Abstract
In a clinical phase I/II trial, pediatric patients with high-risk malignancies were treated with ex vivo IL-2-stimulated donor natural killer (NK) cells after transplantation with haploidentical stem cells. To evaluate the potential negative effects of the immunosuppressive drug mycophenolate mofetil (MMF) used for immunotherapy, the functionality and signaling of ex vivo NK cells was investigated. Our results show that during NK cell expansion, long-term (9 days) incubation with mycophenolic acid (MPA), the active metabolite of MMF, in therapeutically relevant concentrations led to the severe inhibition of NK cell proliferation. This correlated with a significantly reduced cytokine/chemokine secretion and the inhibited acquisition of surface receptors regarding cytotoxicity (e.g., NKp30, NKp44, NKp46, NKG2D), adhesion/migration (e.g., ICAM-1/CD54, LFA-1/CD11a, CD62L, CXCR3) and activation (e.g., CD25). Moreover, MPA prevented phosphorylation of the central signaling molecules STAT-3/-4/-5, AKT and ERK1/2. In contrast, short-term (24 h) MPA incubation of IL-2-stimulated NK cells had no or only marginal effects on the activated NK cell phenotype, including receptor expression, cytokine/chemokine secretion and intracellular signaling. Further, short-term MPA incubation only moderately affected the highly cytotoxic activity of previously IL-2-stimulated NK cells. In conclusion, while long-term MPA incubation significantly compromised ex vivo NK cell functionality, previously IL-2-activated NK cells seemed to be rather resistant to short-term MPA treatment. This finding supports the use of IL-2-activated NK cells as immunotherapy, especially for patients treated with MMF after haploidentical stem cell transplantation.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1556-5) contains supplementary material, which is available to authorized users.
Keywords: MMF, MPA, NK cells, Immunosuppressive therapy, Immunotherapy, Haploidentical stem cell transplantation
Introduction
Natural killer (NK) cells are lymphocytes of the innate immune system that are characterized phenotypically by a CD56+CD3− antigen expression, which can be subdivided into two main subpopulations. The largest population is composed of the CD56dimCD16+ NK cells that perform cytotoxic functions [1], and the smaller group comprises CD56brightCD16dim/− cells that have predominantly immune regulatory functions via their potent cytokine producing capacity [2, 3]. NK cell cytotoxicity is mediated by a balance of inhibitory and activating signals that are mediated by a specific receptor repertoire and various adhesion and co-stimulatory molecules [4]. While most inhibitory signals are transmitted by killer-cell immunoglobulin-like receptors according to the “missing self” hypothesis [5], activating signals are mediated by receptors such as the natural cytotoxicity receptors (NCR) NKp30, NKp44 and NKp46 and the NK group 2D (NKG2D) receptors [6, 7]. The IL-2 stimulation of NK cells leads to a significant up-regulation of all NCRs and NKG2D receptors and to an increase in cytokine secretion, which is associated with enhanced cytotoxic activity against leukemic and tumor cells [8, 9]. Therefore, immunotherapeutic trials with unstimulated and either ex vivo or in vivo IL-2-activated donor NK cells have focused on treating patients with high-risk malignancies in non-transplant settings and after haploidentical stem cell transplantation (haplo-SCT) [10–15]. However, extensive graft-versus-host-disease (GvHD) prophylaxis, predominantly consisting of the immunosuppressive drug mycophenolate mofetil (MMF), early after haplo-SCT with, e.g., CD3-depleted grafts may compromise the beneficial graft-versus-leukemia/tumor (GvL/T) effect of highly activated donor NK cells during immunotherapy.
Mycophenolic acid (MPA), the active metabolite of the prodrug MMF, is a non-competitive, reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH). The selective inhibition of IMPDH, a central enzyme in the de novo pathway of guanosine and deoxyguanosine nucleotide biosynthesis, leads to a decrease in nucleotides, resulting in the inhibition of cell proliferation. Because lymphocytes strongly rely on the de novo pathway for purine biosynthesis, MPA more effectively inhibits the proliferation of lymphocytes than of other cell types [16–18]. While the impairment of T cells has been extensively described in the literature, very little is known about the influence of MMF on NK cells. In this good clinical practice (GCP)-conform phase I/II trial, we treated pediatric patients who have high-risk leukemia and tumors with ex vivo IL-2-stimulated NK cell donor lymphocyte infusions (NK-DLI) at day +40 and +100 after haplo-SCT [10]. However, at the time of NK-DLI, patients receive the immunosuppressive drug MMF to prevent severe GvHD. Therefore, to evaluate the possible consequences for NK cell-based immunotherapy, we investigated the effects of long- and short-term MPA incubation in therapeutically relevant doses on non-activated and previously IL-2-activated NK cells.
Materials and methods
NK cell immunotherapy after haplo-SCT and immunosuppressive therapy
In a clinical phase I/II trial, a total of 16 pediatric patients received unstimulated (n = 9, suppl. Fig. 1a) and/or IL-2-stimulated (n = 9, suppl. Fig. 1b) NK cells from their respective haploidentical stem cell donors (clinicaltrials.gov identifier: NCT01386619), as we have described in detail [8, 10, 15, 19, 20]. Two patients received both unstimulated and IL-2-stimulated NK cells. Because of the progressive stem cell purification procedure from CD34-selection to CD3/CD19-depletion, immunosuppressive therapy was necessary because of residual amounts [up to 1 × 105 kg body weight (BW)] of CD3+ donor T cells in the grafts. Therefore, as GvHD prophylaxis MMF (Cellcept®, Roche Bioscience) was administered beginning 1 day before haplo-SCT with CD3/CD19-depleted grafts. The initial dose of MMF was 30 mg/kg BW and was individually tapered during the following weeks or months, depending on the patients’ condition, until the CD3+ T cells in the peripheral blood (PB) reached a concentration of 100/µl. In the case of acute GvHD, MMF was reintroduced, either alone or with corticosteroids or Cyclosporin A. Written informed consent was obtained from all children, parents or legal guardians according to the guidelines of the medical ethics committee of the University Hospital Frankfurt (Ref.No. 262/03). The material used in the experiments regarding the influence of MMF on IL-2-stimulated NK cells was collected from six donors or patients, whereof two patients dropped out of the study, leaving four to be included in the immunotherapy trail (Table 1).
Table 1.
