Abstract
Tumor-infiltrating lymphocytes play an important role in cell-mediated immune destruction of cancer cells and tumor growth control. We investigated the heterogeneity of immune cell infiltrates between primary non-small cell lung carcinomas (NSCLC) and corresponding metastases. Formalin-fixed, paraffin-embedded primary tumors and corresponding metastases from 34 NSCLC patients were analyzed by immunohistochemistry for CD4, CD8, CD11c, CD68, CD163 and PD-L1. The percentage of positively stained cells within the stroma and tumor cell clusters was recorded and compared between primary tumors and metastases. We found significantly fewer CD4+ and CD8+ T cells within tumor cell clusters as compared with the stromal compartment, both in primary tumors and corresponding metastases. CD8+ T cell counts were significantly lower in metastatic lesions than in the corresponding primary tumors, both in the stroma and the tumor cell islets. Of note, the CD8/CD4 ratio was significantly reduced in metastatic lesions compared with the corresponding primary tumors in tumor cell islets, but not in the stroma. We noted significantly fewer CD11c+ cells and CD68+ as well as CD163+ macrophages in tumor cell islets compared with the tumor stroma, but no difference between primary and metastatic lesions. Furthermore, the CD8/CD68 ratio was higher in primary tumors than in the corresponding metastases. We demonstrate a differential pattern of immune cell infiltration in matched primary and metastatic NSCLC lesions, with a significantly lower density of CD8+ T cells in metastatic lesions compared with the primary tumors. The lower CD8/CD4 and CD8/CD68 ratios observed in metastases indicate a rather tolerogenic and tumor-promoting microenvironment at the metastatic site.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1768-3) contains supplementary material, which is available to authorized users.
Keywords: Non-small cell lung cancer, Primary tumor, Metastasis, Immune cells, Anti-tumor immunity
Introduction
Cancer is a disease characterized by a complex network of interactions between different cell types. It is well established that tumors do not only consist of neoplastic cells but also involve non-cancerous cells such as stromal cells (e.g., fibroblasts), the neovasculature and a variety of tumor-infiltrating immune cells as well as products of these cells, such as cytokines, growth factors and metabolites [1]. The role of tumor-infiltrating immune cells has been a matter of debate for many decades [2–4]. While immune cells may initiate anti-tumor immune responses, which may lead to tumor eradication or control, some immune cells, such as tumor-associated macrophages, mast cells and regulatory T cells have been shown to be rather tolerogenic, thereby promoting tumor growth, angiogenesis and metastasis [3, 5–7]. M1 macrophages characterized by the expression of nitric oxide synthase 2 and proinflammatory cytokines harbor anti-tumoral activity, whereas M2 macrophages support tumor progression by producing pro-angiogenic and growth factors as well as by direct dampening of anti-tumor immunity [8]. An in-depth characterization and subtyping of the different tumor-infiltrating immune cells are clearly warranted to allow for a better understanding of the distinct prognostic and predictive values of individual cell types. In colon cancer, a favorable clinical outcome was associated with a coordinated Th1 polarization and cytotoxic T cell infiltration. In contrast, a low density of T cells was associated with a poor prognosis [9, 10]. Importantly, utilizing a network model representing the entire tumor microenvironment allowed interrogating dynamic networks in the three-dimensional immune landscape along with tumor progression and recurrence [11]. In non-small cell lung cancer (NSCLC), a higher number of tumor-infiltrating CD8+ T cells, natural killer cells and/or dendritic cells have been associated with improved patient survival [12–17]. Along the same [8] line, the frequency of regulatory T cells has recently been shown to be an independent prognostic factor [18].
T cell-targeted immunotherapies, such as antibodies against the co-inhibitory receptors CTLA-4 and PD-1, have demonstrated a statistically significant improvement in overall survival in patients with advanced melanoma [19–21]. Recently, the PD-1 blocking therapeutic antibody nivolumab was shown to improve overall survival in lung adenocarcinoma compared with standard second-line chemotherapy with docetaxel [22]. In addition to their promising therapeutic activity, PD-1/PD-L1 blocking antibodies displayed a favorable safety profile [21, 23, 24]. These clinical successes highlight the potential of immune- and in particular T cell-targeted therapies in oncology.
In order to better understand the cellular mechanisms underlying cancer biology, and to further improve the clinical benefit of patients treated with this novel class of immunotherapeutics, it is crucial to better understand the relative contribution of T cells to anti-tumor immunity in different tumor types. Moreover, we need to delineate the differential contribution and distribution of these cells in the primary tumor, but, equally important, at metastatic deposits. In patients with advanced cancer, immunotherapy also targets metastatic disease, which ultimately determines the clinical outcome.
