Abstract
Spread of head and neck cancer along the cranial nerves is often a lethal complication of this tumour. Current treatment options include surgical resection and/or radiotherapy, but recurrence is a frequent event suggesting that our understanding of this tumour and its microenvironment is incomplete. In this study, we have analysed the nature of the perineural tumour microenvironment by immunohistochemistry with particular focus on immune cells and molecules, which might impair anti-tumour immunity. Moderate to marked lymphocyte infiltrates were present in 58.8 % of the patient cohort including T cells, B cells and FoxP3-expressing T cells. While human leukocyte antigen (HLA) class I and more variably HLA class II were expressed on the tumour cells, this did not associate with patient survival or recurrence. In contrast, galectin-1 staining within lymphocyte areas of the tumour was significantly associated with a poorer patient outcome. Given the known role of galectin-1 in immune suppression, the data suggest that galectin inhibitors might improve the prognosis of patients with perineural spread of cancer.
Keywords: Perineural spread, Perineural tumour microenvironment, Immunohistochemistry, Galectin-1, Lymphocytes
Introduction
Owing to a combination of demographic and geographic factors, subtropical and tropical regions of Australia have the highest prevalence of cutaneous malignancy across the globe [1]. Not surprisingly, patients with cutaneous cancer, particularly cutaneous squamous cell carcinoma (cSCC) of the head and neck region, contribute significantly to the disease burden in this population and may present with perineural spread of cancer along the named nerves (in our experience more so the trigeminal than the facial nerve) in the head and neck region [2–4]. This is a unique form of cancer dissemination in comparison with the more common lymphovascular spread and microscopic perineural invasion typically seen in head and neck cancer. It represents an aggressive form of disease, conferring poor prognosis upon the patient and, if left untreated, progresses centripetally to eventually spill into the subarachnoid space of the central nervous system or extends directly into the brainstem. Diagnosis of perineural spread involves a variety of imaging techniques, particularly magnetic resonance imaging (MRI), but is often delayed due to the slow development of clinical symptoms [5]. Successful imaging of the tumour is important in determining therapeutic options, which include surgical resection and/or radiation treatment [6]. While many studies have focused on the diagnosis and imaging of these tumours, very little is known about the role of the immune system within the local tumour environment.
The tumour microenvironment in perineural cancer consists of both invasive cancer and stromal elements. The latter includes the extracellular matrix (ECM), fibroblasts, immune and inflammatory cells and the neurovascular structures. Amongst the stromal elements, cancer-associated fibroblasts play a key role, expressing various transcriptional factors and providing the malignant cells access to the embryonal programs, thus conferring pleiotropic properties to the cancer [7]. Tumour cells also secrete various proteins into the microenvironment [8, 9]. They include proteinases, which degrade ECM to facilitate the spread of malignant cells; growth factors; and various regulatory molecules that influence cell adhesion, motility and invasiveness. It has been suggested that the ‘secreted protein signature’ of the tumour can be utilised for earlier diagnosis and improved disease prognostication. Galectins are one such group of proteins with a well-recognised link to cancer progression [10, 11].
Galectin-1 is the prototype member of galectins, a group of lectins widely expressed in the animal kingdom, both intra- and extra-cellularly [12, 13]. It is a 14- to 16-kDa protein and a pleiotropic molecule that binds ligands to maintain its extracellular stability and activity. Its function depends on the location of galectin-1 within or outside the cell as well as its concentration [11]. As a group, a wide variety of functions has been attributed to galectins and includes mediation of inflammatory and immune responses, apoptosis and morphogenesis and tissue differentiation [14].
The role of the host immune response in tumourigenesis has been identified as one of the hallmarks of cancer [15]. While, previously, the mere presence of inflammatory and immune cells in the tumour microenvironment was deemed as evidence of host immune responsiveness, the interaction of tumour and immune cells is thought to be much more complex [16]. The immune cells’ role can vary from a coordinated cytotoxic response which eliminates tumour cells to immune suppression mediated by local regulatory T cells (e.g. FoxP3+ Treg) or myeloid-derived suppressor cells. The influence of these suppressor cells can result in immune tolerance [17]. Indeed, the term ‘immune contexture’ has been coined to denote the combined influence of the nature, density, functional orientation and location of the immune cells within the tumour [18].
