Abstract
EBV-transformed lymphoblastoid cell lines (LCL) are potent antigen-presenting cells. To investigate their potential use as cancer testis antigen (CTA) vaccines, we studied the expression of 12 cancer testis (CT) genes in 20 LCL by RT-PCR. The most frequently expressed CT genes were SSX4 (50 %), followed by GAGE (45 %), SSX1 (40 %), MAGE-A3 and SSX2 (25 %), SCP1, HOM-TES-85, MAGE-C1, and MAGE-C2 (15 %). NY-ESO-1 and MAGE-A4 were found in 1/20 LCL and BORIS was not detected at all. Fifteen of 20 LCL expressed at least one antigen, 9 LCL expressed ≥2 CT genes, and 7 of the 20 LCL expressed ≥4 CT genes. The expression of CT genes did not correlate with the length of in vitro culture, telomerase activity, aneuploidy, or proliferation state. While spontaneous expression of CT genes determined by real-time PCR and Western blot was rather weak in most LCL, treatment with DNA methyltransferase 1 inhibitor alone or in combination with histone deacetylase inhibitors increased CTA expression considerably thus enabling LCL to induce CTA-specific T cell responses. The stability of the CT gene expression over prolonged culture periods makes LCL attractive candidates for CT vaccines both in hematological neoplasias and solid tumors.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1412-z) contains supplementary material, which is available to authorized users.
Keywords: Lymphoblastoid cell lines, Cancer testis antigen, DNMT1 inhibitor, HDAC inhibitor, Specific immunotherapy, Cancer vaccine
Introduction
A prerequisite for the development of tumor-specific immunotherapeutic strategies is the existence and identification of tumor antigens, that is, genes that are either exclusively or preferentially expressed in malignant tissues compared with normal tissues. One of the most attractive groups of tumor-associated antigens are the so-called ‘shared’ tumor antigens which are only expressed by a variety of malignant tumors, but not by normal tissues, except for the germ cells in testis. Due to this peculiar expression profile, the term cancer testis (CT) antigens [1] or cancer germline (CG) antigens has been coined. The group of CT antigens comprises more than 40 distinct CT antigen families [2], the most prominent among them are MAGE [3], BAGE [4], and GAGE [5] families, as well as HOM-MEL-40/SSX2 and other SSX family members [6], NY-ESO-1 [7], HOM-TES-14/SCP1 [1, 8], HOM-TES-85/CT-28 [9], and CT-10 [10]. Most of them have been defined using SEREX [11]. Recently, it has become evident that they are also frequently expressed in hematological neoplasias such as multiple myeloma [12], acute leukemias [13, 14], Hodgkin, and non-Hodgkin lymphomas [15, 16].
Resting human B lymphocytes from peripheral blood are easily transformed by Epstein-Barr virus (EBV) into actively proliferating B-lymphoblastoid cell lines (LCL) [17]. These LCL with normal karyotypes have been reported to be ‘immortal’ without becoming tumorigenic. We therefore investigated whether LCL express cancer testis genes, and whether cancer testis antigen expression can be correlated with certain biological characteristics.
Of the large number of peptide epitopes derived from different CTA, most have been shown to elicit either CD8+ cytotoxic T lymphocyte [18] [19] [20] or CD4+ T-helper cell responses [21]. Only two peptide epitopes so far, one derived from NY-ESO-1 and another from SSX2, have been shown to simultaneously elicit CTL as well and T-helper cell activity together with the production of epitope-specific antibodies [22] [23]. Another problem of tumor antigen-derived peptides is their HLA restriction, for example, CD8+ stimulation capacity has rarely been shown for HLA haplotypes other than HLA-A*0201. The same applies for most of the known MHC-II-restricted CD4+ T-helper cell epitopes. One possibility to overcome these problems is the use of the complete CTA-derived protein. However, the costs to produce full-length CTA for clinical trials has precluded their widespread use [24, 25]. CTA-expressing LCL would offer a new perspective for cancer-specific vaccines. LCL are easy to generate in unlimited amounts, and if used as autologous vaccines, they provide a full-house HLA-consensus, and LCL as professional APC stimulate different components of the immune system.
Materials and methods
The study had been approved of by the local ethical review board. Recombinant DNA work was done with the official permission and according to the rules of the State Government of Saarland. All donors gave written informed consent.
Establishment of lymphoblastoid cell lines (LCL)
B cells either were transfected with EBV propagated in the marmoset cell B95-8 as described by Miller et al. [26] or had been growing out spontaneously ex vivo. The LCL ‘MGAR’ and ‘JY’ were obtained from S. Stevanovic (Institute for Immunology, University of Tübingen) and ‘LG2’ from P. van der Bruggen (Ludwig Institute for Cancer Research (LICR), Brussels, Belgium).
Tumor cell lines
Me275 (Melanoma) was kindly provided by D. Valmori (LICR, Lausanne, Switzerland). ESTAB was provided by A. Wadle (University Hospital Zürich, Switzerland). SK-MEL-37, U118 (Glioma), and U266 (Myeloma) were purchased from ATCC (LGC Standards GmbH, Wesel, Germany). Cell lines were cultured in RPMI/10 % FCS/2 mM l-glutamine/1 % penicillin/streptomycin (PAA Laboratories GmbH).
Determination of proliferation rate and cells in S-phase
Ki-67 was stained using antibody clone MIB-1 followed by cell fixation and permeabilization according to the manual (IntraStain kit, DakoCytomation, Glostrup, Denmark). The proliferating cell nuclear antigen (PCNA) as a marker for cells in S-phase was detected using the mouse anti-human PCNA kit according to the manual (Becton–Dickinson Biosciences, Heidelberg, Germany). Cells were counter-stained with antiCD23. For flow cytometric analysis, CD23+ LCL were gated and the proportion of PCNA+ cells was calculated using a FACSCalibur and Cellquest software (Becton–Dickinson Biosciences).
Ploidy analysis
DNA content of the cells was determined by propidium iodide staining (50 μg/ml, 15 min, 4 °C, in the dark) followed fixation (70 % ethanol, 2 h, 4 °C), RNA digest (RNase A 50 μg/ml, 45 min, 4 °C) as described before [27] and finally analyzed by flow cytometry.
Telomere length analysis
Telomere length was determined by the FlowFish method as described previously [28].
Telomerase activity
Telomerase activity was quantified under real-time PCR conditions using a LightCycler system (Roche, Mannheim, Germany) as described before [29]. In brief, protein extracts of 103 cells were incubated with primers and SYBR Green PCR Master Mix at 30 °C for 30 min to allow template elongation by telomerase activity and amplified by 36 PCR cycles carried out with 20 s at 95 °C, 30 s at 60 °C, and 50 s at 72 °C. Telomerase activity was displayed relatively to a standard of 103 HEK 293 cells (Gibco, Karlsruhe, Germany). Positive and negative controls were run in every experiment.
