Abstract
Liposomes are frequently used in cancer therapy to encapsulate and apply anticancer drugs. Here, we show that a systemic treatment of mice bearing skin tumors with empty phosphatidylcholine liposomes (PCL) resulted in inhibition of tumor growth, which was similar to that observed with the synthetic bacterial lipoprotein and TLR1/2 agonist Pam3CSK4 (BLP). Both compounds led to a substantial decrease of macrophages in spleen and in the tumor-bearing skin. Furthermore, both treatments induced the expression of typical macrophage markers in the tumor-bearing tissue. As expected, BLP induced the expression of the M1 marker genes Cxcl10 and iNOS, whereas PCL, besides inducing iNOS, also increased the M2 marker genes Arg1 and Trem2. In vitro experiments demonstrated that neither PCL nor BLP influenced proliferation or survival of tumor cells, whereas both compounds inhibited proliferation and survival and increased the migratory capacity of bone marrow-derived macrophages (BMDM). However, in contrast to BLP, PCL did not activate cytokine secretion and induced a different BMDM phenotype. Together, the data suggest that similar to BLP, PCL induce an antitumor response by influencing the tumor microenvironment, in particular by functional alterations of macrophages, however, in a distinct manner from those induced by BLP.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1444-4) contains supplementary material, which is available to authorized users.
Keywords: Liposomes, Bacterial lipoprotein, Antitumor effects, Macrophages, TAM
Introduction
Liposomes are frequently used to encapsulate and apply drugs, because they both are considered nontoxic and protect the substances from immediate dilution or degradation [1]. Several laboratories have reported the use of liposomes as drug carriers in the treatment of cancer and as vaccine delivery systems. Intense research has already led to the commercialization of several liposome-based therapeutics. Doxil and Lipoplatin, for example, represent liposome-encapsulated doxorubicin and cisplatin, respectively, for systemic cancer therapy [1].
Liposomes can be classified according to their lamellarity, size, charge and preparation method [1]. Neutral liposomes usually consist of phosphatidylcholine (PC). Inclusion of phospholipids, such as phosphatidylinositol, phosphatidylglycerol, phosphatidic acid, phosphatidylserine (PS) and diacetylphosphate, imparts negative charges [2]. Positively charged lipids such as phosphatidylethanolamine and derivatives thereof can be used to prepare cationic liposomes. Since they can interact with negatively charged nucleic acid molecules, they are used as DNA or RNA delivery systems [3]. Other molecules frequently included into liposomes are cholesterol and α-tocopherol. Whereas cholesterol increases the physical stability of liposomes particularly in the presence of biological fluids such as plasma [2], α-tocopherol prevents liposome auto-oxidation, thereby increasing their stability without inducing any toxicity [4]. Furthermore, the addition of polyethylene glycol (PEG)-modified lipids increases the liposome circulation time and pharmacological efficacy of encapsulated drugs [5].
In contrast to other liposomes, PC liposomes (PCL) are thought to have no immunogenic activity [6]. In contrast to PS liposomes, PCL neither induced apoptosis of phagocytic cell lines [7] nor did they modulate inflammatory responses [8]. They also did not mimic the effects of apoptotic cells on macrophages, i.e., inducing the expression of prostaglandin E synthases [9]. However, some immune modulatory capacity of PCL has been recently discussed by Graeser et al. [10], who showed that empty PCL have antimetastatic activity in an orthotopic mouse model of pancreatic cancer. The authors suggested that PCL may activate cellular signaling pathways involved in tumor defense mechanisms in macrophages or endothelial cells.
Macrophages comprise a major population of tumor-infiltrating immune cells [11], and their role in cancer is controversially discussed. In most solid tumors, the existence of macrophages is advantageous for tumor growth and metastasis, and the majority of studies have linked high macrophage numbers to reduced patient survival [12]. These macrophages generally have a so-called M2-like phenotype (also known as “alternatively” activated macrophages), consistent with cancer-related inflammation. The polarization to the M2 phenotype occurs when macrophages are exposed to Th2 cytokines, such as IL-4, IL-13 and IL-10 [13, 14]. On the other hand, macrophages can also exert beneficial roles in cancer [15]. Thus, in vitro studies showed that “classically” activated macrophages can be cytotoxic to tumor cells, but not to normal cells. Macrophages with such a M1 phenotype commit to a proinflammatory response profile upon exposure to the Th1 cytokine IFNγ or to pathogen-associated molecules such as bacterial lipopolysaccharide (LPS) or BLP, i.e., agonists for Toll-like receptor (TLR) 4 and 1/2, respectively. Therefore, macrophages probably can have contrasting roles in cancer depending on their phenotype (for review see [14]). Macrophage phenotypes can be identified by the expression of representative M1 and M2 genes. For example, iNOS and the C–X–C motif chemokine 10 (Cxcl10) are key effector molecules produced by M1 macrophages, whereas arginase 1 (Arg1) and Trem2 (triggering receptor expressed on myeloid cells 2) are typical M2 markers [16].
We made the unexpected observation that systemic treatment of mice bearing basal cell carcinoma (BCC, which is a tumor of the skin) with PCL resulted in a significant tumor growth inhibition as compared to controls. Our analysis revealed that the antitumor effects of PCL were similar to those of BLP that has been shown to inhibit tumor growth in mice and to modulate the activity of macrophages [17, 18]. Furthermore, our data suggest that PCL can alter the function and phenotype of macrophages in a particular manner, which are distinct from those induced by BLP. These data should be considered when PCL are used as drug delivery systems in the diseased organism.
Materials and methods
Preparation of empty liposomes
For the preparation of 40 ml of conventional liposomes soy phosphatidylcholine (4.0 g, Epikuron 200, Lukas Meyer, Hamburg, Germany), cholesterol (0.6 g, Fluka, Buchs, Switzerland) and d,l-α-tocopherol (0.02 g, Merck, Darmstadt, Germany) corresponding to 1:0.3:0.01 mol parts were used. The lipid mixture was solubilized in 20 ml of a physiologic phosphate buffer (20 mM, pH 7.4) supplemented with mannitol (230 mM, PB-Man). The resulting multilamellar vesicles were freeze-thawed in three cycles of liquid nitrogen and water at 40 °C, followed by repetitive (8 ×) filter extrusion through 400 and 200 nm membranes (Nuclepore, Sterico, Dietikon, Switzerland) using a Lipex extruder (Northern Lipids Inc., Burnaby, BC, Canada). Liposome size and homogeneity was measured with a Nicomp laser light scattering particle sizer (Nicomp 370, Sta. Barbara, CA, USA). Routinely prepared small unilamellar liposomes have a mean diameter of 135 ± 55 nm [3, 19].