Characteristics of selected patients in clinical phase I/II NK cell immunotherapy trial
| No. | Sex, age (years) | BW (kg) | Diagnosis, status | Conditioning regime | Graft | CD34+ (106/kg BW) | CD56+ CD3− (106/kg BW) | CD3+ (103/kg BW) | CD56+ CD3− (106/kg BW) | CD56+ CD3+ (103/kg BW) | CD56− CD3+ (103/kg BW) | GvHD prophylaxis | GvHD Grade | Sample collection | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In Stem Cell Graft | In NK-DLI | Cytotox MPA | Cytotox plasma | Cytotox steroid | Density# | Video | ||||||||||||
| 1 | f, 16 | 90 | ALL, NR | Flu/Thio/Mel/OKT3 | CD3/19 | 8.0 | 5.8 | 92.6 | 15.0 | 29.3 | 2.4 | MMF, Steroids | I | × | × | × | ||
| 2 | m, 4 | 14 | NB IV, NR | Treo/Flu/Thio/OKT3 | CD3/19 | 10.0 | 9.0 | 123.4 | – * | – * | – * | MMF | – | × | × | × | × | |
| 3 | f, 16 | 47 | AML, NR | Flu/Thio/Mel/OKT3 | CD3/19 | 8.4 | 55.8 | 143.4 | 6.6 | 8.8 | 0.3 | MMF | – | × | × | × | ||
| 4 | m,15 | 51 | AML, NR | Treo/FluThio/ATG | CD3/19 | 5.5 | 25.9 | 100.1 | 14.6 | 11.3 | 4.1 | MMF | – | × | × | × | ||
| 5 | f, 1 | 7 | AML, NR | Treo/Flu/Thio/ATG | CD3/19 | 12.7 | 17.9 | 44.4 | 14.9 | 20.9 | 31.6 | MMF | – | × | ||||
| 6 | m, 15 | 38 | RMS IV, PR | Flu/Theo/Mel/OKT3 | CD3/19 | 4.8 | 23.4 | 74.6 | 13.7 | 53.7 | 24.4 | MMF | – | × | ||||
| Median | 8.0 | 23.4 | 100.1 | 14.2 | 20.3 | 3.3 | ||||||||||||
ALL acute lymphatic leukemia, AML acute myeloid leukemia, ATG anti-thymocyte globulin, BW body weight, CD3/19 CD3/CD19-depleted stem cell graft, f female, Flu fludarabine, GvHD graft-versus-host-disease, m male, Mel melphalan, MMF mycophenolate mofetil, NB neuroblastoma, NK-DLI NK cell donor lymphocyte infusion, No. number, NR nonremission, OKT3 Orthoclone/muronomab, PR partial remission, RMS rhabdomyosarcoma, Thio Thiotepa, Treo Treosulfan
* patient died before IL-2 stim. NK-DLI was applied, # data not shown
NK cell purification and cultivation
For clinical applications, NK cells were purified by a two-step CD3-depletion/CD56-selection procedure using a CliniMACS device (Miltenyi biotec, Bergisch Gladbach, Germany) (median purity 95 %, range 84.4–98.6) [10] and cultivated for 9–10 days using 1,000 U/ml of IL-2, according to good manufacturing practice (GMP), as we have described in detail [10, 11, 19, 21]. For the study accompanying bench scale experiments, NK cells were isolated from whole blood and purified using a EasySep® Human NK Cell Enrichment Kit (STEMCELL™-Technologies, Vancouver, Canada). NK cells (median purity 97 %, range 94.9–98.7) were cultured under the same conditions as the clinically derived NK cells, at cell culture concentrations of 1–2 × 106/ml in X-VIVO™10 medium (Lonza, Verviers, Belgium) + 5 % heat-inactivated human fresh frozen plasma for 9–10 days using 1,000 U/mL IL-2 at 37 °C and 5 % CO2. For these experiments, MPA (Sigma-Aldrich, Taufkirchen, Germany) or PB plasma from the respective patient, which was collected prior to NK-DLI application, was added to the cell culture at the indicated concentrations.
Sample collection and preparation for ex vivo investigations
For the experiments regarding proliferation (Fig. 1), surface receptor expression (Fig. 3), intracellular signaling (Fig. 4) and cytokine/chemokine secretion (Fig. 5), bench scale purified NK cells of healthy donors were used (n = 10 independent experiments). For the study accompanying experiments, residual amounts of quality control samples of the freshly generated NK-DLI on day 40 after SCT allowed the analysis of the influence of MPA on donor NK cell cytotoxicity (Fig. 2a, NK cell donors for patients No. 1–5, Table 1, n = 5). Additionally, cytotoxicity was investigated following short-term (24 h) incubation with patients’ PB plasma taken prior to NK-DLI (Fig. 2c, NK cell donors for patients No. 1–4, Table 1, n = 4), as was receptor density expression (data not shown, NK cell donors for patients No. 2–4 and 6, Table 1, n = 4). One patient also received steroids (prednisolone, 1 × 2 mg/kg BW) because of more severe adverse reactions, which allowed investigation of the influence of steroid therapy on the cytotoxicity of IL-2-stimulated donor NK cells (Fig. 2d, donor for patient No. 1, Table 1, n = 1). For time-lapse microscopy, the patient’s primary human neuroblastoma (NB) cells were co-incubated with residual amounts of quality control samples of IL-2-stimulated NK-DLI from the donor (Fig. 2b and suppl. video 1+2, patient No. 2, Table 1, n = 1) [22, 23].
Fig. 1.
Dose-dependent inhibition of ex vivo NK cell proliferation by long-term MPA treatment. a Evaluation of potent MPA dose. Overlay histogram plots show a dose-dependent inhibition of the proliferation of CFSE-labeled NK cells indicated by reduced CFSE fluorescence intensity. The right overlay plot gives an overview of the tested MPA concentrations. Here, 10 µM MPA was the lowest concentration that completely inhibited proliferation. Plots gated on viable CD56+CD3− NK cells (red: IL-2-stimulated NK cells + MPA; black: IL-2-stimulated NK cells d9 (positive control); dark gray area: unstimulated NK cells d0 (negative control); light gray area: autofluorescence IL-2-stimulated NK cells). b Inhibition and reversibility of MPA. The proliferation of IL-2-stimulated NK cells was severely inhibited in the first cell divisions by 10 µM MPA. This MPA-mediated inhibition was completely reversed after the removal of MPA from the cell medium on day 3, and these NK cells also reached the 7th cell generation during 9 days of IL-2 stimulation. Plots gated on viable CD56+CD3− NK cells. c Effect in absolute cell count. Following an initial decrease in cell count, NK cells showed a mean 2.7-fold expansion. Long-term treatment with 10 µM MPA resulted in an absolute inhibition of proliferation, which correlated with a complete decrease in viable cell count (n = 10, mean ± SD). Plots gated on viable CD56+CD3− NK cells. d CD56 surface expression. Unstimulated PB NK cells showed a CD56dimCD16+ (85.8 %) and CD56brightCD16dim/− (14.3 %) phenotype. Following IL-2 stimulation, CD56 surface expression became highly up-regulated [mean fluorescence intensity (MFI) 7–73]. This was completely inhibited by long-term MPA incubation (MFI 10). Plots gated on viable CD56+CD3− NK cells. Plots show representative results of n = 10 experiments
Fig. 3.
