Abstract
Trastuzumab, a humanized antibody targeting HER2, exhibits remarkable therapeutic efficacy against HER2-positive breast and gastric cancers; however, acquired resistance presents a formidable obstacle to long-term tumor responses in the majority of patients. Here, we show the mechanism of resistance to trastuzumab in HER2-positive human cancer cells and explore the molecular sensitization by exogenous expression of HER2-extracellular domain (ECD) in HER2-negative or trastuzumab-resistant human cancer cells. We found that long-term exposure to trastuzumab induced resistance in HER2-positive cancer cells; HER2 expression was downregulated, and antibody-dependent cellular cytotoxicity (ADCC) activity was impaired. We next examined the hypothesis that trastuzumab-resistant cells could be re-sensitized by the transfer of non-functional HER2-ECD. Exogenous HER2-ECD expression induced by the stable transfection of a plasmid vector or infection with a replication-deficient adenovirus vector had no apparent effect on the signaling pathway, but strongly enhanced ADCC activity in low HER2-expressing or trastuzumab-resistant human cancer cells. Our data indicate that restoration of HER2-ECD expression sensitizes HER2-negative or HER2-downregulated human cancer cells to trastuzumab-mediated ADCC, an outcome that has important implications for the treatment of human cancers.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-012-1249-x) contains supplementary material, which is available to authorized users.
Keywords: HER2, Extracellular domain, Trastuzumab, ADCC, Adenovirus
Introduction
Human epidermal growth factor receptor 2 (HER2) is a member of a receptor family associated with tumor cell proliferation, adhesion, migration, and differentiation [1]. Trastuzumab, a humanized monoclonal antibody that targets HER2, inhibits the HER2-mediated signaling pathway [2, 3] and also induces antibody-dependent cellular cytotoxicity (ADCC) [4–7]. The randomized clinical trial that led to the approval of trastuzumab for clinical use was conducted in combination with standard cytotoxic chemotherapy [8]. Subsequent trials have confirmed the utility of trastuzumab in HER2-overexpressing breast cancer in various clinical scenarios [9]. Recently, the efficacy and safety of adding trastuzumab to chemotherapy in HER2-positive advanced gastric cancer was evaluated, and the combination therapy was found to be significantly superior to chemotherapy alone [10]. However, HER2 is overexpressed only in approximately 20 % of primary breast and gastric cancers [8, 11–13]. Moreover, even if the HER2 status is positive, the majority of patients that initially respond to trastuzumab eventually develop resistance [9, 14, 15]. Thus, more effective treatments against HER2-overexpressing cancer require a deeper understanding of the mechanisms of resistance to trastuzumab.
Several mechanisms for trastuzumab resistance have been proposed, including the truncation of the HER2 receptor into a constitutively activated form (p95HER2) [16, 17], increased cellular signaling through alternative receptor tyrosine kinases [18–20], and altered intracellular signaling involving the loss of PTEN [21–23], which increases Akt activity. However, the biochemical nature of the resistance mechanism is confusing and controversial. Although the activation of ADCC is an important antitumor mechanism of trastuzumab, few studies have examined the role of ADCC in trastuzumab resistance. ADCC relies on the binding of antigen–antibody complexes to Fcγ receptors expressed on immune cells, and it is mainly attributable to the activation of natural killer (NK) cells. In fact, ADCC and overall NK cell activity were found to correlate with responses to trastuzumab [24]. Tumor cells potentially avoid ADCC attack from therapeutic antibodies by various mechanisms, such as insufficient recruitment of effector cells into tumors and the reduction or elimination of antigen expression on tumor cells [25].
Several strategies have been proposed to re-sensitize resistant tumor cells to therapeutic antibodies. In particular, the modification of heterogeneous or decreased antigen expression in resistant tumor cells might overcome resistance by enhancing ADCC activity. HER2 contains an extracellular ligand-binding domain, a short hydrophobic transmembrane region, and a cytoplasmic tyrosine kinase domain, which is crucial for downstream signaling [26]. Therefore, we hypothesize that truncated HER2 without an intracellular domain could be used as a non-signaling target for ADCC.
In the present study, we analyzed the HER2 surface expression and ADCC susceptibility of HER2-positive human cancer cells following repeated exposure to trastuzumab. We found that the surviving cells had reduced HER2 expression and were consequently less susceptible to ADCC. Moreover, we explored the effect of exogenous overexpression of the extracellular domain (ECD) of HER2, which lacks an intracellular signaling fragment, in HER2-negative and trastuzumab-resistant human cancer cells.
Materials and methods
Cell lines and cell cultures
Three human mammary gland adenocarcinoma cell lines, SKBR3, BT474, and MCF7, were obtained from American Type Culture Collection. SKBR3 was cultured in McCoy’s 5A medium. BT474 was cultured in Leibovitz’s medium. MCF7 was cultured in DMEM supplemented with 2 mmol/ml l-glutamine. The human gastric adenocarcinoma cell lines MKN1 and MKN28 were obtained from Human Science Research Resources Bank and cultured in RPMI1640. Penicillin (100 units/ml), streptomycin (100 μg/ml), and 10 % fetal bovine serum were added to the medium for each cell line.
Construction of plasmids and establishment of stable cell lines
Complementary DNAs of human full-length HER2 (HER2-wt) and truncated HER2 containing extracellular and transmembrane regions (HER2-ECD) were kindly provided by Dr. Mien-Chie Hung (M. D. Anderson Cancer Center). HER2-wt and HER2-ECD cDNAs were subcloned into the multi-cloning sites of the pcDNA3 vector. MCF7 breast cancer cells were transfected with the vectors expressing HER2-wt or HER2-ECD. For selection of stably transfected cells, cells were maintained in medium containing 0.2 mg/ml geneticin (G418), and single colonies were isolated.
Recombinant adenovirus
Replication-deficient adenoviral vector expressing the extracellular and transmembrane domains of HER2 (Ad-HER2-ECD) was constructed. Briefly, the HER2-ECD expression cassette that contains the human cytomegalovirus promoter, HER2-ECD cDNA, and the SV40 early polyadenylation signal was inserted between the XbaI and ClaI sites of pXCJL.1. The HER2-ECD shuttle vector and the recombinant plasmid pJM17 were cotransfected into 293 cells (Ad5-transformed human embryonic kidney cell line). The culture supernatant of 293 cells showing the complete cytopathic effect was collected and used for subsequent infections. This virus was purified by ultracentrifugation in cesium chloride step gradients, and its titer was determined by a plaque-forming assay using 293 cells. Replication-deficient E1A-deleted adenovirus (dl312) was used as control adenovirus. The viruses were stored at −80 °C before use.