Animals and i.p. injection of PCL and BLP in mice
Conditional Ptch floxflox ERT2 +/− mice were randomized into 3 groups (n = 5 per group). BCC were induced by intramuscular injection of 100 μg tamoxifen as described [20]. Fifteen days after tamoxifen-mediated BCC induction, the mice were injected intraperitoneally (i.p.) with PCL at a dose of 550 μg/kg mouse body weight, followed by 275 μg/kg for the subsequent doses every 4th day for 75 days. For this purpose, the liposome stock solution was freshly thawed and diluted each time in PBS to obtain the desired dose in 120 μl for each animal. Control animals were i.p. injected with the respective volume of PBS. Mice were also treated i.p. with 25 μg BLP (from InvivoGen) per mouse dissolved in 120 μl PBS with the same treatment schedule as PCL. Animals were euthanized 24 h after the last dose, and spleen and skin were excised. Parts of each tissue were either frozen and stored at −80 oC until RNA isolation, or formalin-fixed and embedded in paraffin for immunohistological analyses. The remains of the tissues were used for FACS analyses (see below). All animals were treated and housed in accordance with the German animal protection law.
Measurement of tumor size
Basal cell carcinoma size was measured on hematoxylin and eosin (H&E) stained sections using the area calculation tool of the software cellF (Olympus Soft Imaging Solutions GmbH; Germany).
Cell culture experiments
The murine BCC cell line ASZ001 was established from UV-induced BCC of Ptch mutant mice and maintained as described [21]. BMDMs were isolated from femurs of C57BL/6 wildtype mice as previously described in a slightly modified manner [22]. In short, the ends of each femur were cut off with a scissor, and the marrow was flushed out with Pluznik medium (DMEM, 30 % L929 conditioned medium containing CSF, 5 % horse-serum, 0.002 % β-mercaptoethanol) that induces the differentiation of monocytes to macrophages. The single cell suspension was first grown in a coated cell culture dish. This allowed fibroblasts and other sessile cells to attach to the surface of the culture dish. After 24 h, the medium containing mononuclear cells was collected by centrifugation for 10 min at 1,200 rpm. The cells were resuspended in Pluznik medium and seeded in uncoated petri dishes. The medium was refreshed after 3 days, and after 4 additional days, the BMDM were ready to use for further experiments.
For BrdU incorporation assays, 6000 ASZ001 or 8000 BMDM were seeded in each well of a 96-well plate. For Annexin V stainings and cell cycle analyses, 2.4 × 105 cells/well were seeded into 6-well plates. After 24 h, the cells were washed and incubated for 72 h in medium, supplemented with 0.5 μg/ml PCL or 100 ng/ml BLP (this dose is usually applied to activate BMDM’s in vitro [23] or the respective solvent as indicated in the individual experiments). The medium was changed daily.
Cell viability/metabolic activity was determined by addition of WST-1 reagent (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s recommendations after incubation of the cells with 0.5 μg/ml PCL or 100 ng/ml BLP and also with 0.5 μg/ml pure PC (dissolved in ethanol/PBS 1:100) for 24–72 h as indicated in the respective experiments.
Cell proliferation was measured after BrdU pulsing for the last 24 h using a cell proliferation BrdU ELISA (Roche Diagnostics GmbH) on a microplate reader. For cell cycle analysis, cells were resuspended in 100 % ice-cold ethanol and incubated at −20 °C for at least 2 h. Afterward, the cells were centrifuged, washed and incubated with staining solution (10 μg/ml propidium iodide; 100 μg/ml RNaseA in PBS) for 30 min at 37 °C and analyzed on a BD LSR II flow cytometer.
For Annexin V staining, the cells were harvested using accutase (BD Biosciences). After washing with PBS, the cells were resuspended in 100 μl binding buffer (BD Pharmingen) and 2 μl Annexin V-FITC and incubated for 10 min in the dark. Thereafter, the cells were incubated with 1 μl To-Pro-3 Iodide (Invitrogen) for 5 min. Afterward, 200 μl binding buffer was added, and the samples were measured on a BD FACSCalibur.
For the BMDM migration assay, 7.5 × 104 BMDM were seeded onto membrane inserts (translucent track-etched polyethylene terephthalate (PET) membranes with 8 μm pores, BD) and incubated for 18 h in a 24-well plate (companion plate, BD) in 300 μl medium, supplemented with either 0.5 μg/ml PCL or 100 ng/ml BLP or the respective solvent. Afterward, the membrane was separated, and the cells were stained with 5 μm calcein for 1 h at 37 °C. After washing with PBS and removing of cells on top of the membrane (BMDM which had not migrated), the cells at the bottom of the membrane were analyzed on a microscope and counted with the help of the software ImageJ.
Gene expression analysis
Total RNA from tissue was isolated using TriReagent (Sigma-Aldrich, Taufkirchen, Germany). cDNA was synthesized using Superscript II and random hexamers (Invitrogen, Karlsruhe, Germany). Real-time quantitative RT-PCR analysis was carried out using the ABI Prism HT 7900 detection system instrument and software (Applied Biosystems, Darmstadt, Germany). Primer pairs used for expression analysis are shown in supplementary Table S1. The data were analyzed using the standard curve method for relative quantification. Levels of assayed genes were normalized to 18S rRNA expression. All samples were reverse transcribed and measured in two independent experiments in triplicates.