Lung cancer is the leading cause of cancer-related death. About 85 % of lung cancers are NSCLC. At the time of diagnosis, most patients have distant metastases [7, 25]. The outcome of patients with NSCLC is mainly dependent on the extent of metastatic spread. Tumor metastasis is a complex process and characterized by different steps. During tumor cell selection, and in particular during metastasis, cancer cells undergo phenotypic changes through a process termed clonal evolution. In this context, it is important to realize that cancer cells do not evolve as an isolated entity, but in a continuous, bidirectional interaction with the host immune system, both in treatment-naïve patients as well as patients receiving therapy. The immune system preferentially destroys highly immunogenic tumor cells, thereby forcing the selection of less immunogenic tumor cell variants [26–30]. It is particularly important to understand the role of immune cells during tumor progression for patients treated with novel immunotherapies, which recruit the host immune system/require host immune effector cells to be effective. In line with these findings, several well-established chemotherapeutic agents [31–33] and more recently also cytotoxic payloads of antibody drug conjugates [34, 35] have been found to mediate their anti-tumor effect, at least in part, by (re-)activating anticancer immunity.
There is clear evidence that tumor cells derived from the primary tumor and metastatic sites display molecularly distinct characteristics [36, 37]. However, our understanding of the intra- and inter-tumoral heterogeneity between primary NSCLCs tumors and corresponding metastatic lesions with regard to tumor-infiltrating immune cells is poor. This is surprising owing to the prominent role of the immune system in combating cancer as well as the prognostic value of tumor metastasis in NSCLC.
To better understand differences in immune response mechanisms between primary NSCLC lesions and corresponding metastases, we investigated whole sections of the primary tumors and the corresponding metastases from NSCLC patients for site-specific immune cell infiltration. In the light of the arrival of immune checkpoint blocking antibodies in the clinical arena, this is of particular clinical interest, as these therapies critically require the presence of effector T cell at the targeted tumor site.
Materials and methods
Study population
Thirty-four consecutive NSCLC diagnosed between 1998 and 2012 with available resection specimens from both the primary tumor and the corresponding metastases were retrospectively analyzed. The specimens were retrieved from the Institutes of Pathology University Hospital Basel (n = 14) and the Cantonal Hospital Lucerne (n = 20). A pathologist (Spasenija Savic) reviewed all tumor specimens for tissue adequacy and histology.
The 34 primary tumors were derived from 18 lobectomies, nine pneumonectomies, five wedge resections and two bilobectomies. The 34 metastases were derived from the lung (n = 11, 32 %), brain (n = 10, 29 %), liver (n = 3, 9 %), distant lymph nodes (n = 2, 6 %), kidney (n = 2, 6 %), bone (n = 2, 6 %), pleura parietalis (n = 2, 6 %) as well as omentum majus and thyroid (n = 1, 2.5 % each). All lung metastases occurred metachronously and showed the identical histology as the corresponding primary tumors.
Tumor tissues were fixed in 4 % neutral-buffered formalin, paraffin-embedded and stained with hematoxylin eosin and alcian blue periodic acid–Schiff according to routine procedures. Histological classification and stage of the tumors were reassessed based on the 2004 World Health Organization Classification, and the 7th edition of the tumor lymph node metastasis stage (TNM) classification of malignant tumors, respectively [38].
This study was conducted with the approval of the local ethics committee (approval number: 84/11).