Reverse transcriptase PCR
Total RNA was extracted from 106 LCL using Trizol reagent according to the manufacturer’s details (Invitrogen GmbH, Karlsruhe, Germany), primed with an oligo(dT)18 and reverse transcribed with Superscript II (Life Technologies, Inc., Eggenstein, Germany). cDNA obtained was tested for integrity by amplification of p53 transcripts as described elsewhere [11, 30]. For PCR analysis of the expression of individual CT gene transcripts, first-strand cDNA was amplified with transcript-specific oligonucleotides (10 μmol) using 1 unit of AmpliTaq Gold (Perkin Elmer, Weiterstadt, Germany), 10 nmol of each deoxynucleotide triphosphate (dATP, dTTP, dCTP, and dGTP), and 1.67 mM MgCl2 in a 30-μl reaction. The primers (MWG Biotech, Ebersberg, Germany) for the respective CT genes have been reported previously [31–33] and are summarized in the supplemental Table 1.
Real-time RT-PCR
Total RNA from 3 × 106 LCL cells was extracted and reversely transcribed as described above. 1 μg of cDNA was applied for quantitative PCR analysis. Expression was classified negative if the crossing point (Cp) of the run with cDNA template was higher than the Cp of the run without the corresponding template. The quote indicating the increase of the CTA expression after LCL treatment compared to the native LCL was normalized to GAPDH and calculated with:  [34]. Quantitative real-time PCR was run on a LightCycler instrument (Roche Diagnostics GmbH, Mannheim, Germany) using FastStart DNA Master SYBR Green I kit (Roche Diagnostics) referred to the manual.
[34]. Quantitative real-time PCR was run on a LightCycler instrument (Roche Diagnostics GmbH, Mannheim, Germany) using FastStart DNA Master SYBR Green I kit (Roche Diagnostics) referred to the manual.
Western blot
Analysis of CTA protein expression was done by Western blot (WB). Total cellular protein extracts were run on standard 12 % SDS polyacrylamide gels. Antigens of the SSX family (30 μg protein/sample) were detected using supernatant (1:1,000) of the E3AS clone, kindly provided by Diederik de Bruijn (Radboud University, Nijmegen Medical Centre, Department of Human Genetics, Netherlands). MAGE-A antigens (10-μg protein/sample) were detected by antibodies (1:400) of clone 6C1 (Santa Cruz Biotechnologies, Heidelberg, Germany), and NY-ESO-1 antigen (10 μg protein/sample) was detected by antibodies (1:300) of clone E978 (Santa Cruz Biotechnologies).
LCL treatment with 5-azacytidine and HDAC inhibitors
To increase CTA expression, LCL were cultivated in medium supplemented with 5-azacytidine (Aza) alone or in combination either with suberoylanilide hydroxyamic acid (SAHA) or Trichostatin A (TSA) or valproic acid (Val), respectively, (inhibitors from Sigma-Aldrich, Taufkirchen, Germany) according to the scheme given in Table 1. Due to the fact that LCL reach maximal proliferation 24–30 h after splitting, LCL were splitted 24 h before treatment and settled in fresh medium.
Table 1.
Scheme of LCL treatment with DNMT1 inhibitor 5-azacytidin alone or in combination with one respective HDAC inhibitor
| Day 1 | Day 2 | Day 3 | Day 4 | ||||
|---|---|---|---|---|---|---|---|
| 0 h | 12 h | 24 h | 36 h | 48 h | 60 h | 72 h | |
| 5-Azacytidin | 1 μM | 1 μM | 1 μM | 1 μM | 1 μM | 1 μM | Yield |
| Valproate | – | – | 750 μM | – | 750 μM | – | |
| SAHA | – | – | 5 nM | – | 5 nM | – | |
| TSA | – | – | – | – | 65 nM | – | |
Generation of SSX2-derived epitope-specific CD8+ T cell clones
PBMC obtained from a patient with a SSX2-expressing breast cancer were isolated by Ficoll-Paque PLUS separation (GE Healthcare Bio-Sciences AB). For in vitro priming, CD8+ T cells were separated by MACS technology (Miltenyi, Bergisch Gladbach, Germany) and cells of the CD8- fraction were pulsed with the nonamer SSX2 peptide p101-111 and used as antigen-presenting cells (APC). Pulsing, cultivation, and stimulation were performed as described [23]. Responding T cells were isolated from the bulk population by interferon-γ (IFN-γ-based magnetic cell enrichment using a cytokine secretion assay (Miltenyi Biotec Inc.) and cloned by limiting-dilution culture as described [35]. Specifically reacting clones were expanded.
IFN-γ ELISPOT and TNF-α ELISA
IFN-γ release of CD8+ T cells of the SSX2/p101-111-specific clone #30 in response to different batches of LCL ‘JY’ was assessed by ELISPOT assay as described before [23]. As APC 3 × 104 JY cells/well (irradiated with 120 Gy) were co-incubated for 20 h with CD8+ cells of clone #30. T cell responses were scored positive if the number of IFN-γ spots induced by untreated and unloaded LCL ‘JY’ was significantly less (p < 0.05) compared to the different treated batches. Spots were counted using a Bioreader-3000 Pro (Biosys, Karben, Germany). TNF-α secretion of clone #30 cells was assessed by ELISA according to the manual (DTA00C, R&DSystems, Wiesbaden, Germany) under the same conditions used for IFN-γ ELISPOT. All tests were run in triplicates, and mean values and standard deviations were determined.
Assessment of cytotoxicity
Lactate dehydrogenase (LDH) release from targets was determined as extent of the cytotoxicity caused by CD8+ T cells of clone #30 in response to different batches of LCL ‘JY’ using the Cytotoxicity KitPlus according to the manual (Roche Diagnostics).
Results
Study population and validity of the experimental approach
The expression of 12 CT genes (MAGE-A3, MAGE-A4, GAGE, NY-ESO-1, MAGE-C1, MAGE-C2, HOM-TES-85, SSX1, SSX2, SSX4, SCP1, and BORIS) and p53 as positive control was analyzed in 20 LCL by RT-PCR. Intensities of CT gene PCR products were found to be heterogeneous, and some specimens yielded only faint amplicon bands. These were scored positive only, if the result could be reproduced by a repeated RNA extraction and specific PCR from the same LCL resulting in clear bands. Cases with very low transcript levels in two experiments were not regarded as positive. The characteristics of the 20 LCL are shown in Table 2. The time in culture of the established LCL at first analysis of CT gene expression ranged from 10 months to >5 years. All data presented in this study are representative results derived from at least three independent experiments.
Table 2.
