Abstract
Accumulating evidence has demonstrated that myeloid-derived suppressor cells (MDSCs), a heterogeneous population of cells, play an important role in the subversion, inhibition, and downregulation of the immune response to cancer. However, the characteristics of these cells, particularly clinical relevance, in malignant tumors remain unclear due to a lack of specific markers. In this study, we characterized peripheral CD14+HLA-DR-/low cells, a new human MDSC subpopulation, in 89 patients with non-small cell lung cancer (NSCLC). As expected, both frequency and absolute number of CD14+HLA-DR-/low cells were significantly increased in the peripheral blood of NSCLC patients compared with that of the healthy controls and indicated an association with metastasis, response to chemotherapy, and progression-free survival. These cells showed decreased expression of CD16 and CD86 compared with HLA-DR+ monocytes. Unlike classical monocytes, these populations showed significantly decreased allostimulatory activity and showed the ability to inhibit autologous T cell proliferation and IFN-γ production in a cell-contact-dependent manner. Furthermore, we demonstrated that CD14+HLA-DR-/low cells expressed the NADPH oxidase component gp91phox and generated high level of reactive oxygen species (ROS). Moreover, inactivation of ROS reversed their immunosuppressive capacity on T cell response. These results prove, for the first time, the existence of ROS-producing CD14+HLA-DR-/low myeloid-derived suppressor cells in NSCLC patients, which mediate tumor immunosuppression and might thus represent a potential target for therapeutic intervention.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1450-6) contains supplementary material, which is available to authorized users.
Keywords: Non-small cell lung cancer, HLA-DR expression, Myeloid-derived suppressor cells, Immunosuppression, Tumor immunity
Introduction
Lung cancer is one of the most frequently diagnosed malignancies and is the leading cause of cancer death among males in the world [1]. Non-small cell lung cancer (NSCLC) accounts for approximately 85 % of all lung cancer and is often only diagnosed in patients with locally advanced or metastatic disease [2]. Within the NSCLC population, those with distant disease are candidates for non-surgical treatment and usually have poor outcomes because no curative treatment is available for them, and therefore, the development of immunotherapeutic approaches to NSCLC is needed [3, 4]. Many of these immunotherapies are of greater benefit to immunocompetent patients [5], whereas systemic immune suppression is often observed in patients with advanced NSCLC. In order to achieve success in the immunotherapy protocols in NSCLC patients, it is necessary to identify the mechanisms behind this immune suppression.
To date, MDSCs have been characterized as key modulators of immune response suppression in the host, and targeting MDSC can improve antitumor activity [6–8]. MDSCs represent a heterogeneous population of immature myeloid cells which are defined as CD11b+Gr-1+ or CD11b+IL-4Rα+ in mice [8, 9]. However, the phenotypic markers of human MDSCs are still being debated due to the lack of a homologous Gr-1 gene in humans. Various MDSC phenotypes have been described in the peripheral blood of NSCLC patients, including Lin−HLA-DR−, CD33+CD11b+, Lin−HLA-DR− CD33+, CD14+S100A9+, and CD11b+ CD33+ CD14− CD15+ [10–14]. Recent studies have shown that a new subset of MDSCs identified by markers CD14+ and HLA-DR-/low can suppress T cell function in B-cell non-Hodgkin lymphoma (NHL) [15], as well as in melanoma [16–18], hepatocellular carcinoma (HCC) [19], prostate cancer [20], glioblastoma [21], squamous cell carcinoma of the head and neck [22], bladder carcinoma [23], and multiple myeloma [24]. The mechanisms of suppression include the production of arginase 1, oxidative stress, TGF-β, IL-10, CD86, and PD-L1. However, whether this subset of MDSCs exists in NSCLC patients and what associations exist with regard to progression of the disease remain unclear.
In the present study, we found that the frequency and counts of CD14+ HLA-DR-/low MDSCs significantly increased in the peripheral blood of patients with NSCLC and were associated with the occurrence of aggressive disease. These cells also showed aberrant expression of surface markers and had a lower allostimulatory potential than the CD14+HLA-DR+ subset. Furthermore, we demonstrated that CD14+ HLA-DR-/low MDSCs strongly suppressed T cell function mediated by ROS production. Taken together, we have identified a phenotype of suppressive monocytes (CD14+ HLA-DR-/low) in NSCLC as a significant source of host immune deficiency.
Materials and methods
Subjects and samples
A total of 89 patients (Karnofsky performance status ≥70) with histologically proven advanced NSCLC and without other systemic diseases were recruited in this study. None of the subjects with NSCLC were being treated with chemotherapy or immunotherapy before sampling. All patients with malignancy received complete staging according to the criteria of the seventh edition of the American Joint Committee on Cancer (AJCC) [25]. Table 1 shows the clinical characteristics of these NSCLC patients in this study. Whole blood obtained from NSCLC patients was collected prior to surgery, immunotherapy, radiotherapy, or any systemic chemotherapy. Samples were analyzed within 6 h of collection. According to WHO classification of lung tumors [26], these NSCLC patients were grouped by disease types including adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and other carcinomas that lack a small cell component. All of the patients received at least two cycles of first-line platinum-doublet chemotherapy in a randomized manner and were longitudinally followed up (Table 1). Of these 89 patients, 60 patients were successfully followed up until disease progression. Progression-free survival (PFS) was analyzed as the length of time from the start of chemotherapy to documented disease progression. In addition, to investigate the potential role of CD14+HLA-DR-/low MDSCs in advanced NSCLC, 46 patients who had partial response (PR), stable disease (SD), or progressive disease (PD) after receiving platinum-based chemotherapy were further recruited. Their clinical characteristics are summarized in Online Resource Table 1. All of the patients received at least two cycles of first-line chemotherapy every 3 weeks. Blood samples were collected at baseline immediately prior to the first cycle of chemotherapy. Subsequent blood samples for MDSC analysis were collected 3 weeks after the last cycle and immediately prior to receiving the following cycle of chemotherapy. Tumor response was assessed by computed tomography according to RECIST criteria [27]. Patients with coexisting medical illnesses that were likely to impact their immune status were excluded from the study. For comparison, 35 age- and sex-matched healthy controls were recruited as controls (M/F = 23/12, age 38.05 ± 5.49 years). The study protocol was approved by the Ethics Committee of Chinese PLA General Hospital. Written informed consent was obtained from the patients and volunteers. Samples were analyzed within 6 h of collection.
Table 1.