Immunohistochemistry (IHC)
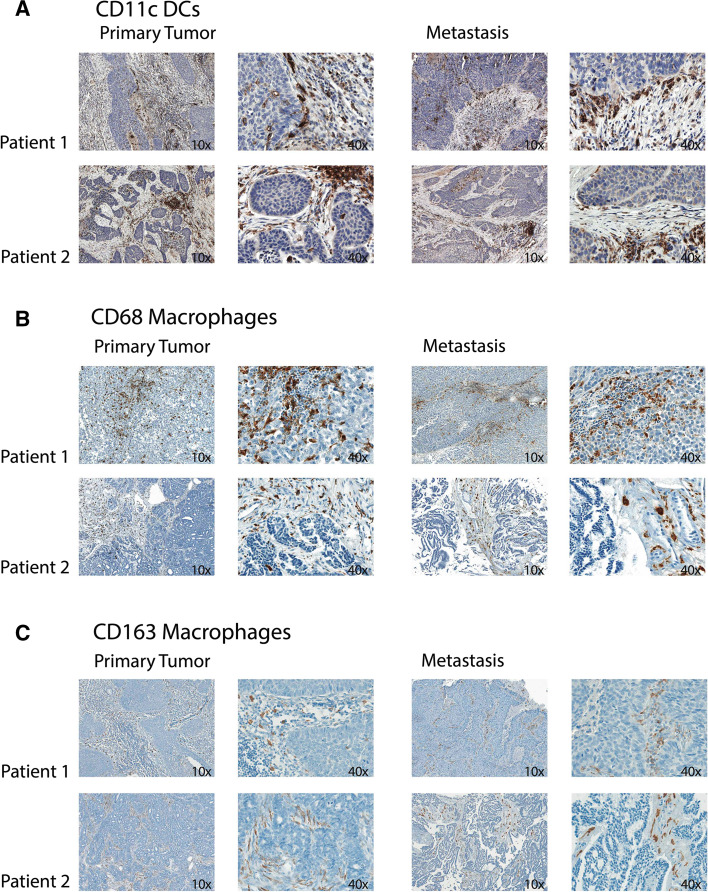
One representative formalin-fixed and paraffin-embedded tumor block each from the primary tumor and the corresponding metastasis was selected for immunohistochemical analyses. The tissue was cut to 4-μm sections and deparaffinized according to routine procedures. IHC was conducted using pre-diluted CD4 (Ventana 790-4423 clone; pretreatment: CC1 16 min; incubation time: 12 min), CD8 (Ventana 790-4460 clone; pretreatment: CC1 16 min; incubation time: 12 min), CD11c (Leica PA0554 clone; pretreatment: 32 min; incubation time: 24 min), CD68 (DAKO IR613 clone; pretreatment: 16 min; incubation time: 12 min), CD163 (Ventana 760-4437 clone; pretreatment: CC1 16 min; incubation time: 16 min) and PD-L1 (Cell Signaling 13684 clone; pretreatment: CC1 24 min; incubation time: 32 min) monoclonal antibodies on the automated immunostainer Benchmark XT (Roche/Ventana Medical Systems, Tucson, USA) with 3,3′-diaminobenzidine as chromogen. IHC protocols were performed according to the recommendations of the respective manufacturer with minor modifications. The CD11c staining protocol did not work on the tumor collective from Lucerne, most likely due to preanalytic reasons. For this reason, only the tumor sections from Basel could be analyzed and included in Fig. 4 for the CD11c staining evaluation. All antibodies have previously been established; tonsillary tissue has been used as positive control.
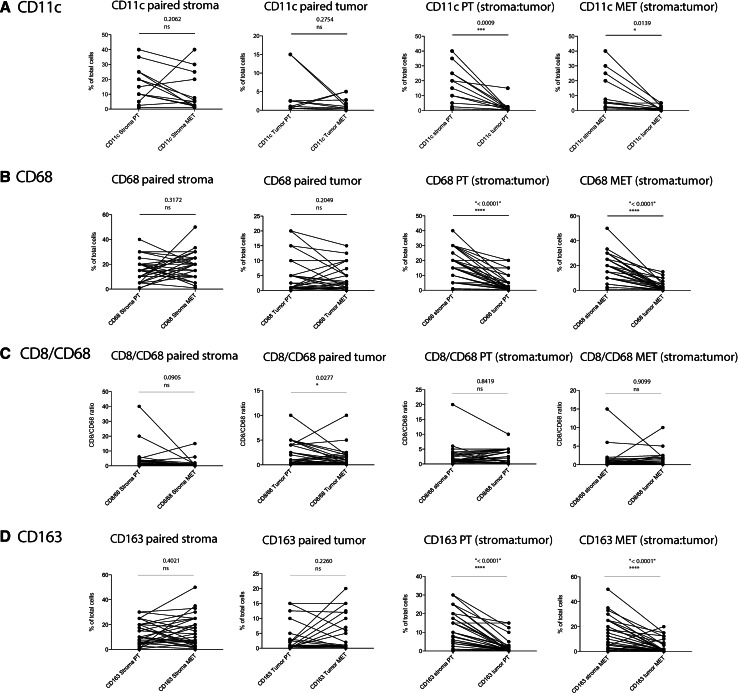
Fig. 4.