Our study aims to characterise perineural infiltrates of lymphocytes in patients with neurotropic head and neck cancer (HNC) that spreads along large, named nerves in the region. The presence of immune cells (CD3 T cells, CD20 B cells, FoxP3 regulatory cells) and immune-related proteins (HLA class I, HLA class II, galectin-1) were analysed alongside patient outcomes. Our study is the first to characterise the tumour-infiltrating lymphocytes of neurotropic large nerve HNC and correlate this with patient outcome measures.
Materials and methods
Immunohistochemistry (IHC)
A review of the histopathology slides was undertaken for the cohort of patients who underwent surgical resection of HNC spreading along the named cranial nerves at the Princess Alexandra Hospital, Brisbane, between 2000 and 2009. Approval was obtained from the institution’s Human Research Ethics Committee (2003/197) to undertake this project.
Formalin-fixed, paraffin-embedded specimens were cut into 5 µm-thick sections and affixed to Menzel Superfrost Plus Adhesive™ slides (Gerhard Menzel GmbH, Brunswick, Germany) and air-dried overnight. This was followed by dewaxing in xylene and rehydration with descending graded alcohols to water. Sections were transferred to phosphate-buffered saline (PBS), and endogenous peroxidase activity was blocked by incubating with 2 % H2O2 for 10 min. The specimens were washed with three changes of water and subjected to 15 min of heat antigen retrieval at 105 °C. After washing with three changes of PBS, nonspecific binding was inhibited using Background Sniper™ blocking reagent (Biocare Medical, Concord CA, USA). The primary antibody (concentration 0.1 mg/ml) was diluted to 1:100 in PBS and applied to the specimen for 60 min at room temperature. Primary antibodies used were anti-human CD3 (Rabbit polyclonal Cat. No. A0452; Dako, Agilent Technologies, Glostrup, Denmark), anti-human CD20 (Clone L26; Dako, Agilent Technologies, Glostrup, Denmark), anti-HLA ABC (Clone EMR8-5; Abcam, Cambridge, USA), anti-HLA DR (Clone KUL/05; Abcam, Cambridge, USA), anti-FoxP3 (Clone 236A/E7; eBioscience, San Diego, USA) and anti-galectin 1 (Rabbit polyclonal Cat. No. ab25138; Abcam, Cambridge, USA). Isotype-matched control antibody staining (both rabbit and mouse IgG) was employed in the immunohistochemistry analysis with no/limited background staining observed. Following primary antibody incubation, specimens were washed and signals were developed using MACH 1 Universal HRP-Polymer Detection kit™ (Biocare Medical, Concord CA, USA). The specimens were washed to remove excess chromogen and lightly counterstained in haematoxylin. Thereafter, they were dehydrated in ascending graded alcohols, cleared in xylene and mounted using appropriate media.
A pathologist specialising in head and neck neoplasms evaluated the IHC slides as well as the haematoxylin and eosin-stained sections blinded to the patients’ clinical history. Areas of large nerve infiltrated with the carcinoma were studied, and the perineural lymphocyte infiltrates and aggregates were graded for overall intensity of the stain and percentage of cells in the region exhibiting the stain. A score was awarded for intensity of the stain (0—none, 1—mild, 2—moderate, 3—intense) and the percentage of cells expressing the stain across the entire section. For the purpose of statistical analysis of the IHC results, the percentage of particular cells stained by IHC were broadly grouped as follows: 1 = 0–33 % stained cells, 2 = 34–66 % stained cells, 3 = 67–100 % stained cells. Examination of haematoxylin and eosin-stained histopathology slides allowed scoring the degree of perineural lymphocyte infiltration: 0—none, 1—mild scattered lymphocytes in and around the tumour, 2—moderate lymphocytic infiltrate and 3—prominent lymphocytic infiltrate with or without ‘pseudo-follicle’ or aggregate formation.
