocytes from tumor-bearing mice, treated with DC/TL/LMW HA, DC/TL/TNF-α, DC/TL, or saline and stimulated for 5 days with mitomycin C-treated CT26 cells. Besides, IL-10 production was evaluated in supernatants from DC derived from CD44 or TLR4 null mice treated or not with LMW HA. The assays were carried out according to the instructions provided by the manufacturer. Three independent experiments were performed, and in each of them, samples were assayed in duplicates.
Mixed leukocyte reaction
The capability of DC/TL/LMW HA, DC/TL/TNF-α, or DC/TL to stimulate the proliferation of allogenic splenocytes was assessed by a mixed leukocyte reaction (MLR) assay in vitro. BALB-c BM-derived mitomycin C-treated DC (104 cells) pulsed with whole tumor lysate were cocultured with allogenic C57BL/6 splenocytes (105 cells) for 5 days, in RPMI-1640 medium. On day 4, 5 μCi/ml [methyl-3H] thymidine (specific activity 20 Ci/mmol; Perkin Elmer) was added to culture, which was further incubated for 18 h. Finally, cells were harvested and radioactivity was determined by using a liquid scintillation counter (Beckman LS 6500). Data are expressed as mean ± SD of c.p.m (measured in triplicates).
Proliferation assays
Suspensions of fresh splenocytes from tumor-bearing mice of different experimental groups (saline, DC/TL/LMW HA, DC/TL/TNF-α, or DC/TL) were isolated by mincing the spleen using the back of a 10-ml syringe. Red blood cells were removed using a lysis solution (0.15 M NH4Cl, 1 mM KHCO3, 0.1 Na2-EDTA). The remnant cells were cultured (105 cells/well; round-bottomed 96-well plates) in RPMI-1640 with 10% FBS, together with mitomycin C-treated CT26 cells (104 cells/well), for 5 days. Cell proliferation was evaluated by [3H] thymidine incorporation assay. Each sample was assayed in triplicate.
In vivo experiments
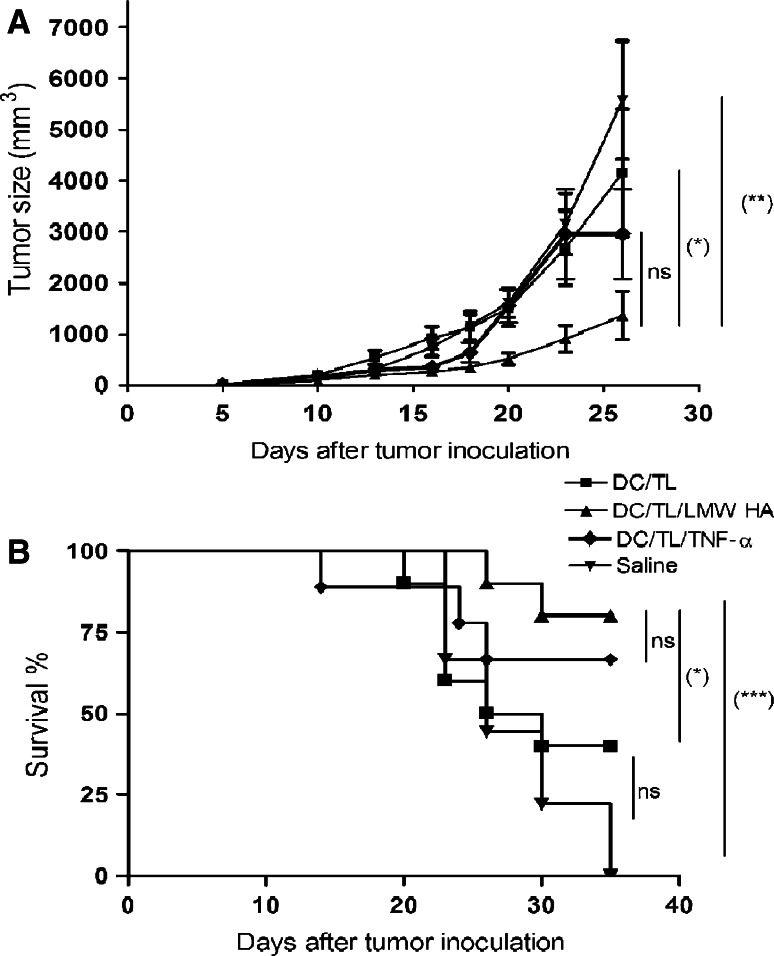
CT26 cells were s.c. injected, at a dose of 5 × 105 cells/animal, into the right flank of BALB/c mice. Tumors were allowed to reach approximately 85 mm3 in size before treatment was started. Animals were distributed in different groups, and then s.c. treated on days 7 and 9 after tumor inoculation with saline, 2.5 × 105 DC/TL/LMW HA, DC/TL/TNF-α, or DC/TL. Tumor length (L) and width (W) were measured with a caliper three times a week, and tumor volume (V) was calculated as V = (L × W2)/2. For proliferation and CTLs assays, animals were killed at 2 weeks after DC inoculation and splenocytes were obtained. To study protective immunity against rechallenge with tumoral cells, animals that were free of disease at 1 month after complete regression of primary tumors were rechallenged with 5 × 105 CT-26 cells at a distal site. Age-matched naive animals received the same amount of cells to serve as controls. Deaths of animals were documented, and results were expressed as percentage of animal survival (Kaplan–Meier, log rank test, P < 0.01).
Cytotoxicity assays
Cytotoxicity assays were performed according to standard protocols [30]. Viable splenocytes (8 × 106/well) from different experimental groups were stimulated in vitro with mitomycin C-treated CT26 cells (8 × 105 cells/well) in 24-well plates. On day 5, cells were harvested and washed, adjusted to 4 × 106/ml, and added to 96-well plate (effector cells). To determine specific CTL cytotoxicity and NK activity, two types of target cells were used (CT26 and YAC-1, respectively) at 4 × 105 cells/ml. After 4 h incubation at 37°C, plates were centrifuged and cell-free supernatants were obtained. Cytotoxicity was evaluated with the LDH Cytotoxicity Detection Kit (Roche Diagnostics, Mannheim, Germany) following manufacturer instructions. Results are expressed as relative values of lysis normalized to non-treated cells.