Quantitative comparison of tumor-infiltrating CD11c+ dendritic cells and CD68+ and CD163+ macrophages, respectively, on large tumor sections as depicted in Fig. 3. A paired analysis of CD11c-positive cells (a), CD68-positive cells (b), the CD8-to-CD68 ratio (c), and CD163-positive cells (d) was performed. From left to right: paired stroma [comparison of infiltrates within the tumor stroma between primary tumor (PT) and metastatic site (MET)]; paired tumor (comparison of infiltrates within the tumor islets between primary tumors and metastatic sites); PT (stroma:tumor) (primary tumor: comparison of infiltrates between the tumor stroma and the tumor islets); MET (stroma:tumor) (metastatic site: comparison of infiltrates between the tumor stroma and the tumor islets). IHC positive events have been depicted as % of total nucleated cells; paired two-sample t test was used to compare paired samples (n = 34 for CD8 and CD68 staining; n = 14 for CD11c staining)
Immunohistochemical evaluation
Stained tumor sections were randomly assembled and evaluated by a pathologist (Spasenija Savic) together with a biologist (Philipp Müller). The evaluation was blinded to the tumor origin (primary tumor vs metastasis). The analysis was done in a semiquantitative manner on whole tumor sections. The percentage of positively stained inflammatory cells, relative to the total number of nucleated cells within the stroma or the tumor cell clusters, were noted for each tumor. Data are presented as the mean and standard error of the mean.
Statistical analysis
Statistical analyses of matched primary tumors and corresponding metastatic lesions were performed using a Student’s paired two-sample t test.
Results
Clinical characteristics
Most patients were male (n = 21, 61.8 %) and had an adenocarcinoma (n = 20, 58.8 %). Fourteen out of 34 patients (41.2 %) had an early stage I disease at time of primary tumor resection. The median time between initial surgery of the primary tumors and resection of the metastases was 19 month (range 5–96 months). The longest interval was in a patient suffering from a metastatic adenocarcinoma who had an excision biopsy of a brain metastasis 96 months after surgical removal of his stage IIIA primary lung carcinoma. Clinicopathologic characteristics are summarized in Supplementary Table 1.
CD4 and CD8 positive T cells
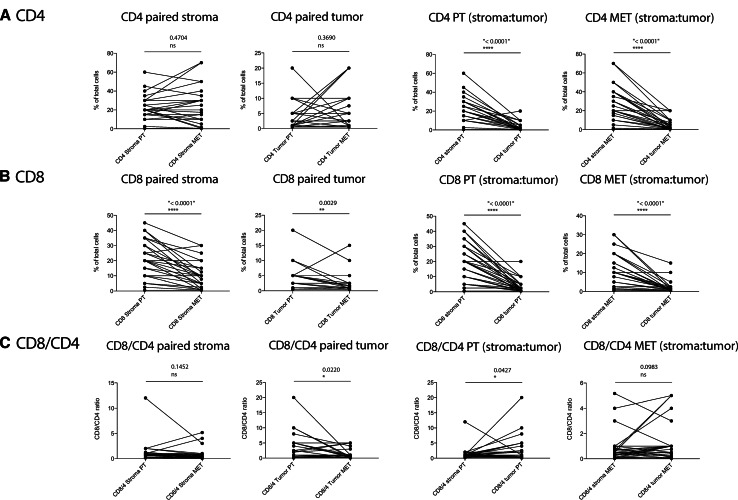
In a first step, we quantitatively compared tumor-infiltrating CD4+ and CD8+ T cells as well as the CD8 to CD4 (contains regulatory T cells) T cell ratio, which is often used as a measure to assess the activation state of anti-tumor immunity [39, 40], in primary tumors and matched metastatic lesions. We further discriminated T cells infiltrating the tumor stroma and tumor cell clusters (islets) within a given malignant lesion [9]. Figure 1 shows two examples from representative patients (panel A: CD4 cells; panel B: CD8 cells). We did not find a significant difference in the frequency of infiltrating CD4+ T cells between primary tumors and metastatic lesions for either the stromal infiltrates or tumor cell islets (Table 1; Fig. 2a). When comparing CD8+ T cells between primary tumors and metastatic lesions, we noted a significant reduction in these cells at the metastatic site both in the stroma and in the tumor islets (Table 1; Fig. 2b). A significantly lower number of CD4+ as well as CD8+ T cells were found within tumor cell islets as compared with the stromal compartment, both in primary tumors as well as the corresponding metastatic lesions.
Fig. 1.
Representative images of immunohistochemical staining results. NSCLC tissue sections from the primary tumor as well as the corresponding metastases were stained for CD4 (a) or CD8 (b). Tumor sections from two independent patients with CD4+ and CD8+ T cell infiltrates are shown (original magnification ×10 and ×40)
Table 1.
