Abstract
The capacity of a low-dose HPV16 synthetic long-peptide vaccine (HPV16-SLP) to induce an HPV16-specific T-cell response as well as to establish long-term immunologic memory in patients with low-grade abnormalities of the cervix was determined in a placebo-controlled, double-blinded phase II study. In addition, the effect of a booster vaccination after 1 year was evaluated. Patients received either the HPV16-SLP or a placebo at the start of the study. After 1 year, the vaccinated patients were again randomized to receive the HPV16-SLP or a placebo. Patients were followed for 2 years. HPV16-specific T-cell responses were determined in pre- and post-vaccination blood samples by ELISPOT, proliferation assay and cytokine assays. We show that the HPV16-specific T-cell responses detected after vaccination are clearly due to vaccination and that reactivity was maintained for at least 2 years. Interestingly, a booster vaccination after 1 year especially augmented the HPV16-specific Th2 response. Furthermore, pre-existing immunity to HPV16 was associated with a stronger response to vaccination and with more side effects, reflected by flu-like symptoms. We conclude that two low-dose injections of HPV16-SLP can induce a strong and stable HPV16-specific T-cell response that lasts for at least 1 year. If booster vaccination is required, then polarizing adjuvant should be added to maintain the Th1 focus of the vaccine-induced T-cell response.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1499-2) contains supplementary material, which is available to authorized users.
Keywords: CIN, HPV16, Immunotherapy, Vaccination, Memory response
Introduction
The development of (pre)cancers of the anogenital tract is associated with persisting human papillomavirus (HPV) infections [1]. The risk of progression of squamous intraepithelial lesions (SIL) is related to the severity of dysplasia. Up to 40 % of low-grade cervical squamous intraepithelial lesions (LSIL) will not spontaneously regress [2]. Small lesions can easily be treated by loop electrosurgical excision procedure (LEEP), yet LEEP of larger lesions can leave positive margins causing lesion recurrence requiring repeated surgery [3]. For the group of patients with a child wish, this can pose a problem due to distortion of the cervix and pre-term delivery [4].
Vaccines have been developed to prevent persistent infection with HPV but these prophylactic vaccines are not effective in patients already infected with HPV16 or HPV18 [5]. Virus-specific, interferon-γ (IFNγ)-producing CD4+ T helper (Th) cells and CD8+ cytotoxic T lymphocytes (CTL) are essential components in controlling chronic viral infections [6, 7].
Healthy donors display relatively robust proliferative T-cell responses against the viral early proteins E2, E6 and E7, characterized by Th cells that produce IFNγ and IL-5 [8–10]. In addition, the majority of subjects who clear HPV16 display HPV16 E6-specific CTL responses [11, 12]. These findings suggest that successful defense against HPV16 infection is associated with a systemic HPV-specific T-cell response. Therapeutic vaccination can be clinically effective in patients with histologically confirmed HPV16+ vulvar epithelial neoplasia grade 3 (VIN3). Complete regression of lesions was seen after vaccination with a protein vaccine [13] or an HPV16 E6/E7 synthetic long-peptide vaccine (HPV16-SLP) [14]. Clinical success correlated with the induction of strong and broad HPV16-specific Th responses and HPV16-specific CD8+ T-cell activity [13–15]. In patients with high-grade cervical squamous intraepithelial lesions (HSIL), immunization with 300 μg per peptide of the HPV16-SLP vaccine-induced robust immune responses [16].
In addition to women with high-grade lesions, also women with persistent low-grade lesions may be treated by therapeutic vaccination. As low-grade cervical lesions are not considered a severe disorder, we decided to immunize such individuals with the lowest dose (50 μg/peptide) previously shown to be immunogenic in patients with cervical cancer [17]. To this end, patients with LSIL or persistent mild cytological cervical abnormalities received either placebo or were vaccinated twice. The group of vaccinated patients was then randomized to receive a placebo or a booster vaccination after 1 year. All patients were followed for 2 years and their HPV-specific immune response was tested at several time points during the study. The aim of this phase II study was threefold. (1) To study the capacity of a low-dose vaccine to induce HPV16-specific T-cell responses in patients with LSIL or persistent mild cytological cervical abnormalities, (2) to evaluate the long-term memory response after vaccination and (3) to study the effect of revaccination after 1 year on the HPV16-specific T-cell response.
Patients and methods
Patients
In this placebo-controlled, double-blinded study, 50 patients with histological evidence of LSIL or persistent mild cytological cervical abnormalities were included from the out-patient departments of the Leiden University Medical Center (Leiden, the Netherlands), the Haga Teaching Hospital (the Hague, the Netherlands) and the Medical Centrum Haaglanden (the Hague, the Netherlands). Patients were included between May 2007 until March 2010 after oral and written informed consent. Eligibility required pre-treatment laboratory findings of leukocytes >3 × 109/L, lymphocytes >1 × 109/L, thrombocytes >100 × 109/L and hematocrit >30 % and no radiotherapy, chemotherapy or other potentially immunosuppressive therapy administered within 4 weeks prior to the immunotherapy. The study was approved by the Dutch Central Committee on Human Research (CCMO, https://toetsingonline.ccmo.nl/ccmo_search.nsf/dossier number NL14057 000 06) and the medical Ethical Committee of the Leiden University Medical Center and the Haga teaching Hospital. Monitoring for adverse events and injection-site reactions, clinical assessments and laboratory tests were performed as described previously [17]. Data were gathered on previous HPV-related disease [PHD, defined as surgical or topical treatment of SIL of the cervix or vulvar intraepithelial neoplasia (VIN)], atopic constitution and smoking habits. Adverse events were classified according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. The flu-like syndrome was defined as having two or more of the following complaints: fever, chills, headache, malaise, fatigue, myalgia, nausea, anorexia, vomiting or diarrhea after vaccination. In most patients, the symptoms subsided within 72 h, any symptoms persisting longer than 72 h or starting 72 h after vaccination were scored separately.
Vaccine and vaccination scheme
The HPV16-synthetic long-peptide vaccine (HPV16-SLP) used in this study consists of two separate drug products, together representing the entire sequence of the E6 and E7 oncoproteins of HPV16. The clinical grade peptides (9 E6 and 4 E7 peptides of 25–35 amino acids long with an overlap of 10–14 amino acids) were synthesized, vialed and formulated at the GMP facility of the department of Clinical Pharmacy and Toxicology of the LUMC as described previously [14–18]. Peptides were dissolved in dimethyl sulfoxide (DMSO) and admixed with 20 mM phosphate buffer (pH 7.5) and Montanide ISA-51 (final volume ratio 20/30/50, respectively), and patients received the vaccine at a dose of 50 μg/peptide. This dose has previously been shown to induce HPV16-specific immunity in end-stage cervical cancer patients [17].