Establishment of trastuzumab-acquired auto-resistance in HER2-positive cancer cells
To establish the trastuzumab-resistant cells, human breast cancer cell lines SKBR3 and BT474 expressing HER2 were exposed to increasing concentrations of the anti-HER2 monoclonal antibody trastuzumab (Chugai Pharmaceutical Co.) for more than 3 months. Briefly, HER2-positive cancer cells were initially exposed to 50 mg/ml trastuzumab for 1 month followed by 100 mg/ml trastuzumab for 2 months. Trastuzumab was administered twice a week. Trastuzumab-resistant cells established by continuous exposure to trastuzumab were maintained in medium with 100 mg/ml trastuzumab. The trastuzumab-resistant cancer cells were cultured in medium without trastuzumab for 5 days before each experiment.
Western blotting analysis
Primary antibodies against HER2-ECD (Thermo Scientific), β-actin (Sigma Chemical, Co.); PTEN (Santa Cruz), HER2-intracellular domain, IGF1-R, pAkt, pmTOR, pHER3, Akt, and mTOR (Cell Signaling Technology) and peroxidase-linked secondary antibodies (Amersham) were used. Proteins were electrophoretically transferred to Hybond-polyvinylidene difluoride transfer membranes (GE Healthcare Life Science) and incubated with primary antibody, followed by peroxidase-linked secondary antibody according to the manufacturer’s protocol. The Amersham ECL chemiluminescence system (GE Healthcare Life Science) was used to detect the peroxidase activity of the bound antibody. In experiments with replication-deficient adenoviral vector, cells were infected with Ad-HER2-ECD or dl312 at a multiplicity of infection (MOI) of 20 for 36 h.
Flow cytometric analysis
In experiments to measure the affinity to trastuzumab, tumor cells were pretreated with 100 μg/ml of trastuzumab for 60 min at 37 °C. Tumor cells were fixed with 4 % paraformaldehyde in PBS for 10 min and then washed with PBS containing 0.5 or 1.5 % BSA. The cells were labeled with APC-conjugated rabbit monoclonal anti-HER2-ECD antibody (R&D Systems Inc.) or APC-conjugated AffiniPure F(ab’)2 fragment goat monoclonal anti-human IgG + IgM (H + L) antibody (Jackson ImmunoResearch Laboratories, Inc.) at room temperature for 45 min and analyzed by FACSAria instrument (BD Biosciences). The cell population was gated on forward scatter and side scatter. The intensity of staining was determined by the BD-FACS Software. In experiments with replication-deficient adenoviral vector, cells were infected with Ad-HER2-ECD at an MOI of 20 for 36 h.
Cell viability assay
Parental or trastuzumab-resistant human breast cancer cells were seeded on 96-well plates at a density of 1 × 103 cells/well for 24 h. Then, trastuzumab was added to every well at the indicated concentration for 5 days. Cell viability was determined 5 days after trastuzumab treatment by using the Cell Proliferation Kit II (Roche Molecular Biochemicals) with the sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulfonic acid hydrate (XTT) assay, according to the manufacturer’s protocol.
Cell proliferation assays
Cells were trypsinized and re-plated in 24-well plates at a density of 1 × 104 cells/well. Parental MCF7 cells, mock vector-treated MCF7 cells, MCF7-HER2-wt cells, and MCF7-HER2-ECD cells were analyzed. Cells were incubated for 12 h to allow for attachment, after which the zero time point was determined. In experiments with adenoviral vector, trastuzumab-resistant SKBR3 or BT-47 cells with downregulated HER2 expression or low HER2-expressing breast and gastric cancer cells were infected with replication-deficient adenovirus (20 MOI) 1 day after the zero time point.
Antibody-dependent cellular cytotoxicity (ADCC) assay
Peripheral blood mononuclear cells (PBMCs) were separated from peripheral blood obtained from healthy donors by Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient centrifugation. After centrifugation, PBMCs were washed three times with PBS and suspended with medium containing 10 % FBS. Target cells were labeled with 50 μCi (1.85 MBq) of Na51 Ci (Parkin Elmer, Waltham, MA, USA) for 60 min. Then, target cells (1 × 104/well) and effector cells at various effector/target ratios were co-incubated in 200 μl of X-VIVO medium in a 96-well U-bottomed plate for 4 h at 37 °C with trastuzumab (2 μg/well; Chugai Pharmaceutical co.) or control antibody, rituximab (2 μg/well; Chugai Pharmaceutical Co.). After 4 h of incubation, the radioactivity of the supernatant (100 μl) was measured with a γ-counter. The percentage of specific lysis = 100 × (experimental count per minute (cpm) − spontaneous cpm)/(maximum cpm − spontaneous cpm). In experiments with replication-deficient adenovirus, target cells were infected with Ad-HER2-ECD or dl312 at an MOI of 20 for 36 h (MCF7 and MDA-MB-231 cells) or 24 h (trastuzumab-resistant SKBR3 or BT474, MKN1, and MKN28 cells) before the ADCC assay was performed.
Statistical analysis
A comparison of continuous variables between two groups for in vitro assays was performed with the two-sided Student’s t test. At least three independent experiments were performed. The differences between groups were considered to be statistically significant when the p values were <0.05. Means and 95 % confidence intervals are reported, unless otherwise indicated. All data were analyzed with the statistical software SPSS 15.0 (SPSS, Inc, Chicago, IL, USA).