Mean percentage of cells staining positively for immune cell markers in primary tumors and matched metastases
| Primary tumor | Metastases | P value (tumor cell islets: primary tumor vs metastases) | P value (stroma: primary tumor vs metastases) | |||
|---|---|---|---|---|---|---|
| Tumor cell islets | Stroma | Tumor cell islets | Stroma | |||
| CD4+ cells | 3.24 (0.67) | 23.09 (1.97) | 4.30 (0.98) | 24.79 (3.39) | 0.3690 | 0.4704 |
| P value | <0.0001 | <0.0001 | ||||
| CD8+ cells | 3.77 (0.68) | 20.37 (1.91) | 1.88 (0.49) | 10.15 (1.39) | 0.0029 | <0.0001 |
| P value | <0.0001 | <0.0001 | ||||
| CD8/4 ratio | 2.84 (0.68) | 1.23 (0.33) | 1.17 (0.24) | 0.79 (0.18) | 0.022 | 0.1452 |
| P value | 0.0427 | 0.0983 | ||||
| CD11c+ cells | 3.27 (1.46) | 16.81 (3.36) | 1.50 (0.47) | 11.33 (3.59) | 0.2754 | 0.2062 |
| P value | 0.0009 | 0.0139 | ||||
| CD68+ cells | 4.07 (0.94) | 16.04 (1.70) | 3.11 (0.66) | 18.34 (1.76) | 0.2049 | 0.3172 |
| P value | <0.0001 | <0.0001 | ||||
| CD8/68 ratio | 2.40 (0.43) | 3.33 (1.22) | 1.32 (0.36) | 1.12 (0.44) | 0.0277 | 0.0905 |
| P value | 0.8419 | 0.9099 | ||||
| CD163+ cells | 2.33 (0.74) | 12.26 (1.53) | 3.5 (0.96) | 14 (2.12) | 0.2260 | 0.4021 |
| P value | <0.0001 | <0.0001 | ||||
Data are the mean (standard error of the mean) percentage of positively staining cells out of the total nucleated cell count
Fig. 2.
Quantitative comparison of tumor-infiltrating CD4+ and CD8+ T cells on large tumor sections as depicted in Fig. 1 showing relationships in individual patients. A paired analysis of CD4-positive cells (a), CD8-positive cells (b), and the CD8-to-CD4 ratio (c) was performed. From left to right: paired stroma [comparison of infiltrates within the tumor stroma between primary tumor (PT) and metastatic site (MET)]; paired tumor (comparison of infiltrates within the tumor islets between primary tumors and metastatic sites); PT (stroma:tumor) (primary tumor: comparison of infiltrates between the tumor stroma and the tumor islets); MET (stroma:tumor) (metastatic site: comparison of infiltrates between the tumor stroma and the tumor islets). IHC positive events have been depicted as % of total nucleated cells; paired two-sample t test was used to compare paired samples (n = 34)
Compared with primary tumors, the CD8-to-CD4 ratio was significantly reduced in metastatic lesions for T cells infiltrating tumor islets but not for those infiltrating the stroma (Table 1; Fig. 2c). When comparing T cell infiltration within the tumor stroma to the infiltrates in tumor islets, we found a weak but significant increase in the CD8-to-CD4 ratio in the tumor islets of primary tumor lesions as compared with the corresponding stromal compartment.
Dendritic cells
In the panel A of Fig. 3, two representative examples of CD11c IHC staining are shown. We could not find a statistically significant difference in tumor-infiltrating CD11c+ dendritic cells between primary tumors and metastatic lesions, neither for cells infiltrating the stroma nor for those infiltrating tumor cell islets (Table 1; Fig. 4a). However, again there was a significantly higher density of CD11c-positive cells in the tumor stroma as compared with the corresponding tumor cell islets, both in primary tumors as well as metastases.
Fig. 3.
Representative images of immunohistochemical staining results. NSCLC tissue sections from the primary tumor as well as the corresponding metastatic site were stained for CD11c (a), CD68 (b) or CD163 (c). Tumor sections from two independent patients with CD11c+ dendritic cell (DC) and CD68+ and CD163+ macrophage infiltrates are shown (original magnification ×10 and ×40)
Macrophages and CD8 T cell-to-macrophage ratio
Panel B of Fig. 3 depicts representative CD68 IHC staining from two patients for macrophages in the primary tumors and the corresponding metastatic lesions. When comparing primary tumors to the corresponding metastases, we could not find a significant difference regarding the abundance of CD68+ cells (Table 1; Fig. 4b). As also shown for other tumor-infiltrating immune cells, macrophages were found in significantly higher numbers in the stromal regions as compared with tumor cell islets in both primary tumors and metastases.
As CD68-positive tumor-associated macrophages have been associated with a rather tolerogenic and therefore immunosuppressive state [8, 41, 42], we also assessed the CD8-to-CD68 ratio. The only significant difference that we observed was between the tumor islets of the primary tumors and the corresponding metastatic lesions, with the CD8-to-CD68 ratio being higher in the primary tumor (Table 1; Fig. 4c).