Clinical characteristics of 89 NSCLC patients who were followed up
| All cases | ≤9.43 % | >9.43 % | |||
|---|---|---|---|---|---|
| Disease progression | Censored data | Disease progression | Censored data | ||
| Case | 89 | 28 | 17 | 32 | 12 |
| Gender (male/female) | 51/38 | 17/11 | 9/8 | 17/15 | 8/4 |
| Age (years) | 46.78 ± 9.60 | 52.5 ± 9.43 | 51.29 ± 10.74 | 51.53 ± 8.81 | 59.67 ± 8.67 |
| Histological type | |||||
| Adenocarcinoma | 57 | 19 | 14 | 17 | 7 |
| Squamous cell carcinoma | 21 | 6 | 1 | 9 | 5 |
| Other non-small cell carcinoma | 11 | 3 | 2 | 6 | 0 |
| AJCC stage | |||||
| III (unresectable) | 23 | 11 | 5 | 6 | 1 |
| IV (M1a) | 23 | 8 | 7 | 5 | 3 |
| IV (M1b) | 43 | 9 | 5 | 21 | 8 |
| 1st line chemotherapy | |||||
| Docetaxel + cisplatin | 31 | 8 | 7 | 14 | 2 |
| Pemetrexed + cisplatin | 42 | 14 | 10 | 11 | 7 |
| Gemcitabine + cisplatin | 16 | 6 | 0 | 7 | 3 |
Flow cytometric analysis
For the surface marker and intracellular cytokine staining, fresh heparinized peripheral blood was added to the following monoclonal antibodies: PE-Cy7-conjugated CD14, PE-Cy5-conjugated HLA-DR, FITC-conjugated CD11b, PE-conjugated CD15, FITC-conjugated CD11c, PE-conjugated CD33, PE-conjugated CD80, FITC-conjugated CD83, FITC-conjugated CD86, FITC-conjugated S100A9, PE-Cy7-conjugated CD11b, FITC-conjugated Lin1 (CD3, 14, 16, 19, 20, 56), and FITC-conjugated IFN-γ (Biolegend, San Diego, CA). After the cells were incubated for 30 min at 4 °C in the dark, they were lysed with RBC Lysis/Fixation solution (Biolegend). For intracellular staining, the cells were permeabilized with Cytofix/Cytoperm Kit (BD Biosciences) and stained with antibodies specific to S100A9. Data acquisition and analysis were performed using a flow cytometer (FC500 MPL, Beckman Coulter) and FlowJo software (TreeStar, Inc, Ashland, OR). Isotype-matched antibodies were used with all the samples as controls. The absolute number of MDSCs was calculated as follows: [total monocyte count (cells/μL) × percentage MDSC]/100 [11].
Cell isolation and sorting
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by Ficoll–Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation. Monocyte counts of these patients were determined by an automated hematology analyzer (KX-21N, Sysmex). PBMCs were stained with antibodies to human CD14 and HLA-DR. In selected assays, CD14-depleted PBMCs, and CD14+HLA-DR-/low and CD14+HLA-DR+ cells were sorted by using MoFlo XDP cell sorter (Beckman Coulter). The purity of the cells after sorting was >97 %.
Allogeneic mixed lymphocyte reaction
Allogeneic PBMCs labeled with CFSE from a healthy control were incubated with various ratios of sorted CD14+HLA-DR+ or CD14+ HLA-DR-/low cells from four NSCLC patients in 96-well plates (Costar, Lowell, MA) in complete RPMI 1640 medium with 10 % fetal bovine serum (Gibco, Carlsbad, CA). PBMCs incubated with anti-CD3/anti-CD28 were used as a positive control. Cells were harvested at 120 h and stained with anti-CD4-PE-Cy5 and anti-CD8-PE. Proliferation of T cells was determined by FACS analysis.
Assay for proliferation and cytokine secretion
To determine the suppressive capacity of MDSCs, CD14-depleted PBMCs were cocultured with CD14+ HLA-DR-/low cells at different ratios in some experiments. Carboxyfluorescein succinimidyl ester (CFSE)-based proliferation assays were performed according to manufacturer’s instructions (Invitrogen, Carlsbad, CA). Cells were stimulated with anti-human CD3 and CD28 (both 1 μg/mL), stained with PE-conjugated CD8 and PE-Cy5-conjugated CD4 at day 5, and further evaluated with CFSE dilution by flow cytometry.
For IFN-γ detection, these cells cocultured as described above were stimulated with phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich; 50 ng/mL) plus ionomycin (Sigma-Aldrich; 1 μg/mL) for 5 h in the presence of monensin. At the end of the stimulation, cells were stained with PE-Cy5-conjugated CD3 and PE-conjugated CD8, further permeabilized with Cytofix/Cytoperm Kit (BD Biosciences), and labeled with intracellular antibody FITC-conjugated IFN-γ.
In some experiments, inhibitors of candidate suppressive molecules were added at the following final concentrations: 100 U/mL catalase (Sigma-Aldrich, St. Louis, MO), 500 μmol/L NG-Methyl-l-arginine acetate salt (L-NMMA, Sigma-Aldrich), 500 μmol/L Nω-Hydroxy-nor-l-arginine, diacetate salt (nor-NOHA, Calbiochem, Darmstadt, Germany), 10 μg/mL neutralizing anti-human TGF-β1 antibody (R&D Systems, Minneapolis, MN), or 10 μg/mL neutralizing anti-human PD-L1 antibody (Biolegend).
ROS detection
CD14+HLA-DR-/low and CD14+HLA-DR+ cells were, respectively, cultured in RPMI1640 medium for 30 min at 37 °C in the presence of 2.5 μM 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA, Molecular Probes/Invitrogen), which could be metabolized to 2′-7′-dichlorofluorescein (DCF) upon oxidation. For activation of ROS production, cells were simultaneously incubated with both CM-H2DCFDA and 30 ng/mL PMA (Sigma-Aldrich). Analysis of ROS production by flow cytometry was then conducted as described above.
Quantitative polymerase chain reaction
Total RNA from CD14+HLA-DR+ or CD14+ HLA-DR-/low cells was extracted using the RNeasy Mini kit (Qiagen, Hilden, Germany), and genomic DNA was removed using the RNase-Free DNase set (Promega, Madison, WI). Complementary DNA (cDNA) was generated using the Reverse Transcription System Kit (Takara). Quantitative PCR (SYBR Green Supermix, iCycler, Bio-Rad) was performed using primer sequences for gene-specific amplifications as previously reported [18, 28]. Reactions were done in duplicate using SYBR-Green (Bio-Rad, München, Germany). Relative transcript levels were determined by 2−ΔΔCT method, in which CT represents the threshold cycle (ABI7500 sequence detection system, Applied Biosystems), and then normalized to β-actin in each sample.
Statistical analysis
The data were summarized and presented as mean ± standard deviation (SD) and were analyzed using SPSS version 13.0 for Windows (SPSS, Chicago, IL). Multiple comparisons were made between different groups with the Kruskal–Wallis H nonparametric test. Comparison between various individuals was performed using the Mann–Whitney U test. Comparison between the same individual was performed using the Wilcoxon matched-pairs t test. Correlation analysis was performed using Spearman’s rank correlation test. The survival curves were estimated by Kaplan–Meier method, and the patient survival times per group were compared by the log-rank test. For all tests, two-sided P < 0.05 was considered to be statistically significant.