In vitro and in vivo migration assays
The in vitro DC migratory capacity of different experimental groups was assayed using a 48-transwell microchemotaxis Boyden Chamber unit (Neuroprobe, Inc., MA, USA). In brief, DC (2.5 × 105 cells/well) treated or not with LMW HA (50 μg/ml), TNF-α (20 ng/ml), LPS (1 μg/ml), or Poly(I:C) (10 μg/ml) were placed in the upper chamber of the transwell unit, which was separated from the lower chamber by 5-μm pore polycarbonate filters (Nucleopore membrane, Neuroprobe, MA, USA). Chemoattractant medium containing 100 ng/ml of CCL21 or CCL19 was placed in the lower chamber of the transwell unit. The system was incubated for 120 min at 37°C in a 5% CO2 humidified atmosphere. After that the membrane was carefully removed and cells on the upper side of the membrane were scraped off with a blade. Cells attached to the lower side of the membrane were fixed in 2% formaldehyde and stained in May–Grünwald–Giemsa. Cells were counted using bright-field microscopy and a 20× objective lens; Ten fields per well were analyzed, and the mean number of cells/field ± SEM were calculated. To assess whether the effect of HA on the ability of DC migration was mediated by CD44 or TLR4, DC derived from CD44−/− and TLR4−/− mice were used. For the in vivo experiments, BALB/c-derived DC (DC/TL and DC/TL/LMW HA) were stained with CMDil™ for flow cytometry analysis or DiR™ (Molecular Probes, Eugene, USA) for Bioluminescence Imaging (BLI), for 5 min at 37°C and then for 15 min at 4°C. Then, DC were washed with PBS and 1 × 106 cells in 75 μl of saline solution were s.c. injected in mice with palpable subcutaneous tumor (9 days approximately), in between the tumor and the ipsilateral inguinal lymph node. In flow cytometric studies, after 48 h, the ipsilateral inguinal lymph node and the tumor were dissected out and separately dissociated by enzymatic digestion with D-collagenase (Calbiochem). They were subsequently fixed in 1% paraformaldehyde and subjected to flow cytometric analysis (FACSCalibur, Becton–Dickinson, BD). Data were processed using WinMDI software. BLI was performed using the Xenogen In Vivo Imaging System (IVIS; Caliper Life Sciences, Hopkinton, MA, USA). Mice injected with DiR™-labeled DC were analyzed at 24 and 48 h after DC/TL application. At 48 h, mice were killed and the ipsilateral inguinal lymph nodes were isolated and analyzed. Images were acquired with an exposure time of 0.5 s for the whole-body image and 0.1 s for the isolated organs. For quantification, a region of interest (ROI) was selected and analyzed with the Living Image version 3.0.3 software (Xenogen). The area of ROI was kept constant, and the intensity was recorded as average photons per second per square centimeter per steridian as previously described [31].
Statistical analyses
Mann–Whitney or Student’s t test (InStat, GraphPad Software) were used for statistical analyses. Differences with P values lower than 0.05 were considered as statistically significant.
Results
In vitro pretreatment with LMW HA increased DC maturation
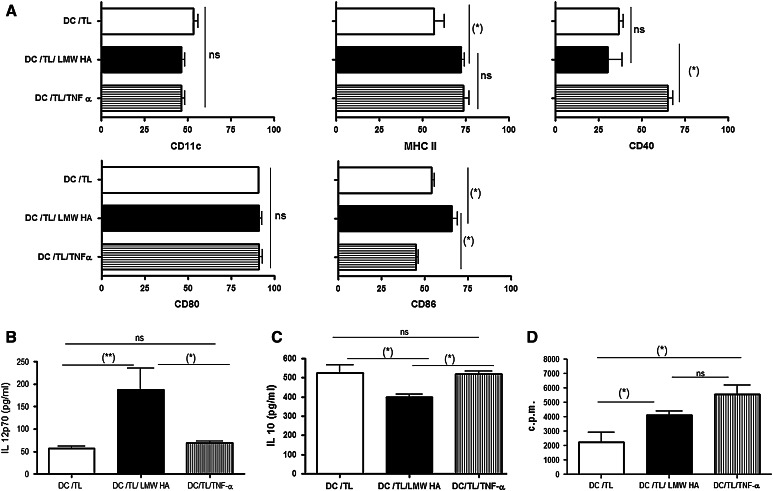
In order to analyze the effect of LMW HA (1 × 105 Da) (endotoxin-free) preconditioning on the maturation of tumor lysate-pulsed DC, BM-derived DC were cultured for 7 days with GM-CSF and at day 3 LMW HA was or not added to culture medium composition. At this moment, DC displayed a relatively immature phenotype (not shown) but became more mature when they were subsequently pulsed, for 18 h, with CT26 whole tumor lysate [32]. When CD11c+ DC/TL were gated, a significant increase in the percentage of expression of MHC class II and CD86 was found in the DC/TL/LMW HA experimental group when compared to DC pulsed with tumor lysate alone (Fig. 1a); similar phenotype of maturation was observed when TNF-α was used for DC activation. This result suggests that pretreatment of DC/TL with LMW HA further increased DC maturation state and their expression of costimulatory molecules with comparable efficacy to TNF-α. Consistently, treatment with LMW HA induced almost a three-time increase (P < 0.05) in the production of the IL-12 p70 heterodimer by DC/TL or DC/TL/TNF-α when compared to LMW HA-untreated DC/TL (56.33 pg/ml vs. 69.53 ± 3.04 vs. 170.8 pg/ml, respectively), as detected in the free-cell supernatant of DC cultured for 24 h (Fig. 1b). To address whether LMW HA treatment may induce a pro-immunogenic response by DC, levels of the immunosuppressive IL-10 were also measured in free-cell culture supernatants from DC cultures. As shown in Fig. 1c, IL-10 levels were significantly reduced in DC/TL/LMW HA (399 pg/ml) cultures when compared to DC/TL controls (527 pg/ml) or DC/TL/TNFα (519.7 ± 15 pg/ml). Moreover, allogeneic C57BL/6 splenocytes cocultured with DC pulsed with tumor lysate presented an enhanced proliferation activity after in vitro treatment with LMW HA in comparison to DC pulsed with tumor lysate alone (Fig. 1d). In agreement with others and our previous work [5, 26, 29, 33], these data strongly suggest that incubation of DC/TL with LMW HA induces an enhanced maturation and activation of DC/TL (that was superior to the effects of TNF-α), favoring a pro-immunogenic and anti-immunosuppressive profile on these cells and resulting in an enhanced DC/TL-mediated MLR activity.
Fig. 1.