Short- and long-term influence of MPA on surface expression of NK cell receptors. a Influence of long- and short-term MPA incubation on surface phenotype. Plots show MFI of central NK cell surface molecules involved in cytotoxicity (NKp30, NKp44, NKp46, NKG2D, NKG2A, DNAM-1), adhesion/migration (CXCR3, CCR5), CD11a/LFA-1, CD62L/L-selectin), and activation (CD25/IL2-Rα). All investigated surface molecules become highly up-regulated upon IL-2 stimulation (white square) compared with unstimulated NK cells at day 0. This was significantly inhibited by long-term MPA incubation (red triangle), while short-term (24 h) MPA treatment (orange triangle) had no effect on previously up-regulated surface receptors (n = 10, plots gated on viable CD56+CD3− NK cells, p < 0.05 and p < 0.01 indicated as * and **, Wilcoxon matched-pairs signed rank test). b Overlay plots show representative results regarding NKG2D, CD11a and CD25 expression following 9 days of IL-2 stimulation (black) ± long- (red) and short-term (orange) MPA incubation compared with unstimulated NK cells at day 0 (gray area), (MFI vs. events, plots gated on viable CD56+CD3− NK cells)
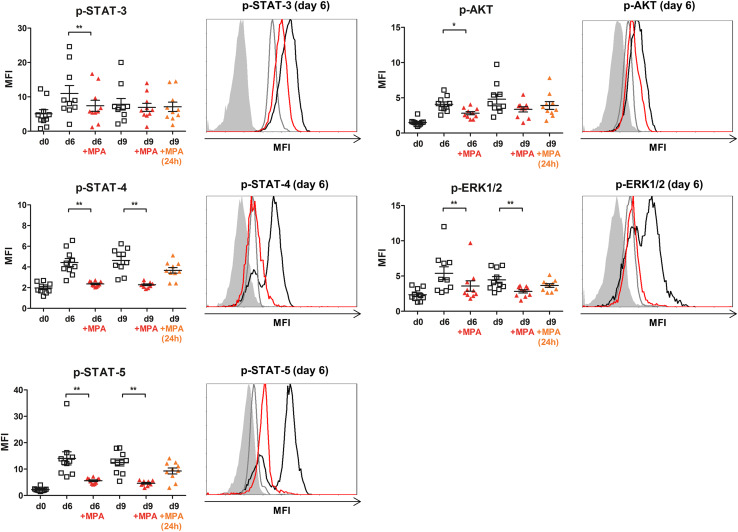
Fig. 4.
Long-term MPA incubation significantly inhibits phosphorylation of central intracellular signaling molecules in NK cells. Influence of long- and short-term MPA incubation on STAT-3, STAT-4, STAT-5, AKT and ERK1/2 phosphorylation. Intracellular signaling molecules became phosphorylated upon IL-2 stimulation with an activation peak at day 6 (STAT-3,-5, ERK1/2) or day 9 (STAT-4, AKT), (white square) compared with unstimulated NK cells at day 0. Long-term MPA incubation (red triangle) significantly inhibited the IL-2-induced phosphorylation/activation, while short-term (24 h) MPA treatment (orange triangle) had no or a minimal effect on previously activated signaling molecules (n = 10, plots gated on CD56+CD3− NK cells, p < 0.05 and p < 0.01 indicated as * and **, Wilcoxon matched-pairs signed rank test). Overlay plots show representative results regarding p-STAT-3, -4, -5, p-AKT and p-ERK1/2 expression following 6d IL-2 stimulation (black) ± long-time (red) MPA incubation compared with unstimulated NK cells at d0 (gray) and autofluorescence (gray area), (MFI vs. events, plots gated on CD56+CD3− NK cells)
Fig. 5.
Ex vivo cytokine and chemokine secretion of IL-2-stimulated NK cells ± MPA. Absolute NK cell count (left Y-axis, black) in relation to cytokine/chemokine concentration in the supernatant of NK cell culture (right Y-axis, green). An initial decrease in viable cell count was followed by the expansion of IL-2-stimulated NK cells (white bars), which was severely decreased by long-term MPA treatment (red bars). The high secretion of IFN-γ, TNF-α, CXCL8, IL-6, CCL2, CCL4 and CCL5 during IL-2 stimulation (green square, black border) was significantly inhibited by the additional treatment with MPA (green square, red border). Left Y-axis: absolute NK cell count; right Y-axis: cytokine/chemokine concentration with different scaling ranging from 0 to 400,000 pg/ml; X-axis: days of cultivation ±10 µM MPA (n = 10, mean ± SEM, gated on viable CD56+CD3− NK cells, p < 0.05 and p < 0.01 indicated as * and **, Wilcoxon matched-pairs signed rank test)
Fig. 2.