Results
Peripheral CD14+HLA-DR-/low cells are significantly increased in NSCLC patients
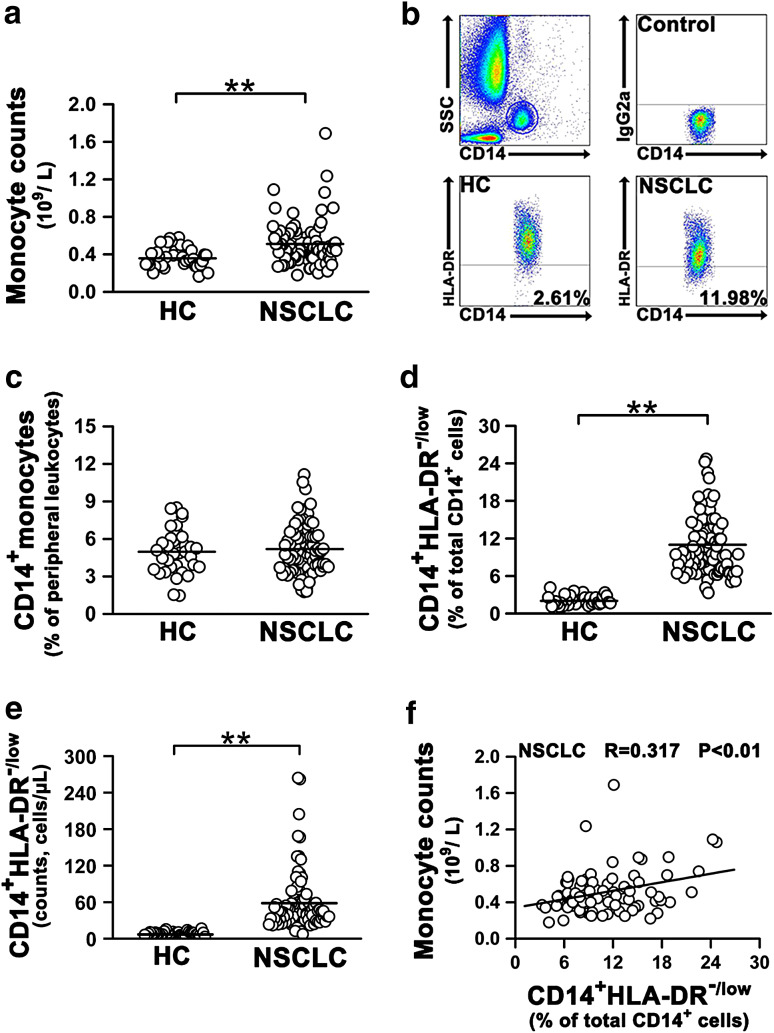
The absolute number of peripheral monocytes was first analyzed in patients with NSCLC and healthy controls (Table 1). We found a significant increase in the absolute number of peripheral monocytes in NSCLC patients compared with healthy controls (Fig. 1a). The CD14+ monocytes were then divided into CD14+HLA-DR+ subset and CD14+HLA-DR-/low subset for HLA-DR expression (Fig. 1b). Although there was no difference in the percentage of CD14+ monocytes in peripheral blood leukocytes of NSCLC patients and healthy controls (Fig. 1c), both percentage and absolute number of circulating CD14+HLA-DR-/low subset were significantly increased in NSCLC patients compared with healthy controls (Fig. 1d, e). A statistically significant positive correlation was observed between CD14+HLA-DR-/low subset frequencies and monocyte counts (Fig. 1f). Moreover, no difference in the percentages of Lin−HLA-DR−CD33+CD11b+, CD11b+CD14−CD33+, and CD15+CD14− could be observed between patients and healthy controls (Online resource, Supplementary Fig. 1).
Fig. 1.
Number and frequency of circulating CD14+HLA-DR-/low cells are significantly increased in NSCLC patients. a Absolute counts of monocytes in peripheral blood of healthy controls and NSCLC patients were analyzed by flow cytometry. b Representative dot plots of a healthy control and a NSCLC patient. Whole blood was stained for monocyte markers, including CD14 and HLA-DR. c The percentage of CD14+ cells in peripheral blood of healthy controls and NSCLC patients. d The percentage of CD14+HLA-DR-/low cells in peripheral blood CD14+ cells in healthy controls and NSCLC patients. e Absolute number of CD14+HLA-DR-/low cells in peripheral blood of healthy controls and NSCLC patients. f The correlation of the percentages of CD14+HLA-DR-/low cells in NSCLC patients with absolute monocyte counts. Healthy controls, n = 35; NSCLC patients, n = 89. Each dot represents one individual. Horizontal bars indicate mean values. All data are shown as mean ±SD. *P < 0.05; **P < 0.01
The phenotypic characterization of CD14+HLA-DR-/low cells
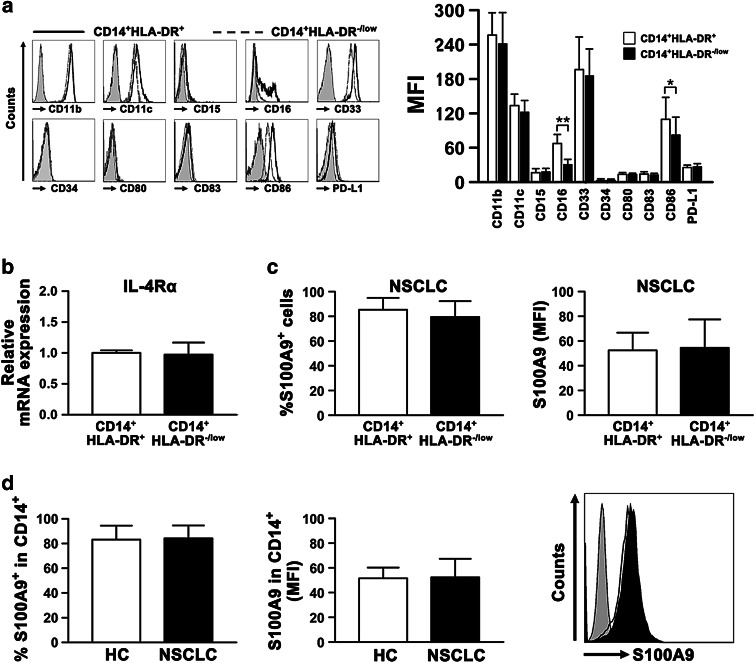
The cell surface activation and monocytic markers in the CD14+HLA-DR-/low population were compared with those in the CD14+HLA-DR+ population in NSCLC patients. Both populations from NSCLC patients were positive for CD11b, CD11c, and CD33, and negative or low for CD15, CD34, CD80, CD83, and PD-L1. Notably, CD14+HLA-DR-/low monocytes expressed lower levels of CD16 and CD86 than the HLA-DR+ monocytes (Fig. 2a). To further investigate the biological characteristics of CD14+HLA-DR-/low cells in NSCLC patients, we analyzed the expression of IL-4Rα and S100A9, both of which have been postulated to be specific markers for monocytic MDSCs, in CD14+HLA-DR-/low cells from NSCLC patients. Compared with the HLA-DR+ monocytes, the expression of IL-4Rα gene was unchanged in CD14+HLA-DR-/low cells (Fig. 2b). In addition, no substantial difference in the expression of S100A9 was observed between CD14+HLA-DR+ and CD14+HLA-DR-/low cells (Fig. 2c) in NSCLC patients or between CD14+ monocytes of healthy controls and NSCLC patients (Fig. 2d).
Fig. 2.