Patients were assigned to one of the two treatment groups (block size 5). Randomization was blinded for patients and the immunomonitoring laboratory. Four out of five patients, in total 40, were randomized to receive two sequential HPV16-SLP vaccinations at a 3-week interval (50 μg/peptide), T = 0 week and T = 3 weeks (Group 1, Fig. 1). They received a mix of nine synthetic long HPV16 E6 peptides in the left arm or thigh and four synthetic long HPV16 E7 peptides in the right arm or thigh. Ten (one out of five) patients were randomized to receive phosphate-buffered saline (PBS) in both arms or thighs in the same regime (Group 2). After 1 year (T = 1 year), a second randomization took place. Half the patients from group 1 were randomized to receive a booster vaccination of 50 μg peptide of the HPV16-SLP (group 1A) and the other half was randomized to receive PBS (group 1B). Patients in group 2 received PBS throughout the study. All vaccinations were performed in the LUMC. Patients stayed at the ward for 1–2 h after vaccination during which any experienced local and/or systemic adverse events were recorded. Patients recorded any adverse events experienced in the weeks between and after vaccinations in a diary or were asked to report any adverse events. Venous blood (70 ml) for immune monitoring was drawn at five time points; before (T = 0 week), 7 weeks (T = 7 weeks) and 1 year (T = 1 year) after first vaccination, 3 weeks after booster vaccination (T = 1 year + 3 weeks) and finally 2 years after first vaccination (T = 2 years; Fig. 1). An extra Pap smear was taken before vaccination and at 1 and 2 years after vaccination for histology and HPV typing.
Fig. 1.
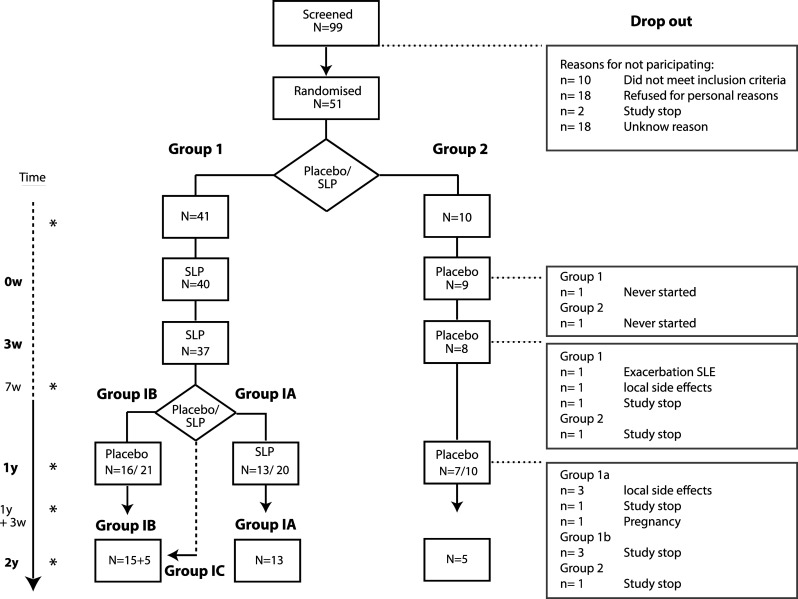
Vaccination scheme and patient flow-chart. To the left is the time line. The stars (asterisk) indicated the time points at which blood was drawn. In group 1 (n = 41), one patient never turned up for vaccination and three patients received only one HPV16-SLP vaccination (one due to an exacerbation of systemic lupus erythematosus after first vaccination, one was lost to follow-up and one stopped due to local side effects). The remaining 37 patients received two HPV16-SLP vaccinations. In group 2, one patient never turned up for vaccination and one did not receive the second vaccination due to a study stop. After 1 year (T = 1 year), within group 1, a second randomization took place. In group 1A (n = 20; HPV16-SLP booster), 13 patients were eventually re-vaccinated (drop-outs: one due to the study stop, three due to local adverse events, one due to an active pregnancy wish). Five patients that were randomized to group 1A did not receive the booster vaccination with HPV16-SLP, yet did give blood at T = 1 year and T = 2 years. These patients formed group 1C and were analyzed together with group 1B at T = 2 year. In group 1B (n = 20; PBS-placebo booster), 16 patients eventually received the placebo (drop-outs: three due to the study stop). In group 2, there were two drop-outs due to the study stop, leaving seven patients who were eventually re-vaccinated with PBS-placebo and five patients could be followed up to 2 years (T = 2 years)
HPV-specific T-cell immunity monitoring
In acknowledgment of the minimal information about T-cell assays (MIATAproject.org) detailed information about the sample, the assay, the data acquisition, the data analysis and the laboratory environment is provided [19, 20]. The peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll (prepared by LUMC pharmacy) gradient centrifugation within 4 h after blood was drawn (70 mL in 9 mL heparine tubes, kept at room temperature). The median PBMC numbers obtained were 56 million and ~10 million cells were used directly in the lymphocyte stimulation test (LST) to test for HPV16 specificity. The proliferative response accompanying cytokines were measured by the cytometric bead array (CBA). The remaining cells were cryopreserved (~5–10 million/vial) in 90 % fetal bovine serum and 10 % DMSO in 1 mL cryovials (Greiner) using a controlled freezing apparatus and immediately stored in the vapor phase of the liquid nitrogen vessel until used (median pre-sample 24 months, post-samples 5 months). Thawed PBMCs were subjected to the IFN-γ-Elispot and geared to determine Th type 1 (Th1) responses. The median cell recovery post-thaw was 73.6 % with a median viability of 76.8 %. Cell counts and viability was obtained using trypan blue (0.4 %, Sigma) staining and counting using the hemocytometer. In this set of complementary T-cell immunomonitoring assays (LST and IFN-γ-Elispot), six pools of 22 amino acid long peptides overlapping by 12 amino acids were used. All tests have previously been described, and positive responses have been pre-defined [21]. For all T-cell assays, a vaccine-induced response was defined as at least a threefold increase in the response after vaccination when compared with the results before vaccination (T = 0 week). Similarly, a booster vaccination-enhanced response was defined as an at least threefold increase in the immune response after the booster vaccination compared with the HPV-specific immune response before booster vaccination. The T-cell assays were performed in the laboratory of the Department of Clinical Oncology (LUMC, Leiden) that operates under exploratory research conditions following standard operating procedure (SOPs) and using trained staff. This laboratory has participated in all proficiency panels of the CIMT Immunoguiding Program (http://www.cimt.eu/workgroups/cip/) as well as in IFNγ-Elispot panels of the Cancer Immunotherapy Consortium (http://www.cancerresearch.org/cic), which both aim to harmonize the assays used for T-cell monitoring and the reporting thereof.
For each different type of immune assay, the strength of the immune response was defined as the median-specific spot count (ELISPOT), stimulation index (LST) or amount of cytokine production (CBA) obtained for all six different peptide pools of all patients in one group. Raw data was stored for verification. Comparisons of the strength of the different types of immune responses at different time points within one group of patients were made by the nonparametric Wilcoxon matched-pairs signed rank test and between groups by the nonparametric Mann–Whitney test using GraphPad InStat Software. For the comparison of the immune responses and patients characteristics, patients were divided into two groups based on the presence or absence of HPV-specific immune response and calculated using the Statistical Package for the Social Sciences (SPSS) software package 17 . All reported P values are two-sided and have not been adjusted for multiple comparisons. P ≤ 0.05 was considered significant.
HPV testing
DNA was isolated from cervical smears or formalin-fixed, paraffin-embedded biopsy samples as previously described [22]. Beta-globin polymerase chain reaction (PCR) was performed using primers RS40 and RS42 http://www.sciencedirect.com/science/article/pii/S0165460807001227—bib21 to determine whether the isolated DNA was suitable for amplification. The DNA was subjected to a short PCR fragment assay using the SPF10 primer set, according to the manufacturer’s instructions (Innogenetics, Ghent, Belgium). Each experiment was performed with separate positive and several negative controls. The presence of HPV was established using a microtiter plate–based hybridization assay, and SPF10-PCR products from HPV DNA-positive cases were directly genotyped using a reverse hybridization line probe assay (Inno-LiPa HPV Genotyping Extra; Innogenetics). With this assay, 28 individual HPV genotypes can be identified simultaneously: HPV 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68, 69, 70, 71, 73, 74 and 82.
