Abstract
The modulation and suppression of anti-tumor immune responses is a characteristic feature of tumor cells to escape immune surveillance. Members of the B7 family are involved in this process, since the level of activation of the anti-tumor immune response depends on the balance between co-stimulatory and co-inhibitory signals. Some molecules are often overexpressed in tumors, which has been associated with the pathogenesis and progression of malignancies as well as their immunological and non-immunological functions. The B7 homologs play a key role in the maintenance of self-tolerance and the regulation of both innate and adaptive immunity in tumor-bearing hosts. Furthermore, the blockade of negative signals mediated by the interaction of co-inhibitory ligands and counter-receptors of the B7 family is currently being studied as a potential immunotherapeutic strategy for the treatment of cancer in humans.
Keywords: Cancer, Co-stimulation, B7 family, PIVAC11, Therapy
Co-stimulatory signaling plays an important role in the initiation and termination of immune cell responses by regulating the T cell priming, growth, maturation, and tolerance. Effective activation of naïve T cells requires two signals: The first is mediated by the recognition of an antigen presented via the major histocompatibility complex (MHC) on antigen-presenting cells (APC) by a corresponding antigen-specific T cell receptor (TCR) and the second is provided by the delivery of a co-stimulatory signal by binding of co-stimulatory molecules to their receptors.
The co-stimulatory pathways have recently gained interest and have been extensively investigated, since they represent elegant strategies to modulate T cell responses in autoimmune diseases, viral infections, and cancers, which are currently therapeutically exploited. In the absence of co-stimulation, the contact between the T cell receptor (TCR) and the human leukocyte antigen (HLA)-presented antigen will result in dysfunction or anergy of T cells, creating an immunological antigen-specific tolerance. In addition, some classical co-inhibitory molecules are also expressed on immune cell populations and may contribute to the escape of tumors to T cell response.
Therefore, it is of interest to understand the balance of co-stimulatory and co-inhibitory molecules, which provide attractive targets to modulate T cell-dependent immune responses in various diseases. In addition, beyond mere co-stimulation/co-inhibition, several components of these pathways can provoke cellular responses without the requirement of antigen presentation and even on a variety of immune cells that may modulate the course of the disease. Augmenting specific anti-tumor T cell responses by blocking the co-inhibitory signals might release the brakes on immune responsiveness leading to tumor elimination.
The following review will therefore focus on the T cell-dependent and -independent involvement of the major co-stimulatory/co-inhibitory components of the increasing group of B7 family members and their important downstream signaling pathways in the pathogenesis of tumors as well as highlights their potential for the treatment of cancer.
The characteristics of the B7 family
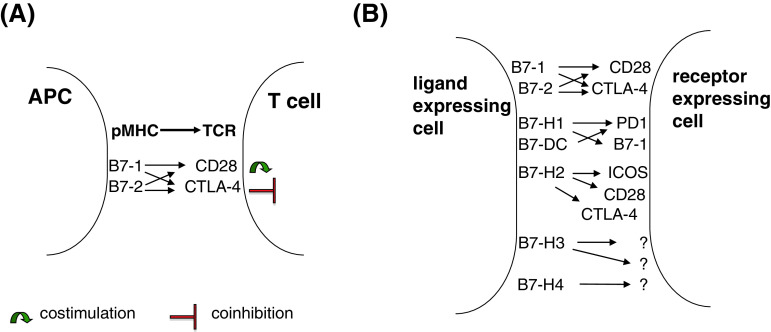
The best characterized co-stimulatory pathway includes members of the growing B7-CD28 superfamily, which are involved in both co-stimulatory and co-inhibitory processes. The B7 superfamily comprises the receptor ligand pairs CD28/CTLA4:B7-1 (CD80)/B7-2 (CD86), ICOS:ICOS-L; PD1:PD-L1/PD-L2; B7-H6:NKp30 [1–6].
For the B7-H3 and B7-H4 (also known as B7x or B7S1) molecules, no human receptors have been so far identified (Fig. 1) [7, 8]. The processes that are initiated upon interaction of the specific receptors with their respective ligands play important roles in the regulation of T cell activation or in the induction of tolerance. These different pathways not only provide critical positive secondary signals that produce and sustain T cell responses, but also contribute to negative signals. It has been suggested that the negative regulation is critical for immune homeostasis and that the interaction of co-inhibitory receptors with their respective ligands induces a negative feedback regulation after the activation of T cells. Thus, they can modulate immune responses by limiting, terminating, and/or attenuating T cell responses and induce either anti-tumor responses or tumor immune escape mechanisms. In general, B7-H molecules primarily execute their functions in peripheral tissues to attenuate immune responses in target organs/tissues [9].
Fig. 1.
Co-stimulatory and co-inhibitory molecules of the B7 family and their receptors. a The classical view of co-stimulation: the first signal originates from pMHC–TCR binding of APC and T cells, and the second signal is provided by co-stimulatory molecules of the B7 family that tune the immune response by generating either co-stimulatory (B7-1/2-CD28 interaction) or co-inhibitory signals (B7-1/2-CTLA-4 interaction) to T cells. b Extension of the classical view: the recently identified, novel B7 family members are widely expressed on different immune cells and/or on cells of non-hematopoietic origin. Within immune cell populations, the B7 family member = ligand expressing cells are not only professional APC (like DC, monocytes, and macrophages), but also B cells, T cells, NK cells, and mast cells. In addition, also mesenchymal stem cells and cells of non-hematopoietic origin do constitutively express some of these molecules. B7 family members are membrane-bound molecules (soluble forms of B7-H1, -H-2, -H-3, and -H-4 exist) that exert their biological function by binding their respective receptors. Receptor-expressing cells are mainly CD4+ and CD8+ T cells, but also B cells, NK cells as well as cells of myeloid origin.
The prototype of co-stimulatory molecules is represented as the B7-1 and B7-2 molecules, which have been described more than two decades ago [10, 11]. Upon binding to their receptor CD28, T cell activation and survival is promoted, while in the absence of the co-stimulatory signal, the ligation of the TCR with the HLA/peptide antigen results in T cell dysfunction and anergy. In addition to the positive co-stimulatory signal augmenting and sustaining T cell responses, co-inhibitory signals could be delivered, which down-regulate T cell activity. Binding of B7-1 or B7-2 molecules to their inhibitory receptor, the cytotoxic T lymphocyte-associated antigen 4 (CTLA4) inhibits T cell responses by blocking IL-2 synthesis and cell cycle progression thereby inducing peripheral tolerance. Thus, CTLA4 constitutes a T cell-intrinsic control mechanism as it competes with CD28 for B7-1 and B7-2 without activating T cells.
In contrast to the defined roles of B7-1 and B7-2 in T cell activation, the function of the novel members of the B7 family has not yet been well characterized. The inducible co-stimulatory receptor (ICOS, CD278) and its respective ligand (ICOS-L, CD275, B7-H2) represent another member of the B7 co-stimulatory molecules [2, 12]. While ICOS is not constitutively expressed on naïve T cells, it could be induced on effector T cells upon activation. Like CD28/B7-1/B7-2, the ICOS/B7-H2 interaction induces cell proliferation, differentiation, and secretion of Th1 and Th2 cytokines. When analysed in highly polarized T cell lines, ICOS blockade reduced the production of Th2, but not of Th1 cytokines [13]. Thus, the ICOS/B7-H2 signal may favor Th2-type responses. Since ICOS has also been found to be constitutively expressed on regulatory T cells (Treg), it exerts a dual role on immune modulatory processes. Recently, B7-H2 has been identified as a ligand of CTLA4 and CD28 on human cells [14].
The programmed death ligands 1 (B7-H1, PD-L1, CD274) and 2 (B7-DC, PD-L2, CD273) belong to the group of co-inhibitory molecules of the B7 family. B7-H1 and B7-DC exert a 41 % amino acid homology [1] and interact with the PD-1 receptor on activated T cells, B cells, and myeloid cells. While B7-H1 is constitutively or upon activation expressed in different cell types including hematopoietic cells, such as B cells, T cells, dendritic cells (DC), and macrophages, but also in non-lymphoid cells, like heart, lung, placenta, kidney, and liver [3], B7-DC expression is more restricted and mainly found in lymphoid tissues and DC. Recently, the importance for B7-H1 expression on activated CD8+ T cells for their survival and anti-tumor immunity has been demonstrated in mice [15]. B7-H1 induces apoptosis of activated T cells and hinders tumor-specific killing of CD8+ cytotoxic T lymphocytes when expressed on tumor cells [16], while Latchman et al. [4] reported that B7-H1 and B7-DC interaction with PD1 inhibited T cell proliferation by blocking cell cycle progression, but not by increasing cell death.