Statistical methods
Kaplan–Meier analyses in conjunction with the log-rank test were used to assess any relationship between survival time and IHC staining percentage scores. The time elapsed between first surgery and death by disease was considered to be the survival time. For those patients who do not experience an event (recurrence and/or death) during the study, the date of the last contact was recorded and the resulting survival time was censored at that point. Progression-free survival was recorded as days since first surgery without recurrence of the malignancy. In absence of recurrence, the date of last contact was recorded and the resulting progression-free survival time was censored at that point.
Statistical analysis was performed using SPSS v.19 (IBM Corporation, Armonk, NY, USA).
Elimination criteria
Tissue removed at the time of surgery was forwarded for histopathological analysis. Additional tissue removed at that time was used for research purposes. Owing to the limited availability of the specimen for this purpose, the slides that were devoid of representative tissue were omitted from microscopic examination and scoring of various stains. These 2 cases were excluded from our current study. Clinical information on two patients was incomplete, and hence they were excluded from the current study. For FoxP3 stain, an additional two patients had insufficient tissue available for analysis.
Results
Patient characteristics
Seventeen patients were included in the study. The data set included demographic details, de-identified clinical details, immunohistochemistry stain scores and review of histopathology slides for the representative neoplasm. Of the 17 patients, there were 3 females and 14 males. The average age at the time of surgery was 59.8 years (Table 1). The cancer recurred in 6 out of 17 patients included in the study; of these, 5 patients died from a disease-related cause, and 1 patient was alive with disease at the time of last recorded follow-up visit. Of the patients who did not have a recurrence (n = 11), one person died of an unrelated disease and the remaining patients (n = 10) were alive and free of disease at the end point of follow-up (data not shown). The trigeminal nerve was the most commonly involved nerve, with at least one of its divisions involved in all 17 cases. Six patients had involvement of a single division of the trigeminal nerve (3 cases each for V1 and V2, respectively). In the remaining 11 cases, multiple cranial nerves were involved. The facial nerve was involved in 5 cases. Most patients (14 out of 17) presented with squamous cell carcinoma and clear surgical margin of excision was obtained in 10 out of 17 patients (Table 1).
Table 1.
Patient characteristics
| Patient characteristics | Observations |
|---|---|
| Mean age | 59.8 years (SD = 12.9) |
| Sex | 14 Male/3 Female |
| Laterality | 10 Left/7 Right |
| Recurrence | 6/17 patients* (5 deaths/6 recurrences)# |
| Nerve involvement | V1 (9); V2 (12); V3 (6); VII (5); Vidian (1); III (1) |
| Zone classification | Peripheral (5), Skull Base (11), Unknown (1) |
| Histology | SCC (14), BCC (1), Adenoid cystic carcinoma (2) |
| Margin | Negative (10); Positive (6); Unknown (1) |
SCC squamous cell carcinoma, BCC basal cell carcinoma
* Median time until recurrence = 74 months
# Median time until death = 89 months
Varying degrees of lymphocyte infiltration is seen in perineural tumours
The relationship between the degree of lymphocyte infiltration (0—none to 3—marked infiltrate) and outcome measures (death/disease recurrence) was evaluated (Fig. 1). Patients with scores of 0 and 1(none or scattered lymphocytes) were grouped as were patients with score of 2 and 3 (moderate to marked infiltrate). The relationship between lymphocyte infiltrate and death or recurrence did not reach statistical significance (death: p = 0.55; recurrence: p = 0.23). That said, interesting trends were borne out of the analysis.