Results
Patients and vaccinations
Between May 2007 and March 2010, 99 patients were screened of whom 51 patients were accrued for the study (Fig. 1). The average age was 40 years. 47 % of the patients had a medical history of PHD (Table 1). The majority (73 %) of the patients was infected by at least one high-risk HPV-type (Table 1) and 33 % were infected with HPV16. Patients were diagnosed with a LSIL at inclusion or had signs of persistent HPV infection (persistent mild cytological cervical abnormalities). The 51 patients were assigned to one of the two treatment groups at the start of the study (Table 1; Fig. 1). Group 1 was assigned to receive the HPV16-SLP twice at T = 0 week and at T = 3 weeks (n = 41) and Group 2 was to receive PBS-placebo (n = 10). The study was temporarily stopped in 2009 and 2010 due to serious adverse events in one of the other HPV16-SLP clinical trials. Figure 1 shows the drop-outs at the different time points. One year after first vaccination, half of group 1 should have been re-vaccinated with the HPV16-SLP (group 1A) and the other half should have received the PBS-placebo (group 1B; Fig. 1). As vaccination with PBS is not considered immunogenic, patients in group 1A who did not receive a booster vaccination at T = 1 year were grouped in group 1C and analyzed together with group 1B at T = 2 years.
Table 1.
Patient characteristics and vaccination scheme
| ID | Age | PHDa | Allergicb constitution | Smokingc | Cytology/Histology | HPVd | Nr of Given injections | Reason for stopping |
T = 0 SLP/Placeboe |
T = 1 year SLP/Placeboe |
Immuno-monitoring 2 yearsf | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes/no | years | T = 0 week | T = 1 year | T = 2 years | T = 0 week | T = 1 year | T = 2 years | |||||||||
| 1001 | 34 | + | UK | − | 15 | Dysplasia/Pap3a | Stopped (SLE) | Stopped (SLE) | 82 | Stopped (SLE) | Stopped (SLE) | 1 | SLE | 1 | 2 | − |
| 1002 | 0 | 1 | 1 | − | ||||||||||||
| 1003 | 54 | + | UK | − | 1.5 | CIN2 (LEEP)/Pap3a | Pap2 | NT | 81 | 54.74 | NT | 3 | 1 | 2 | 1b | |
| 1004 | 32 | + | UK | − | 0.6 | CIN 2–3 (LEEP)/Pap3a | Pap 2 | LTF | 16 | 44.53 | LTF | 3 | 2 | 2 | − | |
| 1005 | 66 | + | UK | − | 1.5 | Pap3a (no dysplasia)/Pap1 | Pap1 | Pap 1 | Neg | Neg | 74 | 3 | 1 | 1 | 1a | |
| 1006 | 45 | − | UK | − | 0 | Pap2, Pap2 | Pap 1 | Pap2 | Neg | Neg | Neg | 3 | 1 | 2 | 1b | |
| 1007 | 33 | + | UK | + | 19 | CIN1 | Pap3a (LEEP CIN1) | Pap2 | 31, 33, 44, (52), (54) | 31, 44, 53, (54) | Neg | 3 | 1 | 2 | 1b | |
| 1008 | 36 | + | + | + | 20 | Pap3a, Pap3a | Pap2 | Pap3a | 31 | 31, 44, (33), (52), (54) | 31, 33, 44, (52), (54) | 3 | 1 | 1 | 1a | |
| 1009 | 27 | + | + | + | 17 | CIN1 | Pap1 | Pap 1 | 70 | 16, 53, 70 | 70 | 3 | 1 | 2 | 1b | |
| 1010 | 50 | − | − | + | 60 | Pap3a (no dysplasia) | Pap1 | Pap2 | 52 | Neg | Neg | 3 | 2 | 2 | 2 | |
| 1011 | 47 | − | UK | + | 1.5 | Pap2, Pap2 | Pap1 | Pap1 | 31.44 | 31, 44, 35, (52), (54) | 52 | 3 | 2 | 2 | 2 | |
| 1012 | 32 | + | UK | + | 1.5 | Pap2, Pap1, VIN, Pap3a | Pap3a | Pap 2 | 31.53 | 31 | 31, 33, 44 | 3 | 1 | 1 | 1a | |
| 1013 | 51 | − | UK | − | 10 | Pap2, Pap2 | Pap1 | Pap1 | Neg | Neg | Neg | 3 | 1 | 1 | 1a | |
| 1014 | 41 | + | UK | + | 18 | Pap3a, Pap3a | Pap2 | Pap3b | 16 | 16 | 16 | 3 | 1 | 2 | 1b | |
| 1015 | 42 | − | UK | − | 9 | Pap2, Pap3a | Pap2 | Pap3a | (52), 53 | 53 | 53.43 | 3 | 1 | 1 | 1a | |
| 1016 | 51 | − | − | + | 10 | CIN1 | Pap3b (LEEP CIN2) | Pap1 | 58 | 16, 74 | LSE | 2 | LSE | 1 | 1 | − |
| 1017 | 28 | + | UK | + | 2 | CIN1 | Pap1 | Pap1 | 39 | Neg | NT | 3 | 2 | 2 | 2 | |
| 1018 | 42 | + | UK | + | 18 | CIN1 | Pap1 | Pap1 | 16.51 | 45.51 | 45.51 | 3 | 1 | 2 | 1b | |
| 1019 | 37 | − | − | − | 0 | Pap3a, Pap3a | Pap1 | Pap1 | 16 | 16 | 16 | 3 | 1 | 2 | 1b | |
| 1020 | 32 | + | − | + | 8.5 | CIN1 | Pap1 | Pap1 | 16 | 16 | 16 | 3 | 1 | 2 | 1b | |
| 1021 | 39 | − | − | − | 12 | Pap2, Pap3a | Pap1 | Pap1 | Neg | Neg | Ntb | 3 | 1 | 2 | 1b | |
| 1022 | 21 | − | + | − | 5 | Pap3a | Pap3a (LEEP CIN2) | Pap1 | 16, 53 | 16 | 54 | 3 | 1 | 2 | 1b | |
| 1023 | 34 | − | − | + | 7.5 | Pap2, Pap2 | Pap1 | Pap1 | 51 | Neg | Neg | 3 | 2 | 2 | 2 | |
| 1024 | 46 | − | + | + | 30 | CIN1 | Pap2 | Pap2 | 56 | 56, 59 | 56 | 3 | 1 | 2 | 1b | |
| 1025 | 43 | − | − | − | 0 | Pap2, Pap2 | Pap1 | Pap1 | Neg | Neg | Neg | 3 | 1 | 2 | 1b | |
| 1026 | 42 | − | + | − | 0 | Pap3a, Pap2 | Pap2 | Pap1 | 16, 51, 66 | 51 | Neg | 3 | 1 | 1 | 1a | |
| 1027 | 36 | − | + | − | 17 | Pap2, Pap2 | Pap1 | Pap1 | 39, 52, 58 | 31.39 | 58,31/54 | 3 | 1 | 2 | 1b | |
| 1028 | 51 | − | − | − | 6 | Pap1, Pap1 | Pap1 | Pap1 | Neg | 66 | Neg | 3 | 1 | 1 | 1a | |
| 1029 | 26 | − | + | + | 7.5 | CIN1 | Pap2 | LSE | 31 | 31.56 | LSE | 2 | LSE | 1 | 1 | 1c |
| 1030 | 46 | − | − | + | 22 | CIN1 | Pap1 | Pap1 | 35, 53, 54 | 35, 53, 54 | 52 | 3 | 2 | 2 | 2 | |
| 1031 | 26 | − | − | + | 1 | Pap3a, Pap3a | Pap1 | Pap1 | 18.35 | 16, 53, 6 | 53 | 3 | 2 | 2 | 2 | |
| 1032 | 32 | − | + | − | 0 | CIN1 | Pap2 | Pap1 | × | × | × | 2 | Pregnancy | 1 | 1 | 1c |
| 1033 | 45 | − | + | − | 0 | CIN1 | Pap1 | Pap1 | 68, (39) | 68 | 18 | 3 | 1 | 1 | 1a | |
| 1034 | 46 | − | + | − | 0 | CIN1 | Pap2 (LEEP CIN2) | Pap2 | 66.82 | NT | 33 | 3 | 1 | 2 | 1b | |