The phenotypic characterization of CD14+HLA-DR-/low cells. a Representative histograms of different cell surface markers expressed on CD14+HLA-DR+ and CD14+HLA-DR-/low cells from NSCLC patients are shown above (left panel). Flow cytometry analysis of the difference in the phenotypic marker expression between CD14+HLA-DR+ and CD14+HLA-DR-/low cells in NSCLC patients (n ≥ 10) was presented as mean fluorescence intensity (MFI) and shown in the right panel. Isotype antibodies were used as a control (filled histograms). b Expression of IL-4Rα in CD14+HLA-DR-/low and CD14+HLA-DR+ cells from NSCLC patients (n = 5) was assessed by qPCR. c Level of S100A9 protein in CD14+HLA-DR-/low and CD14+HLA-DR+ cells from NSCLC patients (n = 8) was determined by flow cytometry. Left panel, the percentage of S100A9+ cells in these two populations; right panel, the MFI of S100A9 in these two populations. d The percentage and MFI of S100A9 in CD14+ monocytes are shown for healthy controls (n = 6) and NSCLC patients (n = 8). Representative histogram for each group is shown in the right panel. All data are shown as mean ±SD. *P < 0.05; **P < 0.01
Frequency of CD14+HLA-DR-/low cells is associated with disease progression and response to treatment in NSCLC
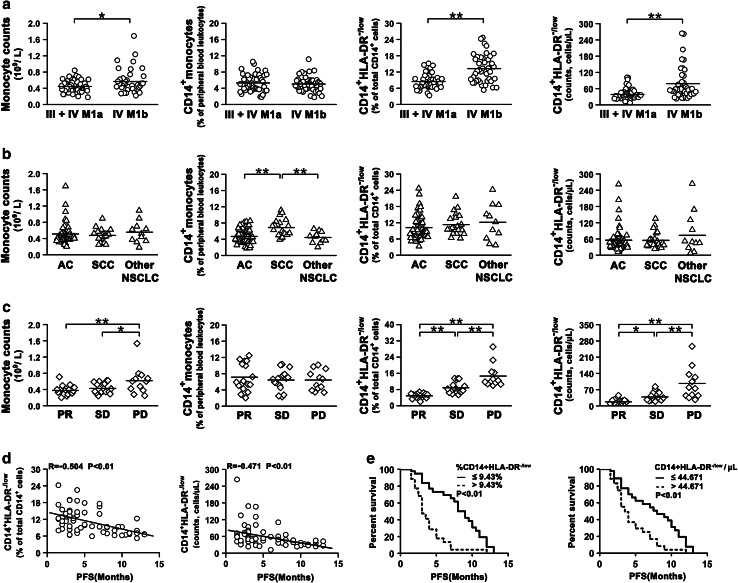
To evaluate the clinical significant of CD14+HLA-DR-/low subset in NSCLC patients, we first analyzed the CD14+HLA-DR-/low cells in patients with different clinical assessment. Within the NSCLC population, the count of total monocytes, but not the percentage of monocytes, in the peripheral blood of patients with stage IV (M1b) disease was higher than the count in patients with stage III or IV (M1a) disease (Fig. 3a). Both percentage and absolute number of CD14+HLA-DR-/low cells were increased in patients with stage IV (M1b) disease compared to patients with stage IV (M1a) or III disease (Fig. 3a). When NSCLC patients were grouped by histological type, there were no statistically significant differences in absolute number of peripheral monocytes, percentage, and absolute number of CD14+HLA-DR-/low cells among all groups (Fig. 3b). However, patients with squamous cell carcinoma (SCC) have the higher ratio of peripheral CD14+ monocytes than other histology groups (Fig. 3b). Furthermore, we determined the distribution of CD14+ monocyte and CD14+HLA-DR-/low cells in the NSCLC patients with various degrees of response to chemotherapy. As shown in Fig. 3c, the absolute number of total monocytes in peripheral blood of patients with PD after chemotherapy was significantly increased in comparison with patients with PR or SD (Online resource, Supplementary Table 1). The percentages of CD14+ monocytes in peripheral blood were almost identical among these three groups (Fig. 3c). Interestingly, both percentage and absolute number of CD14+HLA-DR-/low cells were significantly increased in patients with PD, compared with patients with PR and SD after chemotherapy (Fig. 3c). Patients with SD also had a higher percentage of CD14+HLA-DR-/low cells than those with PR. Of note, baseline percentage and absolute number of CD14+HLA-DR-/low cells were significantly higher in patients experiencing PD in comparison with patients experiencing PR or SD (Online resource, Supplementary Fig. 2).
Fig. 3.
Clinical relevance of circulating CD14+HLA-DR-/low cells in NSCLC patients. a The number and frequency of CD14+ monocyte in peripheral blood leukocytes, and the frequency and absolute number of peripheral CD14+HLA-DR-/low cells in NSCLC patients with various stages were determined by flow cytometry analysis. Stage IV (M1b) disease, n = 43; Stage IV (M1a) or III disease, n = 46. b Distribution of monocyte counts, frequency of CD14+ monocytes, and frequency and absolute number of CD14+HLA-DR-/low cells in NSCLC patients with various histological types (AS, n = 57; SCC, n = 21; other NSCLC, n = 11). AS adenocarcinoma, SCC squamous cell carcinoma. c The number and frequency of CD14+ monocyte, the frequency and absolute number of CD14+HLA-DR-/low cells in NSCLC patients with various response degrees after chemotherapy. PR, n = 17; SD, n = 17; PD, n = 12. d Both frequency and absolute number of CD14+HLA-DR-/low cells were significantly negatively correlated with progression-free survival (n = 60). e Kaplan–Meier curve of PFS after cisplatin-based chemotherapy according to the median values of frequency or absolute number of CD14+HLA-DR-/low cells. The log-rank test was used to compare between the two groups. Each dot represents one individual. Horizontal bars indicate mean values. *P < 0.05; **P < 0.01
Moreover, of total 89 patients, 60 patients were successfully followed up until disease progression (Table 1), and we then found that both frequency and absolute number of CD14+HLA-DR-/low cells negatively correlated with progression-free survival (PFS) (Fig. 3d). To elucidate the validity of circulating CD14+HLA-DR-/low cells in predicting chemotherapy treatment efficacy, patients first were divided into two groups according to the median value of CD14+HLA-DR-/low frequency (9.43 %): high CD14+HLA-DR-/low group (frequency >9.43 %) and low CD14+HLA-DR-/low group (frequency ≤9.43 %). The low CD14+HLA-DR-/low group (PFS median, 9 months) had significantly longer PFS than the high CD14+HLA-DR-/low group (PFS median, 3 months; P < 0.01) (Fig. 3e left panel). These patients were then divided into two groups according to the median value (44.671 cells/μL) of the absolute number of CD14+HLA-DR-/low cells. As expected, absolute number could also predict similarly the poor prognosis of PFS (high vs. low, PFS median 3.5 vs. 8 months, P < 0.01) (Fig. 3e right panel).