NK cell cytotoxicity is significantly reduced by MPA and patient’s plasma obtained during immunosuppressive therapy. a Influence on NK cell cytotoxicity. The high cytotoxic activity of IL-2-stimulated donor NK cells (white) was significantly reduced following short-term incubation (24 h) with 10 µM MPA (red). E:T ratios 1:1, 5:1 and 10:1 against K562 cells (median/range, n = 5 donors in duplicate experiments, Table 1, donors for patients No. 1–5, p < 0.01 indicated as **, Wilcoxon matched-pairs signed rank test). b Influence on NK cell morphology. Representative snapshots of two time-lapse microscopy videos (suppl. material video 1 and 2), magnification 200-fold. Donor NK cells were co-cultured with primary NB cells growing as tumor spheroids (arrow) isolated from a tumor metastasis. IL-2-stimulated NK cells showed a typical polar morphology (left, black inverted triangle) while short-term (24 h) MPA-treated NK cells had a narrowed and rounded morphology (right, red inverted triangle). c Influence of patient PB plasma on NK cell cytotoxicity. The highly cytotoxic activity of the IL-2-activated donor NK cells (white) was significantly reduced following pre-incubation with patient PB plasma for 24 h (red). E:T ratios 1:1, 5:1 and 10:1 against K562 cells (median/range, n = 4 donors in duplicate experiments, Table 1, donors for patients No. 1–4, p < 0.01 indicated as **, Wilcoxon matched-pairs signed rank test). d Influence of steroid application during NK-DLI on NK cell cytotoxicity. Short-term incubation (24 h) of stimulated donor NK cells with patient`s PB plasma collected after single steroid application showed the most prominent reduction in cytotoxicity. The high target killing activity of 66.4 % (white) was reduced to 52.7 % after the patient`s plasma incubation alone (red) and was further reduced to 34.5 % after patient’s plasma incubation including steroids (red pattern), (mean ± SD, n = 1 donor in duplicate experiments, Table 1, donor for patient No. 1, E:T ratio 1:1 against K562 cells, p < 0.05 and p < 0.01 indicated as * and **, t-test)
Flow cytometric analyses
All analyses were performed using a FC500 (Beckman Coulter) and monoclonal antibodies conjugated with FITC, PE, ECD, PC-5 and PC-7 were used against following antigens: CD3, CD11a#, CD16, CD252, CD45, CD54, CD56, CD62L, CD812, CD159a/NKG2A1, CD183/CXCR3#, CD195/CCR52#, CD226/DNAM-1#, CD314/NKG2D, CD335/NKp46, CD336/NKp44, CD337/NKp30, p-STAT-33, p-STAT-41#, p-STAT-5#, p-P44/42MAPK(ERK1/2) and p-AKT3 (all mouse IgG1, other than 1IgG2b, 2IgG2a, 3IgG rabbit), (#BD Biosciences; all other Beckman Coulter). 7-AAD was used to assess cell viability. Absolute lymphocyte subset counts were calculated via single-platform using Flow-Count™ fluorospheres (Beckman Coulter).
Cell proliferation
The CellTrace™ CFSE Proliferation Kit (Molecular Probes™ Eugene, USA) was used according to Quah et al. [24]. Proliferation was analyzed using the CXP v2.2 software (Beckman Coulter) and the FlowJo software 7.6.4 (Tree Star Inc., Ashland, USA).
Cytotoxicity assay
The NK cell cytotoxicity was tested against K562 cells using a 5-color flow cytometric single-platform assay, as we have described previously [25]. The cytotoxicity of IL-2-stimulated donor NK cells was evaluated following the 24 h short-term incubation with 10 µM MPA or with the patients’ PB plasma obtained before the NK-DLI.
Time-lapse microscopy
Primary human NB cells growing as tumor spheroids cultured in a Chamber Slide™ system were co-incubated with IL-2-activated donor NK cells with or without 10 µM MPA for 5–6 h. Microscopy was performed using the Olympus IX71, as we have described earlier [23].
Intracellular signaling
To detect intracellular antigens, NK cells were permeabilized and fixed using formaldehyde/methanol. For staining purposes, 5 × 105/ml cells were washed and then incubated with antibody for 30–60 min. The pellet was resuspended in 500 µl PBS for flow cytometric analyses.
Cytokine/chemokine analysis
Cytokine/chemokine levels in the PB plasma samples were measured using the BD™ Cytometric-Bead-Array and FACSArray™ bioanalyzer (BD Biosciences). The human Flex Set was used to detect IL-2, -6, TNF-α, IFN-γ-, CXCL8/IL-8, CCL2/MCP-1, CCL4/MIP-1β and CCL5/RANTES according to the manufacturer’s instructions.
Results
MPA severely inhibits NK cell proliferation ex vivo in clinically relevant concentrations
Long-term incubation with MPA led to a dose-dependent inhibition of NK cell proliferation. While incubation with 100 and 10 µM MPA resulted in a total blockade of NK cell proliferation, 1 µM MPA led to a partial inhibition, and 100 nM MPA showed no effect compared with untreated IL-2-stimulated NK cells (Fig. 1a). The lowest dose with a total blocking effect, 10 µM MPA, was chosen for further ex vivo experiments. This was in accordance with therapeutically relevant in vivo mean PB plasma levels of MPA [26–28]. NK cells extensively proliferated upon IL-2 stimulation, reaching the 7th cell generation, while proliferation was severely inhibited within the first cell divisions through a long-term (9 days) incubation with 10 µM MPA (Fig. 1b). However, the MPA-mediated inhibition was completely reversed within 6 days after the removal of MPA from the cell medium on day 3, and these NK cells also reached the 7th cell generation.
Within the first 3 days of IL-2 stimulation, the initial decrease in the viable NK cell count was followed by a period of growth and increased viability during the following days of NK cell cultivation (Fig. 1c). The mean NK cell expansion after 9 days of IL-2 stimulation was 2.7-fold. In contrast, proliferation capacity was severely impaired after the long-term treatment with MPA, resulting in an absolute decrease in the viable NK cell count. In addition, the surface expression of CD56 became highly up-regulated upon IL-2 stimulation compared with unstimulated NK cells, leading to a predominant phenotype of CD56brightCD16+/− NK cells (Fig. 1d). This was completely inhibited by the treatment with MPA during the NK cell expansion.
NK cell cytotoxicity and mobility toward tumor cells is significantly reduced by treatment with 10 µM MPA
To investigate the influence of MMF on NK cell cytotoxicity and mobility, we compared the ability of IL-2-stimulated donor NK cells in the presence or absence of short-term (24 h) MPA treatment (1) to lyse K562 cells and (2) to interact with primary human NB cells. NK cell cytotoxicity was significantly reduced (p < 0.01) upon MPA incubation in all of the investigated E:T ratios (Fig. 2a). Although the effect of MPA was statistically significant, short-term MPA treatment resulted in a median reduction of 7 % target killing and, therefore, a moderate reduction in cytotoxic NK cell functionality. To investigate whether the reduced cytotoxicity induced by MPA treatment was associated with alterations in NK cell morphology and mobility, IL-2-activated donor NK cells in the presence or absence of short-term MPA treatment were co-incubated with primary NB cells of the patient. Two time-lapse microscopy movies were performed (supplementary material video 1 and 2), and representative snapshots are shown in Fig. 2b. While IL-2-activated NK cells showed the typical polar phenotype with high motility and cytotoxic activity toward the tumor cells, short-term MPA treatment severely affected NK cell morphology, leading to a narrowed, nonpolar and rounded phenotype with a reduced and undirected motility toward the tumor spheroids.