B7-H3, a B7 homolog sharing approximately 31 % sequence homology with B7-H1, is a type I trans-membrane protein. Despite B7-H3 is broadly transcribed in lymphoid and non-lymphoid organs, its protein expression is more limited. However, the broad expression pattern of B7-H3 suggests not only a more diverse immunological, but also non-immunological function of this molecule. Two possible receptors have been postulated for B7-H3 on T cells, one that gives rise to activating signals, whereas the other exerts inhibitory signals (2). Furthermore, the function of B7-H3 is controversially discussed since both co-stimulatory and co-inhibitory properties have been described for this molecule [7, 17]. B7-H3 could enhance the proliferation of CD4+ and CD8+ T cells, induce cytotoxic T lymphocytes (CTL), and stimulate interferon (IFN)-γ secretion [7], while it has also been shown to inhibit CD4+ and CD8+ T cell proliferation and IFN-γ production in mice and man [17, 18].
Like B7-H3, B7-H4 is a type I trans-membrane molecule, which exerts a 20 % amino acid homology in the extracellular region with other B7 molecules. It is broadly transcribed in human tissues and cells including lung, testis, pancreas, placenta, intestine, stomach, kidney, liver, brain, and ovary, while protein expression is limited [19]. Furthermore, B7-H4 is not constitutively expressed on naïve T and B cells, but it can be modulated by various cytokines or during the differentiation process of APC. While IL-6 and IL-10 induce B7-H4 expression, GM-CSF and IL-4 decrease and TNFα counteracts the IL-10-induced expression [20, 21]. Until now, the receptor of B7-H4 has not yet been identified, although the B and T lymphocyte attenuator (BTLA4) had been postulated as its receptor [22], which could not be confirmed by binding studies [23]. Analogous to B7-H1, B7-H4 negatively interferes with immune responses and blocks T cell proliferation and IL-2 production [8].
The B7-H6 represents the newest member of the B7 family and exerts a sequence homology comparable to that of other B7 members. B7-H6 binds to the NK cell receptor NKp30 and could trigger NK cell mediated to toxicity and cytokine secretion. B7-H6 expression is absent in normal untransformed tissues [6].
In addition to the membrane-bound ligands, soluble B7-H (sB7-H) molecules have been detected, which are mainly involved in pathophysiologic processes and are released by different cell types. sB7-H1 is secreted by mature DC, but not by immature DC, T cells, monocytes, or macrophages, and induces apoptosis when incubated with CD4+ or CD8+ T cells [24]. sB7-H2 is detectable at elevated levels in patients with lupus erythematosus [25], whereas elevated sB7-H3 levels were found in the sera of patients with chronic hepatitis B virus infections and higher levels were associated with increased risk of liver cirrhosis [26]. Additionally, sB7-H3 has been detected in the supernatant of monocytes, DC, and activated T cells and binds the unknown B7-H3 receptor, which opens novel interesting immune regulation pathways [27]. sB7-H4 is described in disease settings, such as autoimmune disease and tumor. In rheumatoid arthritis (RA), serum levels of sB7-H4 are high and correlate with DAS activity, and in a mouse model of RA, a function for sB7-H4 as decoy could be demonstrated as it blocks the membrane-bound B7-H4 binding and thereby exacerbating autoimmunity [28].
B7-H molecule expression on tumors
Although initially discovered as membrane-bound ligands in myeloid lineage cells and activated T and B cells, tumors could aberrantly express several types of B7-H molecules (Table 1). The best studied B7-H family member in tumors is B7-H1. B7-H1 is expressed on a variety of solid and hematopoietic malignancies including breast, bladder, lung, colon, pancreatic, gastric, skin, esophagus, liver, ovary, brain, and kidney cancers, Hodgkin lymphoma; acute myeloid leukemia; and myelodysplastic syndromes [16, 29–44]. In contrast, B7-DC has not yet been analyzed in a large series of tumor samples, but the frequency of B7-DC expression in human tumors appears to be low and not associated with clinical outcome. In contrast to the high frequency of B7-H1 expression in human tumors, B7-H2 expression has been mainly detected in cells of hematologic malignancies [45].
Table 1.
B7 family member expression on tumor cells (lesions) of different tumor entities and functional consequences
| B7 family member | Number pos. cases/total sample number | Tumor entity | Immunological and pathophysiologic consequences |
|---|---|---|---|
| B7-H1 | 14/78 | AML (acute myeloid leukemia) | Protected from CTL-mediated lysis upon TLR trigger, stronger expression upon relapse [43] |
| 65/65 | Bladder urothelial carcinoma | Strong expression correlates with relapse and poor survival [31] | |
| 77/280 | High-grade tumors with increased cell infiltration [67] | ||
| 41/69 | Breast cancer | High-risk grading, Ki-67 expression [30] | |
| 13/31 | Esophageal cancer | Associated with poor prognosis [36] | |
| 43/102 | Gastric carcinoma | Correlated to tumor size, invasion, LN metastasis, and survival [34] | |
| 36/48 | Glioma | Associated with tumor malignancy [40] | |
| 60/60 | Hepatocellular carcinoma | High expression associated with tumor IL-10 [37] | |
| 8/61 | Myelodysplastic syndrome | Associated with high-risk patients [44] | |
|
5/43 prim. 8/20 t meta. |
Melanoma | No difference in overall survival and immune infiltrates [66] | |
| 59/59 | Strong expression 34/59 correlates with Breslow and overall survival [35] | ||
| 24/56 | Longer overall survival in strong B7-H1-expressing melanoma, more TILs in B7-H1 + cases [114] | ||
| 52/52 | NSCLC | Focal expression, no clinical association, but less TILs in B7-H1 + regions [32] | |
| 58/109 | Associates with histological types and with reduced overall survival [63] | ||
| 62/70 | Ovarian cancer | High expression correlates with poor prognosis [38] | |
| 22/40 | Pancreatic cancer | Correlates with poor tumor differentiation and advanced tumor stage [33] | |
| 40/81 | Strong expression correlates with TNM status and poor survival [64] | ||
|
130/196 prim. 17/26 meta. |
RCC | Associates with likelihood of death [65] | |
| 73/306 | Correlates with increased risk of death [41] | ||
| B7-H2 | 9/59 | AML | Poor survival [45] |
| B7-H3 | 55/102 | Colorectal cancer | Correlates with more advanced tumor grade [115] |
| 60/102 | Gastric carcinoma | Associated with longer survival and penetration depth [68] | |
| ns/107 | Endometrial cancer | High expression associates with shorter survival [71] | |
|
29/29 prim. 28/29 meta. |
Melanoma | No impact on clinical parameter [48] | |
| 26/70 | NSCLC | Associated with reduced numbers of TILs and enhanced LN metastasis [49] | |
| 60/68 | Pancreatic cancer | Correlates with prolonged survival [69] | |
| 212/823 | Prostate cancer | Associated with cancer recurrence and death [50] | |
| 67/338 | Fourfold increased risk of cancer progression after surgery [70] | ||
| 126/743 | RCC | Associated with increased risk of death [47] | |
| B7-H4 |
165/173 prim. 240/246 meta. |
Breast cancer | Associated with negative hormone receptor status, independent of tumor stage and grade [54] |
| 107/112 | Esophageal cancer | Strong expression associated with shorter survival [55] | |
| 70/156 | Gastric cancer | Correlates with shorter overall survival [52] | |
|
28/29 prim. 26/29 meta. |
Melanoma | Strong expression correlates with shorter overall survival [48] | |
| 30/70 | NSCLC | Associated with reduced numbers of TILs and LN metastasis [49] | |
|
59/59 prim 30/30 meta |
Ovarian cancer | ND [51] | |
| ns/70 | Tumor cell expression is not associated with clinics [98] | ||
| 33/36 | Pancreatic cancer | ND [53] | |
| 120/823 | Prostate cancer | Associated with cancer recurrence and death [50] | |
| 153/259 | RCC | Associated with tumor size, stage, grade, positive patients are 3 times more likely to die [56] | |
| B7-H6 | 5/43 | Acute lymphoblastic leukemia, T cell lymphoblastic leukemia, marginal zone lymphoma | ND [6] |
ALL acute lymphoblastic leukemia, AML acute myeloid leukemia, MDS myelodysplastic syndrome, MZL marginal zone lymphoma, ND not determined, prim primary tumor, t meta in transit metastasis, LN lymph node, ns not specified, RCC renal cell carcinoma, T-ALL T cell lymphoblastic leukemia
B7-H3 expression has also been found on a variety of human tumors including prostate, non-small lung, pancreatic, gastric, endometrial, skin, colorectal, and urothelial cancer as well as RCC [46]. The frequency of B7-H3 expression in human tumor strongly varied between the tumor entities analyzed, ranging between 17 and 100 % [47, 48].