Results
Continuous exposure to trastuzumab downregulates cell-surface HER2 expression and impairs trastuzumab-mediated ADCC in HER2-positive human cancer cells
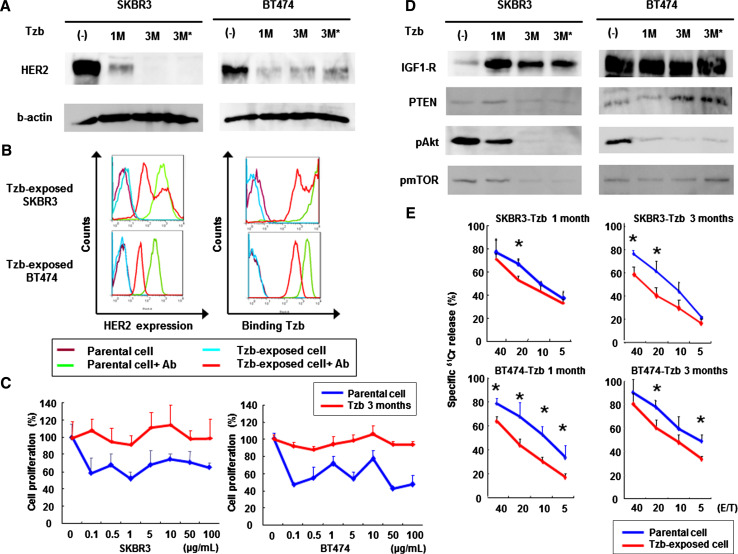
To elucidate the molecular mechanism of developing resistance to trastuzumab, we continuously treated HER2-positive SKBR3 and BT474 breast cancer cells with trastuzumab. Exposure to trastuzumab for 1 month reduced extracellular HER2 levels in both cell lines, and this downregulation was maintained for at least 2 months. HER2 downregulation did not recover following 5 days of incubation in the absence of trastuzumab (Fig. 1a). Intracellular HER2 expression was also reduced by long-term exposure to trastuzumab, although p95HER2 could not be detected (Supplemental Fig. 1). Flow cytometric analysis demonstrated that trastuzumab-exposed SKBR3 and BT474 cells showed decreased HER2 expression as compared with parental lines, leading to reduced affinity to trastuzumab (Fig. 1b).
Fig. 1.
Effects of continuous exposure to trastuzumab in HER2-overexpressing breast cancer cells. a Western blot analysis of HER2 expression. Human breast cancer SKBR3 and BT474 cells were initially incubated with 50 mg/ml trastuzumab (Tzb) for 1 month followed by 100 mg/ml trastuzumab for 2 months. *Cells were cultured in the absence of trastuzumab for 5 days before analysis. Equivalent amounts of protein from whole cell lysates were loaded into each lane. Blots were probed with anti-HER2-ECD antibody and visualized by using an ECL detection system. Equal loading of samples was confirmed by stripping each blot and reprobing with anti-β-actin antibody. b Flow cytometric analysis of HER2 expression and trastuzumab binding. Parental or trastuzumab-exposed cells were stained with APC-conjugated anti-HER2-ECD antibody to measure cell-surface HER2 expression or treated with trastuzumab followed by incubation with APC-conjugated anti-human antibody to measure the amount of bound trastuzumab. c Parental or trastuzumab-exposed cells were further treated with the indicated doses of trastuzumab for 5 days, and cell viability was assessed by XTT assay. d Western blot analysis for assessment of HER2-related signaling pathway. Blots were probed with anti-IGF1-R, anti-PTEN, anti-phosphorylated Akt, or anti-phosphorylated mTOR antibody. e ADCC activity of trastuzumab-exposed SKBR3 or BT474 cells. Parental or trastuzumab-exposed cells were incubated with PBMCs from healthy donors in the presence of 10 μg/ml of trastuzumab, and the cytotoxic activity was assessed by a 4-h standard 51Cr-release assay. Data represent the mean ± SD of 3 wells at four different effector-to-target (E/T) ratios. *p < 0.05
SKBR3 and BT474 cells exposed to trastuzumab for 3 months were apparently more resistant to trastuzumab-mediated growth suppression in vitro (Fig. 1c). Western blotting analysis for assessment of the HER2-related signaling pathway demonstrated that phosphorylated Akt and mTOR expression were downregulated in these resistant cell lines. In contrast, insulin-like growth factor-1 receptor (IGF-1R) expression was notably enhanced following trastuzumab exposure in SKBR3 cells and constitutively high in BT474 cells without trastuzumab treatment (Fig. 1d). These results suggest that the development of resistance to trastuzumab at least partially depends on upregulation of an alternative signaling pathway downstream of other receptor tyrosine kinases such as IGF-1R. We further examined trastuzumab-mediated ADCC against parental and trastuzumab-resistant SKBR3 and BT474 cells by using PBMCs from healthy volunteer donors. Although apparent ADCC activity was observed in parental SKBR3 and BT474 cells, long-term exposure to trastuzumab significantly reduced this activity in both cell lines (Fig. 1e). Thus, impaired ADCC activity might be another possible mechanism contributing to acquired trastuzumab resistance.
Effects of exogenous HER2-ECD expression on in vitro growth and signaling pathways in human cancer cells
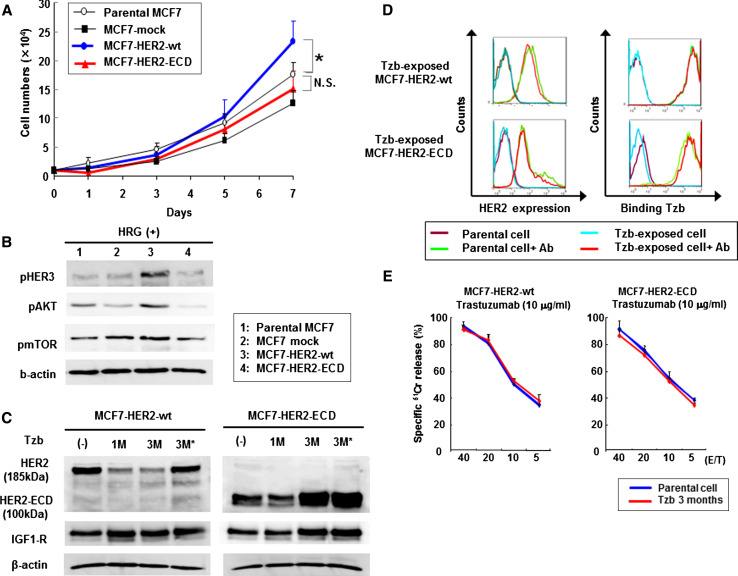
To investigate the effects of exogenous expression of HER2 protein on trastuzumab-mediated antitumor activity, we transfected human full-length HER2 (HER2-wt) and truncated HER2 c DNA containing extracellular and transmembrane regions (HER2-ECD) into low HER2-expressing MCF7 human breast cancer cells. HER2 overexpression contributes to breast cancer carcinogenesis, and studies have indicated that transfection of HER2-wt into mammary epithelial cells induces oncogenic transformation [27]. Indeed, stable HER2-wt-expressing MCF7 cells showed accelerated cell growth compared to parental MCF7 cells, whereas the growth pattern of HER2-ECD-transfected MCF7 cells was similar to that of parental MCF7 cells (Fig. 2a). Furthermore, transfection of HER2-wt, but not HER2-ECD, led to an increase in phosphorylated Akt and mTOR expression in the presence of HER3 ligand, HRG-β; these results suggest that exogenous HER2-ECD expression did not trigger the signaling pathways of HER2/HER3, which is the most potent combination of receptors in human breast cancer cells [28, 29] (Fig. 2b).