Patients’ PB plasma additionally reduces cytotoxicity of IL-2-activated NK cells
The high cytotoxicity of donor NK cells against K562 cells was reduced (p < 0.01) after their short-term incubation with PB plasma that was obtained from each patient before NK-DLI (Fig. 2c). The median reduction in NK cell target killing was 23 % after plasma incubation and was therefore more prominent than target killing after MPA incubation alone, as shown in Fig. 2a. The analyses of the MPA plasma levels revealed therapeutically relevant plasma levels of more than 1 µM MPA in 2 of the 4 patients whom we evaluated. Thus, no differences were observed in the cytotoxic activity in regard to MPA plasma levels within this small cohort. Furthermore, the short-term incubation of donor NK cells with plasma reduced the surface density of the NCRs and NKG2D receptors, which was statistically significant (p < 0.05) concerning NKG2D expression in both NK cell subpopulations (data not shown). The additional steroid treatment in one patient allowed the investigation of in vivo steroid treatment on the cytotoxicity of the highly activated IL-2-stimulated donor NK cells. Incubation with plasma from the patient who was taking steroids led to the most pronounced effect on NK cell functionality; it resulted in a median reduction of 31 % in target killing, leading to a cytotoxic activity of only 34.5 % (Fig. 2d).
Long- but not short-term MPA incubation significantly inhibits IL-2-mediated up-regulation of various surface antigens on NK cells
We further investigated the influence of (1) the long-term MPA treatment of NK cells from the beginning of the 9 day expansion process compared with (2) the short-term MPA treatment for 24 h of previously IL-2-stimulated NK cells. Specifically, the surface expression of several NK cell receptors involved in cytotoxicity (NKp30, NKp44, NKp46, NKG2D, NKG2A, DNAM-1), adhesion/migration (CXCR3, CCR5, CD54/ICAM-1, CD11a/LFA-1, CD62L/L-selectin) and activation (CD25/IL2-Rα) was studied. All investigated surface molecules became highly up-regulated upon IL-2 stimulation, with an expression peak after 6 or 9 days. The up-regulation was significantly inhibited (p < 0.01) by long-term MPA incubation, while short-term MPA treatment had no or only a marginal effect on previously up-regulated surface receptors (Fig. 3).
Long- but not short-term MPA incubation significantly inhibits phosphorylation of intracellular signaling molecules in NK cells
To further evaluate whether the observed impact of MPA on NK cell functionality was associated with an inhibition of the intracellular signaling pathways, we measured the phosphorylation and activation of the signal molecules STAT-3, STAT-4 and STAT-5 in the Jak/STAT pathway. Additionally, we assessed the PI3K/AKT pathway by measuring the phosphorylation of AKT and the activation of the MAPK/ERK pathway by ERK1/2 phosphorylation. All of these signaling molecules became highly phosphorylated after IL-2 stimulation with a peak day of activation at day 6 (STAT-3,-5, ERK1/2) or day 9 (STAT-4, AKT), compared with unstimulated NK cells at day 0. Thus, the long-term (9 days) MPA incubation significantly inhibited (STAT-3, AKT day 3 p < 0.05; STAT-4, STAT-5, ERK1/2 day 3 and day 9 p < 0.01) the IL-2-induced phosphorylation/activation, while short-term MPA treatment (24 h) showed no or only a minimal effect on the previously activated signaling molecules (Fig. 4).
Ex vivo cytokine and chemokine production of NK cells during MPA treatment
IL-2-stimulated NK cells secrete high amounts of the chemokines CXCL8, CCL2, CCL4 and CCL5 and the inflammatory cytokines IL-6, TNF-α and especially IFN-γ (Fig. 5). The cytokine/chemokine concentration peaked at day 3 (CXCL8, IL-2), day 6 (TNF-α, CCL2, CCL4) or day 9 (IFN-γ, IL-6, CCL5) of IL-2 stimulation, correlating with enhanced cellular growth. In contrast, long-term MPA treatment severely decreased the viable cell count, which was accompanied by the significant inhibition of cytokine/chemokine secretion compared with untreated IL-2-stimulated NK cells. However, the 24-h incubation of activated NK cells previously stimulated with IL-2 had no or only a minimal effect on cytokine/chemokine secretion (data not shown). Interestingly, high IL-2 concentrations in the untreated proliferating NK cell culture declined in proportion with the increasing cell counts, while the IL-2 levels in MPA-treated nonproliferating NK cell cultures remained constantly high.
Discussion
There is increasing interest in the use of adoptive NK cell immunotherapy after allogeneic hematopoietic SCT to enhance the potential GvL/T effect. However, prolonged immunosuppressive therapy after haplo-SCT using T cell-depleted grafts may compromise the efficacy of NK cell immunotherapy. While the effect of the immunosuppressive drug MMF and its active metabolite MPA on T cells has been extensively described in the literature, studies regarding the influence of MMF on NK cells are scarce. Accompanying to our clinical phase I/II immunotherapy trial infusing highly activated IL-2-stimulated donor NK cells after haplo-SCT in children, we evaluated the potential negative impact of MPA at clinically relevant concentrations on NK cell functionality. Thereby, we focused on the influence of long-term (9 days) in contrast to short-term (24 h) MPA treatment on ex vivo NK cell effector functions, including the cytotoxic activity against tumor cells, expression of activating receptors and adhesion molecules, cytokine/chemokine secretion, signaling and proliferation capacity.
We have previously shown that ex vivo IL-2 stimulation induces NK cell proliferation leading to a predominant phenotype of CD56brightCD16+/−, an enhanced expression of activating receptors and high cytokine/chemokine secretion, which is accompanied by strong cytotoxic activity [8]. The results presented here confirm our previous findings. Moreover, our data revealed that additional treatment with MPA results in a severe inhibition of all IL-2-mediated NK cell attributes. Specifically, NK cell proliferation was severely inhibited by 1–10 µM MPA. The anti-proliferative effect was reversed when MPA treatment was discontinued, and proliferation recovered completely within the following expansion period. This is in accordance with other ex vivo and in vivo studies showing the strong anti-proliferative effect of MPA or MMF treatment on PBMC (T and B cells) [17, 18].
Moreover, we found that MPA prevented the outgrowth of CD56bright NK cells and significantly suppressed the IL-2-mediated acquisition of important surface antigens involved in activation (CD25/IL-2Rα), adhesion (ICAM-1/CD54, LFA-1/CD11a, CD62L and the chemokine receptors CCR5 and CXCR3) and cytotoxicity (NCRs, NKG2D, NKG2A, DNAM-1). These data agree with data published by Eissens et al. and Ohata et al.[29, 30]. The activation of cytotoxic effector mechanisms is regulated by the dynamic interaction of various surface molecules, among which cell adhesion molecules are involved in verifying the appropriate cell–cell contact and the intracellular signal transduction of the activating signals [31, 32]. Therefore, our results indicate that MPA may have an effect on both target recognition and migration processes such as adhesion and chemotaxis. Our new findings in NK cells are in accordance with previous investigations in T cells reporting the inhibited expression of IL-2Rα, LFA-1 and ICAM-1 [33] and the inhibition of T cell migration by diminishing ICAM-1, VCAM-1, E-selectin and P-selectin in endothelial cells [34]. Furthermore, Allison et al. [35] demonstrated a significant reduction in cell adhesion in T cells and endothelial cells. This reduction was explained by the MPA-mediated depletion of guanosine nucleotides, which led to the suppression of the glycosylation and expression of adhesion molecules.