CD14+HLA-DR-/low cells are potent suppressors of autologous CD4+ and CD8+ T cell response in NSCLC patients
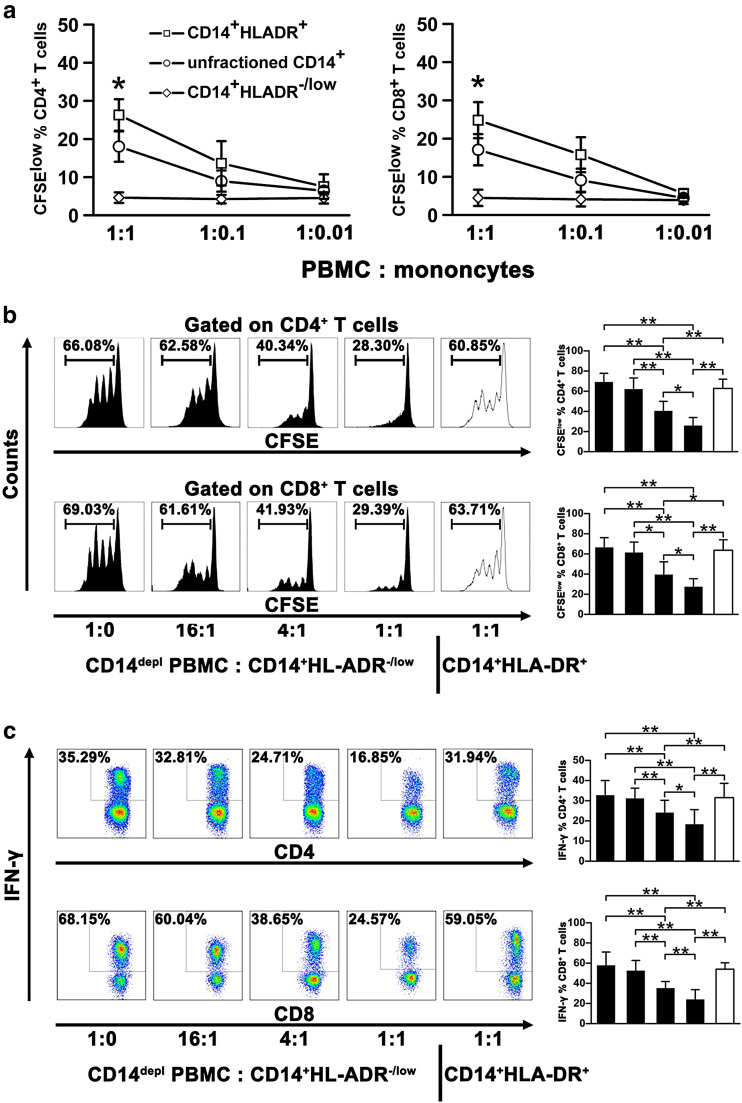
For functional analysis, the contribution of CD14+HLA-DR-/low monocytes as antigen-presenting cells was tested in a mixed lymphocyte reaction. CD14+HLA-DR+ and CD14+HLA-DR-/low cells were isolated from NSCLC PBMCs and cocultured with allogeneic PBMCs from unrelated healthy controls. Unfractioned CD14+ cells also were sorted as a control. CD14+HLA-DR-/low cells did not induce the proliferation of both CD4+ and CD8+ T cells at all cell ratios tested, whereas CD14+HLA-DR+ cells strongly stimulated the allogeneic T cell proliferation in a dose-dependent manner (Fig. 4a). Unfractioned CD14+ cells also moderately stimulated the allogeneic T cell proliferation in a dose-dependent manner (Fig. 4a).
Fig. 4.
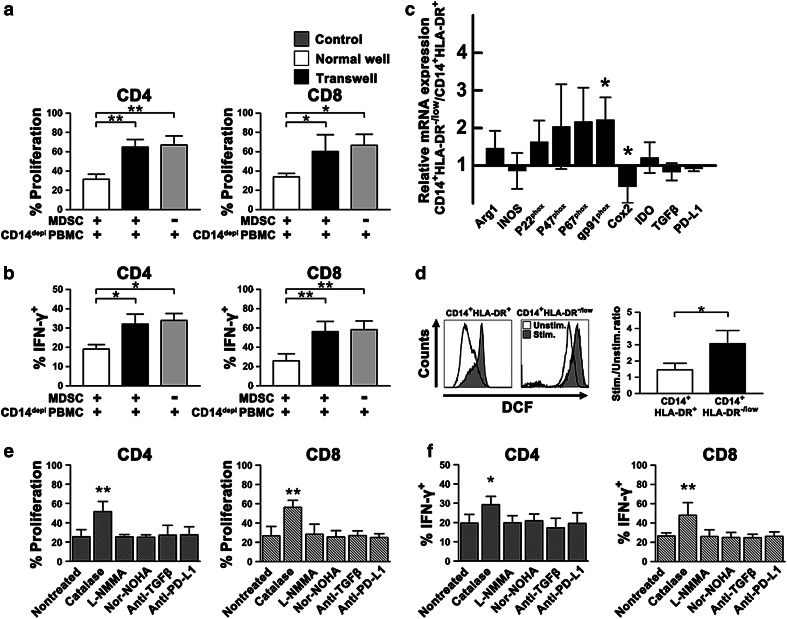
Circulating CD14+HLA-DR-/low cells from NSCLC patients have the impaired allostimulatory activity and effectively suppress autologous CD4+ and CD8+ T cell function. a CD14+HLA-DR-/low and CD14+HLA-DR+ cells from NSCLC patients (n = 4) were cocultured with CFSE-labeled PBMCs from a healthy control for 5 days in different ratios. CFSE dilution was assessed as a measure for CD4+ and CD8 T+ cell proliferation. Unfractioned CD14+ cells were used as a control. b CD14-depleted PBMCs from NSCLC patients were stimulated with anti-CD3/CD28 and added to purified autologous CD14+HLA-DR-/low cells (n = 9) at different ratios. Proliferation of CD4+ and CD8+ T cells was measured by dilution of CFSE staining intensity using flow cytometry. Representative histograms for each condition are shown (left panel), and the frequencies of CFSElow cells are shown (right panel). CD14+HLA-DR+ cells were used as a control. c CD14-depleted PBMCs were stimulated with PMA and ionomycin in the presence of CD14+HLA-DR-/low(n = 6) or CD14+HLA-DR+ cells. The cells were then harvested, and intracellular expression of IFN-γ was determined. The representative histograms (left panel) and summarized results with statistical analysis (right panel) are shown. CD14depl, CD14-depleted. All data are shown as mean values ±SD. *P < 0.05; **P < 0.01
Next, we evaluated the immunosuppressive activities of CD14+HLA-DR-/low cells on autologous T cell proliferation and IFN-γ production. CD14+HLA-DR-/low and CD14+HLA-DR+ subsets were sorted from PBMCs of the same NSCLC patients and, respectively, cocultured with CD14-depleted autologous PBMCs. We then found that CD14+HLA-DR-/low cells suppressed the proliferation of both CD4+ and CD8+ T cells stimulated with anti-CD3/anti-CD28 in a dose-dependent manner (Fig. 4b). Moreover, IFN-γ-producing cells were also significantly decreased in PMA/ionomycin-stimulated T cells cocultured with CD14+HLA-DR-/low cells (Fig. 4c). As expected, CD14+HLA-DR+ cells failed to show suppressive ability on T cell proliferation or IFN-γ production in CD14-depleted autologous PBMCs even at a 1:1 ratio compared with CD14+HLA-DR-/low cells (Figs. 4b, c).
The manner of CD14+HLA-DR-/low cell-mediated suppression of T cells
To investigate the mechanisms of CD14+HLA-DR-/low MDSC-mediated T cell suppression, CD14+HLA-DR-/low MDSCs and CD14-depleted autologous PBMCs were incubated either together or in separate wells of a transwell. There was only marginal suppression of T cell proliferation (Fig. 5a) or IFN-γ production by T cells (Fig. 5b) when MDSCs and CD14-depleted autologous PBMCs were separated using a transwell. These results suggest that MDSC-mediated inhibition of T cell responsiveness relies on either direct cell-to-cell contact or close proximity between MDSCs and T cells.
Fig. 5.