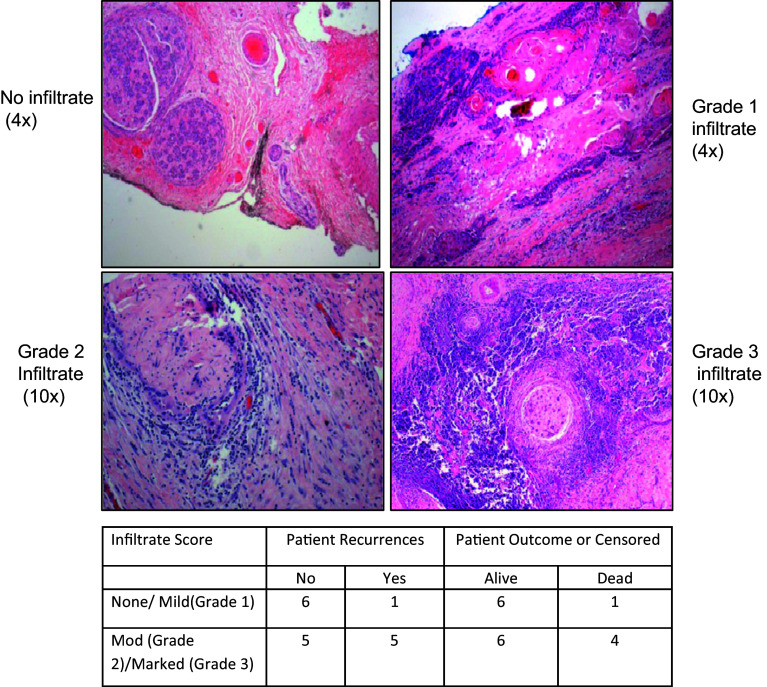
Fig. 1.
Lymphocyte infiltrates are associated with perineural squamous cell carcinoma. Haematoxylin and eosin staining was performed on paraffin-embedded tumour sections to identify graded levels of lymphocyte infiltrate. A summary table of the level of lymphocyte infiltrate associated with different patient outcomes is shown
The trends indicate that the cancer recurred in one out of seven patients (14.2 %) with no or mild perineural lymphocyte infiltrate, and in five out of ten patients (50 %) with moderate to marked lymphocyte infiltrate. A similar trend was observed in the death status with 14.2 % deaths recorded in the none/mild lymphocyte infiltration group and 40 % deaths recorded in the moderate/marked lymphocyte infiltration group. Owing to the small numbers, no statistically significant relationship could be inferred; nonetheless, we believe the trends are noteworthy. The data may suggest that the lymphocyte infiltrate contributes to the pathogenesis of disease or prevents an effective anti-tumour immune response.
T cells, B cells and FoxP3+ cells are present in perineural SCC, along with MHC I and MHC II expression, but do not associate with tumour recurrence or patient outcome
Given the presence of a potentially tumour-promoting lymphocyte infiltrate in the tumour microenvironment, the proportions of T cells, B cells and FoxP3+ T cells were assessed. T cell (CD3+) infiltrates to the tumour site were nil to mild in 11/17 patients (64.7 %) and moderate to prominent in 6/17 patients (35.3 %) (Table 2 and Fig. 2a, left panel) although lymphocytes in these six patients were mostly peritumoural (Fig. 2a). In some patients, CD3+ cells were scattered around the tumour in addition to forming aggregates (Fig. 2a, left panel). In one specimen, no T cell lymphocytic infiltrate could be observed. When present, the tumour B cell infiltrate (CD20+) was nil to mild in 15/17 patients (88.2 %), but prominent in 2/17 pts (11.8 %) including the formation of aggregates (Table 2; Fig. 2a, middle panel). In 10/17 (58.8 %) patients, B cells were located predominantly at the tumour periphery; 5/17 (29.4 %) showed infiltrate both at the periphery and within the tumour; 2/17 specimen (11.7 %) showed no B cell infiltrate (data not shown). No significant relationship existed between death/recurrence and the presence of a T cell or B cell infiltrate. Staining results for FoxP3 were available for 15 patients. Eleven patients had greater than 50 % FoxP3+ cells amongst their T cells (Table 2). In our data, these lymphocyte subsets do not reach statistical significance with respect to the clinical outcomes in terms of survival and recurrence (Table 2).
Table 2.