FACS analyses of tissue macrophages
FACS analysis of immune cells was performed on single cell suspensions of spleen and skin. For this purpose, splenocytes were isolated by squeezing the organ through a nylon mesh (40 μm) into a petri dish containing 10 ml PBS. Single cell suspensions of skin were obtained by dissecting the tumor-bearing ears into very small pieces with a scalpel. After digestion for 1 h at 37 °C in 2 ml HBSS containing 0.2 % collagenase type-II (Worthington), the cells were filtered through a 70 μm nylon filter (BD Bioscience) and the collagenase was inactivated with 10 % FCS in PBS. After a washing step at 400 g for 5 min, cells were resuspended in PBS. Cells (1 × 106) were stained with monoclonal antibodies against CD11b (anti CD11b-FITC, #557396), Gr1 (anti Gr1-PE, #553128) and F4/80 (anti F4/80-Cy5, 15-4801), which were purchased from BD Biosciences and eBiosciences, respectively. At least 10 × 103 viable cells were acquired on the basis of forward and side scattering and quantified by using a BD LSRII cytometer. Data acquisition and analysis were performed using the software BD FacsDiva (BD Biosciences Pharmingen) and FlowJo (Treestar, Ashland, OR), respectively.
Measurement of cytokine production by ELISA
Bone marrow-derived macrophages (1.5 × 104 cells per well) were plated into 96-well plates. The next day, the cells were stimulated with either 0.5 μg/ml PCL or 100 ng/ml BLP or the respective solvent. The supernatant was harvested after 24 h. IL-6, IL-10, MCP-1/CCL2, MIP-1α/CCL3, Rantes/CCL5 and KC/CXCL1 were measured using DuoSet ELISA Development Kits (R&D Systems GmbH, Wiesbaden). TNF-α or IL-12p40 were quantified using ELISA kits from BioLegend (San Diego, CA, USA) and eBioscience (San Diego, CA, USA), respectively [24].
Statistical analyses
If not otherwise indicated, statistical differences were analyzed using Mann–Whitney testing or Student’s t test. Data were considered significant when p < 0.05.
Results
Systemic application of both PCL and BLP results in tumor growth inhibition in a mouse model of BCC
When analyzing the antitumor effects of PCL-encapsulated drugs, we made the unexpected observation that the application of PCL resulted in growth inhibition of BCC in mice. To study this phenomenon in more detail, we induced BCC in Ptch floxflox ERT2 +/− mice and systemically applied PBS, PCL or BLP starting from day 15 after BCC induction. BLP was used as a control substance because it is known to inhibit growth of other tumors in mice [18]. All compounds were applied i.p. every fourth day until day 90 following tumor induction, when mice were killed. Histological analysis of the tumor-bearing skin revealed that tumors of solvent-treated mice were larger when compared to both PCL- and BLP-treated mice (Fig. 1a). This was also evident when the tumor area was calculated using the area calculation tool of the software cellF. Indeed, the differences between solvent- and PCL- or BLP-treated animals were significant (Fig. 1b). These data show that similar to BLP, PCL treatment results in inhibition of tumor growth in the Ptch floxflox ERT2 +/− mouse model for BCC.
Fig. 1.
Systemic application of PCL and BLP results in growth inhibition of BCC in Ptch flox/flox ERT2 +/− mice. a HE-stained skin sections and b relative tumor areas of HE-stained BCC of solvent-, PCL- or BLP-treated mice. The solvent-treated control was normalized to 1. Data represent mean ± SEM. *p < 0.05 by Mann–Whitney U testing
Systemic application of both PCL and BLP decreases the numbers of tissue macrophages
Because tumor growth frequently depends on macrophage infiltration, we first investigated whether systemically applied PCL and BLP had a general effect on the number of macrophages or on related cells derived from the myeloid lineage. For this purpose, we counted the numbers of immune cells isolated from the spleens of PBS-, PCL- or BLP-treated mice. Furthermore, we investigated the numbers of tumor-associated macrophages (TAM) in the tumor-bearing skin in the same cohorts. Single cell suspensions derived from these tissues were stained with antibodies directed against Mac1 (CD11b) and F4/80. Whereas Mac1 is expressed on granulocytes, T-, B- and natural killer (NK)-cells, dendritic cells and monocytes [25] murine macrophages additionally express F4/80 [26].
Compared to PBS-treated mice, the PCL treatment resulted in a significant decrease of Mac1+, F4/80+ and Mac1+F4/80+ cells in the spleen (Table 1). This was similar to BLP treatment, which equally decreased these cell subpopulations. A moderate reduction of these cells was also observed in the skin, however, within statistical variance (Table 1).
Table 1.
Systemic application of PCL and BLP decreases the numbers of tissue macrophages
| Treatment | Positive cells in % (± SEM) | ||
|---|---|---|---|
| Mac1 | F4/80 | Mac1_F4/80 | |
| Spleen | |||
| PBS | 4.89 (0.30) | 0.52 (0.08) | 0.51 (0.08) |
| PCL | 3.50 (0.33)* | 0.29 (0.01)* | 0.26 (0.02)* |
| BLP | 3.05 (0.06)* | 0.24 (0.01)* | 0.23 (0.02)* |
| BCC-bearing skin | |||
| PBS | 4.92 (0.58) | 3.31 (0.10) | 3.06 (0.11) |
| PCL | 3.16 (0.47) | 3.29 (0.39) | 2.38 (0.29) |
| BLP | 3.67 (0.62) | 3.09 (0.39) | 2.33 (0.29) |
The percentage of Mac1+, F4/80+ and Mac1+F4/80+ double-positive cells in spleen and in tumor-bearing skin of Ptch flox/flox ERT2 +/− mice was determined by FACS
* p < 0.05 by Student’s t test
Together, these data demonstrate that systemic PCL or BLP treatment results in reduction of macrophages in the spleen and possibly also in the skin, indicating a change in the tumor microenvironment.