Inhibition of autologous T cell response by CD14+HLA-DR-/low MDSCs requires cell contact and is ROS dependent. a CFSE-labeled CD14-depleted PBMCs were cocultured with CD14+HLA-DR-/low MDSCs (n = 5) as previously described in normal 96-well or in transwell plates. Proliferation of CD4+ or CD8+ T cells was quantified as the percentage of CFSE dilution of gated CD4+ or CD8+ T cells (frequencies of CFSElowCD4+ or CFSElowCD8+ T cells) by using flow cytometry. b CD14-depleted PBMCs cocultured with CD14+HLA-DR-/low MDSCs (n = 5) were stimulated with PMA/ionomycin as described, and transwell inserts were used as indicated. Intracellular expression of IFN-γ was then determined. CD14depl, CD14-depleted. c Comparison of gene expression levels of arginase-1, iNOS, p22phox, p47phox, p67phox, gp91phox, Cox2, IDO, TGF-β, and PD-L1 between CD14+HLA-DR+ and CD14+HLA-DR-/low cells (n = 6). Cox2 cyclooxygenase 2, IDO indoleamine 2, 3-dioxygenase. ROS-producing enzyme NADPH oxidase subunits, p22phox, p47phox, p67phox, and gp91phox. d Intracellular ROS levels (DCF) in both CD14+HLA-DR+ and CD14+HLA-DR-/low cells from patients (n = 5) before and after PMA stimulation. Representative histograms, left panel; MFI, right panel. stim stimulated, nonstim non-stimulated. e and f Effects of variable pharmacological inhibitors and blocking antibodies on the CD14+HLA-DR-/low MDSC suppression of CD4+ and CD8+ T cell proliferation (e) and IFN-γ production (f). L-NMMA, NG-Methyl-L-arginine acetate salt; nor-NOHA, Nω-Hydroxy-nor-l-arginine, diacetate salt. All data are shown as mean ±SD. *P < 0.05; **P < 0.01
In order to further delineate the possible mechanisms as to how NSCLC MDSCs inhibit T cell function, we assessed the expression of candidate factors in CD14+HLA-DR-/low subset compared with the HLA-DR+ monocytes by quantitative PCR. The level of cyclooxygenase 2 (Cox2) transcription was significantly lower in CD14+HLA-DR-/low MDSCs than in CD14+HLA-DR+ cells. No difference in arginase-1, iNOS, TGF-β, indoleamine 2, 3-dioxygenase (IDO), and PD-L1 transcription was observed between both the populations. Interestingly, the expression of gp91phox, a component of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex responsible for ROS production, was significantly upregulated in CD14+HLA-DR-/low MDSCs at the RNA level compared with CD14+HLA-DR+ cells (Fig. 5c). The other NADPH oxidase subunits, p22phox, p47phox, and p67phox, were also modestly upregulated in CD14+HLA-DR-/low MDSCs. Next, we assessed ROS levels in both populations from NSCLC patients by loading the cells with CM-H2DCFDA. CD14+HLA-DR-/low MDSCs produced significantly higher level of ROS following PMA stimulation than the HLA-DR+ monocyte subset (Fig. 5d) stimulated with the same reagent, indicating a more pro-oxidative intracellular milieu in CD14+HLA-DR-/low MDSCs from advanced-stage NSCLC patients. Consistent with the quantitative PCR results and as expected, the addition of ROS-inactivating enzymes, such as catalase, significantly restored T cell proliferation (Fig. 5e) and IFN-γ production by T cells (Fig. 5f). On the contrary, T cell suppression could be still observed after pretreatment with the arginase inhibitor nor-NOHA, the iNOS inhibitor L-NMMA, anti-TGF-β blocking mAb, and anti-PD-L1 blocking mAb. These results strongly suggest that the suppressive effect of CD14+HLA-DR-/low MDSCs on T cells is primarily mediated through the production of ROS.
Discussion
Tumor cells have evolved different mechanisms to evade detection and destruction by the host’s immune system, creating an immunosuppressive environment that may inhibit the effectiveness of immune-enhancing treatments [29]. MDSCs have been shown to impair the efficacy of cancer immunotherapy and have been identified as an important suppressor of antitumor immune responses [15–17, 30]. In this study, we for the first time examined the quantities, frequency, phenotype, and function of CD14+ HLA-DR-/low monocytic MDSCs in patients with advanced NSCLC. The results demonstrated a significant increase in the peripheral CD14+ HLA-DR-/low subset, which was associated with tumor metastasis and degree of response to chemotherapy. These cells showed the impaired potential to stimulate allogeneic proliferation compared with the HLA-DR+ subset and could suppress autologous T cell function ex vivo in a cell-contact-dependent manner. These suppressive cells had upregulated expression of gp91phox, an important component of the ROS-generating NADPH oxidase [31], and the inactivation of ROS was able to abolish the ability of this subset to inhibit T cell responsiveness.
So far, in several human cancers, MDSCs have been defined mainly as lineage, HLA-DR−, CD33+, CD14−, CD11b+, CD15+, and CD16low [10–12, 32]. One of the few well-characterized human MDSC subsets is the monocytic CD14+ cells with decreased HLA-DR expression. We first characterized absolute number and frequency of CD14+ monocytes in a cohort of NSCLC patients and found that changes in monocyte count did not always match the changes in frequency of CD14+ monocytes. Absolute count of other leukocyte subsets would be changed in the peripheral blood of patients. Moreover, in our analysis of the peripheral blood leukocytes, we found that a trend was seen in increased leukocyte count in patients with increased monocyte count (Online resource, Supplementary Fig. 3), which also partly explains why changes in monocyte count were not consistent with monocyte frequency, in agreement with the previous reports of unchanged monocyte frequency in cancer patients [15]. We then observed that absolute number and frequency of CD14+HLA-DR-/low cells were significantly increased in the peripheral blood of NSCLC patients. Additional MDSC subtypes implicated in cancer patients, mainly including Lin−HLA-DR−CD33+ CD11b+, CD11b+CD14−CD33+, and CD15+CD14− cells [8], added complexity to this study. Especially, levels of Lin−CD33+HLA-DR−CD11b+ in patients with gastrointestinal cancer (pancreatic, esophageal, and gastric) were an independent prognostic factor for overall survival [33]. However, we found that the percentages of these MDSC subpopulations in NSCLC were the same as HC. Furthermore, CD14+HLA-DR-/low cell levels correlated positively with the absolute monocyte count. These results suggest that CD14+HLA-DR-/low cells might be the predominant increased MDSCs in NSCLC patients.
Although an increase in CD14+S100A9+ monocytes in NSCLC patients was reported in the past studies, the same results were not observed in our study. CD14+HLA-DR-/low monocytes from NSCLC patients, similar to the HLA-DR+ monocytes, were CD33+CD11b+CD15− cells, in agreement with the previous reports of monocytic MDSCs [19]. CD14+HLA-DR-/low MDSCs expressed a decreased level of CD16 in contrast to the classical HLA-DR+ monocytes. Others have reported that the higher production of reactive oxygen and decreased expression of HLA-DR were observed in CD16low/- monocytes [34, 35]. In addition, Chikamatsu and colleagues [22] showed that the immunosuppressive effects of monocytic MDSCs from patients with squamous cell carcinoma of the head and neck were partly mediated through the upregulation of PD-L1 and CD86. However, in our study, CD14+HLA-DR-/low MDSCs expressed a lower level of CD86 than the HLA-DR+ monocytes, and the expression of PD-L1 was equivalent between both populations. Furthermore, we observed that blocking PD-L1 could not restore the inhibitory function of MDSCs on T cells.