B7-H4 expression was detected in a large variety of tumors of distinct origin, including melanoma, non-small cell lung, prostate, ovary, stomach, pancreas, breast, esophagus, and kidney cancer [48–56]. Immunohistochemical analysis of B7-H4 revealed a heterogeneous expression in tumor types of distinct origin with a frequency ranging between 15 and 100 % [50, 51]. Interestingly, B7-H4 was preferentially expressed in non-dividing tumor cells and in a subset of CD133+ stem cells [57]. The discordant mRNA and protein levels suggest a post-transcriptional control of B7-H4 expression in human tumors.
B7-H6 expression was detected with a relative abundance in tumor cells of distinct origin, but not in normal tissues. B7-H6 was found on different hematologic and solid tumor cells lines and interacts with NKp30 on NK cells, thereby activating NK cells to control tumor cells (Fig. 2 and [6]).
Fig. 2.
B7-H expression and function on tumor cells and on cells of the tumor microenvironment. a Tumor cells of different origin do express B7 molecules and inhibit (B7-H1, B7-DC, B7-H4, partially B7-H3) or stimulate (B7-H2, partially B7-H3) T cell functions upon cognate receptor binding on cytotoxic T lymphocytes (CTL). b Tumor cells also express the more recently identified B7 family member B7-H6. This molecule binds NKp30 on NK cells. NKp30 belongs to the activating NK receptors. B7-H6 binding leads to stimulation of NK cells. NK cells can also express PD-1 and interact with B7-H1 on tumor cells. c Many different cell populations belong to the tumor microenvironment and possibly contribute to the control of tumor cells. These cells do also express members of the B7 family, for example, B7-H1 can be found on MDSC, NK cells, Treg, and TILs in the tumor microenvironment. Tumor DC can express B7-DC. TAM and endothelial cells of the tumor vasculature can express B7-H4. B7-H3 can also be found on the tumor vasculature. All of these molecules can interact with the cognate receptor on T cells, thereby mostly inhibiting T cell function with the exception of B7-H1 on NK cells, which stimulates CTL activity
In addition, soluble B7-H (sB7-H) ligands have been detected in sera of tumor patients (Table 2). For example, sB7-H1 and sB7-H4 have been found in the sera of RCC patients [58, 59], while sB7-H3 and sB7-H4 are discussed as prognostic biomarkers for NSCLC (sB7-H3), ovarian, and renal cell cancer (sB7-H4) [59–61].
Table 2.
B7 family member expression on non-tumor cells and in sera of tumor patients and their functional significance
| B7 family member | Specimen | Number pos. cases/total sample number | Tumor entity | Immunological and pathophysiological consequences |
|---|---|---|---|---|
| B7-H1 | TIL | 18/44 | Breast cancer | Associated with tumor size, grade, and HER-2/neu positivity [116] |
| Serum | 165/172 | RCC | High sB7-H1 levels are correlated to advanced stage and grade and higher risk of death [58] | |
| TIL | 115/196 | More aggressive tumors and increased risk of death [117] | ||
| B7-H3 | Blood | 95/95 | Gastric cancer | Elevated B7-H3 mRNA level correlates with stage and shorter 5-year survival [118] |
| Serum | 98/98 | NSCLC | Elevated sB7-H3 is associated with tumor stage, size, and metastasis, potential use as biomarker [60] | |
| Tumor vasculature | 37/77 | Ovarian cancer | Associated with shorter survival and higher incidence of recurrence [97] | |
| 706/743 | RCC | Strong expression associated with increased risk of death [47] | ||
| B7-H4 | TAM | ns/70 | Ovarian cancer | Associated with higher Treg infiltrates and shorter survival [98] |
| Tumor lysates | 156/251 | Associated with poor prognosis [61] | ||
| Serum | 268/268 | Elevated sB7-H4 levels, potential use as biomarker [73] | ||
| Serum |
53/101 pat. 18/101 con. |
RCC |
Elevated sB7-H4, potential use as biomarker, B7-H4 concentration correlates with tumor stage [59] |
TAM tumor-associated macrophages, ns not specified, pat. patients, con. control, TIL tumor-infiltrating lymphocytes
Of note, as described in the previous chapter, not only tumor cells, but also immune cells could release sB7-H molecules during immune responses including anti-tumor immune reactions. Therefore, the source of sB7-H molecules in the sera of tumor patients is not necessarily exclusively derived from tumor cells.
In this context, it is noteworthy that the function and clinical significance of the sB7-H expression still remain to be determined. Although, it has been suggested that sB7-H molecules independent of their source deliver inhibitory signals that adversely affect anti-tumor responses.
Indeed, expression of B7 family members in non-tumor cells of the tumor microenvironment has been found [62].
Clinical relevance of B7 homolog expression
In order to determine the clinical relevance of the expression of B7 homologs in cancer, their expression levels in tumors, in cells of the tumor microenvironment or the concentration of sB7-H in patients’ serum should be correlated with clinical parameters. Due to the higher expression levels of B7-H1 and B7-H4 in cancer tissues when compared to corresponding normal tissues and their close correlation with tumor stage, grade, pathologic types, and the biologic behavior of tumors, recurrence and survival rate of patients, these molecules might be used as potential diagnostic, prognostic, and predictive markers, for monitoring of treatment efficacy as well as for therapeutic targets. However, the expression pattern of the various B7-H family members has still to be investigated on a larger number of well-defined samples from different tumor types in different centers using the same protocols and antibodies.
The prognostic relevance of B7-H1 expression is in many different tumor entities high, but in some tumor entities controversial results were reported. For example, a significant prognostic value of B7-H1 was found in melanoma, bladder, esophageal, gastric, non-small cell lung cancer (NSCLC), ovarian, pancreatic, and kidney carcinoma [34–36, 38, 43, 63–65]. Discrepant results concerning the number of B7-H1-expressing tumor cases and clinical relevance were found in melanoma [66] and NSCLC [32], which might be at least partially explained by the samples investigated (frozen vs. paraffin embedded) or the antibodies used for the studies. The B7-H1 overexpression in tumor cells was associated with tumor grading and staging in glioma, bladder, breast, and pancreatic cancer [30, 33, 40, 67]. Furthermore, sB7-H1 has been detected in blood samples from tumor patients. In RCC patients, high sB7-H1 levels were associated with an increased risk of death and thus correlate with a poor clinical outcome [58].
Furthermore, for B7-H2, a prognostic relevance has been shown for acute myeloid leukemia [45]. B7-H2 expression is associated with a poor survival of patients, which may be due to stimulation of Tregs or even due to binding of the newly identified partner CTLA-4 [14], thereby dampening anti-tumor responses.
The impact of B7-H3 expression on clinical outcome highly depends on the tumor entity analyzed. Retrospective analyses demonstrated that high levels of B7-H3 expression on the tumor were associated with a prolonged patients’ survival in, for example, gastric and pancreatic cancer [68, 69]. In contrast in RCC, prostate, endometrial, and non-small cell lung carcinoma (NSCLC), an opposite effect was observed, since tumor B7-H3 correlated with an increased risk of death, a higher frequency of recurrence, poor survival of patients, and/or with lymph node metastasis [47, 49, 50, 70, 71]. sB7-H3 in patients with NSCL was also associated with a higher tumor stage, tumor size, and metastasis formation [60].
Most of the studies revealed a correlation between B7-H4 expression on the tumor or in the blood of patients and the TNM stage, pathologic types, patients’ prognosis, and survival [72]. These data suggest that B7-H4 represent a potential prognostic and predictive marker for some tumor entities, such as melanoma, esophageal, gastric, prostate, and renal cancer [48, 50, 52, 55, 56]. In addition to membrane-bound B7-H4, sB7-H4 appears to be also of prognostic relevance at least in ovarian and renal cancer and might serve as prognostic marker in both diseases [59, 73].
So far, there exist no data on the clinical significance of B7-H6, but its tumor-specific expression indicates an up-regulation due to a neoplastic transformation [6].