Fig. 2.
Effects of exogenous HER2-ECD expression in low HER2-expressing MCF7 cells. a MCF7 human breast cancer cells were transfected with a vector expressing human full-length HER2 (HER2-wt) or truncated HER2 containing extracellular and transmembrane regions (HER2-ECD), or empty vector (mock). The cell growth was assessed for parental cells and stable clones. *p < 0.05. b Western blot analysis of phosphorylated HER3, Akt, and mTOR. Cells were stimulated with HER3 ligand, heregulin-β. c Western blot analysis of MCF7 cells expressing HER2-wt (185 kDa) or HER2-ECD (100 kDa) after continuous exposure to trastuzumab for 1 month or 3 months. Cells were prepared as described in the legend for Fig. 1a. d Flow cytometric analysis of HER2 expression and the amount of bound trastuzumab on parental or trastuzumab-exposed MCF7 cells expressing HER2-wt or HER2-ECD. Cells were stained and subjected to the analysis as described in the legend to Fig. 1b. e ADCC activity of parental or trastuzumab-exposed MCF7 cells expressing HER2-wt or HER2-ECD. The cytotoxic activity of PBMCs was assessed in the presence of 10 μg/ml of trastuzumab by a 4-h standard 51Cr-release assay. Data represent the mean ± SD of 3 wells at four different E/T ratios
We next explored whether exogenous expression of HER2-wt or HER2-ECD was altered by long-term exposure to trastuzumab. The expression of 185-kDa full-length HER2 protein was slightly reduced in the presence of trastuzumab, whereas 3-month treatment with trastuzumab resulted in a slight increase in 100-kDa HER2-ECD expression (Fig. 2c). However, flow cytometric analysis demonstrated that neither cell-surface HER2 expression nor trastuzumab binding affinity changed following long-term trastuzumab exposure (Fig. 2d). Furthermore, the ADCC activity of trastuzumab against MCF7 cells transfected with HER2-wt or HER2-ECD was maintained even after a 3-month exposure to trastuzumab (Fig. 2e). These results indicate that exogenous overexpression of HER2-wt or HER2-ECD could overcome trastuzumab-mediated downregulation of endogenous HER2 expression.
Exogenous HER2-ECD expression enhances trastuzumab-mediated ADCC activity in low HER-2-expressing human cancer cells
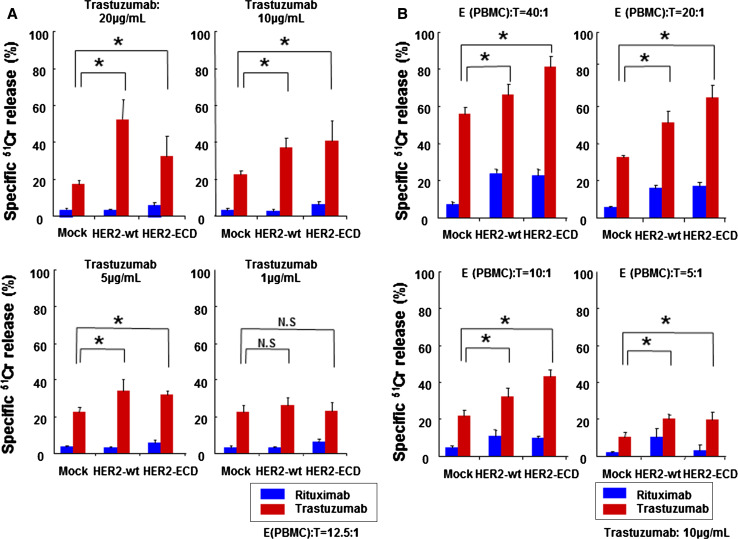
We conducted a standard 4-h 51Cr release assay with PBMCs from healthy volunteer donors to test the hypothesis that trastuzumab-mediated ADCC activity could be enhanced by exogenous overexpression of HER-ECD. With 5, 10, or 20 μg/ml of trastuzumab, ADCC activity was significantly increased in both HER2-ECD- and HER2-wt-expressing MCF7 cells as compared to MCF7 cells transfected with control pcDNA3 vector. A low concentration of trastuzumab (1 μg/ml) failed to enhance ADCC (Fig. 3a). Furthermore, effector cells showed significantly increased ADCC against HER2-ECD- or HER2-wt-expressing MCF7 cells as compared to pcDNA3-transfected cells at the effector/target cell ratios of 5:1, 10:1, 20:1, and 40:1 (Fig. 3b). These results indicate that exogenous overexpression of HER2-ECD may be an appropriate strategy to sensitize human cancer cells with low or reduced expression of HER2 to trastuzumab. Thus, we next examined the most efficient tool for gene transfer.
Fig. 3.
Trastuzumab-mediated ADCC activity on HER2-ECD-expressing MCF7 cells. a The cytotoxic activity against MCF7 human breast cancer cells transfected with vector expressing HER2-wt or HER2-ECD or empty vector (mock) was assessed by a 4-h standard 51Cr-release assay in the presence of the indicated doses of trastuzumab or control rituximab. Data represent the mean ± SD of 3 wells at an E/T ratio of 12.5:1. *p < 0.05. b A 4-h 51Cr-release assay was also performed against MCF7 cells expressing HER2-wt or HER2-ECD, or mock-treated MCF7 cells in the presence of 10 μg/ml of trastuzumab or control rituximab. Data represent the mean ± SD of 3 wells at four different E/T ratios. *p < 0.05
Efficient HER2-ECD overexpression in human cancer cell lines by a recombinant replication-deficient adenovirus vector
Modified adenovirus type 5 (Ad5) vectors have been used as a platform to deliver genes of interest into various types of human cells. We constructed a replication-deficient adenoviral vector containing a gene that encodes the extracellular domain of HER2 plus the transmembrane domain (Ad-HER2-ECD). To assess the efficient exogenous HER2-ECD overexpression by Ad-HER2-ECD infection, we used trastuzumab-resistant and low HER2-expressing human breast and gastric cancer cell lines. Various HER2 tests have demonstrated that the levels of HER2 expression in both MKN1 and MKN28 cells are low and that MKN7 cells overexpress HER2 antigen (Table 1). Indeed, the degree of HER2 expression correlated well with trastuzumab-mediated ADCC activity (Supplemental Fig. 2).