In addition, we were able to show for the first time that intracellular NK cell signaling pathways are inhibited by MPA. The central signaling cascades, Jak/STAT, MAPK/ERK and PI3K/AKT can be activated by either the ligand or the cytokine binding to the corresponding receptors. IL-2, the most prominent activator of NK cells, facilitates survival, proliferation and cytotoxicity [36, 37]. Engagement of the IL-2 receptor (IL-2Rαβγ) induces the phosphorylation of STAT-3 and STAT-5 in NK and T cells [38], while activation of STAT-4 exclusively occurs in NK cells and is related to NK cell cytotoxicity [39]. The activation of the protein kinase AKT is associated with proliferation, growth and especially cell survival, while ERK1/2 plays a crucial role in NK cell cytotoxicity [40, 41]. Our results clearly show that IL-2 stimulation causes the activation of the signal molecules that we studied, leading to an activation peak after either 6 or 9 days. These data correlate with the observed cytokine/chemokine secretion and presume the activation of the signaling cascades involved in cytokine/chemokine production within 6-9 days of IL-2 stimulation. However, the activation of the respective signaling pathways could also be caused by additional cytokine stimuli secreted during NK cell expansion. Additionally, the activation of signaling molecules correlated to the time- and IL-2-dependent expression of IL-2Rα (CD25). Therefore, the development of the high-affinity IL-2Rαβγ was inhibited by MPA. Importantly, we could show that the IL-2-mediated phosphorylation/activation of AKT, STAT-3 and STAT-5 was significantly and the activation of ERK1/2 and STAT-4 was completely inhibited by MPA. This inhibited intracellular signaling was accompanied by a significantly inhibited cytokine/chemokine secretion of IFN-γ, IL-6, TNF-α, CXCL8, CCL2, CCL4 and CCL5. Similarly, reduced IL-2, -3, -4, -5, -6, -10, IFN-γ and TNF-α secretion in human lymphocytes after incubation with MPA for 48 h has also been described [42]. Therefore, our results provide evidence that the MPA-induced inhibition of IL-2-dependent NK cell processes such as proliferation, survival and cytotoxicity is associated with a disturbed activation of these three central signaling pathways.
In contrast to the strong inhibitory effects of long-term MPA incubation, we showed that activated NK cells with highly up-regulated surface receptors seem to be resistant to MPA. Short-term MPA treatment (24 h) of previously IL-2-stimulated NK cells led to no or minimal changes in the expression of cell surface antigens regarding adhesion and migration, activating cytotoxicity receptors, intracellular signaling molecules and cytokine/chemokine secretion. However, the short-term MPA incubation of highly activated NK cells led to a statistically significant but not absolute reduction in NK cell cytotoxic activity against K562 cells and to a distinct inhibition of NK cell motility toward NB tumor spheroids. Therefore, additional mechanisms involved in NK cell cytotoxicity must be affected by MPA. In comparison, Ohata et al. [30] described a 50 % reduction in NK cell activity, resulting in NK cell cytotoxic activity of 15 and 10 % against K562 and Daudi cells when incubated for 7 days with 31.3 and 312.5 µM MPA, respectively. This correlated with a markedly reduced expression of all activating receptors. The discrepancy between these data and our results might be related to their 30-fold higher MPA doses and considerably longer incubation periods of freshly isolated unstimulated NK cells. Our cytotoxicity experiments focused on the short-term MPA treatment of previously stimulated NK cells for our clinical NK cell immunotherapeutic trial and our clinically observed MPA plasma levels.
In this context, we investigated the effect of the patients’ in vivo MMF administration by incubating highly activated IL-2-stimulated donor NK cells with PB plasma from samples collected before NK-DLI. Short-term plasma incubation significantly reduced NKG2D receptor expression and NK cell cytotoxicity. Anyhow, clinically relevant plasma MPA levels of more than 1 µM MPA were present in 2 of the 4 PB plasma samples only, and no differences in cytotoxicity in regard to MPA plasma levels were observed. Because of known significant intra- and inter-patient variability of MPA plasma levels, therapeutic drug monitoring after MMF administration has been controversial and, therefore, has not yet been incorporated into routine clinical practice [27]. In addition, we showed that the incubation of donor NK cells with one patient’s PB plasma obtained during single steroid treatment resulted in the strongest reduction in NK cell cytotoxicity. Severely impaired NK cell cytotoxicity and an inhibited NCR expression have been reported previously when NK cells were treated with the steroid methylprednisolone [43, 44]. Therefore, in addition to MPA, enhanced endogenous cortisol levels produced in response to a patient’s therapy may also dampen the efficacy of NK cell immunotherapy.
In summary, while long-term MPA treatment prevented the activation and functionality of NK cells, already highly cytotoxic IL-2-activated NK cells seem to be resistant to short-term MPA incubation. Therefore, to retain the potential beneficial GvL/T effect of allogeneic NK cells, our data clearly support the use of previously IL-2-activated NK cells for immunotherapy, especially for patients treated with MMF after haplo-SCT.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This project was supported by the “Deutsche Forschungsgemeinschaft (DFG)“(GRK-1172), “Hilfe für krebskranke Kinder Frankfurt e.V.” and the “LOEWE Center for Cell and Gene Therapy Frankfurt” funded by Hessisches Ministerium für Wissenschaft und Kunst, III L 4- 518/17.004 (2010). The authors would like to thank all patients, nurses and physicians and the members of the Laboratory of Stem Cell Transplantation and Immunotherapy for their technical support and Dr. D. Hintereder of the Central Laboratory for the quantification of total MPA in patients’ PB plasma.
Conflict of interest
The authors declare that they have no conflicts of interest.