In addition to the correlation of aberrant expression of B7-H molecules with the clinical outcome of patients, polymorphisms in CD28, ICOS, and CTLA4, which have been shown to influence the protein expression level, were associated with malignancies [74–76].
Molecular mechanisms of aberrant expression of B7 homologs in tumors
So far, little is known about the control of B7 expression in human tumors. It has recently been shown that the regulatory mechanisms of B7-H1 are not uniform, and many signaling pathways are involved in its regulation. Normal human cells contain B7-H1 transcript, but marginally express or lack B7-H1 protein. IFN-γ resulted in an induction of B7-H1, suggesting that the JAK/STAT pathway is involved in the IFN-γ-mediated up-regulation of B7-H1 [77]. Not only IFN-γ, but also TNF-α, could up-regulate B7-H1 expression, which is mediated by the activation of NF-κB [44]). In addition, B7-H1 expression could be post-transcriptionally increased after the loss of the tumor suppressor gene (phosphatase and tensin homolog PTEN) function, which is dependent on the PI3K pathway and S6K1 kinase activation [39]. In PTEN-deficient tumors, IFN-γ treatment resulted in a super-induction of B7-H1 protein [78]. The dependence of B7-H1 on the PI3K pathway and S6K1 kinase could also be confirmed in breast and prostate cancer specimens [79]. Furthermore, the B7-H1 expression could be enhanced via the MyD88-, TNFR6 (tumor necrosis factor receptor-associated factor 6)-, and MEK-dependent pathways after stimulation with IFN-γ and toll-like receptor ligands [80]. In some tumors, the B7-H1 expression is controlled by the MEK/ERK and STAT3 pathway [81], while the MEK/ERK and the p38 mitogen-activated protein kinase (MAPK) pathways participate in the regulation of B7-H1 in other tumor entities [42].
In contrast, the expression of B7-H1 and other B7-H-family members could also be regulated by other mechanisms including structural alterations, for example, polymorphisms and mutations, transcriptional, post-transcriptional, and epigenetic control [82, 83]. In hematopoietic malignancies, chromosomal translocations, gene amplifications, hypermethylation, and microRNA control the B7-H expression [84–87].
The immunologic and non-immunologic function of B7 homolog expression in tumors and the tumor microenvironment
Although the functional role of B7-H expression in tumor cells remains unclear, the aberrant expression of B7-H molecules could be associated with both an increased resistance to immune and pharmacological attack. In in vitro assays, B7-H molecules could inhibit T cell proliferation, cytokine secretion, and the induction of CTL [29]. In in vivo assays, the frequency of T cell infiltration and B7-H expression do not always correlate. An association between B7-H4 expression and T cell infiltration has been reported for some tumor types, for example, NSCLC [49], whereas it was not found in melanoma [48].
In addition to the immunological effects, B7-H1, -H3, and -H4 could regulate tumor cell survival. Thus, these ligands are able to promote tumorigenesis and exert not only immunologic, but also non-immunologic activities.
During the past decade, cancer-related inflammation and the avoidance of immune destruction have been recognized as hallmarks of cancer and play an important role in the tumor development. The effect of tumor invasion, recurrence, and metastasis was investigated by the in situ analysis of immune components. A heterogeneous immune cell infiltration was shown between the different tumor types analyzed and is further diverse from patient to patient. All major immune cells might be present in the tumor including macrophages, DC, naïve and memory B cells, and various subsets of effector T cells and regulatory T cells [88]. In the absence of B7-1 co-stimulation and during low antigen stimulation, which is a situation most likely comparable to the tumor microenvironment, repetitively stimulated CD8+ T cells could become susceptible to inhibition by PD1/PD-L1 interaction [89].
Tumor-associated macrophages (TAM) represent a component of cancer-related inflammation and are involved in tumor growth, angiogenesis, invasion, and metastasis [90]. The B7-H1 expression on hepatocellular carcinoma was up-regulated by TAM, which was dependent on the STAT3 and NF-κB signaling [91]. Furthermore, the tumor microenvironment also constitutes of dysfunctional (e.g., CD4+, CD8+ T cells, and DC) and suppressive immune cells like Treg and myeloid-derived suppressor cells (MDSC), which provide a milieu for tumors to evade anti-tumor immunity [92].
B7-H1 expression in tumor cells increased the apoptosis of tumor-reactive T cells [16]. Recent studies showed that the engagement of B7-H1 leads to a down-regulation of T cell immunity, whereas B7-DC could enhance anti-tumor immunity [93]. The B7-DC-related anti-tumor immunity might depend on a PD-1-independent mechanism [94]. B7-H1 and B7-DC could be expressed on non-transformed cells of the tumor micromilieu, including tumor-infiltrating lymphocytes, NK cells, Treg, MDCS, and tumor-associated DC (Fig. 2). In addition, the number of tumor-infiltrating immune cells, in particular CD8+ T cells, could be increased by using blocking antibodies directed against B7-H1, while antibody-mediated inhibition of B7-DC decreases the number of Tregs. One can speculate that the B7-DC blockade might regulate the suppressive effect of Treg on effector T cells by inhibiting the migration of Treg into tumor cells and thus the direct contact with effector T cells and decrease the intratumoral IL-10. Alternatively, B7-DC might be involved in the expansion of Treg [95]. Furthermore, NK cells also do express PD-1. B7-H1 on multiple myeloma cells interacts with PD-1 on NK cells, thereby restricting the NK cell-mediated anti-tumor response [96]. Thus, tumors could escape anti-tumor immunity by three independent B7-H1/B7-DC controlled mechanisms: decrease in IFN-γ-producing effector T cells by B7-H1, an increase in Treg by B7-DC, and reduced NK cell response.
B7-H3 and B7-H4 expression was found on endothelial cells of the tumor-associated vasculature in ovary cancer and RCC [56, 97], but the underlying molecular mechanisms leading to the aberrant expression of both B7-H molecules have still to be defined. B7-H3-positive tumor vasculature in ovarian carcinoma was associated with a shorter survival of patients and higher incidence of recurrence [97]. These data suggest that one had to destruct or block the tumor vasculature-associated B7-H3 and B7-H4 expression, which might provide a benefit for T cell-based anti-tumor immunity. It is noteworthy that tumor-derived Treg could trigger macrophages to produce IL-6 and IL-10, which are able to stimulate APC to express B7-H4 in an autocrine or paracrine manner [98]. Even more important, B7-H4 expression on TAM is associated with higher frequency of Treg infiltrates and shorter survival of patients [98].
Potential role of B7 molecules regarding their clinical application
Based on the frequency of the expression of co-stimulatory and co-inhibitory molecules on tumors cells and their role in disease development and progression, it could be hypothesized that the expression of a respective B7 co-inhibitory molecule represents an immune escape mechanism by down-regulating the anti-tumor immunity, in particular the T cell response at the level of effector cells. Thus, these molecules might serve as attractive targets for tumor immunotherapy. Since B7-H1 and B7-H4 molecules block T cell function, their inhibition may offer an opportunity to enhance anti-tumor immunity. Targeting co-inhibitory molecules of the B7 family with antagonistic antibodies is a rational approach and has been confirmed by animal experiments. The profound importance of CD28, CTLA4, and PD-1 for the induction of immune tolerance has further led to the development of several human drugs that target the co-stimulatory/co-inhibitory pathways.
Recently, the blockade of the most extensively studied co-inhibitory molecule, CTLA4, has been studied in tumor patients with advanced disease (Table 3). The approval and implementation of fully human anti-CTLA4 monoclonal antibody (mAb) (ipilimumab) that binds to CTLA4 and thus inhibits the binding to B7 for the treatment of patients with metastatic melanoma is an example of a targeted immune-modulatory agent [62, 99, 100]. This treatment resulted in an enhanced recruitment of memory CD4+ and CD8+ T cells, objective tumor regressions, and durable responses both in murine model systems as well as in humans with an extension of the life span of stage 3–4 melanoma from a median of 6.4–10 months [101]. Additionally to melanoma patients, prostate and renal cancer patients also responded to anti-CTLA-4 Ab therapy [102, 103]. As expected from the CTLA4 knock-out mice, which exert an uncontrolled lymphoproliferation, the anti-CTLA4 mAb therapy in humans was associated with severe immunologic side effects. Therefore, additionally, alternative checkpoints with a more favorable profile should be envisaged.
Table 3.