In vitro incubation of tumor lysate-pulsed DC with LMW HA enhances their maturation and modified their cytokine profile expression. Phenotypic analyses of 7-day cultures of BM-derived DC from BALB/c mice. a At days 3 and 5, LMW HA (50 μg/ml) was added to the culture. At day 7, DC were pulsed with CT26-derived whole tumor lysate for 18 h alone (DC/TL), in the presence of LMW HA (DC/TL/LMW HA) or TNF-α (DC/TL/TNF-α). Then, DC were harvested and stained with mAbs anti-CD11c, MHC-II, CD40, CD80, and CD86. The CD11c+ was gated and the co-expression of several markers was analyzed. Data are expressed as mean percentage ± SD. Expression of IL-12 (b) and IL-10 (c) were determined by ELISA assays from free-cell culture supernatant of DC/TL, DC/TL/LMW HA, and DC/TL/TNF-α. Results are shown in pg/ml mean ± SD and correspond to three independent experiments. (*) P < 0.05. d DC cultured alone and with TNF-α or LMW HA, pulsed with tumor lysates and treated with mitomycin C, were cocultured with allogeneic C57BL/6 splenocytes for 5 days, proliferation was determined by [3H] thymidine incorporation. Data are expressed as mean ± SD of c.p.m. (measured in triplicates) (*) P < 0.05; (**) P < 0.01
CCL19/CCL21-triggered migration of DC is dramatically increased in response to LMW HA stimulation
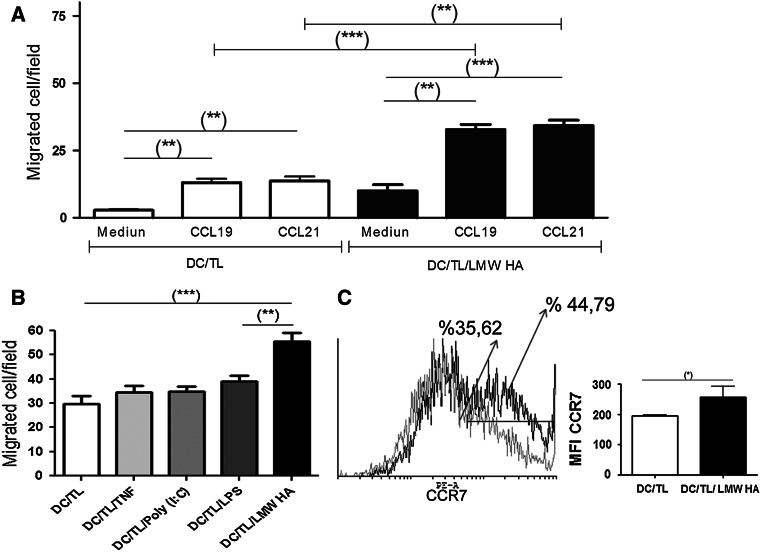
Migration to lymph node and precise positioning of DC is critical to induce immunity against cancer [11, 34] and it is a challenge for DC-based vaccine protocols [35, 36]. Therefore, we decided to assess if preconditioning DC/TL with LMW HA is able to modify the ability of tumor lysate-pulsed DC to migrate in vitro toward the CCR7 ligands, CCL19 and CCL21, in a Boyden chamber. As a result, a significant migration of DC toward these chemokines was found when they were preincubated with LMW HA in comparison with LMW HA non-treated DC (Fig. 2a). This effect of preconditioning DC/TL with LMW HA on migration was compared with other stimulus such as TNF-α, poly (I:C), and LPS, widely used for DC stimulation [18]. DC/TL LMW HA demonstrated a significant increased migration in comparison with DC/TL treated with TNF-α, poly (I:C), or LPS (Fig. 2b). As it was previously shown that DC maturation results in the upregulation of chemokine receptors, such as CCR7, mediating their mobilization toward lymphoid tissues [34], we have then analyzed whether the enhanced migration triggered by LMW HA could be explained by an upregulation of CCR7 expression. LMW HA treatment significantly increased CCR7 expression in DC/TL (71.9% vs. 64.6%; MFI 256 ± 36 vs. 195 ± 3.7), when compared to LMW HA non-treated DC/TL (Fig. 2c).
Fig. 2.
LMW HA enhances chemotactic DC/TL response and modulates their CCR7 expression levels. a Migratory ability of DC alone or treated with LMW HA toward the CCR7 ligands CCL19 and CCL21 was assessed using a 5-μm pore polycarbonate filters in a Boyden chamber system. Briefly, DC treated or not with LMW HA were loaded into the upper well of the chamber and chemokines (CCL19 or CCl21) at a concentration of 200 ng/ml were separately added to the lower chamber. b Migration of DC/TL pretreated with TNF-α, Poly (I:C), or LPS toward CCL21 was compared with DC/TL/LMW HA capacity. The mean number of cells migrated/field ± SEM was compared among groups. (**) P < 0.01 DC/TL/LMW HA versus DC/TL/LPS; (***) P < 0.001 DC/TL/LMW HA versus DC/TL. c Overlays of representative histograms of CCR7 expression on DC from BALB/c wild-type mice showing positively stained DC gated in the CD11c+ region. Gray line represents DC/TL and black line represents DC/TL/LMW HA CCR7 expression. One representative experiment of three independent experiments with similar results is shown (left panel). Mean fluorescence intensity (MFI) from three independent experiments is depicted here (right panel). (*) P < 0.05
LMW HA mediates in vitro DC migration toward CCL21 chemokine in a CD44- and TLR4-independent manner
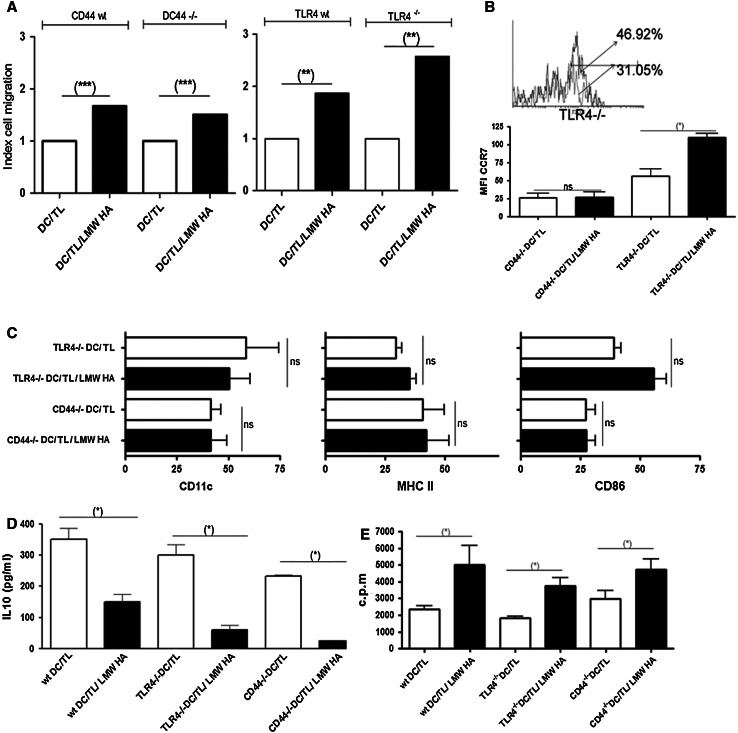
To assess whether CD44, one of the main HA receptors, plays a role in the increased LMW HA-dependent migration, chemotatic response of DC/TL toward CCL21 was studied in the presence in CD44 knockout mice-derived DC. We have observed that these DC have a lower basal migration in comparison with CD44 wild-type derived DC; however, they were able to respond to LMW HA preconditioning increasing their migration toward CCL21 (Fig. 3a). Thus, we concluded that despite CD44 has a role in DC migration as it was thoroughly demonstrated, the effect of LMW HA on the migration process is independent of this receptor, at least under our experimental conditions. From these results, we conclude that LMW HA enhances DC/TL migratory capacity in response to lymph nodes-derived chemokines in a CD44-independent manner.