Systemic PCL application in tumor-bearing mice results in a gene expression profile different to that induced by BLP
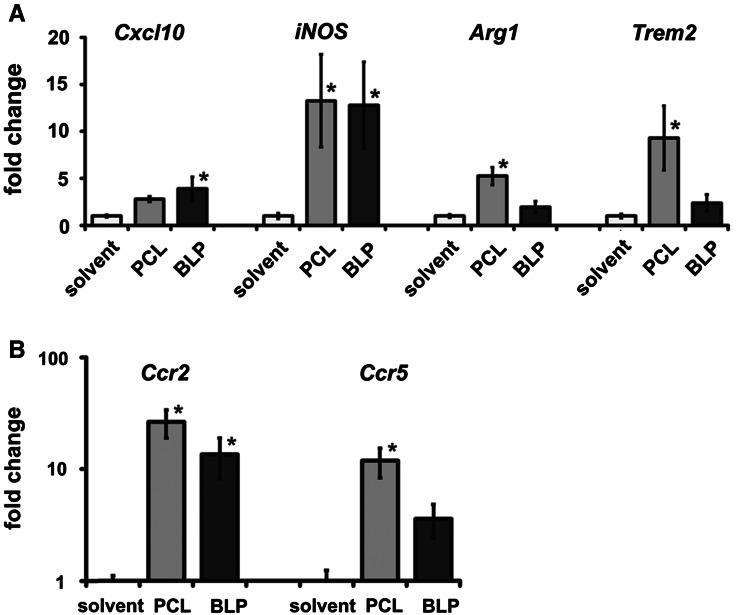
To investigate possible changes in the tumor microenvironment, we analyzed typical macrophage markers in the tumor-bearing skin. These were the M1-specific markers Cxcl10 and iNOS and the M2-specific markers Arg1 and Trem2. In comparison with the control group, BLP significantly augmented iNOS and Cxcl10, whereas PCL significantly increased the expression of iNOS and also the M2 markers Arg1 and Trem2 (Fig. 2a). These data suggest that BLP induced a M1 phenotype, whereas PCL administration induced a more M2-like phenotype. To test whether the moderate decrease in macrophage infiltration after PCL or BLP treatment in BCC-bearing skin is associated with altered expression of chemokine receptors, we analyzed the expression of the chemokine receptors Ccr2 and Ccr5. These receptors have been shown to be involved in the trafficking of monocytes/macrophages [27, 28], and downregulation could explain the moderately reduced macrophage abundance in BCC. However, the analysis of Ccr2 and Ccr5 expression revealed upregulation of both genes in the treated BCC-bearing skin (PCL 26-fold and 12-fold and BLP 14-fold and 4-fold for Ccr2 and Ccr5, respectively: Fig. 2b). The upregulation after BLP treatment was significant for Ccr2, whereas significance was reached for both genes after PCL treatment. To rule out that, increased expression levels of Ccr2 and Ccr5 were caused by infiltrating T cells, we counted CD3-positive cells on tumor sections stained with an appropriate antibody. However, the infiltration with T cells was not different between the treatment groups (data not shown). Collectively, these data indicate a change of the tumor microenvironment after BLP- and PCL treatments that particularly involves a change in the phenotype of TAM.
Fig. 2.
Systemic application of PCL results in a gene expression profile different to that induced by BLP in BCC-bearing skin. Gene expression was measured by qRT-PCR. a Cxcl10, iNOS, Arginase1, Trem2 and b Ccr2 and Ccr5 in BCC-bearing skin from solvent-, PCL- or BLP-treated mice. Values of solvent-treated controls were normalized to 1. Data represent mean ± SEM of at least three independent experiments performed in triplicates. * p < 0.05 by Mann–Whitney U testing
Similar to BLP, PCL inhibit proliferation, induce apoptosis and enhance the migratory capacity of BMDM
To exclude that BLP or PCL suppress tumor cells by direct tumoricidal activity as observed with, e.g., TLR3 agonists [29], we investigated the effects of both compounds on BCC cells in vitro. For this purpose, the BCC cell line ASZ001 was incubated with 0.5 μg/ml of PCL for 72 h. BLP was applied at a concentration of 100 ng/ml. Neither PCL nor BLP affected the level of BrdU incorporation in these cells (Supplementary Fig. S1a). The treatments also did not change the numbers of Annexin V+ ASZ001 cells (Supplementary Fig. S1b). Finally, we also investigated the tumoricidal activity of pure PC by analyzing the toxicity of 0.5 μg/ml PC. However, pure PC also did not impact on viability of ASZ001, although it was used at a slightly higher concentration than the PC present in 0.5 μg/ml PCL (Supplementary Fig. S2). These data indicate that neither PCL nor BLP exerted direct tumoricidal activity and did not inhibit proliferation or survival of BCC cells.
As shown above, PCL and BLP induce changes in the expression of macrophage markers in tumor-bearing skin, suggesting an impact of both substances on the TAM phenotype. Hence, we characterized the effects of PCL and BLP on macrophages in vitro. For this purpose, we generated BMDM and cultured them in the presence or absence of 0.5 μg/ml PLC or 100 ng/ml BLP. PBS was used as a vehicle control.
As shown in Fig. 3a, BLP treatment resulted in larger cells exhibiting a swollen cell body resembling the phenotype of LPS-activated BMDM [30]. In contrast, after treatment with PCL, BMDM preserved the general cellular shape compared to control cells. Consistently, the WST assay measuring cell viability and metabolic activity revealed that BLP significantly enhanced the metabolic activity of BMDM, whereas the increase after PCL treatment was not significant (Fig. 3b).
Fig. 3.
PCL and BLP inhibit proliferation, induce apoptosis and enhance the migratory capacity of BMDM. a Phenotypic appearance of BMDM after incubation with PBS, PCL or BLP for 72 h. b Metabolic activity of PBS-, PCL- or BLP-treated BMDM as measured by a WST-1 assay. Values of solvent-treated controls were set to 100 %. c BrdU incorporation shown as percentage of respective solvent-treated controls and d Annexin V+ cells after treatment with PBS, PCL or BLP for 72 h. e Migratory capacity of BMDM shown as percentage of the values obtained with solvent-treated controls. Data represent mean ± SEM of at least three independent experiments as performed in duplicates. * p < 0.05 by Mann–Whitney U testing
We then analyzed the proliferative capacity of BMDM after treatment with PCL or BLP. BMDM were incubated for 72 h with the compounds. As revealed by the BrdU incorporation assay, both PCL and BLP significantly reduced BMDM proliferation (Fig. 3c). A cell cycle analysis showed that PCL treatment caused a cell cycle arrest at the G1/S boundary, because the percentage of PCL-treated BMDM in the S phase was significantly lower and the fraction of cells in the G0/G1 phase was significantly higher, relative to PBS-treated BMDM (Table 2). In contrast, BLP seems to induce an arrest at the G1/S as well at G2/M boundaries, because treatment significantly decreased the cell numbers in the S phase and additionally increased the number of cells in the G2/M phase (Table 2). These data indicate that PCL and BLP arrest BMDM proliferation in different phases of the cell cycle.