Therapeutic approaches targeting B7 family members or their receptors in anti-tumor therapy
| Therapeutic strategies | Therapeutic agents | Tumor patients | Clinical outcome |
|---|---|---|---|
| Antibodies | Anti-CTLA-4 (Ipilimumab, MDX-010, BMS-734016) | Melanoma | 2/137 complete response (CR), 13/137 partial response (PR), 24/137 stable disease (SD) [101] |
| Prostate Cancer | 2/14 PR [102] | ||
| RCC | 6/41 OR [103] | ||
| Anti-CTLA-4 (Tremelimumab, CP-675206) | Melanoma | 4/29, 2x PR, 2x CR [119] | |
| Anti-PD-1 antibody (MDX-1106) | Mixed tumor entities | 5/39 Mixed, PR or CR [104] | |
| Melanoma | 15/46 PR [105] | ||
| RCC | 7/19 PR or CR [106] | ||
| Anti-PD-1 antibody (CT-011) | Mixed advanced hematological malignancies | Clinical benefit in 6/17 patients, 1 CR [107] | |
| Combination | Anti-CTLA-4 (Ipilimumab) + IL-2 | Melanoma | 6/36 CR, 84-month follow-up [113] |
| Anti-CTLA-4 (Ipilimumab) + gp100 peptide | 4/56 CR, 92-month follow-up [113] | ||
| Anti-CTLA-4 (Ipilimumab) after vaccination with GM-CSF | Ovarian | 6/11, 1x CR, 5x SD [120, 121] | |
| Cellular vaccines | Vaccinia virus encoding B7-1 | Melanoma | 3/12, 1x PR, 2x SD [111] |
| Adenocarcinoma cell line engineered to express B7-1 and HLA-A | NSCLC | 6/19, 1x PR, 5x SD [112] |
CR complete remission/response, OR objective response, PR partial response, SD stable disease
Blocking of the B7-H1 signaling pathway might also represent a promising approach to improve the efficacy of anti-tumor immune responses. Indeed, two fully human-blocking anti-PD1 antibodies (MDX-1106, CT-011) have been developed and are currently tested in clinical trials [104–107]. The antibody administration is well tolerated, and there exists evidence of anti-tumor activity [104]. In experimental models, systemic disruption of PD1 caused autoimmune diseases, while short-term administration exerts no side effects. A more targeted strategy was developed by selective disruption of the PD1 signaling using PD1-specific siRNA, which prevents PD1 expression on the cell surface [108]. The reduced PD1 expression led to improved T cell functions and thus might achieve a long-lasting enhancement of tumor-specific T cell responses.
Antibodies targeting B7-H1 and B7-H3 for use in humans have been developed. There is an initiated clinical trial as phase I multicenter study, still in the patient recruitment phase, with anti-B7-H1 antibody (MDX-1105) for therapy of multiple cancers (web: National Cancer Institute: Clinical trials). Additionally, there is a recruiting phase I study with anti-B7-H3 antibody (MGA271) for therapy of multiple refractory cancers that express B7-H3 (web: National Cancer Institute: Clinical trials).
Increased B7-H4 expression in tumor tissues and in the blood of patients represents a realistic opportunity to design novel immunotherapies, by regulating the immune response through manipulating the expression of B7-H4 and/or its receptor. Furthermore, B7-H4 might be used as a diagnostic, prognostic, or predictive marker, but its expression pattern has still to be investigated in a huge series of tumor samples. So far, efficient neutralizing antibodies directed against B7-H4 are not available. The use of B7-H4-specific siRNA in activated hepatic stellate cells (HSC) has been shown to restore effector T cell function in mice [109], and down modulation of B7-H4 in breast cancer cell lines increased caspase activity and leads to apoptosis of tumor cells [110].
Earlier on, cellular vaccines encoding the normally not expressed B7-1 molecule in melanoma and NSCLC cancer have led to some clinical efficacy with mostly stable disease outcome in some patients [111, 112].
Recently, combinations of immunotherapies targeting the co-inhibitory molecules with co-stimulatory molecules or tumor-associated antigen are investigated, which might lead to an improved immune response. The data in melanoma patients for the combination of anti-CTLA-4 along with IL-2 or with gp100 peptide treatment are very promising: 6/36 complete responder and 4/56 complete responder at 84-month and 92-month follow-up, respectively [113]. Several actively recruiting clinical trials for therapy of RCC and melanoma with combination therapy of B7 receptors are ongoing, such as anti-PD1 combined with anti-CTLA-4 and anti-PD-1 along with sunitinib or pazopanib, both tyrosine kinase inhibitors (web: National Cancer Institute: Clinical trials).
Conclusions and future perspectives
The B7 family members are widely expressed on tumor cells and cell populations of the tumor microenvironment. The co-inhibitory B7-H ligands promote the suppression of host anti-tumor response, whereas the co-stimulatory molecules might affect growth and survival of malignant cells. An increased knowledge of the balance between co-stimulatory and co-inhibitory pathways in tumors and the immune cell infiltration will shed further light on the relationship between immunity and cancer, but also provide insights into the management of malignancies as well as the development of novel immunotherapeutics. In addition, the receptor identification will also be an important issue in order to understand the (patho)physiological role of the B7-H family members.
The improved survival of patients upon modulating these pathways might be both due to the increased destruction of tumor cells and an improved homeostasis of the immune system, which results in a reconstitution of an efficient immune texture with indirect anti-tumor effects.
Acknowledgments
We would like to thank Sylvi Magdeburg for secretarial help and acknowledge the Wilhelm Roux Program of the Martin Luther University Halle-Wittenberg (D.Q.) and a grant from the Mildred Scheel Foundation, Bonn (B.S., D.Q.).
Conflict of interest
The authors declare no conflict of interest.
Abbreviations
- ALL
Acute lymphoblastic leukemia
- BTLA
B and T lymphocyte attenuator
- CTL
Cytotoxic T lymphocyte
- CTLA4
Cytotoxic T lymphocyte-associated antigen 4
- CR
Complete remission/response
- CTL
Cytotoxic T lymphocyte
- HLA
Human leukocyte antigen
- ICOS
Inducible co-stimulatory molecule
- IFN
Interferon
- JAK
Janus kinase
- mAb
Monoclonal antibody
- MAPK
Mitogen-activated protein kinase
- MDS
Myelodysplastic syndrome
- MDSC
Myeloid-derived suppressor cell
- MHC
Major histocompatibility complex
- MZL
Marginal zone lymphoma
- NK
Natural killer cell
- NSCLC
Non-small cell lung cancer
- OR
Objective response
- PR
Partial response
- PTEN
Phosphatase and tensin homolog
- RCC
Renal cell carcinoma
- STAT
Signal transducer and activator of transcription
- T-ALL
T cell lymphoblastic leukemia
- TAM
Tumor-associated macrophage
- TCR
T cell receptor
- TLR
Toll-like receptor
- TNF
Tumor necrosis factor
- Treg
Regulatory T cell
- SD
Stable disease
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Eleventh International Conference on Progress in Vaccination against Cancer (PIVAC 11), held in Copenhagen, Denmark, 10–13 October 2011. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 2.Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM, Scully S, Shahinian A, Shaklee CL, Van G, Mak TW, Senaldi G. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402(6763):827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 3.Dong H, Zhu G, Tamada K, Chen L. B7–H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 4.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 5.Kaifu T, Escaliere B, Gastinel LN, Vivier E, Baratin M. B7-H6/NKp30 interaction: a mechanism of alerting NK cells against tumors. Cell Mol Life Sci. 2011;68(21):3531–3539. doi: 10.1007/s00018-011-0802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, Moretta A, West R, Xu W, Vivier E, Levin SD. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206(7):1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2(3):269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 8.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7–H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18(6):849–861. doi: 10.1016/S1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 9.Kroczek RA, Mages HW, Hutloff A. Emerging paradigms of T-cell co-stimulation. Curr Opin Immunol. 2004;16(3):321–327. doi: 10.1016/j.coi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins MK, Johnson JG. Molecules involved in T-cell costimulation. Curr Opin Immunol. 1993;5(3):361–367. doi: 10.1016/0952-7915(93)90054-V. [DOI] [PubMed] [Google Scholar]