Table 1.
HER2 expression status of gastric cancer cell lines
| Cell lines | HER2 status | ||
|---|---|---|---|
| FACS (MFI) | HercepTest | Western blotting | |
| MKN1 | 13 | – | Weak |
| MKN7 | 106 | 3+ | Strong |
| MKN28 | 30 | – | Negative |
| MKN45 | 23 | 1+ | Weak |
| NUGC3 | 9 | – | Negative |
| KATO-III | 30 | 2+ | Medium |
HER2-expressing status of six different gastric cancer cell lines measured by flow cytometry, immunocytochemistry (HercepTest), and Western blotting analysis
MFI mean fluorescence intensity, FACS fluorescence-activated cell sorting
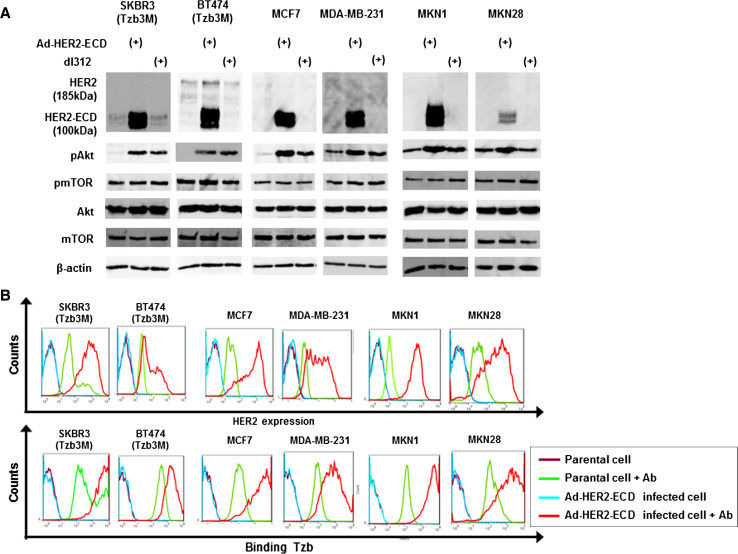
As expected, Ad-HER2-ECD infection at an MOI of 20 for 36 h resulted in a marked increase in the expression of 100-kDa HER2-ECD protein in trastuzumab-resistant breast cancer cells (SKBR3 and BT474), low HER2-expressing breast cancer cells (MCF7 and MDA-MB-231), and low HER2-expressing gastric cancer cells (MKN1 and MKN28) as compared to mock- or control dl312-infected cells (Fig. 4a). Western blot analysis also demonstrated that Ad-HER2-ECD had no apparent effects on the HER2 signaling pathway such as Akt and mTOR expression as well as phosphorylated mTOR expression, although Ad-HER2-ECD and control dl312 induced phosphorylated Akt. Flow cytometric analysis confirmed the cell-surface expression of HER2-ECD in Ad-HER2-ECD-infected cells, which in turn leads to increased trastuzumab binding (Fig. 4b).
Fig. 4.
Efficient HER2-ECD overexpression in human cancer cell lines by a recombinant replication-deficient adenovirus vector. a Western blot analysis of HER2-wt (185 kDa), HER2-ECD (100 kDa), and representative HER2-related signaling proteins in various types of human cancer cells. Trastuzumab-resistant breast cancer cells (SKBR3 and BT474), low HER2-expressing breast cancer cells (MCF7 and MDA-MB-231), and low HER2-expressing gastric cancer cells (MKN1 and MKN28) were infected with replication-deficient adenoviral vector expressing exogenous HER2-ECD (Ad-HER2-ECD) or replication-deficient control adenovirus (dl312) at an MOI of 20 for 36 h. b Flow cytometric analysis of HER2 expression and the amount of bound trastuzumab in cells 36 h after Ad-HER2-ECD infection at an MOI of 20
Direct antitumor effects of Ad-HER2-ECD on trastuzumab-resistant or low HER2-expressing human cancer cells
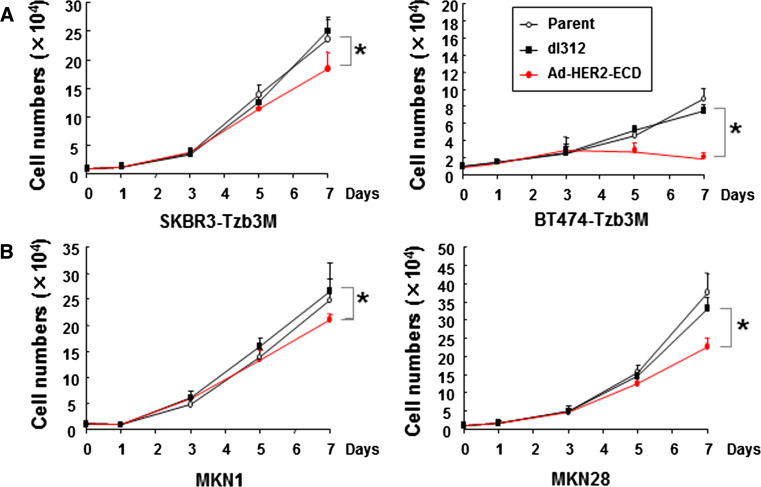
Next, we assessed the cell growth pattern of trastuzumab-resistant SKBR3 and BT474 human breast cancer cells and low HER2-expressing MKN1 and MKN28 human gastric cancer cells following Ad-HER2-ECD infection. MCF7 cells that were stably transfected with the HER2-ECD plasmid showed a growth pattern similar to that of parental or control vector-transfected MCF7 cells (Fig. 2a). However, adenovirus-mediated overexpression of HER2-ECD unexpectedly induced a significant suppression of in vitro growth in all cell lines as compared to uninfected cells or cells infected with control dl312 (Fig. 5). These results suggest that Ad-HER2-ECD had a slight but significant direct antitumor effect on trastuzumab-resistant and low HER2-expressing human cancer cell lines in vitro.