Expression of EBV antigens, cancer testis antigens, and selected characteristics of the LCL analyzed in this study
| LCL | EBNA1 | EBNA2 | LMP1 | Month in culture | Ploidy | Rel. telomerase activity | Telomere length | CT 1 | CT 4 | CT 5 | CT 6 | CT 7 | CT 8 | CT 10 | CT 27 | CT 28 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAGE-A3 | MAGE-A4 | GAGE | SSX1 | SSX2 | SSX4 | NY-ESO-1 | MAGE-C1 | SCP1 | MAGE-C2 | BORIS | HOM-TES-85 | ||||||||
| GS70/126 | n.d. | n.d. | + | 10 | 2n | 0.3 | 6.9 | − | − | − | − | − | + | − | − | − | − | − | − |
| GS125 | n.d. | n.d. | + | 10 | 2n | 0.2 | 6.5 | − | − | + | + | + | − | − | − | − | − | − | − |
| GS133 | + | + | + | 10 | 2n | 1.6 | 4.3 | + | − | + | + | + | + | − | + | + | + | − | − |
| GS134 | n.d. | n.d. | + | 10 | 2n | 0.3 | 5.1 | − | − | − | − | − | + | − | − | + | − | − | − |
| G506 | + | + | + | 18 | 2n | 0.5 | 6.8 | − | − | − | − | − | − | − | − | − | − | − | − |
| G497 | + | + | + | 21 | 2n | 21.3 | 5.0 | − | − | − | + | − | − | − | − | − | − | − | − |
| G476 | n.d. | n.d. | n.d. | 25 | n.d. | 0.3 | n.d. | − | − | − | − | − | − | − | − | − | − | − | − |
| G478 | + | + | + | 25 | 2n | n.d. | 5.6 | − | − | − | − | − | + | − | − | − | − | − | − |
| G468 | n.d. | n.d. | n.d. | 26 | n.d. | 0.4 | 4.6 | − | − | − | − | − | − | − | − | − | − | − | − |
| G418 | + | + | + | 26 | 2n | 3.0 | 4.1 | − | − | − | − | − | − | − | − | − | − | − | − |
| G355 | n.d. | n.d. | + | 35 | 2n | 7.3 | 4.5 | + | − | + | + | − | + | − | + | − | + | − | + |
| G399 | + | + | + | 37 | >2n | 43.3 | 7.8 | − | − | + | − | − | + | − | − | − | − | − | − |
| GS7 | + | + | + | 48 | 2n | 12.6 | 5.4 | − | − | + | + | − | + | − | − | − | − | − | − |
| GS17 | + | + | + | 48 | 2n | 11.5 | 3.9 | − | − | − | − | − | + | − | − | − | − | − | − |
| GS89 | n.d. | n.d. | + | 48 | 2n | 0.4 | 5.3 | + | − | + | − | + | − | − | − | − | − | − | + |
| GS40 | + | + | + | 50 | 2n | 11.1 | 4.1 | − | − | + | − | − | − | − | − | − | − | − | − |
| GS90 | + | + | + | >60 | >2n | 14.8 | 6.3 | − | − | − | + | − | − | − | − | − | − | − | − |
| JY | n.d. | n.d. | + | >60 | >2n | 20.2 | 5.6 | + | − | + | + | + | + | − | − | + | + | − | + |
| MGAR | + | + | + | >60 | 2n | 4.8 | 4.5 | − | − | − | − | − | − | − | − | − | − | − | − |
| LG2 | n.d. | n.d. | + | >60 | 2n | 34.2 | 5.0 | + | + | + | + | + | + | + | + | − | − | − | − |
Expression of Individual CT Gene mRNA by LCL
None of the 12 CT antigens studied was expressed in normal peripheral blood mononuclear cells (n > 20) or other normal tissues. In contrast, except for 5 LCL, most LCL expressed one or more CT gene mRNA. The most frequent was SSX4 in 10/20 LCL (50 %) followed by GAGE in 9/20 LCL. SSX1 was expressed in 8/20, MAGE-A3 and SSX2 in 5/20 of the LCL. SCP1, HOM-TES-85, MAGE-C1, and MAGE-C2 were expressed in 3/20 LCL. NY-ESO-1 and MAGE-A4 were found in 1/20. BORIS was not detected in any of the 20 LCL tested (Fig. 1a). The expression of CT genes by the respective LCL in culture was stable during the study period of >4 years.
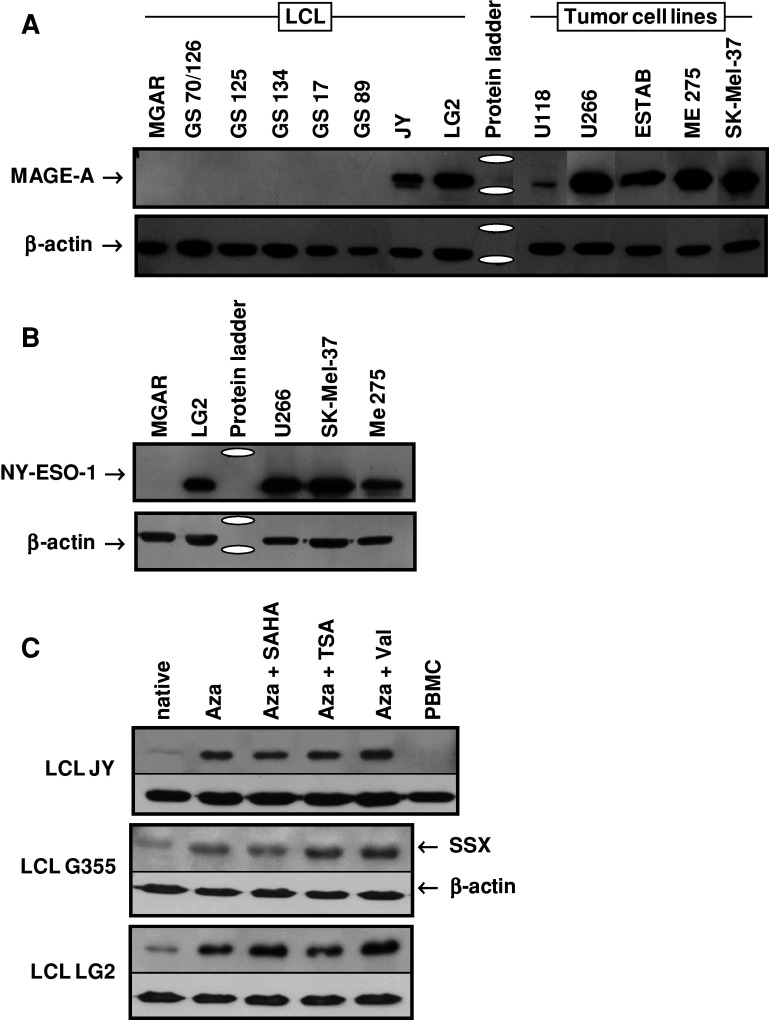
Fig. 1.

Expression of CT genes in EBV-transformed lymphoblastoid cell lines (LCL) and quantitative expression of CT genes in LCL. a Frequency of the expression of 12 CT genes in 20 LCL. b Simultaneous co-expression of the 12 CT genes in 20 LCL. All results assessed by RT-PCR in multiply repeated tests. c Comparison of the strength of CT gene mRNA expression in 16 LCL with 10 tumor cell lines of different origin. The mRNA expression of 6/6 CT gene was higher in the tumor cell lines. d The expression of the different CT gene increased significantly after treatment of the LCL with the DNMT1 inhibitor 5-azacytidine alone or in combination with the HDAC inhibitors SAHA, TSA, or valproic acid. Y-axis (logarithmic scale!) shows the times of increase of CT gene expression in LCL ‘JY’ after treatment compared to the untreated cells with a base line expression factor of 1. Factor variations are caused by the variable efficiency of the 4 different ways of treatment (4 columns per CTA). All results were assessed by LightCycler PCR in multiple repeated tests and normalized to the expression of GAPDH
Co-expression of multiple CT gene mRNAs in LCL
Among the CT gene-expressing LCL, co-expression of multiple CT genes was the rule, with a median expression of five CT genes. The expression of at least one antigen was observed in 15/20 LCL, 9 LCL expressed at least 2 CT genes, 7 LCL at least 3 CT genes, 5 LCL at least 4 CT genes, 4 LCL at least 7 CT genes, 3 LCL expressed ≥8 CT genes, and 2 LCL expressed 9 CT genes simultaneously (Fig. 1b). More than 9 CTA were not detected in any of the LCL. There was no obvious coordination of CT gene expression. However, except for three, all 15 LCL-expressing ≥1 CT gene of the CT 5 family expressed SSX1 or SSX2 only in the presence of SSX4.