| 1035 | 49 | − | − | + | 11 | Pap1, Pap3a (no dysplasia) | Pap3a | Pap1 | Neg | Neg | Ntb | 3 | 1 | 1 | 1a | |
| 1036 | 38 | + | + | − | 0 | CIN2-VAIN-Pap3a | Pap1 | Pap2 | 16 | Neg | Neg | 3 | 1 | 1 | 1a | |
| 1037 | 28 | + | − | + | 8.5 | Pap2, Pap1 | Pap1 | Pap1 | 16 | 16 | Neg | 3 | 1 | 1 | 1a | |
| 1038 | 38 | + | − | + | 1 | Pap2, Pap3a | Pap1 | Pap1 | 56, (74) | Neg | NT | 3 | 1 | 2 | 1b | |
| 1039 | 0 | 2 | 2 | − | ||||||||||||
| 1041 | 49 | + | − | − | 0 | Pap2, Pap3a | Pap1 | Pap1 | Neg | Neg | Neg | 3 | 1 | 2 | 1b | |
| 1042 | 32 | − | + | − | 12 | Pap3a, Pap3a | Pap1 | Pap1 | 52 | 52 | 52 | 3 | 1 | 1 | 1a | |
| 1043 | 36 | + | − | − | 6 | Pap3a, Pap3a | Pap2 | Study stop | 16 | 16 | Study stop | 2 | Study stop | 2 | 2 | − |
| 1044 | 42 | + | − | − | 0 | CIN1 | Pap3a | Hysterectomy | 45 | Neg | Hysterectomy | 2 | Study stop | 1 | 2 | 1c |
| 1045 | 39 | + | − | − | 0 | Pap2, Pap3a | Pap2 | Pap1 | 45 | Neg | LTF | 2 | Study stop | 1 | 1 | − |
| 1046 | 47 | − | − | − | 0 | Pap2, Pap2 | Pap3a | Pap3a | 56 | 56 | 56, 66 | 2 | Study stop | 1 | 2 | 1c |
| 1047 | 46 | + | − | − | 7 | CIN1 | Pap1 | Pap1 | 16, 58 | Study stop | Study stop | 1 | Study stop | 2 | 2 | − |
| 1048 | 28 | − | UK | + | 2.5 | CIN1 | LTF | LTF | 16 | LTF | LTF | 1 | LTF | 1 | 2 | − |
| 1049 | 57 | + | + | + | 14 | Pap2, Pap1 | Pap1 | Pap1 | Neg | LSE | LSE | 1 | LSE | 1 | 1 | − |
| 1050 | 41 | + | − | + | 25 | Pap3a, Pap2 | Pap1 | Pap1 | 35 | 58 | LSE | 2 | LSE | 1 | 1 | 1c |
| 1051 | 49 | − | + | + | 17 | CIN1 | Study stop | Pap1 | 31 | 31, 33, 44 | Study stop | 2 | Study stop | 1 | 2 | − |
Six patient did not strictly fit the inclusion criteria, yet had extensive histories of abnormal cervical lesions or clear indication of HPV infection
The italics indicate patients who did not receive all three vaccinations or missing samples
UK unknown, SLE systemic lupus erymatosus, LSE local side effects, LTF lost to follow-up
aPHD previous HPV-related disease
bAllergic constitution: Atopy (atopic syndrome) is a syndrome characterized by a tendency to be “hyperallergic.” A person presents with one or more of the following: eczema (atopic dermatitis), allergic rhinitis (hayfever), allergic conjunctivitis or allergic asthma
cSmoking at the time of inclusion. 20 cigarettes/day/year is one smoking year
dHPV testing was done by Inno-LiPA that detects high-risk HPV genotypes (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82), a number of low-risk HPV genotypes (6, 11, 40, 43, 44, 54, 70) and some additional types (69, 71, 74). HPV X indicates that the HPV type was not found by Inno-LiPA. Neg indicates no HPV
eAt T = 0, patients were randomized to receive two vaccination with HPV16 SLP (1) or a placebo (2). At T = 1 year, patients in group 1 were again randomized to receive the HPV16-SLP or placebo
fFor the immunomonitoring at 2 years patients who had received 3× HPV16-SLP were group 1a; 2× HPV16-SLP and 1× placebo group 1b; 2× HPV16-SLP vaccination followed by nothing at 1 year were allotted to group 1c and analyzed together with group 1b. Patients who had received 2 or 3× placebo were allotted to group 2
Adverse events
In total, 40 patients received one or more vaccinations with the HPV16-SLP. Of these, five patients discontinued the study pre-maturely because of adverse events four because of local adverse events, and one patient (1001) with a history of systemic lupus erythematosus who developed an acute exacerbation of cutaneous lupus erythematosus (LE) 3 days after the first vaccination. Adverse effects did not exceed grade 2 (Table 2). The most frequent systemic adverse event after vaccination was the flu-like syndrome (FLS; 26 %). Fifty-four percent of the patients experienced a second flare of systemic and/or local side effects several days (5–21 days) after the initial reaction had subsided. In the placebo group, no side effects exceeded grade 1 (Table 2). After booster vaccination with the HPV16-SLP, the flu-like syndrome (23 %) was the most frequently experienced adverse event, but it did not exceed grade 1 (Group 1A). Almost all patients in group 1 had grade 2 or 3 injection-site reactions in the weeks following vaccination with swellings beyond 8 cm (Table 2) accompanied by redness, pain and/or itching. Patients were evaluated for remaining local adverse events at 1 and 2 years after vaccination. A painless swelling was still palpable in 41 % of the patients after 1 year and in 48 % after 2 years (56 % in group 1A and 41 % in group 1B). Three patients developed an ulcer at the site of injection. The first developed the ulcer (<2 cm) 6 months after first vaccination. Wound culture revealed a secondary infection with Staphylococcus aureus. After 2 years, there was scarring at the site of injection. Two other ulcers developed within the second year of the study. Both were sterile ulcers, showing a granulomatous infection as seen in foreign body reactions. Ulcers took a longtime to resolve with periods of healing followed by renewed sterile drainage. In one patient, we were able to do a skin test that revealed a type IV allergic reaction to the montanide.