Abbreviations
- BW
Body weight
- GCP
Good clinical practice
- GMP
Good manufacturing practice
- GvL/T
Graft-versus-leukemia/tumor
- GvHD
Graft-versus-host-disease
- haplo-SCT
Haploidentical stem cell transplantation
- IMPDH
Inosine monophosphate dehydrogenase
- MFI
Mean fluorescence intensity
- MMF
Mycophenolate mofetil
- MPA
Mycophenolic acid
- NB
Neuroblastoma
- NCR
Natural cytotoxicity receptors
- NK-DLI
Natural killer cell donor lymphocyte infusion
- NKG2D
NK group 2D
- PB
Peripheral blood
References
- 1.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–640. doi: 10.1016/S1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 2.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006;20(3):123–137. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7(5):329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 4.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 5.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11(7):237–244. doi: 10.1016/0167-5699(90)90097-S. [DOI] [PubMed] [Google Scholar]
- 6.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 7.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2(11):850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 8.Huenecke S, Zimmermann SY, Kloess S, Esser R, Brinkmann A, Tramsen L, Koenig M, Erben S, Seidl C, Tonn T, Eggert A, Schramm A, Bader P, Klingebiel T, Lehrnbecher T, Passweg JR, Soerensen J, Schwabe D, Koehl U. IL-2-driven regulation of NK cell receptors with regard to the distribution of CD16 + and CD16- subpopulations and in vivo influence after haploidentical NK cell infusion. J Immunother. 2010;33(2):200–210. doi: 10.1097/CJI.0b013e3181bb46f7. [DOI] [PubMed] [Google Scholar]
- 9.Sivori S, Parolini S, Marcenaro E, Castriconi R, Pende D, Millo R, Moretta A. Involvement of natural cytotoxicity receptors in human natural killer cell-mediated lysis of neuroblastoma and glioblastoma cell lines. J Neuroimmunol. 2000;107(2):220–225. doi: 10.1016/S0165-5728(00)00221-6. [DOI] [PubMed] [Google Scholar]
- 10.Brehm C, Huenecke S, Quaiser A, Esser R, Bremm M, Kloess S, Soerensen J, Kreyenberg H, Seidl C, Becker PS, Muhl H, Klingebiel T, Bader P, Passweg JR, Schwabe D, Koehl U. IL-2 stimulated but not unstimulated NK cells induce selective disappearance of peripheral blood cells: concomitant results to a phase I/II study. PLoS ONE. 2011;6(11):e27351. doi: 10.1371/journal.pone.0027351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koehl U, Sorensen J, Esser R, Zimmermann S, Gruttner HP, Tonn T, Seidl C, Seifried E, Klingebiel T, Schwabe D. IL-2 activated NK cell immunotherapy of three children after haploidentical stem cell transplantation. Blood Cells Mol Dis. 2004;33(3):261–266. doi: 10.1016/j.bcmd.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 13.Passweg JR, Koehl U, Uharek L, Meyer-Monard S, Tichelli A. Natural-killer-cell-based treatment in haematopoietic stem-cell transplantation. Best Pract Res Clin Haematol. 2006;19(4):811–824. doi: 10.1016/j.beha.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui CH, Leung W. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28(6):955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stern M, Passweg JR, Meyer-Monard S, Esser R, Tonn T, Soerensen J, Paulussen M, Gratwohl A, Klingebiel T, Bader P, Tichelli A, Schwabe D, Koehl U. Pre-emptive immunotherapy with purified natural killer cells after haploidentical SCT: a prospective phase II study in two centers. Bone Marrow Transplant. 2013;48(3):433–438. doi: 10.1038/bmt.2012.162. [DOI] [PubMed] [Google Scholar]
- 16.Allison AC, Eugui EM. Immunosuppressive and other effects of mycophenolic acid and an ester prodrug, mycophenolate mofetil. Immunol Rev. 1993;136:5–28. doi: 10.1111/j.1600-065X.1993.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 17.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47(2–3):85–118. doi: 10.1016/S0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 18.Allison AC, Eugui EM. Mechanisms of action of mycophenolate mofetil in preventing acute and chronic allograft rejection. Transplantation. 2005;80(2 Suppl):S181–S190. doi: 10.1097/01.tp.0000186390.10150.66. [DOI] [PubMed] [Google Scholar]
- 19.Koehl U, Esser R, Zimmermann S, Tonn T, Kotchetkov R, Bartling T, Sorensen J, Gruttner HP, Bader P, Seifried E, Martin H, Lang P, Passweg JR, Klingebiel T, Schwabe D. Ex vivo expansion of highly purified NK cells for immunotherapy after haploidentical stem cell transplantation in children. Klin Padiatr. 2005;217(6):345–350. doi: 10.1055/s-2005-872520. [DOI] [PubMed] [Google Scholar]
- 20.Passweg JR, Stern M, Koehl U, Uharek L, Tichelli A. Use of natural killer cells in hematopoetic stem cell transplantation. Bone Marrow Transplant. 2005;35(7):637–643. doi: 10.1038/sj.bmt.1704810. [DOI] [PubMed] [Google Scholar]
- 21.Koehl U, Brehm C, Huenecke S, Zimmermann SY, Kloess S, Bremm M, Ullrich E, Soerensen J, Quaiser A, Erben S, Wunram C, Gardlowski T, Auth E, Tonn T, Seidl C, Meyer-Monard S, Stern M, Passweg J, Klingebiel T, Bader P, Schwabe D, Esser R. Clinical grade purification and expansion of NK cell products for an optimized manufacturing protocol. Front Oncol. 2013;3:118. doi: 10.3389/fonc.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esser R, Muller T, Stefes D, Kloess S, Seidel D, Gillies SD, Aperlo-Iffland C, Huston JS, Uherek C, Schonfeld K, Tonn T, Huebener N, Lode HN, Koehl U, Wels WS. NK cells engineered to express a GD2 -specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J Cell Mol Med. 2012;16(3):569–581. doi: 10.1111/j.1582-4934.2011.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kloess S, Huenecke S, Piechulek D, Esser R, Koch J, Brehm C, Soerensen J, Gardlowski T, Brinkmann A, Bader P, Passweg J, Klingebiel T, Schwabe D, Koehl U. IL-2-activated haploidentical NK cells restore NKG2D-mediated NK-cell cytotoxicity in neuroblastoma patients by scavenging of plasma MICA. Eur J Immunol. 2010;40(11):3255–3267. doi: 10.1002/eji.201040568. [DOI] [PubMed] [Google Scholar]