Summary of lymphocyte subset staining
| Patient outcome | No. of patients with graded CD3+ infiltrate* | No. of patients with graded CD20+ infiltrate* | No. of patients with % FoxP3+ amongst T cells* | |||
|---|---|---|---|---|---|---|
| Grade 0/1 | Grade 2/3 | Grade 0/1 | Grade 2/3 | <50 % | >50 % | |
| Censored | 7 | 5 | 10 | 2 | 1 | 9 |
| Death | 4 | 1 | 5 | 0 | 3 | 2 |
| Log-rank test (p value) | 0.8 | 0.54 | 0.69 | |||
| Recurrence | 4 | 2 | 6 | 0 | 3 | 3 |
| No recurrence | 7 | 4 | 9 | 2 | 1 | 8 |
| Log-rank test (p value) | 0.81 | 0.39 | 0.56 | |||
* Median survival times not calculated due to small number of events in each group
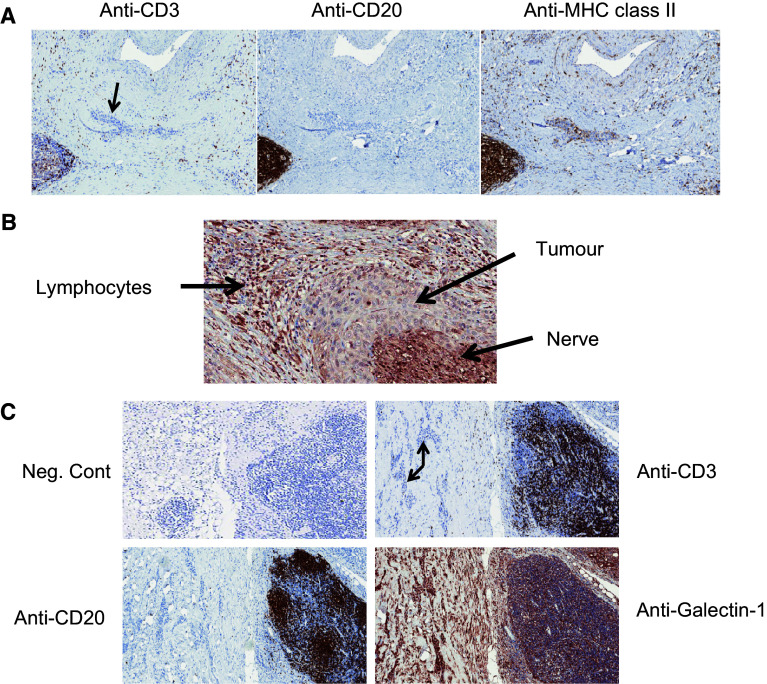
Fig. 2.
Immunostaining patterns for CD3, CD20, MHC II and galectin-1 in perineural SCC. a Consecutive tissue sections from a single patient were stained with antibodies directed against CD3 (left panel), CD20 (middle panel) and MHC class II (right panel). Tumour tissue is indicated by the arrow. Magnification ×9 for all images. b Galectin-1 staining from a single patient showing the relationship between lymphocytes, tumour and nerve tissue. Magnification ×18. c Consecutive tissue sections from a single patient were stained with antibodies directed against CD3, CD20 and galectin-1. Tumour tissue is indicated by the arrow. Magnification ×7
The functional role of HLA class I and HLA class II on the tumour cell surface is related to the presentation of cancer-derived peptides allowing for T cell receptor recognition and activation of effector T cell function. Loss of either HLA class I or HLA class II on tumours has been associated with tumour escape from the immune system. In our study, the distribution of HLA class II was variable in tumour cells and lymphocytes. In 70.6 % specimens, less than one-third of the tumour cells expressed HLA class II. HLA class II was expressed by lymphocytes to some degree in 15 out of 17 (88.2 %) cases (Table 3; Fig. 2a, right panel).
Table 3.
HLA class II staining
| HLA class II staining score | Tumour | Lymphocytes | ||
|---|---|---|---|---|
| % patients with staining (no. of patients) | Cumulative % patients with staining | % patients with staining (no. of patients) | Cumulative % patients with staining | |
| 0 | 11.8 (2) | 11.8 | 11.8 (2) | 11.8 |
| 1 | 58.8 (10) | 70.6 | 35.3 (6) | 47.1 |
| 2 | 23.5 (4) | 94.1 | 41.2 (7) | 88.2 |
| 3 | 5.9 (1) | 100 | 11.8 (2) | 100 |
HLA class I was avidly expressed by the tumour cells in all specimens. In 88.2 % cases, over two-thirds of the tumour cells expressed HLA class I and between one-third and two-thirds of the tumour cells expressed HLA class I in the remaining 11.8 % cases (data not shown). A similar expression pattern of HLA class I expression was seen in the lymphocyte population, with HLA class I expressed by over two-thirds of the visualised lymphocytes in 88.2 % cases (data not shown).