Fig. 3.
LMW HA enhances chemotactic DC/TL responses in a CD44- and TLR4-independent manner. a To assess wether CD44 or TLR4 plays a role in the increased LMW HA-dependent migration, chemotatic response of DC/TL toward CCL21 of DC was assessed on DC/TL derived from CD44−/− and TLR4−/− mice. Results are expressed as index of cell migration with respect to nontreated DC/TL. Representative experiment of three independent experiments with similar results is shown. (**) P < 0.01. b Overlay of representative histograms of CCR7 expression from TLR4−/−-derived DC gated in the CD11c+ region. Gray line represents DC/TL and the black line represents DC/TL/LMW HA CCR7 expression. One of three independent experiments with similar results is shown (upper panel). The MFI for CCR7 from CD44−/−- and TLR4−/−-derived DC is shown in the lower panel. c DC derived from CD44−/− and TLR4−/− mice were harvested and stained with mAbs anti-CD11c, MHC-II, and CD86 alone or after incubation with LMW HA. The CD11c+ was gated, and the co-expression of several markers was analyzed. Data are expressed as mean percentage ± SD. IL-10 (d) expression was determined by ELISA from free-cell culture supernatant of tumor lysate-pulsed DC from wild type, CD44−/− and TLR4−/− treated or not with LMW HA. Results are shown in pg/ml mean ± SD and correspond to three independent experiments. e These cells were treated with mitomycin C and cocultured with allogeneic C57BL/6 splenocytes for 5 days. Proliferation was determined by [3H] thymidine incorporation. Data are expressed as mean ± SD of c.p.m. (measured in triplicates). (*) P < 0.05; (**) P < 0.01; (***) P < 0.001
It was previously described that oligosaccharides of HA were able to induce DC activation through TLR4 receptor [29]. In order to address whether binding of LMW HA to TLR4 was also mechanistically involved in the enhanced migratory behavior previously shown, DC were derived from TLR4 knockout mice. In these mice, LMW HA preconditioning of DC/TL was found to increase their migration toward CCL21 compared to the DC/TL experimental group, suggesting that LMW HA effects are at least largely independent of TLR4 (Fig. 3b). In addition, DC from TLR4 knockout or wild type mice showed similar expression changes in CCR7 when they were treated with LMW HA (Fig. 3b). Besides, we also analyzed DC maturation status in BM-derived DC from CD44 and TLR4 null mice treated or not with LMW HA (Fig. 3c–e). The surface maturation markers did not showed significant differences (Fig. 3c) but IL-10 production (Fig. 3d) and MLR activity (Fig. 3e) were similar in comparison with DC (wild type) treated with LMW HA.
LMW HA increased migration of ex vivo generated DC vaccine in CRC-bearing mice
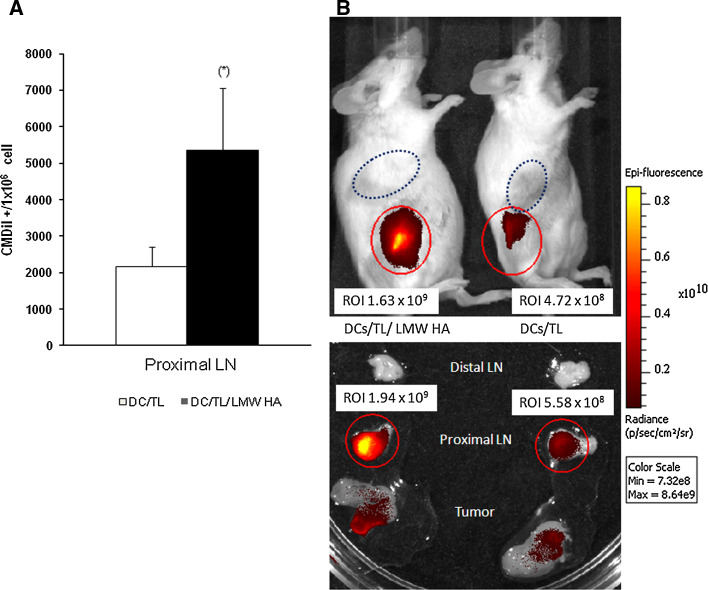
We have then asked whether or not the enhancement of DC/TL migratory behavior by LMW HA preconditioning could also be shown in vivo, using a CT26 tumor-bearing BALB/c mice model. To this end, CM-DiI-labeled DC were injected s.c., in between the tumor nodule and regional lymph node, and their recruitment into the draining lymph nodes (LN) was analyzed by flow cytometry 24h later. Remarkably, the number of DC-CM-DiI+ cells recovered from LN was significantly higher in DC/TL/LMW HA-treated animals when compared to DC/TL ones (Fig. 4a). Besides, in vivo migration of DiR-stained DC were also visualized using a bioluminometer in living mice as indicated by a stronger LN signal in DC/TL/LMW HA-treated animals in comparison with the DC/TL-treated group (Fig. 4b). From these results, we conclude that LMW HA treatment of DC/TL significantly enhances their in vivo migration and recruitment into LN, which correlates with their increased maturation, stimulation of T cell proliferation and CTL activities, and ultimately with their efficient antitumoral effects when applied as vaccines.