Correlation of CT gene expression with LCL characteristics
As can be seen from Table 2, the expression of CT genes did not correlate with the length of in vitro culture. The 5 LCL without any CT gene expression (G506, G497, G468, G418, and MGAR) had been kept in culture for 18, 21, 26, and >60 months, respectively. On the other hand, the other LCL kept in culture for more than 5 years (GS90, JY, and LG2) co-expressed 3, 8, and 9 CT genes, respectively. Similarly, CT gene expression did not correlate with proliferation rate as determined by Ki-67 staining (data not shown), nor with ploidy of the LCL. The three hyperdiploid LCL (G399, GS90, and JY) expressed 2, 3, and 8 CT genes, respectively, and the numbers of CT genes expressed by the diploid cell lines were within the same range (Table 2). There was also no correlation between CT gene expression and telomerase activity, which, as expected, showed a tendency to be higher in long-term cultures (Table 2) and stronger proliferating cell lines. Telomere length ranged from 3.9 to 7.8 kb (median 5.3 kb) and, again, was not correlated with CT gene expression. Concerning the CTA expression pattern, there was no difference between spontaneous outgrowing LCL and those derived from in vitro EBV-transformed B cells.
Strength of expression of individual CT genes
Besides the high frequency of the expression of different CT genes in LCL, it was interesting to compare the strength of their expression with cell lines derived from different tumors. For this purpose, we quantified the mRNA content of 6 CT genes (MAGE-A3, MAGE-A4, SSX1, SSX2, SSX4, and NY-ESO-1) in 16 LCL and 10 tumor cell lines by LightCycler–based PCR. In each cell line, only those CT genes were analyzed by LightCycler PCR which were known to be expressed according to the previous RT-PCR results. The mean crossing points for each of the 6 CT gene were determined by LightCycler PCR in two independent experiments (starting with new mRNA isolation) and the difference between the mean crossing points of tumor cell lines and LCL was calculated. As can be seen in Fig. 1c, CT mRNA contents in tumor cell lines were in the mean 19 × higher compared to those expressed in LCL. The biggest difference referred to the SSX1 expression, which was about 50 times stronger in SSX1+ tumor cell lines. However, there were also 3 strongly CT gene-expressing LCL: GS133 expressed SSX mRNAs stronger than the SSX-expressing tumor cell lines. LG2 expressed 3/6 CT genes slightly stronger than the average of the corresponding tumor lines, and cells derived from the LCL ‘JY’ also reached the SSX4 mRNA level of the SSX4+ tumor lines (data not shown). In summary, LCL did indeed express a considerable number of different CT genes, but most of them rather weakly.
Strength of expression of individual CTA at the protein level
To determine the level of protein expression of three selected CTA by LCL in comparison with tumor cell lines, we used SDS-PAGE-based separation and Western blots (WB) for the detection of specific CTA. Due to the fact that reliable specific antibodies are available only for a few CTA, we restricted these analyses to SSX, MAGE-A, and NY-ESO-1. With the exception of MGAR which was used as a CT gene-negative control, all cell lines used for these analyses were known to express the respective CT genes on the basis of PCR results. As can be seen in Fig. 2a, only LCL ‘JY’ and ‘LG2’ which had a strong MAGE-A RNA message had a MAGE-A protein expression detectable by Western blot. In particular, in LG2 cells, MAGE-A protein expression was strong and comparable to the expression of this protein by tumor cells. The analysis of NY-ESO-1 protein expression showed a similar pattern (Fig. 2b). A very weak SSX antigen was detected in cells of the LCL ‘GS 89, G355, JY, GS133, and LG2’ (data not shown). Thus, Western blot analyses paralleled the results of the quantitative PCR in a way that only those LCL with low crossing points expressed detectable levels of the corresponding protein.
Fig. 2.
Expression of CT antigens at the protein level. Protein expression of the corresponding CT genes was analyzed by Western blot using β-actin as loading control. a With MGAR as CTA-negative control, all others LCL expressed MAGE-A3 and/or MAGE-A4 as assessed by RT-PCR. However, only cells from JY and LG2 also had these antigens at detectable protein levels. These levels were lower compared to those of the MAGE-A3/A4+ tumor cell lines. Antibody clone 6C1, which was used for detection, allowed no differentiation between MAGE-A3 and MAGE-A4. Blots of the different cell lines were analyzed in different runs and mounted to this slide. b LG2 was the only LCL of the 20 lines in this study which expressed NY-ESO-1 as assessed by RT-PCR. MGAR was used as NY-ESO-1-negative control LCL. c After treatment with 5-azacytidine to inhibit DNMT1 and different HDAC inhibitors, the expression of CTA was amplified in 3/3 LCL. Antibody of clone E3AS was used to detect SSX (a. o. SSX1, SSX2, and SSX4) as an example to demonstrate that the gene amplification leads to increased levels of the protein. In all LCL, the combined use of 5-azacytidine and valproic acid showed the strongest effect. For this analysis, PBMC were used as a CTA-negative control. All experiments were run twice and achieved the same results
Induction of CTA expression by 5-azacytidine and HDAC inhibitors
CTA expression can be influenced either by DNMT1 inhibition alone or in combination with HDAC inhibition. 5-azacytidine (Aza) is mostly used to reduce DNMT1 activity. Because there are 3 different classes of HDAC it is necessary to use also different inhibitors to address the complete spectrum of their activity. Therefore, we used SAHA, TSA, and valproic acid in combination with 5-azacytidin for the induction of CTA in 4 LCL (G355, JY, LG2, and MGAR). The induction of the expression of 5 CTA (MAGE-A3, MAGE-A4, SSX2, SSX4, and BORIS) was determined by RT-PCR, LightCycler PCR and Western blot after culture with Aza only, with Aza + SAHA, Aza + TSA, and Aza + Val. As proof of principle we included the LCL ‘MGAR’ because this line did not express any of the CT genes under native conditions and the CTA ‘BORIS,’ which was never expressed natively. However, after treating cells of this LCL either with Aza alone or in combination with any of the HDAC inhibitors all 5 CT genes were expressed simultaneously as assessed by RT-PCR (data not shown). In the other cell lines (G355, JY, LG2) we found the following inhibitor-induced expression enhancement (Table 3): MAGE-A3 and MAGE-A4 protein expression was only slightly increased and MAGE-A4 was not induced at all in LCL G355. BORIS showed a consistently increased and strong expression with the smallest variability. The induction of the SSX4 gene varied from weak (LG2) over moderate (G355) to strong (JY). SSX2 expression increased most after treatment. After 4 days under Aza and Val, SSX2-mRNA copies in G355 increased more than 1,000-times. The strongest CTA induction was observed in JY (Table 3; Fig. 1d). Although the differences between the different ways of LCL treatment were mostly non-significant (p ≥ 0.05) the strongest effect on CTA expression was achieved by the combined use of 5-azacytidine and Val. In most cases, the use of the DNMT1 inhibitor 5-azacytidine was enough to increase the CT expression, and combinations of 5-azacytidin with any of the three HDAC inhibitors did not result in an additional significant gain. A typical example of the effects of the various treatments on CT gene expression is shown for the LCL ‘JY’ in Fig. 1d. To test, whether an increase of CT gene mRNA was paralleled by an increased protein expression, SSX, MAGE-A, and NY-ESO-1 expression in 3 LCL was analyzed by Western blot after DNMT1/HDAC inhibition. Figure 2c shows that the SSX protein expression in untreated cells from all 3 LCL increased considerably after DNMT1 inhibition alone as well as after combined inhibition by DNMT1 and HDAC. Similar results were obtained for NY-ESO-1 and MAGE-A (data not shown).