Fig. 5.
Antitumor effects of Ad-HER2-ECD on trastuzumab-resistant or low HER2-expresssing human cancer cells. Trastuzumab-resistant SKBR3 and BT474 breast cancer cells (a) and low HER2-expressing MKN1 and MKN28 gastric cancer cells (b) cultured as a monolayer were infected with Ad-HER2-ECD or control dl312 at an MOI of 20. The cell growth was determined by counting cell numbers each day after infection. The mean ± SD of three different wells is shown. *p < 0.05
Adenovirus-mediated HER2-ECD overexpression sensitizes trastuzumab-resistant or low HER2-expressing human cancer cells to trastuzumab-mediated ADCC
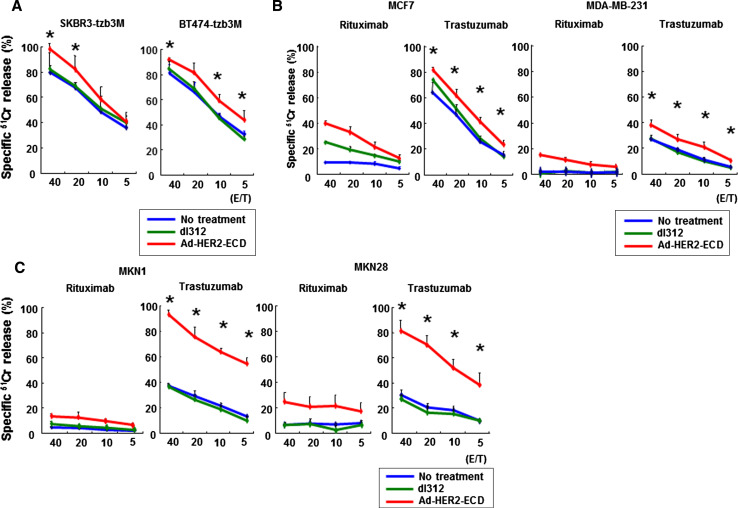
Finally, we examined whether Ad-HER2-ECD infection could overcome acquired resistance to trastuzumab-mediated ADCC in SKBR3 and BT474 human breast cancer cells. Enhancement of ADCC activity by Ad-HER2-ECD infection was also assessed in low HER2-expressing human breast and gastric cancer cell lines. Following Ad-HER2-ECD infection, trastuzumab-resistant (Fig. 6a) as well as low HER2-expressing cells (Fig. 6b, c) were more efficiently killed by ADCC, and a significant difference was detected at all effector/target ratios in all cell lines, except trastuzumab-resistant SKBR3 cells, as compared to mock- or control dl312-infected cells. Thus, Ad-HER2-ECD-mediated exogenous expression of HER2-ECD could sensitize trastuzumab-resistant HER2-downregulated cells or low HER2-expressing cells to trastuzumab through ADCC activation in vitro.
Fig. 6.
Molecular sensitization of human cancer cells to trastuzumab by Ad-HER2-ECD-mediated exogenous expression of HER2-ECD. The cytotoxic reactivity of PBMCs against HER2-downregulated SKBR3 or BT474 cells (a), low HER2-expressing MCF7 or MDA-MB-231 human breast cancer cells (b), or low HER2-expressing MKN1 or MKN28 human gastric cancer cells (c) was assessed after Ad-HER2-ECD or dl312 infection in the presence of 10 μg/ml of trastuzumab or control rituximab by a 4-h 51Cr-release assay. Data represent the mean ± SD of 3 wells at four different E/T ratios
Discussion
The nature of acquired resistance to trastuzumab is an area of active research in both the laboratory and the clinic. In the present study, we exposed HER2-positive breast cancer cells to trastuzumab continuously in vitro to induce resistance against this antibody and investigate the mechanisms responsible for this resistance. Some studies indicated that trastuzumab treatment does not alter the cell-surface HER2 expression status [30, 31]. However, we have demonstrated that continuous exposure to trastuzumab results in HER2 downregulation in HER2-overexpressing breast cancer cell lines in vitro. Previous studies also showed that alternative receptor tyrosine kinase signaling may play a role in trastuzumab resistance [18–20]. In fact, trastuzumab-exposed SKBR3 cells exhibited upregulated IGF-1R expression, suggesting that an alternative signaling pathway was enhanced to protect cells from trastuzumab-mediated HER2 signaling inhibition.
We also found that trastuzumab-exposed HER2-overexpressing breast cancer cells developed impaired trastuzumab-mediated ADCC activity in vitro. The ability of trastuzumab to mediate ADCC activity is strictly related to HER2 density [7]. In addition, Mimura et al. [32] previously reported that the HER2 status determined by flow cytometry is well correlated with trastuzumab-mediated ADCC activity in esophageal squamous cell carcinoma cell lines in vitro. Taking into account these reports, we conclude that the impaired trastuzumab-mediated ADCC activity in trastuzumab-exposed HER2-positive human cancer cells was due to the downregulation of HER2 expression on the cell surface. These results led us to examine whether exogenous expression of the HER2 receptor on the cell surface could re-sensitize HER2-downregulated human cancer cells to trastuzumab via ADCC re-activation.
HER2 overexpression is a significant prognostic factor in terms of nodal status, tumor grade, overall survival and probability of relapse in breast cancer patients [33, 34]. Although reports are conflicting, some studies have suggested that HER2-positive status in gastric cancer is associated with poor outcomes and aggressive disease [12, 13]. As expected, human cancer cells transfected with the full-length functional HER2 showed accelerated cell growth as compared to parental cells, whereas the cell growth pattern of HER2-ECD-transfected low HER2-expressing human cancer cells was similar to that of parental cells. Furthermore, we showed that HER2-ECD transfection of low HER2-expressing human cancer cells did not enhance the HER2/HER3 signaling pathway, which is the major oncogenic signal in HER2-overexpressing breast tumors [35, 36]. Although transfection of HER2-ECD-expressing plasmid did not influence cell growth, adenovirus-mediated exogenous HER2-ECD expression significantly suppressed the tumor cell growth in vitro, suggesting that the growth inhibition associated with HER2-ECD overexpression might be due to its levels on the cell surface. Therefore, Ad-HER2-ECD infection showed slightly enhanced cytotoxic activity against some types of human cancer cells even with the control antibody rituximab in the 51Cr release assay. The mechanism of Ad-HER2-ECD-mediated cell growth inhibition is unclear; however, it is likely to be caused by the restriction of other HER family receptors through the formation of heterodimers with exogenously expressed HER2-ECD that lacks the downstream signaling pathway.