Table 2.
Treatment of BMDM with PCL causes cell cycle arrest in the G1/S phase
| Treatment | Counts in % (± SEM) | ||
|---|---|---|---|
| G0/G1 | S phase | G2/M | |
| PBS | 58.20 (1.95) | 26.00 (0.77) | 11.30 (0.95) |
| PCL | 73.50 (1.44)* | 11.90 (1.17)* | 11.40 (0.37) |
| BLP | 62.00 (0.90) | 13.20 (0.21)* | 15.00 (0.30)* |
BMDM were incubated with the compounds or solvent for 72 h, stained with propidium iodide and analyzed by FACS
* p < 0.05 by student’s t test
The effects of PCL and BLP on BMDM apoptosis were investigated by FACS analysis of Annexin V/PI-stained cells. As demonstrated in Fig. 3d, both treatments resulted in a significant increase of Annexin V+ cells. However, the degree of apoptotic cells was higher with BLP than with PCL treatment (26 vs. 13 % Annexin V+ cells, respectively) (Fig. 3d). When we distinguished between early (Annexin V+/PI−) and late (Annexin V+/PI+) apoptotic cells, we found that both compounds increased either of these cellular subsets in a similar fashion (data not shown). These results suggest that PCL induce apoptosis of BMDM. This is similar to BLP that is known for its proapoptotic activity on the monocytic cell line THP-1 [31].
Together, these findings suggest that both PCL and BLP inhibit survival and proliferation of BMDM, while keeping the cells in a state of high metabolic activity.
Finally, we analyzed the migratory capacity of PCL- and BLP-treated BMDM. As shown in Fig. 3e, both substances enhanced the migratory capacity, and the effect was significant for PCL (Fig. 3e).
PCL do not induce cytokine secretion in BMDM
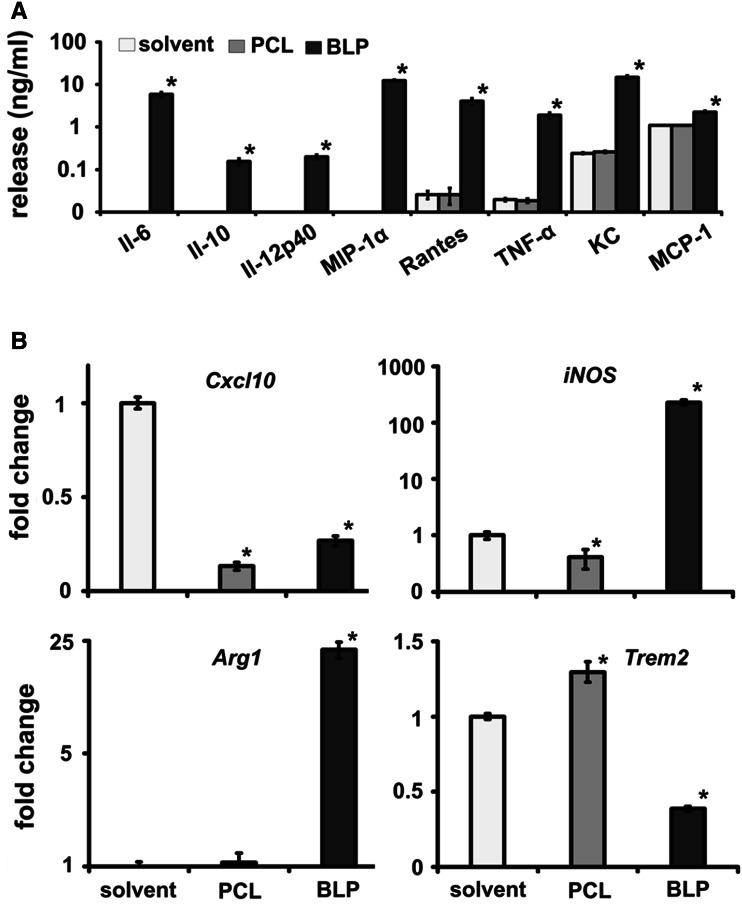
So far, the results showed that PCL treatment switches the TAM phenotype, decreases the survival and proliferative capacity of macrophages, but results in an increased migratory capacity. The latter finding, together with the high metabolic activity of the BMDM (see Fig. 3b), prompted us to investigate whether PCL induced the secretion of cytokines in BMDM. Surprisingly and in contrast to BLP, PCL did not induce cytokine production. BLP induced Rantes/CCL5 and KC/CXCL1, factors known as chemoattractants of neutrophils, immature dendritic cells, NK cells and activated T cells [32]. Further, BLP-treated BMDM produced the pro-inflammatory cytokines TNFα and MCP-1/CCL2, and the latter being involved in migration of monocytes, memory T lymphocytes and natural killer cells [33]. BLP-treated BMDM also secreted IL-6, IL-10, IL-12p40 and MIP-1α/CCL3. All these proteins were not detected in the supernatant of PCL-treated BMDM or were not elevated beyond solvent treatment (Fig. 4a). Hence, the impact of BLP and PCL on proliferation, migration and apoptosis is apparently not related to these cytokines implying that the impact of BLP and PCL on the functional behavior of BMDM is not intimately coupled to a concomitant organization of a cyto/chemokine release spectrum.
Fig. 4.