Table 3.
Increased CT gene expression by LCL after treatment with 5-azacytidine and different HDAC inhibitors amplification factors of CT gene expression after treatment compared to the untreated LCL with a stated expression factor of 1
| BORIS | MAGE-A3 | MAGE-A4 | SSX2 | SSX4 | |
|---|---|---|---|---|---|
| LCL G355 | 132–280 | 1.8–6.3 | Negative | 525–1,032 | 34–81 |
| LCL JY | 250–320 | 10–16 | 21–32 | 411–756 | 241–406 |
| LCL LG2 | 215–360 | 5.2–11.3 | 3.6–7.8 | 28–70 | 7.5–44 |
Factor variations are caused by the variable efficiency of the different ways of treatment
Induction of CTL responses by LCL
To test whether CTA-expressing LCL are suitable antigen-presenting cells (APC), we used differently treated batches of the SSX2+ and HLA-A*0201+ JY cells as APC in cytokine secretion assays against the CD8+ T cell clone #30. This clone has been established and characterized in a former study as specific for the SSX2-derived peptide epitope p101-111 in the context of HLA-A*0201. As shown in Fig. 3a, clone #30 did not respond to native LCL ‘JY’. However, after treatment with the DNMT1 inhibitor Aza alone or in combination with one of the three HDAC inhibitors CD8+ T cells of clone #30 secreted IFN-γ as well as TNF-α during incubation with these targets demonstrating that after treatment enough SSX2 peptide was processed and presented in the context of HLA-A*0201 on the cell surface of the LCL. The strength of the T cell response increased after treatment with Aza alone and was strongest after treatment with Aza/Val. Clone #30 responded also by killing the corresponding JY targets as shown in Fig. 3b exemplarily after combined Aza/Val treatment compared to native JY. External loading with peptide p101-111 was required to increase cytotoxicity of the native LCL to a level after treatment.
Fig. 3.
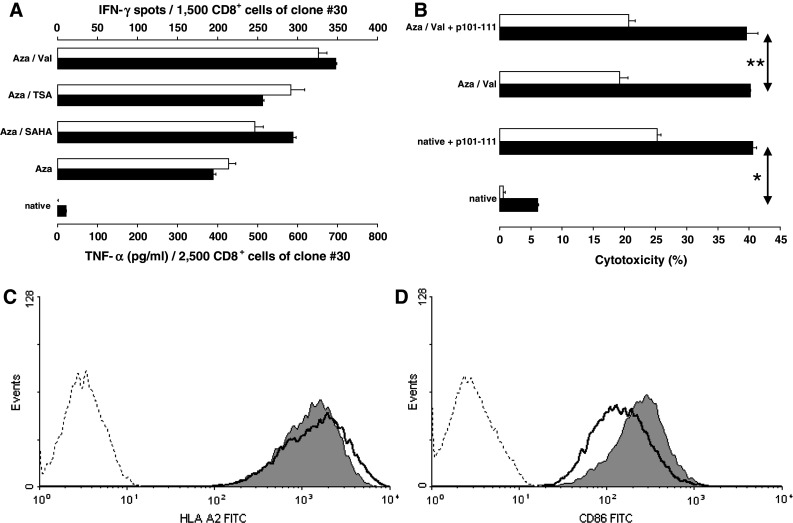
Induction of T cell responses using LCL as CTA-expressing APC. a CD8+ T-cell clone #30, specific for the SSX2 epitope p101-111 in the context of HLA-A*0201, did not respond to untreated LCL ‘JY’ (HLA-A*0201 +) used as APC. However, after treatment with the DNMT1 inhibitor, Aza alone or in combination with one of the three HDAC inhibitors as described, T cells of clone #30, responded to these targets by secreting IFN-γ (unfilled bars) as well as TNF-α (black bars). Results were assessed by IFN-γ ELISPOT and TNF-α ELISA, respectively. b T cells of the same clone were induced to lyse native JY cells used as targets only after additional external loading with 2 μM peptide p101-111 (*p = 0.005). The difference between peptide-loaded or unloaded LCL disappeared after these LCL had been treated with a combination of Aza and Val (**p > 0.05) (p values determined by F test followed by 2-sided Student’s t test). Cytotoxicity was assessed by LDH release and calculated relatively compared to maximum lysis induced by lysing solution. Two different effector/target ratios, 4/1 (black bars) and 2/1 (unfilled bars), were applied. c The expression of MHC-I molecules under DNMT1/HDAC inhibition was analyzed by flow cytometry exemplarily for HLA-A2 (BB7.2 antibody). A small but significantly (∆GMean p = 0.00002) reduced expression of HLA-A2 molecules on LCL after treatment with valproic acid was detected (dotted line = isotype control, wide line = native JY, and gray-filled graph = Val-treated JY). d The expression of costimulatory CD86 molecules increased significantly (∆GMean p = 0.003) after Aza/Val treatment also assessed by flow cytometry (FUN-1 antibody)
Expression of MHC and costimulatory molecules after DNMT1/HDAC inhibition
Expression of MHC and costimulatory molecules under DNMT/HDAC inhibition was analyzed by flow cytometry. As shown in Fig. 3c, combined treatment of LCL ‘JY’ with Aza and Val caused a slightly reduced expression of MHC-I molecules. Nevertheless, the expression remains on a very high level. Same results were obtained analyzing MHC-II expression (data not shown). In contrast, expression of CD86, the most important T cell costimulatory molecule, increased when JY LCL had been cultured with Aza/Val (Fig. 3d). Similar could be seen for the expression of CD40 (data not shown).