The percentage of galectin-1 expression in tumoural zones with lymphocyte infiltration negatively associates with survival and recurrence
Although lymphocyte subsets did not correlate with death or recurrence, it was possible that the function of these cells might better reflect disease outcome. Galectin-1 can function as an immune-suppressive molecule within the tumour microenvironment and was chosen for further study in our experiments.
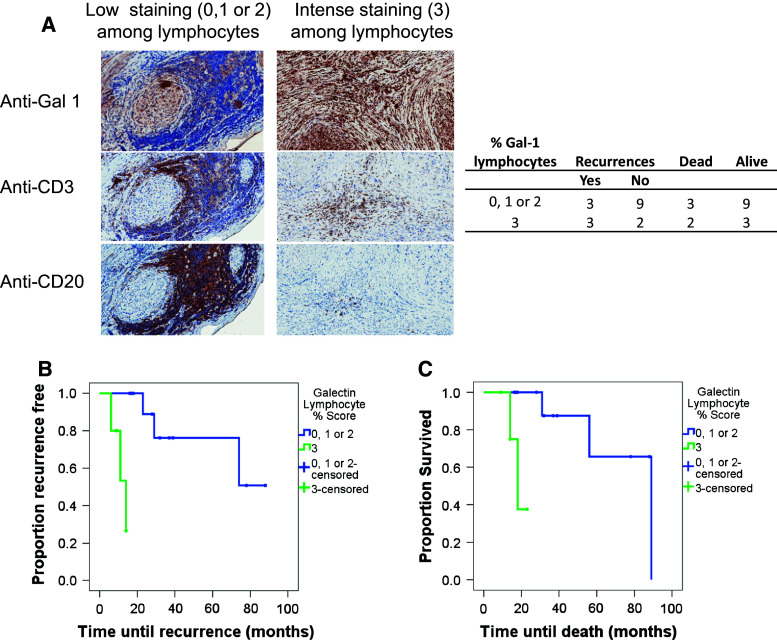
In 12 cases (70.6 %), galectin-1 was expressed by over two-thirds of the cancer cells contained in the specimen. In patient samples expressing intense galectin staining, it is evident that galectin-1 can be expressed widely across many cells in the tissue (Figs. 2b, 3a). When we focused on galectin-1 expression within lymphocyte areas of the tumour, 5 cases (29.4 %) demonstrated a high proportion (over two-thirds) of the lymphocyte area with galectin-1 staining, while in the remaining 12 cases, galectin-1 was expressed over a smaller proportion of the lymphocyte region. An example of high galectin staining and its relationship with tumour and nerve (Fig. 2b) or T cells and B cells is shown (Fig. 2c). In some patients, the presence of aggregates of T and B cells was observed (shown in Fig. 2c). Further, both high and low galectin staining in tumour tissue and the spatial relationship with lymphocytes (both T and B cells) are illustrated (Fig. 3a). In terms of the actual number of recurrences and deaths, there were three recurrences each and three and two deaths, respectively, in the low galectin 1 group (score 0, 1 or 2) and high galectin 1 group (score 3) for fraction of the lymphocyte area staining with galectin-1 (Fig. 3a—table). A significant relationship was noted between percentage of galectin-1 staining in lymphocyte areas of the tumour and both time until recurrence and time until death (Fig. 3b, c). Patients with over two-thirds of the lymphocyte area staining for galectin-1 (i.e. score of 3) had shorter survival times and shorter time till death compared to patients with scores of 0, 1 or 2 (p = 0.001 and p = 0.006, respectively; Fig. 3b, c). Due to these small numbers, it is not possible to accurately estimate median time until death or median time until recurrence. We have not assessed the relationship between galectin staining and other immune cell subsets, but no relationship was observed between the proportion of tumour cells expressing galectin-1 and patient outcome measures.