In contrast to BLP, PCL alter the phenotype of BMDM without inducing cytokine release. a Cytokine concentrations were measured by ELISA in BMDM culture supernatants after treatment with solvent, PCL or BLP. b qRT-PCR analysis of the M1-specific markers Cxcl10 and iNOS and of the M2-specific markers Arg1 and Trem2 in RNA isolated from solvent-, PCL- or BLP-treated BMDM. Values of solvent-treated controls were set to 1. Data represent mean ± SEM of at least two independent experiments performed in triplicates. * p < 0.05 by Mann–Whitney U testing
PCL and BLP induce different macrophage phenotypes
To further support the in vivo data shown in Fig. 2, we incubated BMDM with either PCL or BLP and measured the expression of M1 and M2 markers. In BMDM, both PCL and BLP significantly inhibited expression of the M1-specific marker Cxcl10. PCL also inhibited iNOS expression, whereas iNOS was upregulated by BLP. The expression levels of the M2-specific markers Arg1 or Trem2 were upregulated or downregulated, respectively, by BLP. In contrast, PCL treatment resulted in a significant upregulation of Trem2, whereas the expression level of Arg1 remained unchanged (Fig. 4b). These data indicate that PCL or BLP induce different macrophage phenotypes. Second, the in vivo situation seems to be more complex than the in vitro situation, because TAM showed a different expression of M1/M2 markers after treatment compared to BMDM. This could be due to the particular composition of the tumor microenvironment. Finally, the distinct expression profiles induced by either compound both in vivo and in vitro indicate that the inhibition of tumor growth cannot be assigned to a simple phenotypic macrophage orientation and must have been provoked by alternative mechanisms. Most importantly, however, PCL as commonly used drug encapsulation tool deliver their own intrinsic tumor-cell-targeted activity.
Discussion
Our data show that the systemic application of empty PCL induces an antitumor response against BCC in mice. The antitumor response is similar to that achieved by BLP, although the response of TAM to either compound seems to be different.
The antitumor activity of TLR2 agonists is well known. Indeed, TLR2 agonists can be beneficial in the prevention of cancer relapse, and the therapeutic effects of Bacillus Calmette-Guerin (BCG), which is used in the treatment of human bladder cancer, are mainly TLR2 mediated [34].
During the last years, it has emerged that BLP induces an immunotherapeutic activity on a variety of tumors in mice [18]. Zhang et al. showed that the antitumor effect of BLP was not mediated by direct tumoricidal activity. This is similar to the findings in our study, in which apoptosis and proliferation of tumor cells were not influenced by BLP. The authors showed that the anticancer effects of BLP involved a reduction of the suppressive function of Foxp3-expressing regulatory T cells (Tregs) and a concomitant enhancement of the cytotoxicity of tumor-specific CTL in vitro and in vivo. Because BLP lacked an antitumor effect in immune-deficient SCID mice, the authors concluded that the BLP-mediated tumor remission was not mediated by innate immune cells [18]. This is in contrast to our observations. Admittedly, we have not investigated the function of T cell populations in our setting. Although these cells may have contributed to the antitumor activity of BLP in our experiments, our data suggest an additional involvement of macrophages. This assumption is based on the BLP-induced reduction of macrophages numbers in the in vivo setting. Indeed, BLP also exerted a strong antiproliferative and proapoptotic effect on macrophages in culture and induced cell cycle arrest. In addition, BLP treatment modulated the expression of M1 and/or M2 markers in vivo and in vitro.
Importantly, our data show that already empty PCL modulate innate immune cells and inhibit tumor growth. This is surprising because the antitumor effects of empty PCL reported so far were insignificant in both immune-deficient and immune-competent mice [19, 35]. However, because the tumors and tumor cell lines used in these studies were fast-growing, it is possible that differences in tumor growth and progression after PCL treatment have been overlooked. Indeed, a moderate tumor growth inhibition after PCL treatment is demonstrated in the work of Zeisberger et al. [19], who used the teratocarcinoma F9 cells to study antitumor effects of PCL-encapsulated drugs. In addition, one recent report described antimetastatic activity of PCL in a mouse model of pancreatic cancer. Since the PCL used in the latter study contained fully hydrogenated palmitic and stearic fatty acids, the authors ascribed the antimetastatic activity in their model to these particular lipids. They also discussed the potential influence of these fatty acids on lipid metabolism, which could impede the generation of lipid second messengers being crucial for the metastatic process [10]. However, the PCL used in our study did not contain hydrogenated fatty acids. This excludes the possibility that hydrogenated fatty acids were responsible for its antitumor effects. Our data also strongly argue against a role of pure PC in the antitumor effects of PCL. Indeed, Sakakima et al. demonstrated that PC by itself is able to directly induce the inhibition of the growth of hepatic cancer cells. Furthermore, intragastrical administration of PC significantly reduced the number of macroscopic hepatic tumor nodules in rats submitted to hepatocarcinogenesis by diethylnitrosamine and phenobarbital co-administration [36]. Additionally, other authors demonstrated that PC induces apoptosis in different cell lines such as colon cancer cells [37], vascular endothelial cells [38] and 3T3-L1 pre-adipocytes [39]. However, the fact that the viability of the BCC cell line ASZ001 was not altered by PC indicates that this substance probably was not the cause for the antitumor effects of PCL in the BCC mouse model. Because PCL in our experiments were applied intraperitoneally, we also can exclude metabolism of PC by microbes of the gut, which has been reported to result in the formation of deleterious catabolites that equally could have inhibited tumor growth [40].