Discussion
Cancer testis antigens are normally expressed by gametes and trophoblasts and are aberrantly expressed in a range of human tumors [36, 37]. An important point concerning the therapeutic use of CT antigens is that vaccines targeting CT antigens will not affect germ cells, because they lack the expression of MHC molecules that are necessary for the presentation of the respective antigenic peptides. Therefore, CT antigens are considered to be ideal candidates for vaccine approaches. A range of vaccine formulations containing one ore more CT antigens has been demonstrated to induce cellular and antibody responses in clinical trials [38], but to date with only rare clinical responses [37, 38]. Dendritic cells (DC) loaded with CT proteins or peptides are among the vaccine formulations which showed the strongest immune responses in vitro and in vivo. However, the preparation and maturation of DC is demanding and costly. Investigating the antigen-presenting capacity of the more easily available autologous LCL [39], we demonstrated that autologous LCL transfected with mutated ras induce strong T cell responses in patients with pancreatic cancer carrying the respective mutant of the ras oncogene. In a study of 7 patients using autologous LCL transfected with mutated ras, a strong delayed-type hypersensitivity reaction and the induction of both CD8+- and CD4+-mediated specific anti-mutated ras immune response has been observed [40]. Hence, autologous LCL-expressing CT genes also expressed by the malignant cells of patients with cancer would represent a simple source for cellular vaccine approaches. To test whether LCL can be used as CT antigen-presenting cells, we investigated the expression of CT genes in 20 LCL. We selected those CT genes which have been previously shown to be frequently expressed in human cancers and hence are prime candidates for vaccine approaches. To the best of our knowledge, the expression of CT antigens in LCL has not been described before. Our results show that 75 % of established LCL expressed at least one of the CT genes investigated, and the majority of the LCL co-expressed several CT antigens. The spectrum and frequency of the CT genes expressed by LCL is similar to what has been observed in human malignant tumors such as bladder cancer, ovarian cancer, hepatocellular carcinoma [32], lung cancer, breast cancer [33], pancreatic cancer [31], melanoma, and multiple myeloma [12], suggesting that similar mechanisms are operative in malignant tumors and B cells after EBV transformation. For example, global DNA hypomethylation, gene-specific hypomethylation, and regional hypomethylation occur during tumorigenesis, and global hypomethylation has been correlated with the expression of many [41–43], but not all CT genes [42]. Nevertheless, hypomethylation alone is obviously not sufficient for the induction of CT gene expression, as DNA in colon cancer is universally hypomethylated, even though CT gene expression is rare in this type of tumor. Recently, the role of HDAC inhibitors for the induction of CTA expression has been demonstrated [44–46].
We could not find a correlation between the expression of CTA and specific characteristics of the LCL such as duration in culture, telomerase activity, aneuploidy, or proliferation. The lack of correlation of CT gene expression with any of the biological characteristics of the individual LCL studied here suggests that the mechanisms underlying CT gene expression in malignant transformation are complex. However, the fact that CTA expression starts in the course of EBV transformation of B cells (always CTA-negative) to LCL provides a new model to investigate the epigenetic basis of CTA expression.
The most important finding of our study is that stable expression of many CTA by LCL is obtained after DNMT1 inhibition alone and in combination with HDAC inhibition and that this induced expression is strong enough to elicit CT antigen-specific responses by CD8+ T lymphocytes, demonstrating the feasibility of using autologous LCL as a source for CT gene–specific vaccine strategies without HLA restrictions. Moreover, EBV-transformed LCL are highly immunogenic and can be established and maintained with simple logistics [39]. In this study, only 4 LCL had been included in the DNMT1/HDAC inhibition experiments. This may be the reason why not the complete CTA spectrum could be induced or amplified. In our clinical trial [40], it was easy to establish a lot of different spontaneous LCL from each EBV+ patient. Treating all these LCL with additional/different DNMT1/HDAC inhibitors, it should be possible to close this gap of expression. Application of spontaneous LCL after high-dose irradiation should exclude the risk of lymphoproliferative disorders.
With regards to the therapy of diseases such as Acute Myeloid Leukemia or Myelodysplastic Syndrome, where combinations of 5-azacytidine and HDAC inhibitors are already used [47], equally pretreated LCL could provide an interesting 2nd line therapy supplement. In the context of these malignancies, Goodyear et al. [48] observed an increase of CTA-specific CTL under therapy with Aza/Na-valproate. Although many of the CTA-responding patients achieved clinical responses, both drugs finally were not curative. Complementary vaccination with pretreated autologous LCL could enhance the therapy-induced T cell responses. In contrast to previous clinical vaccination studies using single tumor-associated antigens (TAA) combined with different adjuvants, recent vaccination strategies against TAA were reported to achieve stronger anti-tumor responses by using (recombinant) immunogenic vectors derived from viruses such as vaccinia or fowlpox [49], parasites such as trypanosomas [50], or mixed bacterial vaccine (Coley’s toxin) [51]. Since LCL are also highly immunogenic CTA-expressing targets equipped with professional antigen-presenting properties, they are attractive candidates for the use as efficient and inexpensive cellular anti-cancer vaccines.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank Evi Regitz, Claudia Schormann, and Cora Stephan for their excellent technical support. We also thank Diederik de Bruijn for providing the SSX-specific antibody clone E3AS. This work was supported by grants of LICR, HOM-FOR, German Research Society (DFG), and Deutsche Krebshilfe.
Conflict of interest
None of the authors declares any conflict of interest.