Table 2.
Safety and toxicity
| ID | Systemic toxicitya | Local toxicityb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | Booster | T = 7 weeks | T = 1 year | Booster | T = 2 years | ||||||
| Grade 1 | Grade 2 | Grade 1 | Grade2 | Grade 1 | Grade2 | Swellingc | Pigmentation | |||||
| Group 1a | 1035 | 3, 4 | – | 4 | 1 | 1 | – | 3 | 0 | 2 | 0 | 0 |
| 1037 | – | – | – | 1 | 13 | – | 2 | 0 | 1 | 1 | 0 | |
| 1040 | – | – | 3 | 1, 2 | 1 | – | 2 | 1 | 2 | Ulcer | 1 | |
| 1042 | 11 | – | – | 1, 2 | – | – | 2 | 0 | 1 | 0 | 0 | |
| 1015 | 4 | – | – | 3 | 3 | – | 1 | 0 | 1 | 0 | 0 | |
| 1012 | – | 1 | – | – | – | – | 2 | 1 | 1 | 1 | 1 | |
| 1008 | – | 3 | 2 | – | 3, 7 | – | 2 | 1 | 1 | 1 | 1 | |
| 1005 | – | – | – | – | 4 | – | 2 | 0 | 1 | LTF | ||
| 1013 | – | – | 14 | – | – | – | 2 | 0 | 1 | 0 | 0 | |
| 1026 | – | – | – | – | – | – | 2 | 1 | 0 | 0 | 0 | |
| 1028 | – | – | – | – | – | – | 2 | 1 | 1 | 1 | 0 | |
| 1033 | – | – | 1 | – | 1, 5 | – | 2 | 0 | 1 | 0 | 0 | |
| 1036 | – | – | – | – | – | – | 3 | 0 | 1 | 1 | 0 | |
| 1016 | 7 | – | – | 1 | 2 | 2 | Ulcer | |||||
| 1029 | 9 | – | 1 | – | 1 | Ulcer | ||||||
| 1032 | 9 | – | 1 | – | 3 | 1 | 1 | 1 | ||||
| 1045 | 3 | – | 3 | – | 2 | 0 | ||||||
| 1050 | – | – | – | – | 2 | 1 | 2 | 1 | ||||
| 1049 | 13 | – | 2 | 1 | ||||||||
| Group 1b | 1003 | – | – | – | – | – | – | 2 | 0 | LTF | LTF | |
| 1006 | – | – | – | 1, 5 | 1 | – | 3 | 1 | 0 | 1 | 0 | |
| 1007 | – | – | 6 | – | – | 3 | 0 | 0 | 0 | 0 | ||
| 1009 | – | – | 3 | – | – | – | 2 | 0 | 0 | 0 | 0 | |
| 1014 | – | – | – | – | – | – | 2 | 0 | 0 | 0 | 0 | |
| 1018 | 1 | – | 1 | 6, 8 | – | – | 2 | 0 | 0 | 1 | 0 | |
| 1019 | 4 | – | 4 | – | – | – | 3 | 0 | 0 | 0 | 1 | |
| 1020 | 9 | – | – | 1 | – | – | 2 | 1 | 0 | 1 | 1 | |
| 1021 | – | – | – | – | – | – | 1 | 0 | 0 | 0 | 0 | |
| 1022 | 3 | – | – | – | – | – | 2 | 1 | 0 | UK | UK | |
| 1024 | 1, 9 | – | 9 | – | – | – | 2 | 1 | 0 | 1 | 0 | |
| 1025 | – | – | 9, 11 | – | – | – | 1 | 0 | 0 | 1 | 1 | |
| 1027 | 1, 5, 3 | – | 1 | 5, 3 | 4 | – | 2 | 0 | 0 | 0 | 0 | |
| 1034 | 3, 4 | – | 4 | 1 | – | – | 2 | 0 | 1 | 0 | 0 | |
| 1038 | 1 | – | – | – | – | – | 2 | 1 | 0 | 1 | 1 | |
| 1041 | 1 | – | – | – | – | – | 1 | 1 | 0 | 0 | 0 | |
| 1044 | – | – | – | – | 1 | 1 | 0 | 0 | ||||
| 1046 | – | – | – | – | 3 | 0 | 0 | 0 | ||||
| 1051 | 3 | – | 1 | 2, 8 | 3 | 1 | 1 | |||||
| 1001 | Exacerbation SLE | UK | ||||||||||
| 1048 | LTF | LTF | UK | |||||||||
| Group 2 | 1004 | – | – | – | – | – | – | 1 | 0 | 1 | LTF | LTF |
| 1010 | – | – | – | – | – | – | 0 | 0 | 0 | 0 | 0 | |
| 1011 | – | – | – | – | – | – | 0 | 0 | 0 | 0 | 0 | |
| 1017 | – | – | – | – | – | – | 0 | 0 | 0 | 0 | 0 | |
| 1023 | 3 | – | – | – | – | – | 0 | 0 | 0 | 0 | 0 | |
| 1030 | – | – | – | – | – | – | 0 | 0 | 0 | 0 | 0 | |
| 1031 | 4 | – | – | – | – | – | 0 | 0 | 0 | 0 | 0 | |
| 1043 | 10 | – | 10 | – | 0 | 0 | ||||||
| 1047 | – | – | 0 | |||||||||
Up to ID 1014, no diaries were handed out to patient
LTF lost to follow-up, UK unknown
a1 Flu-like syndrome; 2 rash; 3 fatigue; 4 headache; 5 depression; 6 pruritis; 7 hotflushes; 8 hand and foot syndrome; 9 myalgia; 10 anorexia; 11 throat ache; 12 constipation; 13 nausea and 14 other
bThe swelling shortly after up to 3 weeks after vaccination are noted here. If more than one vaccinations sit reacted, the biggest was recorded. Grade 0: no swelling, grade 1: <4 cm; grade: 2 ≥4 cm; grade 3: ≥8 cm
cSwelling <4 cm
HPV-specific memory T-cell responses are detected at 1 year after vaccination
Blood samples were drawn before vaccination (T = 0 week), at 7 weeks (T = 7 weeks) and 1 year (T = 1 year) after the first vaccination for immunomonitoring (Fig. 2). Pre-vaccination, 35 % of the patients in group 1 displayed an HPV16-specific T-cell response against a median of two peptide pools (out of 6) as detected by IFNγ-Elispot. At T = 7 weeks and T = 1 year, this response was significantly boosted and 97 % of the patients reacted against a median of five peptide pools (P value <0.0001; Fig. 2a). In the placebo group, no HPV16-specific responses were found at T = 0 week or T = 7 weeks by IFNγ-Elispot. At T = 1 year, two patients in group 2 had developed a response (against 1 and 4 peptide pools). The proliferation assay (LST) revealed an HPV16-specific T-cell response in 49 % of the patients in group 1 against a median of two peptide pools at T = 0. After vaccination, all patients (100 %) displayed a significantly increased proliferative response against a median of five peptide pools at T = 7 weeks and T = 1 year (P < 0.0001; Fig. 2a). HPV16-specific cytokine production was also boosted by vaccination (P < 0.0001; Fig. 2a), albeit that the levels of cytokines were low in most patients rendering the median level under the cutoff value for all cytokines except IL-5 (Fig. 2a, dashed lines). Representative data for the responses measured by these assays are shown for one patient in Supplemental figure 1. In order to define which of the peptide pools were responsible for the HPV16-SLP-induced response in group 1 patients, the response was analyzed with respect to the individual peptide pools (Fig. 2b). This showed an HPV16-specific T-cell response that was detected against all peptide pools as measured by both IFNγ-Elispot and LST, with peptide pool E6.Two being the most immunogenic, and peptide pools E6.1 and E7.2 the least. There was no association between pre-existing HPV16-specific T-cell responses and various patient characteristics: i.e., HPV16 status at T = 0 (n = 11), PHD, allergic constitution and smoking (data not shown). There was no difference in response between patients with or without an active HPV16 infection in response to the HPV16-SLP.