Fig. 4.
Incubation with LMW HA of tumor lysate-pulsed DC increases their capability to migrate toward regional lymph nodes of tumor-bearing mice. a BALB/c mice bearing CT26 implanted tumors for 7 days were injected s.c. between the tumor nodule and regional lymph node with CM-DiI-stained DC/TL/LMW HA or DC/TL cells. Twenty-four hours later, cell suspensions from draining lymph nodes and tumor nodules were collected and analyzed by flow cytometry (106 cells). Bars represent the number of DC-CM-DiI+ cells recovered from LN from three different mice. Results are representative of three independent experiments. b Similarly, BALB/c mice bearing CT26 tumors were s.c. injected with DiR-stained DC/TL/LMW HA or DC/TL cells. DiR-stained DC were visualized using a bioluminometer in living mice showing increased LN signal in animals injected with DC/TL/LMW HA cells in comparison with DC/TL-treated ones (upper panel). When lymph node samples were excised and analyzed similar results were achieved (lower panel)
Therapeutic vaccination of CRC-bearing mice with DC plus LMW HA induced a potent antitumoral response
We asked then whether the enhanced in vitro activation of DC by LMW HA may have the ability to exert efficient antitumoral effects in vivo in a therapeutic vaccination protocol scheme. For this purpose, we have s.c. injected DC/TL, DC/TL/TNF-α, or DC/TL/LMW HA into 7 days CT26 tumor-bearing mice in 2 doses at days 7 and 9 and found that DC/TL/LMW HA and DC/TL/TNF-α, but not DC/TL, significantly reduced tumor growth (P < 0.05) compared to control mice, injected with saline (Fig. 5a). Treatments were well tolerated with no signs of toxicity (data not shown). In addition, LMW HA added to DC/TL or DC/TL/TNF-α, but not DC/TL, significantly increased animals survival when compared to saline control mice (Log rank test *P < 0.01) (Fig. 5b). To determine whether treatment of tumor nodules with DC/TL/LMW HA can induce lasting immunological memory, we rechallenged animals that were free of tumors after DC/TL/LMW HA treatment with a new s.c. administration of CT26 cells at 1 month after complete regression of primary tumors. All animals rejected the s.c. implantation of 5 × 105 parental CT26 cells.
Fig. 5.
LMW HA preconditioning of DC/TL induces a potent in vivo antitumoral response and significantly prolongs animal survival. a BALB/c mice were s.c. inoculated with 5 × 105 CT26 cells; on day 7 and 9, mice were s.c injected with 2.5 × 105 DC treated or not with LMW HA, TNF-α, or with saline. Tumor volumes were measured three times a week over 30-days period. Results are representative of three independent experiments (mean ± SD, n = 10). **Versus Saline; *Versus DC/TL. *P < 0.05; **P < 0.01; b Kaplan–Meier 40-days survival curve of the mice bearing CT26 tumor and treated with DC/TL, DC/TL/LMW HA, DC/TL/TNF-α, or saline. Log rank test *P < 0.05; ***P < 0.001
Splenocytes derived from LMW HA-treated mice changed their cytokine production profile and showed a potent specific CTL activity
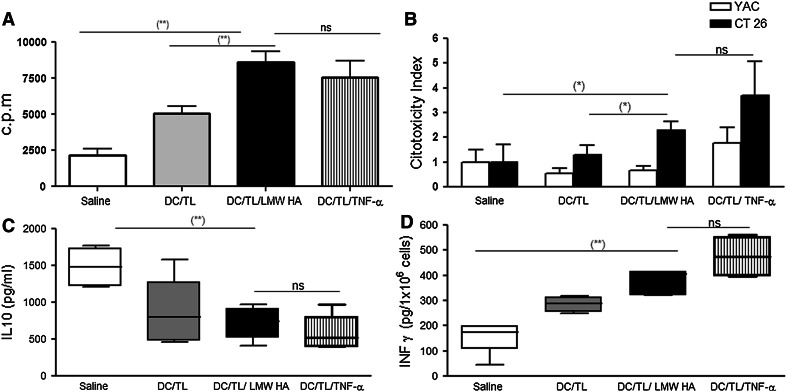
Splenocytes derived from animals treated with DC/TL/LMW HA or TNF-α showed similar increased proliferation rate upon in vitro stimulation with mitomycin C-treated CT26 cells for 5 days in comparison with DC/TL (Fig. 6a). We next investigated whether the antitumor effect induced by HA treatment was mediated, at least in part, by activation of NK and/or cytotoxic T lymphocytes. As shown in Fig. 6b, treatment of tumor-bearing mice with DC/TL was found to elicit a cytotoxic activity against CT26 cells. However, splenocytes harvested from mice receiving DC/TL/LMW HA or TNF-α treatment displayed a significant higher lytic activity against CT26 after 5 days of in vitro restimulation (P < 0.05) in comparison with DC/TL or saline control. The specificity of cytotoxicity was confirmed by the NK-sensitive target YAC-1 (Fig. 6b). Our data strongly suggest that LMW HA treatment of DC/TL was able to enhance the specific CTL activity against CT26 CRC tumor cells. In addition, animals treated with either DC/TL/LMW HA or DC/TL showed a significant reduction in the production of IL-10 by splenocytes when compared to the saline group (745 ± 124.5 pg/ml vs. 806 ± 202.3 pg/ml vs. 1,484 ± 94.88 pg/ml, respectively) (Fig. 6c). DC/TL/LMW HA therapeutic vaccination is capable to inhibit CRC tumors through a potent induction of efficient immune responses. To confirm this possibility, we have analyzed the IFN-γ production by splenocytes from CT26 tumor-bearing mice and observed that DC/TL/LMW HA-treated animals showed higher IFN-γ production in comparison with those treated with DC/TL alone (368 ± 26.48 pg/ml vs. 284 ± 16.9 pg/ml) (Fig. 6d). Overall, from these results, we can conclude that LMW HA priming of DC/TL induces significantly enhanced specific CTL responses against CRC comparable to TNF-α.
Fig. 6.