Abbreviations
- CTA
Cancer testis antigen
- RT-PCR
Reverse transcriptase PCR
References
- 1.Chen YT, Gure AO, Tsang S, Stockert E, Jager E, Knuth A, Old LJ. Identification of multiple cancer/testis antigens by allogeneic antibody screening of a melanoma cell line library. Proc Natl Acad Sci USA. 1998;95:6919–6923. doi: 10.1073/pnas.95.12.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4:1. [PubMed] [Google Scholar]
- 3.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De PE, Van den Eynde BJ, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. J Immunol. 2007;178:2617–2621. [PubMed] [Google Scholar]
- 4.Boel P, Wildmann C, Sensi ML, Brasseur R, Renauld JC, Coulie P, Boon T, van der Bruggen P. BAGE: a new gene encoding an antigen recognized on human melanomas by cytolytic T lymphocytes. Immunity. 1995;2:167–175. doi: 10.1016/S1074-7613(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 5.Van den Eynde B, Peeters O, De BO, Gaugler B, Lucas S, Boon T. A new family of genes coding for an antigen recognized by autologous cytolytic T lymphocytes on a human melanoma. J Exp Med. 1995;182:689–698. doi: 10.1084/jem.182.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gure AO, Wei IJ, Old LJ, Chen YT. The SSX gene family: characterization of 9 complete genes. Int J Cancer. 2002;101:448–453. doi: 10.1002/ijc.10634. [DOI] [PubMed] [Google Scholar]
- 7.Chen YT, Scanlan MJ, Sahin U, Tureci O, Gure AO, Tsang S, Williamson B, Stockert E, Pfreundschuh M, Old LJ. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tureci O, Sahin U, Zwick C, Koslowski M, Seitz G, Pfreundschuh M. Identification of a meiosis-specific protein as a member of the class of cancer/testis antigens. Proc Natl Acad Sci USA. 1998;95:5211–5216. doi: 10.1073/pnas.95.9.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tureci O, Sahin U, Koslowski M, Buss B, Bell C, Ballweber P, Zwick C, Eberle T, Zuber M, Villena-Heinsen C, Seitz G, Pfreundschuh M. A novel tumour associated leucine zipper protein targeting to sites of gene transcription and splicing. Oncogene. 2002;21:3879–3888. doi: 10.1038/sj.onc.1205481. [DOI] [PubMed] [Google Scholar]
- 10.Gure AO, Stockert E, Arden KC, Boyer AD, Viars CS, Scanlan MJ, Old LJ, Chen YT. CT10: a new cancer-testis (CT) antigen homologous to CT7 and the MAGE family, identified by representational-difference analysis. Int J Cancer. 2000;85:726–732. doi: 10.1002/(SICI)1097-0215(20000301)85:5<726::AID-IJC21>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 11.Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci USA. 1995;92:11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Duin M, Broyl A, de Knegt Y, Goldschmidt H, Richardson PG, Hop WC, van der Holt B, Joseph-Pietras D, Mulligan G, Neuwirth R, Sahota SS, Sonneveld P. Cancer testis antigens in newly diagnosed and relapse multiple myeloma: prognostic markers and potential targets for immunotherapy. Haematologica. 2011;96:1662–1669. doi: 10.3324/haematol.2010.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quintarelli C, Dotti G, Hasan ST, De AB, Hoyos V, Errichiello S, Mims M, Luciano L, Shafer J, Leen AM, Heslop HE, Rooney CM, et al. High-avidity cytotoxic T lymphocytes specific for a new PRAME-derived peptide can target leukemic and leukemic-precursor cells. Blood. 2011;117:3353–3362. doi: 10.1182/blood-2010-08-300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochsenreither S, Majeti R, Schmitt T, Stirewalt D, Keilholz U, Loeb KR, Wood B, Choi YE, Bleakley M, Warren EH, Hudecek M, Akatsuka Y, et al. Cyclin-A1 represents a new immunogenic targetable antigen expressed in acute myeloid leukemia stem cells with characteristics of a cancer-testis antigen. Blood. 2012;119:5492–5501. doi: 10.1182/blood-2011-07-365890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang S, Preuss KD, Xie X, Regitz E, Pfreundschuh M. Analysis of the antibody repertoire of lymphoma patients. Cancer Immunol Immunother. 2002;51:655–662. doi: 10.1007/s00262-002-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie X, Wacker HH, Huang S, Regitz E, Preuss KD, Romeike B, Parwaresch R, Tiemann M, Pfreundschuh M. Differential expression of cancer testis genes in histological subtypes of non-Hodgkin’s lymphomas. Clin Cancer Res. 2003;9:167–173. [PubMed] [Google Scholar]
- 17.Sugimoto M, Tahara H, Ide T, Furuichi Y. Steps involved in immortalization and tumorigenesis in human B-lymphoblastoid cell lines transformed by Epstein-Barr virus. Cancer Res. 2004;64:3361–3364. doi: 10.1158/0008-5472.CAN-04-0079. [DOI] [PubMed] [Google Scholar]
- 18.Wagner C, Neumann F, Kubuschok B, Regitz E, Mischo A, Stevanovic S, Friedrich M, Schmidt W, Rammensee HG, Pfreundschuh M. Identification of an HLA-A*02 restricted immunogenic peptide derived from the cancer testis antigen HOM-MEL-40/SSX2. Cancer Immun. 2003;3:18. [PubMed] [Google Scholar]
- 19.Smith HA, McNeel DG. Vaccines targeting the cancer-testis antigen SSX-2 elicit HLA-A2 epitope-specific cytolytic T cells. J Immunother. 2011;34:569–580. doi: 10.1097/CJI.0b013e31822b5b1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karbach J, Gnjatic S, Bender A, Neumann A, Weidmann E, Yuan J, Ferrara CA, Hoffmann E, Old LJ, Altorki NK, Jager E. Tumor-reactive CD8+ T-cell responses after vaccination with NY-ESO-1 peptide, CpG 7909 and Montanide ISA-51: association with survival. Int J Cancer. 2010;126:909–918. doi: 10.1002/ijc.24850. [DOI] [PubMed] [Google Scholar]
- 21.Neumann F, Wagner C, Preuss KD, Kubuschok B, Schormann C, Stevanovic S, Pfreundschuh M. Identification of an epitope derived from the cancer testis antigen HOM-TES-14/SCP1 and presented by dendritic cells to circulating CD4+ T cells. Blood. 2005;106:3105–3113. doi: 10.1182/blood-2005-04-1487. [DOI] [PubMed] [Google Scholar]
- 22.Odunsi K, Qian F, Matsuzaki J, Mhawech-Fauceglia P, Andrews C, Hoffman EW, Pan L, Ritter G, Villella J, Thomas B, Rodabaugh K, Lele S, et al. Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc Natl Acad Sci USA. 2007;104:12837–12842. doi: 10.1073/pnas.0703342104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neumann F, Kubuschok B, Ertan K, Schormann C, Stevanovic S, Preuss KD, Schmidt W, Pfreundschuh M. A peptide epitope derived from the cancer testis antigen HOM-MEL-40/SSX2 capable of inducing CD4(+) and CD8(+) T-cell as well as B-cell responses. Cancer Immunol Immunother. 2011;60:1333–1346. doi: 10.1007/s00262-011-1030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atanackovic D, Altorki NK, Cao Y, Ritter E, Ferrara CA, Ritter G, Hoffman EW, Bokemeyer C, Old LJ, Gnjatic S. Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. Proc Natl Acad Sci USA. 2008;105:1650–1655. doi: 10.1073/pnas.0707140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawada J, Wada H, Isobe M, Gnjatic S, Nishikawa H, Jungbluth AA, Okazaki N, Uenaka A, Nakamura Y, Fujiwara S, Mizuno N, Saika T, et al. Heteroclitic serological response in esophageal and prostate cancer patients after NY-ESO-1 protein vaccination. Int J Cancer. 2012;130:584–592. doi: 10.1002/ijc.26074. [DOI] [PubMed] [Google Scholar]