To our knowledge, it has not been demonstrated that CD14+HLA-DR-/low subset from peripheral blood was associated with distant metastasis in NSCLC patients. Others have reported that there was a substantial difference in prognosis between patients with spread within the thorax and those with extrathoracic metastasis [36]. In our study, we observed for the first time that both the absolute number and percentage of CD14+HLA-DR-/low cells were increased in NSCLC patients with metastasis, indicating a causal relationship between NSCLC disease progression and the presence of CD14+HLA-DR-/low MDSCs. Furthermore, in keeping with the previous study in renal cell cancer patients [37], both percentage and absolute number of CD14+HLA-DR-/low cells prior to therapy negatively correlated with clinical response and PFS for cisplatin-based chemotherapy in advanced NSCLC patients, suggesting that increased immunosuppressive CD14+HLA-DR-/low cells might be associated with poor prognosis of NSCLC.
In keeping with the previous reports [15, 19], the present study showed that the antigen-presenting capacity of CD14+HLA-DR-/low monocytes was potentially impaired in advanced NSCLC patients in contrast to CD14+HLA-DR+ monocytes. Moreover, CD14+HLA-DR-/low cells seemed to be able to inhibit allogeneic T cell response [38]. In patients with colon cancer, S100A9 can be utilized as a specific marker to identify CD14+HLA-DR-/low MDSCs [39]. Nevertheless, our findings showed that the mean fluorescence intensity (MFI) of S100A9 was almost identical between CD14+HLA-DR+ and CD14+HLA-DR-/low cells in the blood of patients with advanced NSCLC, in agreement with the previous study in advanced melanoma [18]. Therefore, it remains elusive whether S100A9 is a specific marker for CD14+HLA-DR-/low MDSCs. IL-4Rα has also been postulated to be a potential marker of MDSCs [9, 40]. Activation of IL-4Rα/STAT6 pathway is required for the production of arginase 1, TGF-β, and iNOS [8]. We detected no difference in IL-4Rα expression between CD14+HLA-DR+ and CD14+HLA-DR-/low cells in the peripheral blood of NSCLC patients, suggesting that IL-4Rα might not be a key regulator of MDSCs [41].
In our study, we further observed that T cell responses of NSCLC patients were significantly suppressed by the CD14+HLA-DR-/low monocyte subset rather than the CD14+HLA-DR+ subset. Supporting our data, CD14+HLA-DR-/low cells, but not CD14+HLA-DR+, have recently been reported to display a clear immunosuppressive capacity on T cells in several human carcinomas [15–22]. However, in contrast to our study, Feng and colleagues [13] have shown that all CD14+CD11b+ cells, which represented a mixture of the HLA-DR-/low and HLA-DR+ monocyte subsets, had a more powerful suppressive activity on T cells than CD14+HLA-DR-/low cells in NSCLC patients. We postulated that the difference in suppressive capacity of MDSCs might result from various histological types in NSCLC individuals. These findings indicate that the characteristics of MDSCs might be diverse in cancer patients, probably depending on different tumor-derived factors.
CD14+HLA-DR-/low MDSCs have been reported to exert their immunosuppressive function through numerous mechanisms in a wide variety of tumors [42], but these suppressive mechanisms seem to be controversial and contradictory. For instance, in patients with melanoma, the suppressive mechanisms of CD14+HLA-DR-/low have been shown to be different among three previous studies [16–18]. In this study, CD14+HLA-DR-/low MDSC-mediated suppressions were cell contact dependent, which could exclude a functional role for cytokines in these cells. We found that CD14+HLA-DR-/low cells from NSCLC patients suppress T cell proliferation and IFN-γ production via extracellular ROS, similar to the previous reports in several human diseases [18, 43]. Recent studies have implicated that S100A9 through NAPDH oxidase upregulates the production of ROS [44, 45]. In keeping with the two pervious reports on human cancers [13, 18], we found that S100A9 was almost expressed in over 80 % of total CD14+ cells. There was no substantial differential expression of S100A9 between CD14+HLA-DR+ and CD14+HLA-DR-/low subsets in NSCLC patients. However, we detected that gp91phox, an important subunit of the ROS-generating NADPH oxidase, was expressed significantly higher in CD14+HLA-DR-/low cells than in CD14+HLA-DR+ cells. Recently, CD14+gp91phox+ cells have been identified in patients with acute myeloid leukemia [46]. These cells could produce extracellular ROS through the NADPH oxidase complex and inactivate antileukemic lymphocytes in a ROS-dependent manner. The extremely short half-life of ROS also explains why suppression of T cell responses by CD14+HLA-DR-/low cells requires cell-to-cell contact.
Taken together, our data demonstrated, for the first time, that CD14+HLA-DR-/low cells act as pivotal modulators in antitumor immune response and were associated with tumor metastasis and response to treatment in NSCLC patients. The capacity of CD14+HLA-DR-/low cells in NSCLC patients to inhibit T cell function was mediated by a functional NADPH oxidase, as shown by the expression of the oxidase component gp91phox and ROS production by these monocytic MDSCs. Thus, understanding the role of MDSCs in systemic immune suppression may contribute to the development of immunologic strategies for NSCLC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by grants from the Ministry of Science and Technology of China (“973” projects) (No. 2012CB917104) and the National Natural Science Foundation of China (No. 81172853 and No. 81201605).
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- MDSCs
Myeloid-derived suppressor cells
- AS
Adenocarcinoma
- SCC
Squamous cell carcinoma
- RECIST
Response Evaluation Criteria in Solid Tumors
- PFS
Progression-free survival
- L-NMMA
NG-Methyl-l-arginine acetate salt
- nor-NOHA
Nω-Hydroxy-nor-l-arginine, diacetate salt
- CD14depl
CD14-depleted
- Arg1
Arginase-1
- iNOS
Inducible nitric oxide synthase
- Cox2
Cyclooxygenase 2
- IDO
Indoleamine 2, 3-dioxygenase
- NADPH
Nicotinamide adenine dinucleotide phosphate
- DCF
2′,7′-dichlorofluorescein
- ROS
Reactive oxygen species
Footnotes
Ang Huang and Bo Zhang contributed equally to this work as first authors.
Contributor Information
Ke-Xing Fan, Phone: +86-21-81870801, FAX: +86-21-81870801, Email: kexingfan@gmail.com.