Currently, it is believed that the innate immune system is not affected by liposomes [41, 42]. Indeed, PCL do not induce cytokine production of bone marrow-derived cells in culture (as found in this study and as described by Faisal et al. [43]), which is in support of this assumption. However, our results also revealed that PCL substantially decrease the number of splenic and tumor-associated macrophages. This data suggest that PCL directly affect these cell populations. Indeed, intravenously injected liposomes are mainly found in macrophages of the spleen, in macrophages in inflamed tissue [44] and in tumor-associated macrophages [19, 45]. Thus, our data suggest that application of PCL may have an impact on the innate immune system by affecting survival or proliferation of these cells. On the one hand, it is possible that PCL reduced the recruitment of macrophages to the tumor cells in our setting. The proapoptotic and antiproliferative effects of PCL on cultured macrophages as well as the increased migratory capacity of PCL-treated BMDM support both assumptions. Furthermore, PCL seem to modulate expression of markers specific for both the M1 and M2 type of macrophages: in contrast to BLP, which induced a significant M1-biased expression pattern, i.e., iNOS and Cxcl10, PCL also resulted in a significant upregulation of Arg1 and Trem2 in the tumor-bearing skin. Because the expression of these genes was not induced in ASZ001 (data not shown), and because Arg1 and Trem2 are specifically expressed by macrophages [46, 47], the PCL treatment apparently also triggers a phenotype switch toward TAM of an alternatively activated phenotype in the tumor-bearing skin. Although M2 macrophages in general are thought to promote tumor progression, TAM can have a very variable phenotype and their relative abundance varies with the tumor type. For example, macrophages with neither M1 nor M2 characteristics have been identified in mammary tumor models. Moreover, the macrophage phenotype also varies in different areas of a tumor. Different phenotypes also can depend on the applied anticancer drugs. These studies emphasize the plasticity and heterogeneity of macrophage functional states and indicate that typical M1 and M2 phenotypes are extremes of a broad spectrum of functional states (reviewed in [15, 45]). The effects of PCL and BLP on the expression of M1- and M2-specific markers were also different on BMDM in culture. In addition, the expression of these markers differed substantially between TAM in vivo and BMDM in vitro. The most obvious explanation for latter discrepancy is the fact that TAM interact with many other cellular components, such as tumor cells, epithelial cells, B and T cells or fibroblasts [48]. All these cells may govern functional adjustments in TAM in vivo, but are absent in BMDM cultures. Conceivably, Ccr2 and Ccr5 could be involved in the recruitment of, e.g., T and B cells as both chemokines regulate trafficking of these cells [49, 50]. However, a first analysis indicates that T cell trafficking may not be affected by the compounds tested.
Collectively, our data demonstrate that the application of empty liposomes has antitumor activity in a mouse model of BCC. Furthermore, our data suggest that the antitumor activity of PCL is mediated by the tumor microenvironment, in particular by TAM. As resident immune and tumor-associated cells, they are capable of liposome phagocytosis, which in turn can apparently influence a whole variety of processes within the tumor microenvironment. Although the exact mechanisms remain to be established, PCL-mediated antitumor effects correlate with a macrophage phenotype different from that induced by BLP. However, most important is the finding that application of empty PCL alters function and phenotype of macrophages which should be considered when PCL are used as drug delivery systems.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We are grateful to Susan Peter for excellent animal care and Anke Frommhold for technical assistance. The work is supported by the DFG (FOR942 HA 2197/5-2 to Heidi Hahn).
Conflict of interest
None
References
- 1.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1:297–315. doi: 10.2217/17435889.1.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riaz M. Review article: stability and uses of liposomes. Pak J Pharm Sci. 1995;8:69–79. [PubMed] [Google Scholar]
- 3.Schwendener RA. Liposomes in biology and medicine. Adv Exp Med Biol. 2007;620:117–128. doi: 10.1007/978-0-387-76713-0_9. [DOI] [PubMed] [Google Scholar]
- 4.Alipour M, Smith MG, Pucaj K, Suntres ZE. Acute toxicity study of liposomal antioxidant formulations containing N-acetylcysteine, alpha-tocopherol, and gamma-tocopherol in rats. J Liposome Res. 2012;22(2):158–167. doi: 10.3109/08982104.2012.662654. [DOI] [PubMed] [Google Scholar]
- 5.Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci USA. 1991;88:11460–11464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Rooijen N, van Nieuwmegen R. Liposomes in immunology: multilamellar phosphatidylcholine liposomes as a simple, biodegradable and harmless adjuvant without any immunogenic activity of its own. Immunol Commun. 1980;9:243–256. doi: 10.3109/08820138009065997. [DOI] [PubMed] [Google Scholar]
- 7.Takano S, Aramaki Y, Tsuchiya S. Physicochemical properties of liposomes affecting apoptosis induced by cationic liposomes in macrophages. Pharm Res. 2003;20:962–968. doi: 10.1023/A:1024441702398. [DOI] [PubMed] [Google Scholar]
- 8.Ma HM, Wu Z, Nakanishi H. Phosphatidylserine-containing liposomes suppress inflammatory bone loss by ameliorating the cytokine imbalance provoked by infiltrated macrophages. Lab Invest. 2011;91:921–931. doi: 10.1038/labinvest.2011.54. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Fujii S, Wu Z, Hashioka S, Tanaka Y, et al. Involvement of COX-1 and up-regulated prostaglandin E synthases in phosphatidylserine liposome-induced prostaglandin E2 production by microglia. J Neuroimmunol. 2006;172:112–120. doi: 10.1016/j.jneuroim.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Graeser R, Bornmann C, Esser N, Ziroli V, Jantscheff P, et al. Antimetastatic effects of liposomal gemcitabine and empty liposomes in an orthotopic mouse model of pancreatic cancer. Pancreas. 2009;38:330–337. doi: 10.1097/MPA.0b013e31819436e6. [DOI] [PubMed] [Google Scholar]
- 11.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 12.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 13.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 16.Ydens E, Cauwels A, Asselbergh B, Goethals S, Peeraer L, et al. Acute injury in the peripheral nervous system triggers an alternative macrophage response. J Neuroinflammation. 2012;9:176. doi: 10.1186/1742-2094-9-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liew FY, Patel M, Xu D. Toll-like receptor 2 signalling and inflammation. Ann Rheum Dis. 2005;64(Suppl 4):iv104–iv105. doi: 10.1136/ard.2005.042515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Luo F, Cai Y, Liu N, Wang L, et al. TLR1/TLR2 agonist induces tumor regression by reciprocal modulation of effector and regulatory T cells. J Immunol. 2011;186:1963–1969. doi: 10.4049/jimmunol.1002320. [DOI] [PubMed] [Google Scholar]