The scavenger receptor CD163 is expressed on M2 macrophages that are associated with tumor progression and immune tolerance. Panel C of Fig. 3 shows representative CD163 IHC staining from two patients. CD163 staining showed a similar distribution as CD68. We detected a significantly lower rate of CD163 positivity in tumor cell islets compared with the tumor stroma. Comparing primary tumors to metastatic lesions, we could not detect a statistically significant difference for the infiltration of CD163-positive cells (Table 1; Fig. 4d).
Programmed cell death ligand 1 (PD-L1)
Figure 5a shows two representative samples of PD-L1 IHC staining from primary tumors and the corresponding metastasis. Comparing primary tumors and corresponding metastases revealed no significant difference in PD-L1 positivity of tumor or myeloid cells (Fig. 5b).
Fig. 5.
a Representative images of immunohistochemical staining results. NSCLC tissue sections from the primary tumor as well as the corresponding metastatic site were stained for PD-L1. Tumor sections from two independent patients with PD-L1-positive tumor cells and PD-L1-positive tumor-infiltrating myeloid cells are shown (original magnification ×10 and ×40). b Quantitative comparison of PD-L1 expression on tumor and myeloid cells on large tumor sections as depicted in a of this figure. A paired analysis of PD-L1-positive tumor cells and PD-L1-positive myeloid cells in primary tumors and metastatic lesions was performed. IHC positive events have been depicted as % of total nucleated cells; paired two-sample t test was used to compare paired samples (n = 34)
Results are summarized in Table 1 and Supplementary Fig. 1.
Discussion
The increasing knowledge and in-depth understanding of the role that tumor-infiltrating immune cells, and in particular T cells, play during tumor development have revolutionized our view of cancer and paved the road for new immune-directed treatment strategies. It is well established that tumor-infiltrating immune cells are heterogeneously distributed between tumor types, and show a wide inter-patient variability. Their prognostic significance has been demonstrated for different tumor entities [43–45]. Recently, B cell density has been established as a new prognostic marker in NSCLC patients demonstrating the existence of a protective B cell-mediated immunity [46]. It has further been shown that the immune composition within malignant lesions may even predict the therapeutic efficacy of specific chemotherapeutic drugs in colorectal and breast cancer [47, 48]. However, though the presence of distinct immune cell populations within tumors can serve as a prognostic or even predictive marker in some tumor entities, there are additional important parameters, such as location, density, and functional differentiation, which are crucial for effective anti-tumor immunity. The global immune composition of tumors has recently been termed the “Tumor Immunome” [11, 49–51].
To the best of our knowledge, our study is the first to investigate differences regarding tumor-infiltrating immune cells between primary tumors and the matched metastatic lesions from NSCLC patients. Furthermore, we compared the frequencies of different immune cell subsets in the tumor stroma to that in tumor cell islets.
Our results show that the number of T cells, dendritic cells and macrophages is significantly higher in the tumor stroma as compared with the tumor cell islets, which is also true for M2 macrophages. This is in line with the results from previous studies and may reflect the difficulty that immune cells which have been attracted to the tumor site have overcoming the physical and endothelial barriers in order to penetrate into tumor cell islets [52]. We found a significantly higher density of CD8+ T cells, in both compartments of primary tumors as compared with the corresponding metastatic lesions. This observation may have important implications for novel immunotherapeutic interventions in patients with metastatic versus localized disease, as therapies such as PD-1 blockade specifically target these effector T cells. PD-L1 is expressed in several solid tumors and seems to be associated with worse prognosis in NSCLC [53]. In our study, the expression of PD-L1 on tumor cells or tumor-infiltrating lymphocytes is not different between primary tumors and metastatic lesions. However, the significance of PD-L1 positivity as predictive marker for immune checkpoint inhibitors targeting PD-1/PD-L1 has not been clarified so far. Interestingly, it has been shown that preexisting CD8+ T cells distinctly located at the invasive tumor margin may predict response to anti-PD-1 therapy and are associated with expression of the PD-1/PD-L1 immune inhibitory axis [54]. Different immunophenotypes of human cancer have been correlated with cytokine/chemokine profiles and may require a different type of therapeutic intervention and/or pretreatment to increase the number of effector T cells infiltrating the malignant lesions, to facilitate immunotherapeutic interventions [55, 56]. Furthermore, a higher CD8-to-CD4 ratio was detected in the tumor cell islets of primary tumors as compared with the tumor cell islets of the corresponding metastatic lesions. No significant differences were detected in the tumor stroma. However, there are no significant differences for CD4+ T cells located in primary tumor and metastases, respectively. The CD8-to-CD4 ratio was further elevated within the primary tumor islets as compared with the primary tumor stroma. Regulatory T cells (CD4+) play a key role in the suppression of anti-tumor immunity by dampening cytotoxic T lymphocyte responses (mostly CD8+ T cells) [57]. The reduced CD8-to-CD4 ratio in tumor islets at the metastatic site may therefore indicate a rather tolerogenic local immune environment, which hampers anti-tumor immunity and favors tumor growth [39, 40]. We demonstrated a similar correlation for the CD8-to-CD68 ratio with a lower ratio in metastatic lesions, which exhibited a significant difference in the tumor cell islets only. As tumor-associated macrophages are generally associated with local immune suppression [41, 42, 58], these findings further support a scenario that the metastatic deposits represent functionally different and rather tolerogenic sites as compared with the corresponding primary tumors.