Some previous studies demonstrated that primary or acquired resistance to trastuzumab often results from preventing the binding of antibody to the HER2 protein by proteins such as membrane-associated glycoprotein mucin-4 [37, 38]. In our study, even after a long-term exposure to trastuzumab, trastuzumab-mediated ADCC activity on stably HER2-ECD-expressing MCF7 cells was significantly enhanced compared to mock-treated MCF7 cells, and, furthermore, HER2-downregulated or low HER2-expressing human cancer cells could be re-sensitized to trastuzumab via re-activation of trastuzumab-mediated ADCC. These results indicate that the degree of antibody-mediated ADCC activity is likely to be correlated with the cell-surface expression levels of HER2. These results suggest that the HER2-downregulated or low HER2-expressing human cancer cells exogenously overexpressing HER2-ECD is hard to develop resistance to trastuzumab in terms of the importance of ADCC activity in antitumor effects of this antibody.
A previous study has demonstrated that heterogeneity and incomplete membranous immunoreactivity for HER2 were more common in gastric cancer than in breast cancer [39], suggesting that the gastric tumors diagnosed as HER2-positive by immunohistochemistry or fluorescent in situ hybridization are more likely to be residual and re-grow under trastuzumab treatment. Therefore, molecular sensitization to trastuzumab through the expression of HER2-ECD is thought to be effective even against HER2-positive gastric cancer. We would like to examine whether the ADCC activation by exogenous HER2-ECD expression functions in vivo; however, since murine NK cells do not recognize trastuzumab, which is a humanized antibody, the in vivo experiments are hard to be performed. The genetically engineered fluorescent tumor cells as well as the whole-body fluorescent imaging technology may be available for such kinds of in vivo studies [40, 41].
Although the strategy for molecular sensitization to trastuzumab via ADCC activation by using an adenoviral vector is considered to be effective, some limitations exist; for example, there are variations in the efficiency of viral infection and the expression levels of exogenous HER2-ECD. As we used a replication-deficient adenovirus vector, the viral spread might be less than ideal after intratumoral administration. We previously developed a telomerase-specific oncolytic adenovirus that causes cell death in human cancer cells with telomerase activities. These oncolytic viruses engineered to replicate in tumor cells but not in normal cells could be used as tumor-specific vectors carrying therapeutic genes such as HER2-ECD. Moreover, ADCC activity of PBMCs from cancer patients is likely to be impaired due to immunosuppression and NK cell dysfunction, as previously reported for gastric cancer patients [42, 43]. The immunosuppressive state is associated with immunosuppressive cytokines such as IL-10 and TGF-β. These cytokines are produced within the tumor microenvironment and suppress the activity of NK cells, monocytes, and T cells [43–46]. Therefore, to sufficiently enhance the effect of trastuzumab-mediated ADCC activity in cancer patients, supportive immunotherapy such as the administration of immune-stimulating cytokines may be required.
In conclusion, our data demonstrate that HER2 downregulation and impaired ADCC activity may be one mechanism of trastuzumab resistance. We also show that exogenous overexpression of non-signaling HER2-ECD could sensitize HER2-downregulated or HER2-negative human cancer cells via ADCC activation, an outcome that has important implications for the treatment of human cancers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr. Mien-Chie Hung (M.D. Anderson Cancer Center) for supplying complementary DNAs of human full-length HER2 (HER2-wt) and truncated HER2 containing extracellular and transmembrane regions (HER2-ECD). We also thank Tomoko Sueishi for her excellent technical support. This work was supported by grants-in-aid from the Ministry of Education, Science, and Culture, Japan (T. F.), and grants from the Ministry of Health and Welfare, Japan (T. F.).
Conflict of interest
All authors state that they have no potential conflicts of interest.
Abbreviations
- HER2
human epidermal growth factor receptor 2
- ADCC
antibody-dependent cellular cytotoxicity
- NK
natural killer
- ECD
extracellular domain
- MOI
multiplicity of infection
- IGF-1R
insulin-like growth factor-1 receptor
- Ad5
adenovirus type 5
- tzb
trastuzumab
References
- 1.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 2.Sarup JC, Johnson RM, King KL, Fendly BM, Lipari MT, Napier MA, Ullrich A, Shepard HM. Characterization of an anti-p185HER2 monoclonal antibody that stimulates receptor function and inhibits tumor cell growth. Growth Regul. 1991;1:72–82. [PubMed] [Google Scholar]
- 3.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- 4.Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E, Castiglioni F, Villani L, Magalotti C, Gibelli N, Oliviero B, Ballardini B, Da Prada G, Zambelli A, Costa A. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10:5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 5.Cooley S, Burns LJ, Repka T, Miller JS. Natural killer cell cytotoxicity of breast cancer targets is enhanced by two distinct mechanisms of antibody-dependent cellular cytotoxicity against LFA-3 and HER2/neu. Exp Hematol. 1999;27:1533–1541. doi: 10.1016/S0301-472X(99)00089-2. [DOI] [PubMed] [Google Scholar]
- 6.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 7.Lewis GD, Figari I, Fendly B, Wong WL, Carter P, Gorman C, Shepard HM. Differential responses of human tumor cell lines to anti-p185HER2 monoclonal antibodies. Cancer Immunol Immunother. 1993;37:255–263. doi: 10.1007/BF01518520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 9.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 10.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 11.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 12.Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 13.Tanner M, Hollmen M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, Elenius K, Isola J. Amplification of HER-2 in gastric carcinoma: association with topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- 14.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.20.3.719. [DOI] [PubMed] [Google Scholar]