- 11.Frauwirth KA, Thompson CB. Activation and inhibition of lymphocytes by costimulation. J Clin Invest. 2002;109(3):295–299. doi: 10.1172/JCI14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397(6716):263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 13.Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo JA, Gosselin M, Owen LR, Rudd CE, Gutierrez-Ramos JC. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13(1):95–105. doi: 10.1016/S1074-7613(00)00011-X. [DOI] [PubMed] [Google Scholar]
- 14.Yao S, Zhu Y, Zhu G, Augustine M, Zheng L, Goode DJ, Broadwater M, Ruff W, Flies S, Xu H, Flies D, Luo L, Wang S, Chen L. B7-h2 is a costimulatory ligand for CD28 in human. Immunity. 2011;34(5):729–740. doi: 10.1016/j.immuni.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pulko V, Harris KJ, Liu X, Gibbons RM, Harrington SM, Krco CJ, Kwon ED, Dong H. B7-H1 expressed by activated CD8 T cells is essential for their survival. J Immunol. 2011;187(11):5606–5614. doi: 10.4049/jimmunol.1003976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 17.Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, Wakeham A, Itie A, Chung S, Da Costa J, Arya S, Horan T, Campbell P, Gaida K, Ohashi PS, Watts TH, Yoshinaga SK, Bray MR, Jordana M, Mak TW. The B7 family member B7–H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4(9):899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 18.Leitner J, Klauser C, Pickl WF, Stockl J, Majdic O, Bardet AF, Kreil DP, Dong C, Yamazaki T, Zlabinger G, Pfistershammer K, Steinberger P. B7–H3 is a potent inhibitor of human T-cell activation: no evidence for B7–H3 and TREML2 interaction. Eur J Immunol. 2009;39(7):1754–1764. doi: 10.1002/eji.200839028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi IH, Zhu G, Sica GL, Strome SE, Cheville JC, Lau JS, Zhu Y, Flies DB, Tamada K, Chen L. Genomic organization and expression analysis of B7–H4, an immune inhibitory molecule of the B7 family. J Immunol. 2003;171(9):4650–4654. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- 20.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W. B7–H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203(4):871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng C, Qu QX, Shen Y, Lv YT, Zhu YB, Zhang XG, Huang JA. Overexpression of B7–H4 in tumor infiltrated dendritic cells. J Immunoassay Immunochem. 2011;32(4):353–364. doi: 10.1080/15321819.2011.578190. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, Murphy TL, Russell JH, Allison JP, Murphy KM. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4(7):670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 23.Compaan DM, Gonzalez LC, Tom I, Loyet KM, Eaton D, Hymowitz SG. Attenuating lymphocyte activity: the crystal structure of the BTLA–HVEM complex. J Biol Chem. 2005;280(47):39553–39561. doi: 10.1074/jbc.M507629200. [DOI] [PubMed] [Google Scholar]
- 24.Frigola X, Inman BA, Krco CJ, Liu X, Harrington SM, Bulur PA, Dietz AB, Dong H, Kwon ED. Soluble B7–H1: differences in production between dendritic cells and T cells. Immunol Lett. 2012;142(1–2):78–82. doi: 10.1016/j.imlet.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Her M, Kim D, Oh M, Jeong H, Choi I. Increased expression of soluble inducible costimulator ligand (ICOSL) in patients with systemic lupus erythematosus. Lupus. 2009;18(6):501–507. doi: 10.1177/0961203308099176. [DOI] [PubMed] [Google Scholar]
- 26.Luan Y, Ju J, Luo L, Zhang Z, Wang J, Zhu DM, Cheng L, Zhang SY, Chen L, Wang FS, Wang S. Potential role of soluble B7–H3 in liver immunopathogenesis during chronic HBV infection. J Viral Hepat. 2012;19(1):23–31. doi: 10.1111/j.1365-2893.2010.01421.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang G, Hou J, Shi J, Yu G, Lu B, Zhang X. Soluble CD276 (B7–H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123(4):538–546. doi: 10.1111/j.1365-2567.2007.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azuma T, Zhu G, Xu H, Rietz AC, Drake CG, Matteson EL, Chen L. Potential role of decoy B7–H4 in the pathogenesis of rheumatoid arthritis: a mouse model informed by clinical data. PLoS Med. 2009;6(10):e1000166. doi: 10.1371/journal.pmed.1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 30.Ghebeh H, Tulbah A, Mohammed S, Elkum N, Bin Amer SM, Al-Tweigeri T, Dermime S. Expression of B7–H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. Int J Cancer. 2007;121(4):751–758. doi: 10.1002/ijc.22703. [DOI] [PubMed] [Google Scholar]
- 31.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7–H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56(8):1173–1182. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7–H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10(15):5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 33.Geng L, Huang D, Liu J, Qian Y, Deng J, Li D, Hu Z, Zhang J, Jiang G, Zheng S. B7–H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. J Cancer Res Clin Oncol. 2008;134(9):1021–1027. doi: 10.1007/s00432-008-0364-8. [DOI] [PubMed] [Google Scholar]
- 34.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108(1):19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116(7):1757–1766. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 36.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11(8):2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 37.Geng L, Deng J, Jiang G, Song P, Wang Z, Jiang Z, Zhang M, Zheng S. B7-H1 up-regulated expression in human hepatocellular carcinoma tissue: correlation with tumor interleukin-10 levels. Hepatogastroenterology. 2011;58(107–108):960–964. [PubMed] [Google Scholar]
- 38.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8 + T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO. Loss of tumor suppressor PTEN function increases B7–H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 40.Yao Y, Tao R, Wang X, Wang Y, Mao Y, Zhou LF. B7–H1 is correlated with malignancy-grade gliomas but is not expressed exclusively on tumor stem-like cells. Neuro Oncol. 2009;11(6):757–766. doi: 10.1215/15228517-2009-014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, Blute ML, Sebo TJ, Cheville JC, Kwon ED. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto R, Nishikori M, Tashima M, Sakai T, Ichinohe T, Takaori-Kondo A, Ohmori K, Uchiyama T. B7–H1 expression is regulated by MEK/ERK signaling pathway in anaplastic large cell lymphoma and Hodgkin lymphoma. Cancer Sci. 2009;100(11):2093–2100. doi: 10.1111/j.1349-7006.2009.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berthon C, Driss V, Liu J, Kuranda K, Leleu X, Jouy N, Hetuin D, Quesnel B. In acute myeloid leukemia, B7–H1 (PD-L1) protection of blasts from cytotoxic T cells is induced by TLR ligands and interferon-gamma and can be reversed using MEK inhibitors. Cancer Immunol Immunother. 2010;59(12):1839–1849. doi: 10.1007/s00262-010-0909-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kondo A, Yamashita T, Tamura H, Zhao W, Tsuji T, Shimizu M, Shinya E, Takahashi H, Tamada K, Chen L, Dan K, Ogata K. Interferon-gamma and tumor necrosis factor-alpha induce an immunoinhibitory molecule, B7–H1, via nuclear factor-kappaB activation in blasts in myelodysplastic syndromes. Blood. 2010;116(7):1124–1131. doi: 10.1182/blood-2009-12-255125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamura H, Dan K, Tamada K, Nakamura K, Shioi Y, Hyodo H, Wang SD, Dong H, Chen L, Ogata K. Expression of functional B7-H2 and B7.2 costimulatory molecules and their prognostic implications in de novo acute myeloid leukemia. Clin Cancer Res. 2005;11(16):5708–5717. doi: 10.1158/1078-0432.CCR-04-2672. [DOI] [PubMed] [Google Scholar]
- 46.Loos M, Hedderich DM, Friess H, Kleeff J. B7–h3 and its role in antitumor immunity. Clin Dev Immunol. 2010;2010:683875. doi: 10.1155/2010/683875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crispen PL, Sheinin Y, Roth TJ, Lohse CM, Kuntz SM, Frigola X, Thompson RH, Boorjian SA, Dong H, Leibovich BC, Blute ML, Kwon ED. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14(16):5150–5157. doi: 10.1158/1078-0432.CCR-08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quandt D, Fiedler E, Boettcher D, Marsch W, Seliger B. B7-H4 expression in human melanoma: its association with patients’ survival and antitumor immune response. Clin Cancer Res. 2011;17(10):3100–3111. doi: 10.1158/1078-0432.CCR-10-2268. [DOI] [PubMed] [Google Scholar]