Fig. 2.

Strong vaccine-induced T-cell immunity was seen after two vaccinations with HPV16-SLP vaccine at 50 μg/peptide. a HPV16-specific T-cell reactivity was determined using PBMCs before (T = 0 week), 7 weeks (T = 7 weeks) and 1 year (T = 1 year) after vaccination as determined by IFNγ-Elispot, lymphocyte stimulation test (LST) and cytometric bead array (CBA). The median (line), interquartile range (boxes) and 10–90 % range (bars) of the HPV16-specific T-cell responses are shown for patients in group 1 (HPV16-SLP; n = 37) and group 2 (PBS-placebo; n = 8). The Wilcoxon matched-pairs signed rank test shows a significant increase after vaccination of HPV16-specific responses in all tests at T = 7 weeks and T = 1 year in group 1 (*0.01 < P < 0.05; **0.001 < P < 0.01; ***P < 0.001). In group 2, no differences in responses were seen except between T = 0 and T = 1 year in the IFNγ-Elispot. b Analysis of the individual peptide pools in PBMCs from patients of group 1 shows broad responses with the greatest immunogenicity against E6.2, E6.4 and E7.2 and hardly any responses to E6.1
The occurrence of a flu-like syndrome is correlated to the strength of the HPV16-specific T-cell response after vaccination
One of the major adverse events seen was the occurrence of the flu-like syndrome in group 1. The Mann–Whitney test was used to determine the association between the FLS and the HPV16-specific response. Patients who had a FLS displayed a significantly higher HPV16-specific T-cell response by all tests after vaccination at T = 7 weeks than patients with no FLS (P < 0.0001; Fig. 3a). Interestingly, patients with FLS also displayed a stronger pre-existing proliferative response associated with the production of cytokines to HPV16. This observation was not made using the IFNγ-ELISPOT assay, probably because of the shorter assay length. The assay time of the proliferation test and associated cytokine production allows low-magnitude T-cell response to expand before measurement. The same correlations were found with the immune response to MRM, suggesting a correlation between the occurrence of FLS, a stronger response to vaccination and the overall immune status of patients (supplemental figure 2).
Fig. 3.
a Stronger HPV16-specific T-cell responses were seen in patients that had the Flu-like syndrome (FLS) after vaccination compared with patients with no FLS in patients of group 1. The median (line), interquartile range (boxes) and 10–90 % range (bars) of the HPV16-specific T-cell response by IFNγ-Elispot, lymphocyte stimulation test (LST) and cytometric bead array (CBA) are shown for both groups. Patients with FLS had significantly stronger responses after vaccination by all tests (*0.01 < P < 0.05; ** 0.001 < P < 0.01; ***P < 0.001). This difference was already seen before vaccination in the LST and CBA. b A booster vaccination-enhanced response was defined as a threefold increase in the immune response after the booster vaccination compared with the HPV-specific immune response before booster vaccination. Booster vaccination significantly increased the response in the IFNγ-Elispot assay and IL-5 production
Re-vaccination at 1 year boosts the immune response
One year after first vaccination, 13 patients from group 1 were re-vaccinated with the HPV16-SLP (group 1A) and 16 received the PBS-placebo (group 1B; Fig. 1). Five patients did not receive booster vaccination and were grouped into group 1C and analyzed together with group 1B at T = 2 years (Fig. 1; Table 1, supplemental Figure 3). A significant effect of the booster vaccination was seen on the HPV16-specific responses as measured by IFNγ-Elispot and IL-5 production in the patients in group 1A (Fig. 3b). No significant increase was seen after booster vaccination on HPV16-specific proliferative responses and the associated produced cytokines IFNγ, TNFα and IL-10 compared with patients in group 1B + 1C (data not shown). Interestingly, analysis of the HPV16-specific cytokine responses revealed that patients who received a booster vaccination at T = 1 year maintained a Th1 response, but also started to develop a Th2 response, indicated by the increased production of IL-5. This did not occur in patients who did not receive a booster vaccination at 1 year. Patients in group 2 did not show any significant increase in any of the tests for the duration of the study (supplemental Figure 4).
Clinical and virological follow-up
Clinical and virological responses were not endpoints of this study but all patients were followed according to standard clinical practice. HPV typing was performed at three time points. At T = 1 year in group 1, 51 % (19/37) had regressed to a Pap1, 43 % (16/37) had a Pap2/3a (of whom three patients had a LEEP performed after diagnosing 2× a CIN2 and 1× a CIN1) and 3 % (1/37) had progressed to a Pap3b after two vaccinations (which after LEEP excision turned out to be a CIN2) (Table 1). In group 2, 78 % (7/9) of the patients had returned to a Pap1 and 22 % (2/9) still had a Pap2 at T = 1 year. At 2 years follow-up, 69 % (9/13) had a Pap1 in group 1A and 30 % (4/13) a Pap2/3a. In group 1B and 1C, 63 % (15/24) had a Pap1 at T = 2 years, 21 % (5/24) a Pap2/3a and 4 % (1/24) a Pap3b. Four percent (1/24) had undergone a hysterectomy (myoma) and 8 % (2/24) was not tested. At T = 0 year, nine patients in group 1 tested HPV16 positive. In the patients that were followed up for 1–2 years, the clearance rate at T = 1 year was 3/8 and T = 2 years 5/8. In group 2, two patients tested positive at T = 0, the clearance rate was 1/2 and 1/1 at T = 1 year and T = 2 years, respectively. For comparison, six patients in group 1 tested HPV31 positive at T = 0 and the clearance rate was 0/6 and 2/4 at T = 1 year and T = 2 years. In group 2, one patient tested positive for HPV31 at T = 0, the clearance rate was 0/1 and 1/1 at T = 1 year and T = 2 years, respectively.
Discussion
In this randomized trial, patients received either a placebo or two vaccinations of a therapeutic HPV16-SLP vaccine with or without a booster vaccination after 1 year. The differences in the HPV16-specific T-cell responses detected between the patients in the vaccine and placebo groups clearly showed that the HPV16-SLP vaccine is responsible for a strong HPV16-specific T-cell response after vaccination. Furthermore, the study showed that the most immunogenic parts of the vaccine are E6 peptide pool two (amino acid 41–92), pool four (amino acid 111–158) and E7 pool two (amino acid 41–98). These regions are similar to those that are recognized by the spontaneously induced HPV16-specific T-cell response found in healthy volunteers [9, 23].