- 19.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, et al. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zibat A, Uhmann A, Nitzki F, Wijgerde M, Frommhold A, et al. Time-point and dosage of gene inactivation determine the tumor spectrum in conditional Ptch knockouts. Carcinogenesis. 2009;30:918–926. doi: 10.1093/carcin/bgp068. [DOI] [PubMed] [Google Scholar]
- 21.So PL, Langston AW, Daniallinia N, Hebert JL, Fujimoto MA, et al. Long-term establishment, characterization and manipulation of cell lines from mouse basal cell carcinoma tumors. Exp Dermatol. 2006;15:742–750. doi: 10.1111/j.1600-0625.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 22.Francke A, Herold J, Weinert S, Strasser RH, Braun-Dullaeus RC. Generation of mature murine monocytes from heterogeneous bone marrow and description of their properties. J Histochem Cytochem. 2011;59:813–825. doi: 10.1369/0022155411416007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Buckley JM, Redmond HP, Wang JH. ST2 negatively regulates TLR2 signaling, but is not required for bacterial lipoprotein-induced tolerance. J Immunol. 2010;184:5802–5808. doi: 10.4049/jimmunol.0904127. [DOI] [PubMed] [Google Scholar]
- 24.Scheffel J, Regen T, Van Rossum D, Seifert S, Ribes S, et al. Toll-like receptor activation reveals developmental reorganization and unmasks responder subsets of microglia. Glia. 2012;60:1930–1943. doi: 10.1002/glia.22409. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen JW, Cello J, Gil H, Forestal CA, Furie MB, et al. Mac-1 + cells are the predominant subset in the early hepatic lesions of mice infected with Francisella tularensis . Infect Immun. 2006;74:6590–6598. doi: 10.1128/IAI.00868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferron M, Vacher J. Targeted expression of cre recombinase in macrophages and osteoclasts in transgenic mice. Genesis. 2005;41:138–145. doi: 10.1002/gene.20108. [DOI] [PubMed] [Google Scholar]
- 27.D’Ambrosio D, Panina-Bordignon P, Sinigaglia F. Chemokine receptors in inflammation: an overview. J Immunol Methods. 2003;273:3–13. doi: 10.1016/S0022-1759(02)00414-3. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Q. Dual targeting of CCR2 and CCR5: therapeutic potential for immunologic and cardiovascular diseases. J Leukoc Biol. 2010;88:41–55. doi: 10.1189/jlb.1009671. [DOI] [PubMed] [Google Scholar]
- 29.Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol. 2006;176:4894–4901. doi: 10.4049/jimmunol.176.8.4894. [DOI] [PubMed] [Google Scholar]
- 30.Geutskens SB, Nikolic T, Dardenne M, Leenen PJ, Savino W. Defective up-regulation of CD49d in final maturation of NOD mouse macrophages. Eur J Imun. 2004;34:3465–3476. doi: 10.1002/eji.200425259. [DOI] [PubMed] [Google Scholar]
- 31.Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J. 2000;19:3325–3336. doi: 10.1093/emboj/19.13.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luster AD. The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol. 2002;14:129–135. doi: 10.1016/S0952-7915(01)00308-9. [DOI] [PubMed] [Google Scholar]
- 33.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murata M. Activation of Toll-like receptor 2 by a novel preparation of cell wall skeleton from mycobacterium bovis BCG Tokyo (SMP-105) sufficiently enhances immune responses against tumors. Cancer Sci. 2008;99:1435–1440. doi: 10.1111/j.1349-7006.2008.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng N, Xia T, Han Y, He QJ, Zhao R, et al. Synergistic antitumor effects of liposomal honokiol combined with cisplatin in colon cancer models. Oncol Lett. 2011;2:957–962. doi: 10.3892/ol.2011.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakakima Y, Hayakawa A, Nagasaka T, Nakao A. Prevention of hepatocarcinogenesis with phosphatidylcholine and menaquinone-4: in vitro and in vivo experiments. J Hepatol. 2007;47:83–92. doi: 10.1016/j.jhep.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 37.Fukunaga K, Hossain Z, Takahashi K. Marine phosphatidylcholine suppresses 1,2-dimethylhydrazine-induced colon carcinogenesis in rats by inducing apoptosis. Nutr Res. 2008;28:635–640. doi: 10.1016/j.nutres.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Cheng Y, Zhao Q, Liu X, Araki S, Zhang S, et al. Phosphatidylcholine-specific phospholipase C, p53 and ROS in the association of apoptosis and senescence in vascular endothelial cells. FEBS Lett. 2006;580:4911–4915. doi: 10.1016/j.febslet.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Lee JH, Kim SY, Yun HY, Baek KJ, et al. Phosphatidylcholine induces apoptosis of 3T3-L1 adipocytes. J Biomed Sci. 2011;18:91. doi: 10.1186/1423-0127-18-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harokopakis E, Hajishengallis G, Michalek SM. Effectiveness of liposomes possessing surface-linked recombinant B subunit of cholera toxin as an oral antigen delivery system. Infect Immun. 1998;66:4299–4304. doi: 10.1128/iai.66.9.4299-4304.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richards RL, Rao M, Wassef NM, Glenn GM, Rothwell SW, et al. Liposomes containing lipid A serve as an adjuvant for induction of antibody and cytotoxic T-cell responses against RTS, S malaria antigen. Infect Immun. 1998;66:2859–2865. doi: 10.2307/1366431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faisal SM, Chen JW, McDonough SP, Chang CF, Teng CH, et al. Immunostimulatory and antigen delivery properties of liposomes made up of total polar lipids from non-pathogenic bacteria leads to efficient induction of both innate and adaptive immune responses. Vaccine. 2011;29:2381–2391. doi: 10.1016/j.vaccine.2011.01.110. [DOI] [PubMed] [Google Scholar]
- 44.Laverman P, Dams ET, Storm G, Hafmans TG, Croes HJ, et al. Microscopic localization of PEG-liposomes in a rat model of focal infection. J Controlled Release. 2001;75:347–355. doi: 10.1016/S0168-3659(01)00402-3. [DOI] [PubMed] [Google Scholar]
- 45.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Sonoki T, Nagasaki A, Gotoh T, Takiguchi M, Takeya M, et al. Coinduction of nitric-oxide synthase and arginase I in cultured rat peritoneal macrophages and rat tissues in vivo by lipopolysaccharide. J Biol Chem. 1997;272:3689–3693. doi: 10.1074/jbc.272.6.3689. [DOI] [PubMed] [Google Scholar]
- 47.Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M, et al. Cutting edge: TREM-2 attenuates macrophage activation. J Immunol. 2006;177:3520–3524. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 48.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flaishon L, Becker-Herman S, Hart G, Levo Y, Kuziel WA, et al. Expression of the chemokine receptor CCR2 on immature B cells negatively regulates their cytoskeletal rearrangement and migration. Blood. 2004;104:933–941. doi: 10.1182/blood-2003-11-4013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.