- 49.Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, Wang X. B7–H3 and B7–H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53(2):143–151. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, Scardino PT, Sharma P, Allison JP. B7–H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci USA. 2007;104(49):19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tringler B, Liu W, Corral L, Torkko KC, Enomoto T, Davidson S, Lucia MS, Heinz DE, Papkoff J, Shroyer KR. B7–H4 overexpression in ovarian tumors. Gynecol Oncol. 2006;100(1):44–52. doi: 10.1016/j.ygyno.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 52.Jiang J, Zhu Y, Wu C, Shen Y, Wei W, Chen L, Zheng X, Sun J, Lu B, Zhang X. Tumor expression of B7–H4 predicts poor survival of patients suffering from gastric cancer. Cancer Immunol Immunother. 2010;59(11):1707–1714. doi: 10.1007/s00262-010-0900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Awadallah NS, Shroyer KR, Langer DA, Torkko KC, Chen YK, Bentz JS, Papkoff J, Liu W, Nash SR, Shah RJ. Detection of B7–H4 and p53 in pancreatic cancer: potential role as a cytological diagnostic adjunct. Pancreas. 2008;36(2):200–206. doi: 10.1097/MPA.0b013e318150e4e0. [DOI] [PubMed] [Google Scholar]
- 54.Tringler B, Zhuo S, Pilkington G, Torkko KC, Singh M, Lucia MS, Heinz DE, Papkoff J, Shroyer KR. B7–h4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res. 2005;11(5):1842–1848. doi: 10.1158/1078-0432.CCR-04-1658. [DOI] [PubMed] [Google Scholar]
- 55.Chen LJ, Sun J, Wu HY, Zhou SM, Tan Y, Tan M, Shan BE, Lu BF, Zhang XG. B7–H4 expression associates with cancer progression and predicts patient’s survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2011;60(7):1047–1055. doi: 10.1007/s00262-011-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci USA. 2006;103(27):10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao Y, Wang X, Jin K, Zhu J, Wang Y, Xiong S, Mao Y, Zhou L. B7–H4 is preferentially expressed in non-dividing brain tumor cells and in a subset of brain tumor stem-like cells. J Neurooncol. 2008;89(2):121–129. doi: 10.1007/s11060-008-9601-x. [DOI] [PubMed] [Google Scholar]
- 58.Frigola X, Inman BA, Lohse CM, Krco CJ, Cheville JC, Thompson RH, Leibovich B, Blute ML, Dong H, Kwon ED. Identification of a soluble form of B7–H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17(7):1915–1923. doi: 10.1158/1078-0432.CCR-10-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson RH, Zang X, Lohse CM, Leibovich BC, Slovin SF, Reuter VE, Cheville JC, Blute ML, Russo P, Kwon ED, Allison JP. Serum-soluble B7x is elevated in renal cell carcinoma patients and is associated with advanced stage. Cancer Res. 2008;68(15):6054–6058. doi: 10.1158/0008-5472.CAN-08-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang G, Xu Y, Lu X, Huang H, Zhou Y, Lu B, Zhang X. Diagnosis value of serum B7–H3 expression in non-small cell lung cancer. Lung Cancer. 2009;66(2):245–249. doi: 10.1016/j.lungcan.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 61.Simon I, Katsaros D, Rigault de la Longrais I, Massobrio M, Scorilas A, Kim NW, Sarno MJ, Wolfert RL, Diamandis EP. B7–H4 is over-expressed in early-stage ovarian cancer and is independent of CA125 expression. Gynecol Oncol. 2007;106(2):334–341. doi: 10.1016/j.ygyno.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 62.Fu T, He Q, Sharma P. The ICOS/ICOSL pathway is required for optimal antitumor responses mediated by anti-CTLA-4 therapy. Cancer Res. 2011;71(16):5445–5454. doi: 10.1158/0008-5472.CAN-11-1138. [DOI] [PubMed] [Google Scholar]
- 63.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28(3):682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 64.Wang L, Ma Q, Chen X, Guo K, Li J, Zhang M. Clinical significance of B7–H1 and B7–1 expressions in pancreatic carcinoma. World J Surg. 2010;34(5):1059–1065. doi: 10.1007/s00268-010-0448-x. [DOI] [PubMed] [Google Scholar]
- 65.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Chen L, Zincke H, Blute ML, Leibovich BC, Kwon ED. Costimulatory molecule B7–H1 in primary and metastatic clear cell renal cell carcinoma. Cancer. 2005;104(10):2084–2091. doi: 10.1002/cncr.21470. [DOI] [PubMed] [Google Scholar]
- 66.Gadiot J, Hooijkaas AI, Kaiser AD, van Tinteren H, van Boven H, Blank C. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer. 2011;117(10):2192–2201. doi: 10.1002/cncr.25747. [DOI] [PubMed] [Google Scholar]
- 67.Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, Fradet Y, Lacombe L, Kwon ED. PD-L1 (B7–H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109(8):1499–1505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- 68.Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu KF, Zhao JM, Zhang GB, Zhang XG. Relationship between co-stimulatory molecule B7–H3 expression and gastric carcinoma histology and prognosis. World J Gastroenterol. 2006;12(3):457–459. doi: 10.3748/wjg.v12.i3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loos M, Hedderich DM, Ottenhausen M, Giese NA, Laschinger M, Esposito I, Kleeff J, Friess H. Expression of the costimulatory molecule B7–H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer. 2009;9:463. doi: 10.1186/1471-2407-9-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, Krambeck AE, McKenney ME, Karnes RJ, Blute ML, Cheville JC, Sebo TJ, Kwon ED. B7–H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67(16):7893–7900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 71.Brunner A, Hinterholzer S, Riss P, Heinze G, Brustmann H. Immunoexpression of B7–H3 in endometrial cancer: relation to tumor T-cell infiltration and prognosis. Gynecol Oncol. 2012;124(1):105–111. doi: 10.1016/j.ygyno.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 72.He C, Qiao H, Jiang H, Sun X. The inhibitory role of B7-h4 in antitumor immunity: association with cancer progression and survival. Clin Dev Immunol. 2011;2011:695834. doi: 10.1155/2011/695834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simon I, Zhuo S, Corral L, Diamandis EP, Sarno MJ, Wolfert RL, Kim NW. B7–h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res. 2006;66(3):1570–1575. doi: 10.1158/0008-5472.CAN-04-3550. [DOI] [PubMed] [Google Scholar]
- 74.Sun T, Hu Z, Shen H, Lin D. Genetic polymorphisms in cytotoxic T-lymphocyte antigen 4 and cancer: the dialectical nature of subtle human immune dysregulation. Cancer Res. 2009;69(15):6011–6014. doi: 10.1158/0008-5472.CAN-09-0176. [DOI] [PubMed] [Google Scholar]
- 75.Karabon L, Pawlak E, Tomkiewicz A, Jedynak A, Passowicz-Muszynska E, Zajda K, Jonkisz A, Jankowska R, Krzakowski M, Frydecka I. CTLA-4, CD28, and ICOS gene polymorphism associations with non-small-cell lung cancer. Hum Immunol. 2011;72(10):947–954. doi: 10.1016/j.humimm.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 76.Piras G, Monne M, Uras A, Palmas A, Murineddu M, Arru L, Bianchi A, Calvisi A, Curreli L, Gaviano E, Lai P, Murgia A, Latte GC, Noli A, Gabbas A. Genetic analysis of the 2q33 region containing CD28-CTLA4-ICOS genes: association with non-Hodgkin’s lymphoma. Br J Haematol. 2005;129(6):784–790. doi: 10.1111/j.1365-2141.2005.05525.x. [DOI] [PubMed] [Google Scholar]
- 77.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54(4):307–314. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han SJ, Ahn BJ, Waldron JS, Yang I, Fang S, Crane CA, Pieper RO, Parsa AT. Gamma interferon-mediated superinduction of B7–H1 in PTEN-deficient glioblastoma: a paradoxical mechanism of immune evasion. NeuroReport. 2009;20(18):1597–1602. doi: 10.1097/WNR.0b013e32833188f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crane CA, Panner A, Murray JC, Wilson SP, Xu H, Chen L, Simko JP, Waldman FM, Pieper RO, Parsa AT. PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene. 2009;28(2):306–312. doi: 10.1038/onc.2008.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, Saudemont A, Quesnel B. Plasma cells from multiple myeloma patients express B7–H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110(1):296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 81.Wasik MA, Zhang Q, Marzec M, Kasprzycka M, Wang HY, Liu X. Anaplastic lymphoma kinase (ALK)-induced malignancies: novel mechanisms of cell transformation and potential therapeutic approaches. Semin Oncol. 2009;36(2 Suppl 1):S27–S35. doi: 10.1053/j.seminoncol.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 82.Scandiuzzi L, Ghosh K, Zang X. T cell costimulation and coinhibition: genetics and disease. Discov Med. 2011;12(63):119–128. [PMC free article] [PubMed] [Google Scholar]