An important factor for the clinical efficacy of a therapeutic vaccine is its capacity to induce a CD4-mediated Th1 cell response [24]. The first two vaccinations augmented the HPV16-specific Th1 response and this response is still detected after 1 year, albeit that the strength of the response is somewhat lower. The group of patients receiving a booster vaccination, not only showed a threefold increase in the number of HPV16-specific IFNγ-producing T cells as detected by IFNγ-Elispot but also an increase in the HPV16-specific production of IL-5 (compare groups 1A vs 1B/1C). While the combination of HPV16-specific production of both IFNγ and IL-5 is commonly found in the spontaneous T-cell response to HPV infection in healthy volunteers and also in the vaccine-induced response of patients clinically responding to HPV16-SLP vaccination [15, 17, 18], the specific rise in a Th2 type cytokine indicates an undesirable polarization toward a Th2 response. The addition of an adjuvant with the capacity to skew toward a Th1 response, therefore, seems warranted. Recently, IFNα has been used as adjuvant in a clinical trial in which colorectal cancer patients were injected with a p53-SLP vaccine. This trial showed that the addition of IFNα enhanced the frequency of IFNγ-producing p53-specific T-cells in vaccinated patients [25], and thus may also be used as adjuvant in HPV16-SLP vaccines.
A second concern about this vaccination scheme in patients with pre-cancerous lesions is the adverse events observed during vaccination, in particular the delayed local reactions at the vaccination sites occurring several weeks to months after vaccination. In our other studies using the HPV16-SLP and Montanide ISA51, though the adverse events did not exceed grade 2, the local injection-site reactions could be severe with large swelling formation with itching, redness and pain [14, 16, 17]. Therefore, in this study, a low-dose vaccine (50 μg/peptide) was administered with Montanide ISA51 as adjuvant. Montanide ISA 51 is not a component of any approved human vaccine, but has been used in many previous trials of candidate HIV, malaria and cancer vaccines and has been shown to cause severe injection-site reactions with occasional sterile abscess formation [26–29]. For patients with low-grade cervical disease, the short- and long-term local adverse events of the HPV16-SLP vaccine are difficult to accept [16]. To be successful in this patient group, the formulation of the HPV16-SLP should be changed in such a way that it remains effective, yet with reduction in the adverse events. A potential strategy to reach this goal is intradermal vaccination [30] or to use SLP formulations were an adjuvant is directly coupled to the peptide allowing Montanide to be omitted. Recently, Pam3Cys-conjugated SLP were reported to be highly immunogenic and to display low toxicity at the low doses that are still effective in pre-clinical trials [31].
An interesting finding in this trial was the association between an overall higher active immune system—as based on the higher response to recall antigens—the appearance of FLS and a stronger response to vaccination. Apparently, such a profile associates with a stronger response to vaccination with more adverse events. We did not find this in our previous HPV16-SLP vaccination trials in patients with malignant disease, suggesting that this phenomenon specifically becomes apparent in patients with low-grade disease with a more alert immune system.
In conclusion, vaccination with 50 μg/peptide of HPV16-SLP induces a broad and strong immune response in patients with low-grade pre-malignant disorders of the uterine cervix. This response remains at a steady state high level for at least 2 years. A booster vaccination after 1 year specifically increases Th2 responses. Future use of such a vaccination scheme thus may require better adjuvant to steer the immune response toward the desirable Th1 response. Notably, for this group of patients with pre-malignant lesions of the cervix, the often long lasting local adverse events make vaccination with HPV16-SLP as currently formulated inappropriate. The use of other adjuvant, peptide formulations or injection routes may overcome these problems.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was financially supported by The Netherlands Organization for Health Research and Development (ZonMW 92003425).
Conflict of interest
This study has been conducted by the Leiden University Medical Center (LUMC), which holds a patent on the use of synthetic long peptides as vaccine (US 7.202.034). Cornelis J.M. Melief and Sjoerd H. van der Burg are named as inventors on this patent. The LUMC does not share the financial benefit from this patent with its employees. Cornelis J.M. Melief has been employed part-time (75 %) since January 20, 2008, by ISA Pharmaceuticals, which exploits this long-peptide vaccine patent, and has been granted options on ISA Pharmaceuticals stock. All other authors declare that they have no conflict of interest.
References
- 1.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 2.Schiffman M, Kjaer SK. Chapter 2: natural history of anogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Monogr. 2003;31:14–19. doi: 10.1093/oxfordjournals.jncimonographs.a003476. [DOI] [PubMed] [Google Scholar]
- 3.Murdoch JB, Morgan PR, Lopes A, Monaghan JM. Histological incomplete excision of CIN after large loop excision of the transformation zone (LLETZ) merits careful follow up, not retreatment. Br J Obstet Gynaecol. 1992;99:990–993. doi: 10.1111/j.1471-0528.1992.tb13704.x. [DOI] [PubMed] [Google Scholar]
- 4.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367:489–498. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 5.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, Schiller JT, Gonzalez P, Dubin G, Porras C, Jimenez SE, Lowy DR. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 6.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zajac AJ, Murali-Krishna K, Blattman JN, Ahmed R. Therapeutic vaccination against chronic viral infection: the importance of cooperation between CD4+ and CD8+ T cells. Curr Opin Immunol. 1998;10:444–449. doi: 10.1016/S0952-7915(98)80119-2. [DOI] [PubMed] [Google Scholar]
- 8.de Jong A, van der Burg SH, Kwappenberg KM, van der Hulst JM, Franken KL, Geluk A, van Meijgaarden KE, Drijfhout JW, Kenter G, Vermeij P, Melief CJ, Offringa R. Frequent detection of human papillomavirus 16 E2-specific T-helper immunity in healthy subjects. Cancer Res. 2002;62:472–479. [PubMed] [Google Scholar]
- 9.Welters MJ, de Jong A, van den Eeden SJ, van der Hulst JM, Kwappenberg KM, Hassane S, Franken KL, Drijfhout JW, Fleuren GJ, Kenter G, Melief CJ, Offringa R, van der Burg SH. Frequent display of human papillomavirus type 16 E6-specific memory t-Helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 2003;63:636–641. [PubMed] [Google Scholar]