LMW HA treatment induces splenocytes proliferation and specific CTL activities, while reducing their release of IL-10. a Splenocytes from different experimental groups were stimulated in vitro with mitomycin C-treated CT26 cells for 5 days. Cell proliferation was evaluated by 3H-thymidine incorporation assay at day 5. b Subcutaneous injection with BM-derived DC cocultured in the presence of LMW HA induces tumor-specific CTLs. Cytolytic activity in 4 h LDH assays of CTL cultures derived from three/four pooled spleens of CT26 tumor-bearing BALB/c treated on day 7 after tumor inoculation with DC alone, DC/TL, DC/TL/TNF-α, or DC/TL/LMW HA and harvested 7 days later was measured against labeled CT26 and YAC-1 cells at different effector-to-target ratios. Results are representative of two independent experiments. Cytotoxicity was evaluated with the LDH Cytotoxicity detection Kit. Results are expressed as relative values of lysis with respect to non-treated target cells. IL-10 (c) and IFN-γ (d) expression were determined by ELISA from free-cell culture supernatant from animals treated with saline, DC/TL, DC/TL/TNF-α, or DC/TL/LMW HA. (*) P < 0.05; (**) P < 0.01
Discussion
A number of immunostimulatory strategies for the treatment of CRC are currently under preclinical and clinical evaluation [3]. At present, one of the most promising approaches to induce immunity against cancer consists in the use of DC as cancer vaccines [12, 37]. The activation state and migration capacity of DC play a key role for the induction of a potent and specific T cell response [12, 38]. Our previous results showed that systemic administration of LMW HA has the ability to induce immunity against CRC in mice, with an in vivo DC activation being one of the mechanisms involved in the antitumoral effects found [5]. Therefore, we thought reasonable to explore whether LMW HA might have a direct effect on DC, which may sufficiently explain the reported inhibition of CRC tumor growth in vivo. Indeed, the present work shows that pre-incubating DC with LMW HA was able to strongly increase DC activation and their specific migration toward secondary lymphoid organs, ultimately resulting in a potent in vivo antitumoral effect. Thus, the immunophenotypic change induced by LMW HA was able to enhance IL-12 and to reduce IL-10 production as well as to generate specific CTL responses and protective immunity. Thus, we were herein able to show for the first time that pre-incubation of DC with LMW HA, but not LMW HA-untreated DC, as part of a vaccination protocol scheme, was able to significantly improve their ability to inhibit tumor growth and to increase animal survival.
Lately, it was repeatedly reported that oligosaccharides of HA, but not HMW HA, can be used to stimulate immune responses [39]. In this sense, it has been described that small HA fragments are potent activators of DC derived from both humans and mice [26, 29, 33]. These stimulatory effects were shown to be highly specific for HA, in a CD44-independent and TLR4-dependent way [29]. Nevertheless, on previous studies, there is no data published on LMW HA effects on DC/TL migratory response toward lymph node-derived signals and the effector pathway mechanisms therein involved. Thus, our results add significant information to the current knowledge on mechanisms driven by LMW HA when used as adjuvant for DC vaccines in the treatment of experimental CRC. The enhancement in DC/TL migratory behavior likely explains to a large extent the efficient antitumoral responses elicited by LMW HA or its fragments that we and others have previously shown [5, 40].
As previously described by different authors, our work showed that LMW HA is able to further induce the maturation of CRC tumor lysate-pulsed DC, as shown by an enhanced upregulation of the surface markers MHC class II and CD86 when compared with LMW HA non-treated DC/TL [26, 29, 33]. In addition, LMW HA-treated DC showed an increased ability to stimulate alloreactive T cells and to produce higher levels of IL-12. Moreover, DC pre-incubated with LMW HA released lower levels of the immunosuppressive IL-10 consistent with a potent activation state of DC upon LMW HA stimulation.
Different cocktails of cytokines and factors including TNF-α, IL-1β, IL-6, TNF-α, IFN-α, IFN-γ, poly(I:C), or PGE2 have been used to induce DC maturation [18, 36, 41]. Although PGE2 is believed to be critical for DC migration to LN, there are reports showing that PGE2 is able to induce IDO (indoleamine-2,3-dioxygenase) expression and a tolerogenic state on DC [18]. Poly (I:C) has been extensively used to induce DC maturation and production of IL-12 secretion in vitro [18]. However, a recent study showed that TLR3 triggering with poly(I:C) alone can induce IDO expression and did not offer a superior migratory ability in comparison with other stimuli [18]. Moreover, recent studies showed that LPS and poly(I:C) can exert inhibitory effects on DC by the activation of the so-called suppressors of cytokine signaling (SOCS) [42, 43].
Migration of activated DC is a key step to induce immunity against cancer cells [15, 34]. Therefore, one of the concerns regarding ex vivo generated DC is how to ensure effective migration to the T cell areas in the peripheral lymph node after s.c. or intravenous injection, wherein an efficient antigenic presentation can take place [12, 44]. Efforts to facilitate the migration of injected DC into draining lymph nodes in cancer might be of paramount importance. According to this, we thought in the possibility of that LMW HA treatment in the preparation of the DC vaccine might increase DC migration toward signals derived from lymphoid areas of antigenic presentation to T cells and this was in fact the case; in vitro preconditioning significantly increase their migration toward the CCR7 ligands, CCL19 and CCL21. Interestingly, this effect was confirmed when DC were obtained from CD44 knockout mice suggesting that increased migration was, at least under our experimental conditions, independent on HA-CD44 interaction.
TLR, which is another known HA receptor, has the ability to induce phenotypic maturation of DC through TLR2 and/or TLR4 [26, 29, 45]. Although the precise mechanism by which LMW HA induces the expression of inflammatory genes is unclear, it is possible that differences in HA sizes are the reasons of the discrepancy among results. Nevertheless, in our hands, TLR was found not to be involved in the effects induced by LMW HA on DC/TL migration, since DC generated from TLR4-deficient mice behaved similarly than their counterparts derived from TLR4-wild type littermates. It is clear that DC from TLR4-wild type littermates showed increased migration toward chemotactic stimuli upon LMW HA pre-incubation but in a less amount than TLR-deficient mice; reasons for this difference are under study.