- 24.Quah BJ, Parish CR (2010) The use of carboxyfluorescein diacetate succinimidyl ester (CFSE) to monitor lymphocyte proliferation. J Vis Exp (44). doi:10.3791/2259 [DOI] [PMC free article] [PubMed]
- 25.Zimmermann SY, Esser R, Rohrbach E, Klingebiel T, Koehl U. A novel four-colour flow cytometric assay to determine natural killer cell or T-cell-mediated cellular cytotoxicity against leukaemic cells in peripheral or bone marrow specimens containing greater than 20% of normal cells. J Immunol Methods. 2005;296(1–2):63–76. doi: 10.1016/j.jim.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Baudard M, Vincent A, Moreau P, Kergueris MF, Harousseau JL, Milpied N. Mycophenolate mofetil for the treatment of acute and chronic GVHD is effective and well tolerated but induces a high risk of infectious complications: a series of 21 BM or PBSC transplant patients. Bone Marrow Transplant. 2002;30(5):287–295. doi: 10.1038/sj.bmt.1703633. [DOI] [PubMed] [Google Scholar]
- 27.Bhatia M, Militano O, Jin Z, Figurski M, Shaw L, Moore V, Morris E, Tallamy B, van deVen C, Ayello J, Baxter-Lowe L, Satwani P, George D, Bradley MB, Garvin J, Cairo MS. An age-dependent pharmacokinetic study of intravenous and oral mycophenolate mofetil in combination with tacrolimus for GVHD prophylaxis in pediatric allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2010;16(3):333–343. doi: 10.1016/j.bbmt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 28.van Hest RM, Doorduijn JK, de Winter BC, Cornelissen JJ, Vulto AG, Oellerich M, Lowenberg B, Mathot RA, Armstrong VW, van Gelder T. Pharmacokinetics of mycophenolate mofetil in hematopoietic stem cell transplant recipients. Ther Drug Monit. 2007;29(3):353–360. doi: 10.1097/FTD.0b013e31805d8816. [DOI] [PubMed] [Google Scholar]
- 29.Eissens DN, Van Der Meer A, Van Cranenbroek B, Preijers FW, Joosten I. Rapamycin and MPA, but not CsA, impair human NK cell cytotoxicity due to differential effects on NK cell phenotype. Am J Transplant. 2010;10(9):1981–1990. doi: 10.1111/j.1600-6143.2010.03242.x. [DOI] [PubMed] [Google Scholar]
- 30.Ohata K, Espinoza JL, Lu X, Kondo Y, Nakao S. Mycophenolic acid inhibits natural killer cell proliferation and cytotoxic function: a possible disadvantage of including mycophenolate mofetil in the graft-versus-host disease prophylaxis regimen. Biol Blood Marrow Transplant. 2011;17(2):205–213. doi: 10.1016/j.bbmt.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Biassoni R (2009) Human natural killer receptors, co-receptors, and their ligands. Curr Protoc Immunol Chapter 14:Unit 14 10. doi:10.1002/0471142735.im1410s84 [DOI] [PubMed]
- 32.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barten MJ, van Gelder T, Gummert JF, Shorthouse R, Morris RE. Novel assays of multiple lymphocyte functions in whole blood measure: new mechanisms of action of mycophenolate mofetil in vivo. Transpl Immunol. 2002;10(1):1–14. doi: 10.1016/S0966-3274(01)00041-7. [DOI] [PubMed] [Google Scholar]
- 34.Blaheta RA, Leckel K, Wittig B, Zenker D, Oppermann E, Harder S, Scholz M, Weber S, Encke A, Markus BH. Mycophenolate mofetil impairs transendothelial migration of allogeneic CD4 and CD8 T-cells. Transplant Proc. 1999;31(1–2):1250–1252. doi: 10.1016/S0041-1345(98)01984-8. [DOI] [PubMed] [Google Scholar]
- 35.Allison AC, Kowalski WJ, Muller CD, Eugui EM. Mechanisms of action of mycophenolic acid. Ann N Y Acad Sci. 1993;696:63–87. doi: 10.1111/j.1749-6632.1993.tb17143.x. [DOI] [PubMed] [Google Scholar]
- 36.Theze J, Alzari PM, Bertoglio J. Interleukin 2 and its receptors: recent advances and new immunological functions. Immunol Today. 1996;17(10):481–486. doi: 10.1016/0167-5699(96)10057-C. [DOI] [PubMed] [Google Scholar]
- 37.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6(8):595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 38.Yu CR, Lin JX, Fink DW, Akira S, Bloom ET, Yamauchi A. Differential utilization of Janus kinase-signal transducer activator of transcription signaling pathways in the stimulation of human natural killer cells by IL-2, IL-12, and IFN-alpha. J Immunol. 1996;157(1):126–137. [PubMed] [Google Scholar]
- 39.Wang KS, Ritz J, Frank DA. IL-2 induces STAT4 activation in primary NK cells and NK cell lines, but not in T cells. J Immunol. 1999;162(1):299–304. [PubMed] [Google Scholar]
- 40.Jiang K, Zhong B, Gilvary DL, Corliss BC, Hong-Geller E, Wei S, Djeu JY. Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat Immunol. 2000;1(5):419–425. doi: 10.1038/80859. [DOI] [PubMed] [Google Scholar]
- 41.Wei S, Gamero AM, Liu JH, Daulton AA, Valkov NI, Trapani JA, Larner AC, Weber MJ, Djeu JY. Control of lytic function by mitogen-activated protein kinase/extracellular regulatory kinase 2 (ERK2) in a human natural killer cell line: identification of perforin and granzyme B mobilization by functional ERK2. J Exp Med. 1998;187(11):1753–1765. doi: 10.1084/jem.187.11.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagy SE, Andersson JP, Andersson UG. Effect of mycophenolate mofetil (RS-61443) on cytokine production: inhibition of superantigen-induced cytokines. Immunopharmacology. 1993;26(1):11–20. doi: 10.1016/0162-3109(93)90062-U. [DOI] [PubMed] [Google Scholar]
- 43.Chiossone L, Vitale C, Cottalasso F, Moretti S, Azzarone B, Moretta L, Mingari MC. Molecular analysis of the methylprednisolone-mediated inhibition of NK-cell function: evidence for different susceptibility of IL-2- versus IL-15-activated NK cells. Blood. 2007;109(9):3767–3775. doi: 10.1182/blood-2006-07-037846. [DOI] [PubMed] [Google Scholar]
- 44.Vitale C, Chiossone L, Cantoni C, Morreale G, Cottalasso F, Moretti S, Pistorio A, Haupt R, Lanino E, Dini G, Moretta L, Mingari MC. The corticosteroid-induced inhibitory effect on NK cell function reflects down-regulation and/or dysfunction of triggering receptors involved in natural cytotoxicity. Eur J Immunol. 2004;34(11):3028–3038. doi: 10.1002/eji.200425418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.