- 26.Miller G, Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci USA. 1973;70:190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahyar-Roemer M, Roemer K. p21 Waf1/Cip1 can protect human colon carcinoma cells against p53-dependent and p53-independent apoptosis induced by natural chemopreventive and therapeutic agents. Oncogene. 2001;20:3387–3398. doi: 10.1038/sj.onc.1204440. [DOI] [PubMed] [Google Scholar]
- 28.Widmann TA, Willmann B, Pfreundschuh M, Beelen DW. Influence of telomere length on short-term recovery after allogeneic stem cell transplantation. Exp Hematol. 2005;33:1257–1261. doi: 10.1016/j.exphem.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Wege H, Chui MS, Le HT, Tran JM, Zern MA. SYBR Green real-time telomeric repeat amplification protocol for the rapid quantification of telomerase activity. Nucleic Acids Res. 2003;31:E3. doi: 10.1093/nar/gng003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tureci O, Chen YT, Sahin U, Gure AO, Zwick C, Villena C, Tsang S, Seitz G, Old LJ, Pfreundschuh M. Expression of SSX genes in human tumors. Int J Cancer. 1998;77:19–23. doi: 10.1002/(SICI)1097-0215(19980703)77:1<19::AID-IJC4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 31.Kubuschok B, Xie X, Jesnowski R, Preuss KD, Romeike BF, Neumann F, Regitz E, Pistorius G, Schilling M, Scheunemann P, Izbicki JR, Lohr JM, et al. Expression of cancer testis antigens in pancreatic carcinoma cell lines, pancreatic adenocarcinoma and chronic pancreatitis. Int J Cancer. 2004;109:568–575. doi: 10.1002/ijc.20006. [DOI] [PubMed] [Google Scholar]
- 32.Luo G, Huang S, Xie X, Stockert E, Chen YT, Kubuschok B, Pfreundschuh M. Expression of cancer-testis genes in human hepatocellular carcinomas. Cancer Immun. 2002;2:11. [PubMed] [Google Scholar]
- 33.Mischo A, Kubuschok B, Ertan K, Preuss KD, Romeike B, Regitz E, Schormann C, de Bruijn D, Wadle A, Neumann F, Schmidt W, Renner C, et al. Prospective study on the expression of cancer testis genes and antibody responses in 100 consecutive patients with primary breast cancer. Int J Cancer. 2006;118:696–703. doi: 10.1002/ijc.21352. [DOI] [PubMed] [Google Scholar]
- 34.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.zum Buschenfelde CM, Metzger J, Hermann C, Nicklisch N, Peschel C, Bernhard H. The generation of both T killer and Th cell clones specific for the tumor-associated antigen HER2 using retrovirally transduced dendritic cells. J Immunol. 2001;167:1712–1719. doi: 10.4049/jimmunol.167.3.1712. [DOI] [PubMed] [Google Scholar]
- 36.Jungbluth AA, Chen YT, Stockert E, Busam KJ, Kolb D, Iversen K, Coplan K, Williamson B, Altorki N, Old LJ. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856–860. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 37.Marchand M, van Baren N, Weynants P, Brichard V, Dreno B, Tessier MH, Rankin E, Parmiani G, Arienti F, Humblet Y, Bourlond A, Vanwijck R, et al. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer. 1999;80:219–230. doi: 10.1002/(SICI)1097-0215(19990118)80:2<219::AID-IJC10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 38.Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, Chen Q, Dimopoulos N, Luke T, Murphy R, Scott AM, Maraskovsky E, et al. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci USA. 2004;101:10697–10702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubuschok B, Schmits R, Hartmann F, Cochlovius C, Breit R, Konig J, Pistorius G, Schilling M, Renner C, Pfreundschuh M. Use of spontaneous Epstein-Barr virus-lymphoblastoid cell lines genetically modified to express tumor antigen as cancer vaccines: mutated p21 ras oncogene in pancreatic carcinoma as a model. Hum Gene Ther. 2002;13:815–827. doi: 10.1089/10430340252898993. [DOI] [PubMed] [Google Scholar]
- 40.Kubuschok B, Pfreundschuh M, Breit R, Hartmann F, Sester M, Gärtner B, König J, Murawski N, Held G, Zwick C, Neumann F. Mutated ras-transfected, EBV-transformed lymphoblastoid cell lines as a model tumor vaccine for boosting T-cell responses against pancreatic cancer: a pilot trial. Hum Gene Ther. 2012;23:1224–1236. doi: 10.1089/hum.2011.153. [DOI] [PubMed] [Google Scholar]
- 41.De SC, Lurquin C, Lethe B, Martelange V, Boon T. DNA methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Mol Cell Biol. 1999;19:7327–7335. doi: 10.1128/mcb.19.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loriot A, De PE, Boon T, De SC. Transient down-regulation of DNMT1 methyltransferase leads to activation and stable hypomethylation of MAGE-A1 in melanoma cells. J Biol Chem. 2006;281:10118–10126. doi: 10.1074/jbc.M510469200. [DOI] [PubMed] [Google Scholar]
- 43.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 44.Leclercq S, Gueugnon F, Boutin B, Guillot F, Blanquart C, Rogel A, Padieu M, Pouliquen D, Fonteneau JF, Gregoire M. A 5-aza-2′-deoxycytidine/valproate combination induces cytotoxic T-cell response against mesothelioma. Eur Respir J. 2011;38:1105–1116. doi: 10.1183/09031936.00081310. [DOI] [PubMed] [Google Scholar]
- 45.Oi S, Natsume A, Ito M, Kondo Y, Shimato S, Maeda Y, Saito K, Wakabayashi T. Synergistic induction of NY-ESO-1 antigen expression by a novel histone deacetylase inhibitor, valproic acid, with 5-aza-2′-deoxycytidine in glioma cells. J Neurooncol. 2009;92:15–22. doi: 10.1007/s11060-008-9732-0. [DOI] [PubMed] [Google Scholar]
- 46.Wischnewski F, Pantel K, Schwarzenbach H. Promoter demethylation and histone acetylation mediate gene expression of MAGE-A1, -A2, -A3, and -A12 in human cancer cells. Mol Cancer Res. 2006;4:339–349. doi: 10.1158/1541-7786.MCR-05-0229. [DOI] [PubMed] [Google Scholar]
- 47.Kuendgen A, Bug G, Ottmann OG, Haase D, Schanz J, Hildebrandt B, Nachtkamp K, Neukirchen J, Dienst A, Haas R, Germing U, Gattermann N. Treatment of poor-risk myelodysplastic syndromes and acute myeloid leukemia with a combination of 5-azacytidine and valproic acid. Clin Epigenet. 2012;2:389–399. doi: 10.1007/s13148-011-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodyear O, Agathanggelou A, Novitzky-Basso I, Siddique S, McSkeane T, Ryan G, Vyas P, Cavenagh J, Stankovic T, Moss P, Craddock C. Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myeloidplasia. Blood. 2010;116:1908–1918. doi: 10.1182/blood-2009-11-249474. [DOI] [PubMed] [Google Scholar]
- 49.Odunsi K, Matsuzaki J, Karbach J, Neumann A, Mhawech-Fauceglia P, Miller A, Beck A, Morrison CD, Ritter G, Godoy H, Lele S, duPont N, et al. Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma patients. Proc Natl Acad Sci USA. 2012;109:5797–5802. doi: 10.1073/pnas.1117208109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Junqueira C, Santos LI, Galvao-Filho B, Teixeira SM, Rodrigues FG, DaRocha WD, Chiari E, Jungbluth AA, Ritter G, Gnjatic S, Old LJ, Gazzinelli RT. Trypanosoma cruzi as an effective cancer antigen delivery vector. Proc Natl Acad Sci USA. 2011;108:19695–19700. doi: 10.1073/pnas.1110030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karbach J, Neumann A, Brand K, Wahle C, Siegel E, Maeurer MJ, Ritter E, Tsuji T, Gnjatic S, Old LJ, Ritter G, Jager E. Phase I clinical trial of Mixed Bacterial Vaccine (Coley’s Toxins) in patients with NY-ESO-1 expressing cancers: immunological effects and clinical activity. Clin Cancer Res. 2012;18:5449–5459. doi: 10.1158/1078-0432.CCR-12-1116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.