- 15.Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27:5838–5847. doi: 10.1200/JCO.2009.22.1507. [DOI] [PubMed] [Google Scholar]
- 16.Scaltriti M, Rojo F, Ocana A, Anido J, Guzman M, Cortes J, Di Cosimo S, Matias-Guiu X, Ramon y Cajal S, Arribas J, Baselga J. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 17.Molina MA, Saez R, Ramsey EE, Garcia-Barchino MJ, Rojo F, Evans AJ, Albanell J, Keenan EJ, Lluch A, Garcia-Conde J, Baselga J, Clinton GM. NH(2)-terminal truncated HER-2 protein but not full-length receptor is associated with nodal metastasis in human breast cancer. Clin Cancer Res. 2002;8:347–353. [PubMed] [Google Scholar]
- 18.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, Moasser MM. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Y, Zi X, Pollak M. Molecular mechanisms underlying IGF-I-induced attenuation of the growth-inhibitory activity of trastuzumab (Herceptin) on SKBR3 breast cancer cells. Int J Cancer. 2004;108:334–341. doi: 10.1002/ijc.11445. [DOI] [PubMed] [Google Scholar]
- 20.Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 21.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, Hortobagyi GN, Hung MC, Yu D. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Depowski PL, Rosenthal SI, Ross JS. Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Mod Pathol. 2001;14:672–676. doi: 10.1038/modpathol.3880371. [DOI] [PubMed] [Google Scholar]
- 23.Pandolfi PP. Breast cancer—loss of PTEN predicts resistance to treatment. N Engl J Med. 2004;351:2337–2338. doi: 10.1056/NEJMcibr043143. [DOI] [PubMed] [Google Scholar]
- 24.Beano A, Signorino E, Evangelista A, Brusa D, Mistrangelo M, Polimeni MA, Spadi R, Donadio M, Ciuffreda L, Matera L. Correlation between NK function and response to trastuzumab in metastatic breast cancer patients. J Transl Med. 2008;6:25. doi: 10.1186/1479-5876-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reslan L, Dalle S, Dumontet C. Understanding and circumventing resistance to anticancer monoclonal antibodies. MAbs. 2009;1:222–229. doi: 10.4161/mabs.1.3.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 27.Pierce JH, Arnstein P, DiMarco E, Artrip J, Kraus MH, Lonardo F, Di Fiore PP, Aaronson SA. Oncogenic potential of erbB-2 in human mammary epithelial cells. Oncogene. 1991;6:1189–1194. [PubMed] [Google Scholar]
- 28.Wallasch C, Weiss FU, Niederfellner G, Jallal B, Issing W, Ullrich A. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J. 1995;14:4267–4275. doi: 10.1002/j.1460-2075.1995.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin BJ, Sela M, Yarden Y. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- 30.Austin CD, De Maziere AM, Pisacane PI, van Dijk SM, Eigenbrot C, Sliwkowski MX, Klumperman J, Scheller RH. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell. 2004;15:5268–5282. doi: 10.1091/mbc.E04-07-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hommelgaard AM, Lerdrup M, van Deurs B. Association with membrane protrusions makes ErbB2 an internalization-resistant receptor. Mol Biol Cell. 2004;15:1557–1567. doi: 10.1091/mbc.E03-08-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mimura K, Kono K, Hanawa M, Kanzaki M, Nakao A, Ooi A, Fujii H. Trastuzumab-mediated antibody-dependent cellular cytotoxicity against esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11:4898–4904. doi: 10.1158/1078-0432.CCR-04-2476. [DOI] [PubMed] [Google Scholar]
- 33.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 34.Berger MS, Locher GW, Saurer S, Gullick WJ, Waterfield MD, Groner B, Hynes NE. Correlation of c-erbB-2 gene amplification and protein expression in human breast carcinoma with nodal status and nuclear grading. Cancer Res. 1988;48:1238–1243. [PubMed] [Google Scholar]
- 35.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, Sliwkowski MX, Stern HM. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 37.Price-Schiavi SA, Jepson S, Li P, Arango M, Rudland PS, Yee L, Carraway KL. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer. 2002;99:783–791. doi: 10.1002/ijc.10410. [DOI] [PubMed] [Google Scholar]
- 38.Nagy P, Friedlander E, Tanner M, Kapanen AI, Carraway KL, Isola J, Jovin TM. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65:473–482. [PubMed] [Google Scholar]
- 39.Hofmann M, Stoss O, Shi D, Buttner R, van de Vijver M, Kim W, Ochiai A, Ruschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto N, Jiang P, Yang M, Xu M, Yamauchi K, Tsuchiya H, Tomita K, Wahl GM, Moossa AR, Hoffman RM. Cellular dynamics visualized in live cells in vitro and in vivo by differential dual-color nuclear-cytoplasmic fluorescent-protein expression. Cancer Res. 2004;64:4251–4256. doi: 10.1158/0008-5472.CAN-04-0643. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman RM. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat Rev Cancer. 2005;5:796–806. doi: 10.1038/nrc1717. [DOI] [PubMed] [Google Scholar]
- 42.Gafter U, Sredni B, Segal J, Kalechman Y. Suppressed cell-mediated immunity and monocyte and natural killer cell activity following allogeneic immunization of women with spontaneous recurrent abortion. J Clin Immunol. 1997;17:408–419. doi: 10.1023/A:1027372409361. [DOI] [PubMed] [Google Scholar]
- 43.Kono K, Takahashi A, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Impaired antibody-dependent cellular cytotoxicity mediated by herceptin in patients with gastric cancer. Cancer Res. 2002;62:5813–5817. [PubMed] [Google Scholar]
- 44.Hsiao YW, Liao KW, Hung SW, Chu RM. Tumor-infiltrating lymphocyte secretion of IL-6 antagonizes tumor-derived TGF-beta 1 and restores the lymphokine-activated killing activity. J Immunol. 2004;172:1508–1514. doi: 10.4049/jimmunol.172.3.1508. [DOI] [PubMed] [Google Scholar]
- 45.Webb BJ, Bochan MR, Montel A, Padilla LM, Brahmi Z. The lack of NK cytotoxicity associated with fresh HUCB may be due to the presence of soluble HLA in the serum. Cell Immunol. 1994;159:246–261. doi: 10.1006/cimm.1994.1311. [DOI] [PubMed] [Google Scholar]
- 46.Tsuruma T, Yagihashi A, Hirata K, Torigoe T, Araya J, Watanabe N, Sato N. Interleukin-10 reduces natural killer (NK) sensitivity of tumor cells by downregulating NK target structure expression. Cell Immunol. 1999;198:103–110. doi: 10.1006/cimm.1999.1586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.