- 83.Yi KH, Chen L. Fine tuning the immune response through B7–H3 and B7–H4. Immunol Rev. 2009;229(1):145–151. doi: 10.1111/j.1600-065X.2009.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA, Wasik MA. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7–H1) Proc Natl Acad Sci USA. 2008;105(52):20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steidl C, Shah SP, Woolcock BW, Rui L, Kawahara M, Farinha P, Johnson NA, Zhao Y, Telenius A, Neriah SB, McPherson A, Meissner B, Okoye UC, Diepstra A, van den Berg A, Sun M, Leung G, Jones SJ, Connors JM, Huntsman DG, Savage KJ, Rimsza LM, Horsman DE, Staudt LM, Steidl U, Marra MA, Gascoyne RD. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471(7338):377–381. doi: 10.1038/nature09754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gong AY, Zhou R, Hu G, Li X, Splinter PL, O’Hara SP, LaRusso NF, Soukup GA, Dong H, Chen XM. MicroRNA-513 regulates B7–H1 translation and is involved in IFN-gamma-induced B7–H1 expression in cholangiocytes. J Immunol. 2009;182(3):1325–1333. doi: 10.4049/jimmunol.182.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu H, Cheung IY, Guo HF, Cheung NK. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7–H3: potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69(15):6275–6281. doi: 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 89.Kaiser AD, Schuster K, Gadiot J, Borkner L, Daebritz H, Schmitt C, Andreesen R, Blank C. Reduced tumor-antigen density leads to PD-1/PD-L1-mediated impairment of partially exhausted CD8(+) T cells. Eur J Immunol. 2012;42(3):662–671. doi: 10.1002/eji.201141931. [DOI] [PubMed] [Google Scholar]
- 90.Allavena P, Mantovani A. Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin Exp Immunol. 2012;167(2):195–205. doi: 10.1111/j.1365-2249.2011.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen J, Li G, Meng H, Fan Y, Song Y, Wang S, Zhu F, Guo C, Zhang L, Shi Y. Upregulation of B7–H1 expression is associated with macrophage infiltration in hepatocellular carcinomas. Cancer Immunol Immunother. 2012;61(1):101–108. doi: 10.1007/s00262-011-1094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Filipazzi P, Huber V, Rivoltini L. Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunol Immunother. 2012;61(2):255–263. doi: 10.1007/s00262-011-1161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shin T, Yoshimura K, Crafton EB, Tsuchiya H, Housseau F, Koseki H, Schulick RD, Chen L, Pardoll DM. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005;201(10):1531–1541. doi: 10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shin T, Kennedy G, Gorski K, Tsuchiya H, Koseki H, Azuma M, Yagita H, Chen L, Powell J, Pardoll D, Housseau F. Cooperative B7–1/2 (CD80/CD86) and B7-DC costimulation of CD4+ T cells independent of the PD-1 receptor. J Exp Med. 2003;198(1):31–38. doi: 10.1084/jem.20030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Okudaira K, Hokari R, Tsuzuki Y, Okada Y, Komoto S, Watanabe C, Kurihara C, Kawaguchi A, Nagao S, Azuma M, Yagita H, Miura S. Blockade of B7–H1 or B7-DC induces an anti-tumor effect in a mouse pancreatic cancer model. Int J Oncol. 2009;35(4):741–749. doi: 10.3892/ijo_00000387. [DOI] [PubMed] [Google Scholar]
- 96.Benson DM, Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK, Greenfield CN, Porcu P, Devine SM, Rotem-Yehudar R, Lozanski G, Byrd JC, Caligiuri MA. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116(13):2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zang X, Sullivan PS, Soslow RA, Waitz R, Reuter VE, Wilton A, Thaler HT, Arul M, Slovin SF, Wei J, Spriggs DR, Dupont J, Allison JP. Tumor associated endothelial expression of B7–H3 predicts survival in ovarian carcinomas. Mod Pathol. 2010;23(8):1104–1112. doi: 10.1038/modpathol.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, Chen L, Coukos G, Zou W. Relationship between B7–H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67(18):8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 99.Cameron F, Whiteside G, Perry C. Ipilimumab: first global approval. Drugs. 2011;71(8):1093–1104. doi: 10.2165/11594010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 100.Peggs KS, Quezada SA, Allison JP. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224:141–165. doi: 10.1111/j.1600-065X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 101.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13(6):1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 103.Yang JC, Beck KE, Blansfield JA, Tran KQ, Lowy I, Rosenberg SA (2005) Tumor regression in patients with metastatic renal cancer treated with a monoclonal antibody to CTLA4 (MDX-010). J Clin Oncol 2005 ASCO Annual meeting proceedings vol 23, no. 16S, Part I of II (June 1 Supplement), 2005: 2501
- 104.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sznol M (2011) Toxicity and activity of anti-PD-1 in phase 1 clinical trials. American association for cancer research (AACR) 102nd annual meeting. Orlando, FL; April 2–6
- 106.McDermott DF Drake CG, Sznol M, Sosman JA, Smith DC, Powderly JD (2011) A phase I study to evalute safety and antitumor activity of biweekly BMS-936558 (Anti-PD-1, MDX-1106/ONO-4538) in patients with RCC and other advanced refractory malignancies (abstract). American Society for Clinical Oncology (ASCO) February 17–19, Orlando, FL. (J Clin Oncol 29:2011): (sippl 7; abstr. 331)
- 107.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 108.Borkner L, Kaiser A, van de Kasteele W, Andreesen R, Mackensen A, Haanen JB, Schumacher TN, Blank C. RNA interference targeting programmed death receptor-1 improves immune functions of tumor-specific T cells. Cancer Immunol Immunother. 2010;59(8):1173–1183. doi: 10.1007/s00262-010-0842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chinnadurai R, Grakoui A. B7–H4 mediates inhibition of T cell responses by activated murine hepatic stellate cells. Hepatology. 2010;52(6):2177–2185. doi: 10.1002/hep.23953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salceda S, Tang T, Kmet M, Munteanu A, Ghosh M, Macina R, Liu W, Pilkington G, Papkoff J. The immunomodulatory protein B7–H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res. 2005;306(1):128–141. doi: 10.1016/j.yexcr.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 111.Kaufman HL, Deraffele G, Mitcham J, Moroziewicz D, Cohen SM, Hurst-Wicker KS, Cheung K, Lee DS, Divito J, Voulo M, Donovan J, Dolan K, Manson K, Panicali D, Wang E, Horig H, Marincola FM. Targeting the local tumor microenvironment with vaccinia virus expressing B7.1 for the treatment of melanoma. J Clin Invest. 2005;115(7):1903–1912. doi: 10.1172/JCI24624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Raez LE, Cassileth PA, Schlesselman JJ, Sridhar K, Padmanabhan S, Fisher EZ, Baldie PA, Podack ER. Allogeneic vaccination with a B7.1 HLA-A gene-modified adenocarcinoma cell line in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2004;22(14):2800–2807. doi: 10.1200/JCO.2004.10.197. [DOI] [PubMed] [Google Scholar]
- 113.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18(7):2039–2047. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with b7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun J, Chen LJ, Zhang GB, Jiang JT, Zhu M, Tan Y, Wang HT, Lu BF, Zhang XG. Clinical significance and regulation of the costimulatory molecule B7–H3 in human colorectal carcinoma. Cancer Immunol Immunother. 2010;59(8):1163–1171. doi: 10.1007/s00262-010-0841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah A, Ajarim D, Al-Tweigeri T, Dermime S. The B7–H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8(3):190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, Zincke H, Blute ML, Strome SE, Leibovich BC, Kwon ED. Costimulatory B7–H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA. 2004;101(49):17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arigami T, Uenosono Y, Hirata M, Yanagita S, Ishigami S, Natsugoe S. B7–H3 expression in gastric cancer: a novel molecular blood marker for detecting circulating tumor cells. Cancer Sci. 2011;102(5):1019–1024. doi: 10.1111/j.1349-7006.2011.01877.x. [DOI] [PubMed] [Google Scholar]
- 119.Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, Comin-Anduix B, Reuben JM, Seja E, Parker CA, Sharma A, Glaspy JA, Gomez-Navarro J. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23(35):8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 120.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, Korman A, Lautz D, Russell S, Jaklitsch MT, Ramaiya N, Chen TC, Neuberg D, Allison JP, Mihm MC, Dranoff G. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105(8):3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, Padera R, Jaklitsch MT, Shankar S, Chen TC, Korman A, Allison JP, Dranoff G. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;100(8):4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]