Our results prompted us to test whether or not ex vivo-derived activated DC might have a similar effect in vivo. For this purpose, we have s.c. injected DC/TL/LMW HA to CT26 tumor-bearing mice and observed a significant antitumoral effect and prolonged animal survival without any sign of toxicity or autoimmunity. These results are of particular importance because in our CT26 tumor model, LMW HA non-treated DC pulsed with tumor lysate failed to induce any significant antitumor response stressing that LMW HA exerts a potent adjuvant effect in our vaccination protocol. Additionally, splenocytes from animals treated with DC/TL/LMW HA were shown to have increase proliferation activity and production of IFN-γ, to produce lower levels of IL-10 and, importantly, to elicit a more potent and specific CTL response against CT26 cells than those derived from animals not receiving LMW HA-treated cells. Moreover, the protection obtained by activation of antitumoral immunity was also evident in experiments showing that animals that eliminated the tumor after s.c. administration of DC/TL/LMW HA resisted a new challenge with tumor cells administered 1 month after the disappearance of the initial tumor.
We next decided to reproduce in vivo the in vitro results on LMW HA-mediated DC/TL migratory behavior and found that, after their s.c. injection, the number of DC recovered from LN was significantly higher in animals receiving LMW HA preconditioned DC/TL when compared with animals receiving LMW HA non-treated DC/TL. These observations underscore the role of LMW HA on DC migration. Moreover, an increase in CCR7 expression levels was found in DC/TL/LMW HA derived from wild type and TLR4 knockout mice, which partially might explain the changes in DC migratory responses.
In summary, we have herein shown that addition of a simple and nontoxic LMW HA in the preparation of DC vaccines against an experimental model of CRC was able to induce an efficient antitumoral effect. This effect was found to be mediated by an enhancement in DC maturation and activation and in their capacity to sense and respond to signals produced in lymphoid areas of T cell antigenic presentation proximal in vitro and in vivo. In comparison with other molecules such as LPS, Poly(I:C), and TNF-α, LMW HA was superior at inducing DC migration. The mechanisms involved in LMW HA induced DC chemoattraction were shown to be CD44- and TLR4-independent. From our results, we suggest the LMW HA priming of tumor lysate-pulsed DC as a promising potential tool for the treatment of patients with advanced CRC.
Acknowledgments
We would like to thank Soledad Arregui and Guillermo Gastón for expert technical assistance; Marcela Bolontrade for BLI experiments and Infant Foundation for providing us with TLR4 knockout mice. This work was supported by grants from Mizutani Foundation for Glycoscience (80072), Austral University, Agencia Nacional de Promoción Científica y Tecnológica (PICT-2006-1882; PICT-2005-34788, PICT 2007-00736, PICTO-CRUP 2005-31179), CTE-06 and AECI 2008. P.S. and I.E. are supported by “UTE-project CIMA”.
Conflict of interest
The authors indicate no potential conflicts of interest.
Abbreviations
- TL
Tumor lysate
- LMW
Low molecular weight
- HMW
High molecular weight
- HA
Hyaluronan
Footnotes
Manglio Rizzo and Mariana G. Garcia equally contributed to this work.
Contributor Information
Laura Alaniz, Phone: +54-2322-482618, FAX: +54-2322-482204, Email: laualaniz@yahoo.com.ar.
Guillermo Mazzolini, Phone: +54-2322-482618, FAX: +54-2322-482204, Email: gmazzoli@cas.austral.edu.ar.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Lee JJ, Chu E. An update on treatment advances for the first-line therapy of metastatic colorectal cancer. Cancer J. 2007;13(5):276–281. doi: 10.1097/PPO.0b013e3181570062. [DOI] [PubMed] [Google Scholar]
- 3.Dalerba P, Maccalli C, Casati C, Castelli C, Parmiani G. Immunology and immunotherapy of colorectal cancer. Crit Rev Oncol Hematol. 2003;46(1):33–57. doi: 10.1016/S1040-8428(02)00159-2. [DOI] [PubMed] [Google Scholar]
- 4.Mazzolini G, Murillo O, Atorrasagasti C, Dubrot J, Tirapu I, Rizzo M, Arina A, Alfaro C, Azpilicueta A, Berasain C, Perez-Gracia JL, Gonzalez A, Melero I. Immunotherapy and immunoescape in colorectal cancer. World J Gastroenterol. 2007;13(44):5822–5831. doi: 10.3748/wjg.v13.i44.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alaniz L, Rizzo M, Malvicini M, Jaunarena J, Avella D, Atorrasagasti C, Aquino JB, Garcia M, Matar P, Silva M, Mazzolini G. Low molecular weight hyaluronan inhibits colorectal carcinoma growth by decreasing tumor cell proliferation and stimulating immune response. Cancer Lett. 2009;278(1):9–16. doi: 10.1016/j.canlet.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 6.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106(3):255–258. doi: 10.1016/S0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 7.Lin CC, Wang TE, Liu CY, Lin CP, Liu TP, Chen MJ, Chang WH, Lin JC, Chang KM, Chu CH, Shih SC, Chao KS, Chen YJ. Potentiation of the immunotherapeutic effect of autologous dendritic cells by pretreating hepatocellular carcinoma with low-dose radiation. Clin Invest Med. 2008;31(3):E150–E159. doi: 10.25011/cim.v31i3.3472. [DOI] [PubMed] [Google Scholar]
- 8.Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49(1):124–132. doi: 10.1002/hep.22626. [DOI] [PubMed] [Google Scholar]
- 9.Mazzolini G, Alfaro C, Sangro B, Feijoo E, Ruiz J, Benito A, Tirapu I, Arina A, Sola J, Herraiz M, Lucena F, Olague C, Subtil J, Quiroga J, Herrero I, Sadaba B, Bendandi M, Qian C, Prieto J, Melero I. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J Clin Oncol. 2005;23(5):999–1010. doi: 10.1200/JCO.2005.00.463. [DOI] [PubMed] [Google Scholar]
- 10.Nestle FO, Farkas A, Conrad C. Dendritic-cell-based therapeutic vaccination against cancer. Curr Opin Immunol. 2005;17(2):163–169. doi: 10.1016/j.coi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Tuyaerts S, Aerts J, Corthals J, Neyns B, Heirman C, Breckpot K, Thielemans K, Bonehill A. Current approaches in dendritic cell generation and future implications for cancer immunotherapy. Cancer Immunol Immunother. 2007;56(10):1513–1537. doi: 10.1007/s00262-007-0334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palucka AK, Ueno H, Fay J, Banchereau J. Dendritic cells: a critical player in cancer therapy? J Immunother. 2008;31(9):793–805. doi: 10.1097/CJI.0b013e31818403bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong W, Fei M, Zhu Y, Zhang X. Transcriptional profiles during the differentiation and maturation of monocyte-derived dendritic cells, analyzed using focused microarrays. Cell Mol Biol Lett. 2009;14(4):587–608. doi: 10.2478/s11658-009-0023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messmer D, Messmer B, Chiorazzi N. The global transcriptional maturation program and stimuli-specific gene expression profiles of human myeloid dendritic cells. Int Immunol. 2003;15(4):491–503. doi: 10.1093/intimm/dxg052. [DOI] [PubMed] [Google Scholar]