In conclusion, this study confirms previous reports that T cells, dendritic cells and macrophages are found at higher frequencies within the tumor stroma of NSCLC lesions as compared with the adjacent tumor cell islets. For the first time, we show a distinct pattern of immune cell infiltration in primary tumors versus matched metastatic lesions. Further studies using independent cohorts of patients will have to confirm our findings and prove the potential prognostic value of these different, local immune microenvironments in primary tumors and metastases. The different immune profiles observed in primary and metastatic tumors indicate that immunotherapies, which require high numbers of functional effector T cells (CD8+) to be effective, would be more likely to control the growth of the primary tumor mass but not metastatic deposits. Our findings may therefore have important implications for novel immune-based therapies targeting T cells, such as the recently approved PD-1 blockade. They may further be crucial for designing personalized combination immunotherapy treatment regimens that are optimized depending on the immune phenotype of the metastatic sites.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by grants from the Swiss National Science Foundation, the Wilhelm Sander-Foundation, the Cancer League Basel, the Huggenberger-Bischoff Foundation for Cancer Research, the Research Fonds of the University Basel and the Freiwillige Akademische Gesellschaft Basel.
Abbreviation
- NSCLC
Non-small cell lung cancer
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Philipp Müller and Sacha I. Rothschild have contributed equally to this work.
Spasenija Savic and Alfred Zippelius have contributed equally to this work.
Contributor Information
Philipp Müller, Email: ph.mueller@unibas.ch.
Alfred Zippelius, Phone: +41 61 265 50 74, Email: alfred.zippelius@usb.ch.
References
- 1.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin A, Schildknecht A, Nguyen LT, Ohashi PS. Dendritic cells integrate signals from the tumor microenvironment to modulate immunity and tumor growth. Immunol Lett. 2010;127:77–84. doi: 10.1016/j.imlet.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Chen JJ, Lin YC, Yao PL, Yuan A, Chen HY, Shun CT, Tsai MF, Chen CH, Yang PC. Tumor-associated macrophages: the double-edged sword in cancer progression. J Clin Oncol. 2005;23:953–964. doi: 10.1200/JCO.2005.12.172. [DOI] [PubMed] [Google Scholar]
- 4.Loose D, Van de Wiele C. The immune system and cancer. Cancer Biother Radiopharm. 2009;24:369–376. doi: 10.1089/cbr.2008.0593. [DOI] [PubMed] [Google Scholar]
- 5.Audia S, Nicolas A, Cathelin D, et al. Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes. Clin Exp Immunol. 2007;150:523–530. doi: 10.1111/j.1365-2249.2007.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theoharides TC, Conti P. Mast cells: the Jekyll and Hyde of tumor growth. Trends Immunol. 2004;25:235–241. doi: 10.1016/j.it.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Gavert N, Ben-Ze’ev A. Epithelial-mesenchymal transition and the invasive potential of tumors. Trends Mol Med. 2008;14:199–209. doi: 10.1016/j.molmed.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 9.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 10.Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 11.Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 13.Dieu-Nosjean MC, Antoine M, Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 14.Hiraoka K, Miyamoto M, Cho Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhuang X, Xia X, Wang C, Gao F, Shan N, Zhang L, Zhang L. A high number of CD8+ T cells infiltrated in NSCLC tissues is associated with a favorable prognosis. Appl Immunohistochem Mol Morphol. 2010;18:24–28. doi: 10.1097/PAI.0b013e3181b6a741. [DOI] [PubMed] [Google Scholar]
- 16.Takanami I, Takeuchi K, Giga M. The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. 2001;121:1058–1063. doi: 10.1067/mtc.2001.113026. [DOI] [PubMed] [Google Scholar]
- 17.Villegas FR, Coca S, Villarrubia VG, Jiménez R, Chillón MJ, Jareño J, Zuil M, Callol L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–28. doi: 10.1016/S0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa T, Suzuki H, Yamaura T, et al. Prognostic value of peripheral and local forkhead box P3 regulatory T cells in patients with non-small-cell lung cancer. Mol Clin Oncol. 2014;2:685–694. doi: 10.3892/mco.2014.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 21.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2014;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 22.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 26.Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982;217:998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- 27.Talmadge JE, Wolman SR, Fidler IJ. Evidence for the clonal origin of spontaneous metastases. Science. 1982;217:361–363. doi: 10.1126/science.6953592. [DOI] [PubMed] [Google Scholar]