- 10.de Jong A, van Poelgeest MI, van der Hulst JM, Drijfhout JW, Fleuren GJ, Melief CJ, Kenter G, Offringa R, van der Burg SH. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. 2004;64:5449–5455. doi: 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa M, Stites DP, Farhat S, Sisler JR, Moss B, Kong F, Moscicki AB, Palefsky JM. Cytotoxic T lymphocyte responses to E6 and E7 proteins of human papillomavirus type 16: relationship to cervical intraepithelial neoplasia. J Infect Dis. 1997;175:927–931. doi: 10.1086/513992. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa M, Stites DP, Patel S, Farhat S, Scott M, Hills NK, Palefsky JM, Moscicki AB. Persistence of human papillomavirus type 16 infection is associated with lack of cytotoxic T lymphocyte response to the E6 antigens. J Infect Dis. 2000;182:595–598. doi: 10.1086/315706. [DOI] [PubMed] [Google Scholar]
- 13.Daayana S, Elkord E, Winters U, Pawlita M, Roden R, Stern PL, Kitchener HC. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br J Cancer. 2010;102:1129–1136. doi: 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, van der Burg SH, Melief CJ. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 15.Welters MJ, Kenter GG, van Steenwijk PJ, Lowik MJ, Berends-van der Meer DM, Essahsah F, Stynenbosch LF, Vloon AP, Ramwadhdoebe TH, Piersma SJ, van der Hulst JM, Valentijn AR, Fathers LM, Drijfhout JW, Franken KL, Oostendorp J, Fleuren GJ, Melief CJ, van der Burg SH. Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proc Natl Acad Sci USA. 2010;107:11895–11899. doi: 10.1073/pnas.1006500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Vos van Steenwijk PJ, Ramwadhdoebe TH, Lowik MJ, van der Minne CE, Berends-van der Meer DM, Fathers LM, Valentijn AR, Oostendorp J, Fleuren GJ, Hellebrekers BW, Welters MJ, van Poelgeest MI, Melief CJ, Kenter GG and van der Burg SH (2012) A placebo-controlled randomized HPV16 synthetic long-peptide vaccination study in women with high-grade cervical squamous intraepithelial lesions. Cancer Immunol Immunother 61:1485–1492 [DOI] [PMC free article] [PubMed]
- 17.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, Offringa R, van der Burg SH, Melief CJ. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 2008;14:169–177. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 18.Welters MJ, Kenter GG, Piersma SJ, Vloon AP, Lowik MJ, Berends-van der Meer DM, Drijfhout JW, Valentijn AR, Wafelman AR, Oostendorp J, Fleuren GJ, Offringa R, Melief CJ, van der Burg SH. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res. 2008;14:178–187. doi: 10.1158/1078-0432.CCR-07-1880. [DOI] [PubMed] [Google Scholar]
- 19.Britten CM, Janetzki S, van der Burg SH, Huber C, Kalos M, Levitsky HI, Maecker HT, Melief CJ, O’Donnell-Tormey J, Odunsi K, Old LJ, Pawelec G, Roep BO, Romero P, Hoos A, Davis MM. Minimal information about T cell assays: the process of reaching the community of T cell immunologists in cancer and beyond. Cancer Immunol Immunother. 2011;60:15–22. doi: 10.1007/s00262-010-0940-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janetzki S, Britten CM, Kalos M, Levitsky HI, Maecker HT, Melief CJ, Old LJ, Romero P, Hoos A, Davis MM. “MIATA”-minimal information about T cell assays. Immunity. 2009;31:527–528. doi: 10.1016/j.immuni.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Poelgeest MI, Welters MJ, van Esch EM, Stynenbosch LF, Kerpershoek G, van Persijn van Meerten EL, van den Hende M, Lowik MJ, Berends-van der Meer DM, Fathers LM, Valentijn AR, Oostendorp J, Fleuren GJ, Melief CJ, Kenter GG, van der Burg SH. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J Transl Med. 2013;11:88. doi: 10.1186/1479-5876-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krul EJ, van de Vijver MJ, Schuuring E, Van Kanten RW, Peters AA, Fleuren GJ. Human papillomavirus in malignant cervical lesions in Surinam, a high-risk country, compared to the Netherlands, a low-risk country. Int J Gynecol Cancer. 1999;9:206–211. doi: 10.1046/j.1525-1438.1999.99020.x. [DOI] [PubMed] [Google Scholar]
- 23.van der Burg SH, Ressing ME, Kwappenberg KM, de Jong A, Straathof K, de Jong J, Geluk A, van Meijgaarden KE, Franken KL, Ottenhoff TH, Fleuren GJ, Kenter G, Melief CJ, Offringa R. Natural T-helper immunity against human papillomavirus type 16 (HPV16) E7-derived peptide epitopes in patients with HPV16-positive cervical lesions: identification of 3 human leukocyte antigen class II-restricted epitopes. Int J Cancer. 2001;91:612–618. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1119>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 24.van der Burg SH, Melief CJ. Therapeutic vaccination against human papilloma virus induced malignancies. Curr Opin Immunol. 2011;23:252–257. doi: 10.1016/j.coi.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Zeestraten EC, Speetjens FM, Welters MJ, Saadatmand S, Stynenbosch LF, Jongen R, Kapiteijn E, Gelderblom H, Nijman HW, Valentijn AR, Oostendorp J, Fathers LM, Drijfhout JW, van de Velde CJ, Kuppen PJ, van der Burg SH, Melief CJ. Addition of interferon-alpha to the p53-SLP(R) vaccine results in increased production of interferon-gamma in vaccinated colorectal cancer patients: a phase I/II clinical trial. Int J Cancer. 2013;132:1581–1591. doi: 10.1002/ijc.27819. [DOI] [PubMed] [Google Scholar]
- 26.Graham BS, McElrath MJ, Keefer MC, Rybczyk K, Berger D, Weinhold KJ, Ottinger J, Ferarri G, Montefiori DC, Stablein D, Smith C, Ginsberg R, Eldridge J, Duerr A, Fast P, Haynes BF. Immunization with cocktail of HIV-derived peptides in montanide ISA-51 is immunogenic, but causes sterile abscesses and unacceptable reactogenicity. PLoS ONE. 2010;5:e11995. doi: 10.1371/journal.pone.0011995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, Fay MP, Narum D, Rausch K, Miles AP, Aebig J, Orcutt A, Muratova O, Song G, Lambert L, Zhu D, Miura K, Long C, Saul A, Miller LH, Durbin AP. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS ONE. 2008;3:e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaida M, Morita-Hoshi Y, Soeda A, Wakeda T, Yamaki Y, Kojima Y, Ueno H, Kondo S, Morizane C, Ikeda M, Okusaka T, Takaue Y, Heike Y. Phase 1 trial of Wilms tumor 1 (WT1) peptide vaccine and gemcitabine combination therapy in patients with advanced pancreatic or biliary tract cancer. J Immunother. 2011;34:92–99. doi: 10.1097/CJI.0b013e3181fb65b9. [DOI] [PubMed] [Google Scholar]
- 29.Kakimi K, Isobe M, Uenaka A, Wada H, Sato E, Doki Y, Nakajima J, Seto Y, Yamatsuji T, Naomoto Y, Shiraishi K, Takigawa N, Kiura K, Tsuji K, Iwatsuki K, Oka M, Pan L, Hoffman EW, Old LJ, Nakayama E. A phase I study of vaccination with NY-ESO-1f peptide mixed with Picibanil OK-432 and Montanide ISA-51 in patients with cancers expressing the NY-ESO-1 antigen. Int J Cancer. 2011;129:2836–2846. doi: 10.1002/ijc.25955. [DOI] [PubMed] [Google Scholar]
- 30.van den Hende M, van Poelgeest MIE, van der Hulst JM, de Jong JDJW, Fleuren GJ, Wafelman AS, G. MCJM, Offringa R, van der Burg SH, Kenter GG, van Poelgeest MI, van der Hulst JM, de JJ, Drijfhout JW, Fleuren GJ, Valentijn AR, Wafelman AR, Slappendel GM, Melief CJ, Offringa R, van der Burg SH and Kenter GG (2008) Skin reactions to human papillomavirus (HPV) 16 specific antigens intradermally injected in healthy subjects and patients with cervical neoplasia. Int J Cancer 123:146–152 [DOI] [PubMed]
- 31.Zom GG, Khan S, Filippov DV, Ossendorp F. TLR ligand-peptide conjugate vaccines: toward clinical application. Adv Immunol. 2012;114:177–201. doi: 10.1016/B978-0-12-396548-6.00007-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