Ya-Jun Guo, Email: yjguo@smmu.edu.cn.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Molina JR, Yang PG, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shepherd FA, Douillard JY, Blumenschein GR. Immunotherapy for non-small cell lung cancer novel approaches to improve patient outcome. J Thorac Oncol. 2011;6(10):1763–1773. doi: 10.1097/JTO.0b013e31822e28fc. [DOI] [PubMed] [Google Scholar]
- 4.Alberts WM. Diagnosis and management of lung cancer executive summary: ACCP evidence-based clinical practice guidelines (2nd Edition) Chest. 2007;132(3 Suppl):1S–19S. doi: 10.1378/chest.07-1860. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava MK, Bosch JJ, Wilson AL, Edelman MJ, Ostrand-Rosenberg S. MHC II lung cancer vaccines prime and boost tumor-specific CD4+ T cells that cross-react with multiple histologic subtypes of nonsmall cell lung cancer cells. Int J Cancer. 2010;127(11):2612–2621. doi: 10.1002/ijc.25462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68(8):2561–2563. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava MK, Zhu L, Harris-White M, Kar U, Huang M, Johnson MF, Lee JM, Elashoff D, Strieter R, Dubinett S, Sharma S. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One. 2012;7(7):e40677. doi: 10.1371/journal.pone.0040677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116(10):2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166(1):678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava MK, Bosch JJ, Thompson JA, Ksander BR, Edelman MJ, Ostrand-Rosenberg S. Lung cancer patients’ CD4(+) T cells are activated in vitro by MHC II cell-based vaccines despite the presence of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2008;57(10):1493–1504. doi: 10.1007/s00262-008-0490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng PH, Lee KY, Chang YL, Chan YF, Kuo LW, Lin TY, Chung FT, Kuo CS, Yu CT, Lin SM, Wang CH, Chou CL, Huang CD, Kuo HP. CD14+ S100A9+ monocytic myeloid-derived suppressor cells and their clinical relevance in non-small cell lung cancer. Am J Respir Crit Care Med. 2012;186(10):1025–1036. doi: 10.1164/rccm.201204-0636OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, Wu YC, Chu Y, Chung FT, Kuo CH, Lee KY, Lin SM, Lin HC, Wang CH, Yu CT, Kuo HP. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(−)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2010;136(1):35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB. Immunosuppressive CD14+ HLA-DR(low)/- monocytes in B-cell non-Hodgkin lymphoma. Blood. 2011;117(3):872–881. doi: 10.1182/blood-2010-05-283820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Corbelli A, Fais S, Parmiani G, Rivoltini L. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66(18):9290–9298. doi: 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- 17.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor based antitumor vaccine. J Clin Oncol. 2007;25(18):2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 18.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14(+)HLA-DR-/low cells in melanoma patients are Stat3(hi) and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70(11):4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 19.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135(1):234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Vuk-Pavlovic S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao XH, Dietz AB. Immunosuppressive CD14(+)HLA-DRlow/- monocytes in prostate cancer. Prostate. 2010;70(4):443–455. doi: 10.1002/pros.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gustafson MP, Lin Y, New KC, Bulur PA, O’Neill BP, Gastineau DA, Dietz AB. Systemic immune suppression in glioblastoma: the interplay between CD14+ HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010;12(7):631–644. doi: 10.1093/neuonc/noq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chikamatsu K, Sakakura K, Toyoda M, Takahashi K, Yamamoto T, Masuyama K. Immunosuppressive activity of CD14(+) HLA-DR- cells in squamous cell carcinoma of the head and neck. Cancer Sci. 2012;103(6):976–983. doi: 10.1111/j.1349-7006.2012.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan XK, Zhao XK, Xia YC, Zhu X, Xiao P. Increased circulating immunosuppressive CD14(+)HLA-DR-/low cells correlate with clinical cancer stage and pathological grade in patients with bladder carcinoma. J Int Med Res. 2011;39(4):1381–1391. doi: 10.1177/147323001103900424. [DOI] [PubMed] [Google Scholar]
- 24.Brimnes MK, Vangsted AJ, Knudsen LM, Gimsing P, Gang AO, Johnsen HE, Svane IM. Increased level of both CD4+ FOXP3+ regulatory T cells and CD14+ HLA-DR(-)/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand J Immunol. 2010;72(6):540–547. doi: 10.1111/j.1365-3083.2010.02463.x. [DOI] [PubMed] [Google Scholar]
- 25.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7. New York: Springer; 2010. [Google Scholar]
- 26.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40(2):90–97. doi: 10.1053/j.ro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 28.Rebelato E, Mares-Guia TR, Graciano MF, Labriola L, Britto LR, Garay-Malpartida HM, Curi R, Sogayar MC, Carpinelli AR. Expression of NADPH oxidase in human pancreatic islets. Life Sci. 2012;91(7–8):244–249. doi: 10.1016/j.lfs.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber RD, Old LJ, Smyth MJ. Cancer Immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 30.Poschke I, Mao Y, Adamson L, Salazar-Onfray F, Masucci G, Kiessling R. Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol Immunother. 2012;61(6):827–838. doi: 10.1007/s00262-011-1143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu L, Quinn MT, Cross AR, Dinauer MC. Gp91(phox) is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc Natl Acad Sci USA. 1998;95(14):7993–7998. doi: 10.1073/pnas.95.14.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi J, Suh B, Ahn YO, Kim TM, Lee JO, Lee SH, Heo DS. CD15(+)/CD16(low) human granulocytes from terminal cancer patients: granulocytic myeloid-derived suppressor cells that have suppressive function. Tumour Biol. 2012;33(1):121–129. doi: 10.1007/s13277-011-0254-6. [DOI] [PubMed] [Google Scholar]
- 33.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60(10):1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81(3):584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 35.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D’Cruz D, Casanova JL, Trouillet C, Geissmann F. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33(3):375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 37.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, Hilf N, Schoor O, Fritsche J, Mahr A, Maurer D, Vass V, Trautwein C, Lewandrowski P, Flohr C, Pohla H, Stanczak JJ, Bronte V, Mandruzzato S, Biedermann T, Pawelec G, Derhovanessian E, Yamagishi H, Miki T, Hongo F, Takaha N, Hirakawa K, Tanaka H, Stevanovic S, Frisch J, Mayer-Mokler A, Kirner A, Rammensee HG, Reinhardt C, Singh-Jasuja H. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18(8):1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 38.Vollbrecht T, Stirner R, Tufman A, Roider J, Huber RM, Bogner JR, Lechner A, Bourquin C, Draenert R. Chronic progressive HIV-1 infection is associated with elevated levels of myeloid-derived suppressor cells. Aids. 2012;26(12):F31–F37. doi: 10.1097/QAD.0b013e328354b43f. [DOI] [PubMed] [Google Scholar]
- 39.Zhao F, Hoechst B, Duffy A, Gamrekelashvili J, Fioravanti S, Manns MP, Greten TF, Korangy F. S100A9 a new marker for monocytic human myeloid-derived suppressor cells. Immunology. 2012;136(2):176–183. doi: 10.1111/j.1365-2567.2012.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, Zanon A, Rossi CR, Nitti D, Bronte V, Zanovello P. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol. 2009;182(10):6562–6568. doi: 10.4049/jimmunol.0803831. [DOI] [PubMed] [Google Scholar]
- 41.Sinha P, Parker KH, Horn L, Ostrand-Rosenberg S. Tumor-induced myeloid-derived suppressor cell function is independent of IFN-gamma and IL-4Ralpha. Eur J Immunol. 2012;42(8):2052–2059. doi: 10.1002/eji.201142230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Filipazzi P, Huber V, Rivoltini L. Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunol Immunother. 2012;61(2):255–263. doi: 10.1007/s00262-011-1161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tacke RS, Lee HC, Goh C, Courtney J, Polyak SJ, Rosen HR, Hahn YS. Myeloid suppressor cells induced by hepatitis C virus suppress T-cell responses through the production of reactive oxygen species. Hepatology. 2011;55(2):343–353. doi: 10.1002/hep.24700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benedyk M, Sopalla C, Nacken W, Bode G, Melkonyan H, Banfi B, Kerkhoff C. HaCaT keratinocytes overexpressing the S100 proteins S100A8 and S100A9 show increased NADPH oxidase and NF-kappaB activities. J Invest Dermatol. 2007;127(8):2001–2011. doi: 10.1038/sj.jid.5700820. [DOI] [PubMed] [Google Scholar]
- 45.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205(10):2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aurelius J, Thoren FB, Akhiani AA, Brune M, Palmqvist L, Hansson M, Hellstrand K, Martner A. Monocytic AML cells inactivate antileukemic lymphocytes: role of NADPH oxidase/gp91(phox) expression and the PARP-1/PAR pathway of apoptosis. Blood. 2012;119(24):5832–5837. doi: 10.1182/blood-2011-11-391722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.