- 28.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 30.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 31.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 32.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 33.Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 34.Müller P, Martin K, Theurich S, et al. Microtubule-depolymerizing agents used in antibody-drug conjugates induce antitumor immunity by stimulation of dendritic cells. Cancer Immunol Res. 2014;2:741–755. doi: 10.1158/2326-6066.CIR-13-0198. [DOI] [PubMed] [Google Scholar]
- 35.Martin K, Müller P, Schreiner J, Prince SS, Lardinois D, Heinzelmann-Schwarz VA, Thommen DS, Zippelius A. The microtubule-depolymerizing agent ansamitocin P3 programs dendritic cells toward enhanced anti-tumor immunity. Cancer Immunol Immunother. 2014;63:925–938. doi: 10.1007/s00262-014-1565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Guo N, Yu C, Wang H, Zhang Y, Xia H, Yu J, Lu J. Differential expression of ERCC-1 in the primary tumors and metastatic lymph nodes of patients with non-small cell lung cancer adenocarcinoma. Tumour Biol. 2012;33:2209–2216. doi: 10.1007/s13277-012-0482-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimizu K, Yukawa T, Hirami Y, Okita R, Saisho S, Maeda A, Yasuda K, Nakata M. Heterogeneity of the EGFR mutation status between the primary tumor and metastatic lymph node and the sensitivity to EGFR tyrosine kinase inhibitor in non-small cell lung cancer. Target Oncol. 2013;8:237–242. doi: 10.1007/s11523-012-0241-x. [DOI] [PubMed] [Google Scholar]
- 38.Tanoue LT, Detterbeck FC. New TNM classification for non-small-cell lung cancer. Expert Rev Anticancer Ther. 2009;9:413–423. doi: 10.1586/era.09.11. [DOI] [PubMed] [Google Scholar]
- 39.Ries CH, Cannarile MA, Hoves S, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 41.Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res. 2012;4:376–389. [PMC free article] [PubMed] [Google Scholar]
- 42.Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, Pamer EG, Li MO. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–160. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 44.Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 45.Zitvogel L, Kepp O, Aymeric L, Ma Y, Locher C, Delahaye NF, André F, Kroemer G. Integration of host-related signatures with cancer cell-derived predictors for the optimal management of anticancer chemotherapy. Cancer Res. 2010;70:9538–9543. doi: 10.1158/0008-5472.CAN-10-1003. [DOI] [PubMed] [Google Scholar]
- 46.Germain C, Gnjatic S, Tamzalit F, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189:832–844. doi: 10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- 47.Halama N, Michel S, Kloor M, et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71:5670–5677. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 48.Ladoire S, Mignot G, Dabakuyo S, et al. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol. 2011;224:389–400. doi: 10.1002/path.2866. [DOI] [PubMed] [Google Scholar]
- 49.Galon J, Fridman WH, Pagès F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 2007;67:1883–1886. doi: 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- 50.Angell H, Galon J. From the immune contexture to the immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25:261–267. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 51.Restifo NP. A “big data” view of the tumor “immunome”. Immunity. 2013;39:631–632. doi: 10.1016/j.immuni.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beyer I, van Rensburg R, Lieber A. Overcoming physical barriers in cancer therapy. Tissue Barriers. 2013;1:e23647. doi: 10.4161/tisb.23647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang A, Wang HY, Liu Y, Zhao MC, Zhang HJ, Lu ZY, Fang YC, Chen XF, Liu GT. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol. 2015;41:450–456. doi: 10.1016/j.ejso.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 54.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bald T, Landsberg J, Lopez-Ramos D, et al. Immune cell-poor melanomas benefit from PD-1 blockade after targeted type I IFN activation. Cancer Discov. 2014;4:674–687. doi: 10.1158/2159-8290.CD-13-0458. [DOI] [PubMed] [Google Scholar]
- 57.Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, Kaiser LR, June CH. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 58.Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol. 2011;41:2522–2525. doi: 10.1002/eji.201141894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.