- 15.Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 16.Sozzani S, Allavena P, D’Amico G, Luini W, Bianchi G, Kataura M, Imai T, Yoshie O, Bonecchi R, Mantovani A. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J Immunol. 1998;161(3):1083–1086. [PubMed] [Google Scholar]
- 17.Yen J-H, Khayrullina T, Ganea D. PGE2-induced metalloproteinase-9 is essential for dendritic cell migration. Blood. 2008;111(1):260–270. doi: 10.1182/blood-2007-05-090613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moller I, Michel K, Frech N, Burger M, Pfeifer D, Frommolt P, Veelken H, Thomas-Kaskel AK. Dendritic cell maturation with poly(I:C)-based versus PGE2-based cytokine combinations results in differential functional characteristics relevant to clinical application. J Immunother. 2008;31(5):506–519. doi: 10.1097/CJI.0b013e318177d9e5. [DOI] [PubMed] [Google Scholar]
- 19.Toole BP. Hyaluronan is not just a goo! J Clin Invest. 2000;106(3):335–336. doi: 10.1172/JCI10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alaniz L, Garcia M, Rizzo M, Piccioni F, Mazzolini G. Altered hyaluronan biosynthesis and cancer progression: an immunological perspective. Mini Rev Med Chem. 2009;9(13):1538–1546. doi: 10.2174/138955709790361485. [DOI] [PubMed] [Google Scholar]
- 21.Boregowda RK, Appaiah HN, Siddaiah M, Kumarswamy SB, Sunila S, Thimmaiah KN, Mortha K, Toole B, Banerjee S. Expression of hyaluronan in human tumor progression. J Carcinog. 2006;5:2. doi: 10.1186/1477-3163-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21(1):25–29. doi: 10.1016/S0945-053X(01)00184-6. [DOI] [PubMed] [Google Scholar]
- 23.McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996;98(10):2403–2413. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4(1):33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 25.Termeer CC, Hennies J, Voith U, Ahrens T, Weiss JM, Prehm P, Simon JC. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol. 2000;165(4):1863–1870. doi: 10.4049/jimmunol.165.4.1863. [DOI] [PubMed] [Google Scholar]
- 26.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177(2):1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 27.Ruffell B, Johnson P. Hyaluronan induces cell death in activated T cells through CD44. J Immunol. 2008;181(10):7044–7054. doi: 10.4049/jimmunol.181.10.7044. [DOI] [PubMed] [Google Scholar]
- 28.Bollyky PL, Falk BA, Wu RP, Buckner JH, Wight TN, Nepom GT. Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4+ CD25+ regulatory T cells. J Leukoc Biol. 2009;86(3):567–572. doi: 10.1189/jlb.0109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195(1):99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malvicini M, Rizzo M, Alaniz L, Pinero F, Garcia M, Atorrasagasti C, Aquino JB, Rozados V, Scharovsky OG, Matar P, Mazzolini G. A novel synergistic combination of cyclophosphamide and gene transfer of interleukin-12 eradicates colorectal carcinoma in mice. Clin Cancer Res. 2009;15(23):7256–7265. doi: 10.1158/1078-0432.CCR-09-1861. [DOI] [PubMed] [Google Scholar]
- 31.Bhaumik S, Gambhir SS. Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc Natl Acad Sci USA. 2002;99(1):377–382. doi: 10.1073/pnas.012611099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5(11):1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 33.Do Y, Nagarkatti PS, Nagarkatti M. Role of CD44 and hyaluronic acid (HA) in activation of alloreactive and antigen-specific T cells by bone marrow-derived dendritic cells. J Immunother. 2004;27(1):1–12. doi: 10.1097/00002371-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Martin-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: Impact on T lymphocyte traffic and priming. J Exp Med. 2003;198(4):615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adema GJ, de Vries IJ, Punt CJ, Figdor CG. Migration of dendritic cell based cancer vaccines: in vivo veritas? Curr Opin Immunol. 2005;17(2):170–174. doi: 10.1016/j.coi.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Skalova K, Mollova K, Michalek J. Human myeloid dendritic cells for cancer therapy: does maturation matter? Vaccine. 2010;28(32):5153–5160. doi: 10.1016/j.vaccine.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 37.Dauer M, Schnurr M, Eigler A. Dendritic cell-based cancer vaccination: quo vadis? Expert Rev Vaccines. 2008;7(7):1041–1053. doi: 10.1586/14760584.7.7.1041. [DOI] [PubMed] [Google Scholar]
- 38.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y-J, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18(1):767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 39.Mummert ME. Immunologic roles of hyaluronan. Immunol Res. 2005;31(3):189–206. doi: 10.1385/IR:31:3:189. [DOI] [PubMed] [Google Scholar]
- 40.Zeng C, Toole BP, Kinney SD, Kuo JW, Stamenkovic I. Inhibition of tumor growth in vivo by hyaluronan oligomers. Int J Cancer. 1998;77(3):396–401. doi: 10.1002/(SICI)1097-0215(19980729)77:3<396::AID-IJC15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 41.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5(4):296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7(6):454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 43.Bartz H, Avalos NM, Baetz A, Heeg K, Dalpke AH. Involvement of suppressors of cytokine signaling in toll-like receptor-mediated block of dendritic cell differentiation. Blood. 2006;108(13):4102–4108. doi: 10.1182/blood-2006-03-008946. [DOI] [PubMed] [Google Scholar]
- 44.Allavena P, Sica A, Vecchi A, Locati M, Sozzani S, Mantovani A. The chemokine receptor switch paradigm and dendritic cell migration: its significance in tumor tissues. Immunol Rev. 2000;177:141–149. doi: 10.1034/j.1600-065X.2000.17714.x. [DOI] [PubMed] [Google Scholar]
- 45.Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR. The role of hyaluronan degradation products as innate alloimmune agonists. Am J Transplant. 2006;6(11):2622–2635. doi: 10.1111/j.1600-6143.2006.01537.x. [DOI] [PubMed] [Google Scholar]