Fig. 3.
Elevated levels of galectin-1 staining cells amongst lymphocytes within the tumour is associated with poor patient prognosis. a Consecutive, paraffin-embedded tissue sections from two perineural SCC patient tumour samples, representative of either high Gal-1 or low Gal-1 expression, were stained with anti-human galectin-1 antibody (anti-Gal-1; ×20 magnification), anti-CD3 [×12.8 magnification (left image) and ×20 magnification (right image)] and anti-CD20 antibodies [×13 magnification (left image) and ×20 magnification (right image)]. The table summarises patient outcomes relative to the % galectin-1 staining amongst lymphocyte areas (B and T cells) of the tumour. The % galectin-1 staining within the lymphocyte areas of the tumour was plotted in survival curves against tumour recurrence (b) and patient survival (c)
Overall, the data show that a high proportion of the lymphocyte area staining for galectin-1 (over two-thirds of the lymphocyte area expressing galectin-1 in our series) is associated with poorer outcomes for the patients relative to other immune markers.
Discussion
Our study has demonstrated that a number of different lymphocyte subsets are able to infiltrate perineural spread of carcinoma. A link between the percentage of galectin-1 staining within lymphocyte areas and patient outcomes was established with the proportion of the lymphocyte area expressing galectin-1 associated with both the disease-free and overall survival time (p = 0.001 and p = 0.006 respectively). The major limitation of our study is the small sample size, which has impacted the statistical analysis when comparing various immunohistochemical stains with the disease related outcomes. Despite this obvious shortfall, the observed relationship with galectin-1 highlights the potential importance of immune-suppressive molecules in this particular subset of HNC. While other studies have discussed the association of galectin-1 in aggressive phenotypes of head and neck malignancies [19–22] to the best of our knowledge, this study is the first attempt to draw a relationship between galectin-1 and large nerve/clinical perineural spread of HNC. Many studies have demonstrated galectin-1 expression by tumour cells as the key regulator of anti-tumour immunity or tumour aggressiveness and acquisition of metastasis [23–25]. The pro-carcinogenic, immuno-modulatory role of galectin-1 was studied in pancreatic cancer where the high expression of galectin-1 in pancreatic stellar cells was associated with T cell apoptosis and Th-2 cytokine secretion [26]. Another study demonstrated that galectin-1 fostered an immunosuppressive environment at the tumour site by modulating T cell survival (growth arrest and apoptosis of activated T cells) and impairing the effector T cell function [27]. Reviewing the function of galectins in regulation of immune cell homeostasis, galectin-1-secreting cancer cells disrupt the glycosylation-dependent regulatory pathways and form immune-privileged sites through mechanisms that include reduction of T cell survival and impairment of dendritic cell function [28]. Interaction of galectin-1 is not confined to the acquired immune responses. Galectin-1 from lung cancer cell lines or sera of lung cancer patients impaired the function of monocyte-derived dendritic cells and increased the expression of interleukin 10 [29]. Galectin-1 expression by Reed–Sternberg cells in Hodgkin’s lymphoma has been implicated in causing the skew towards an immunosuppressive host response that is dominated by Th-2 cells/Treg [30]. Tumour-derived galectin-1 is more crucial in promoting tumour growth and metastasis than host-derived galectin [31]. Since the molecule produced by host and tumour cells is identical, these functional differences were explained by the presence of quantitatively higher amount of galectin-1 expressed by tumour cells. The same study also demonstrated that immunomodulatory effects of galectin-1 are more critical than its pro-angiogenic effects, and the latter can be abrogated in an immune-deficient environment. Expression of galectin-1 enhances in presence of hypoxia, a condition which is more likely to exist within solid tumour deposits [32]. Expression of galectin-1 in tumour-associated stroma rather than tumour cells was also shown to be important in prostate cancer [33]. Here it was an independent predictor of prostate-specific antigen recurrence (p < 0.0001) and aggressiveness of the tumour. They theorised that presence of peri-tumoural galectin-1 may act as an immunologic shield by inducing activated T cell apoptosis. Our study suggests that intratumoural lymphocytes may represent another important source of galectin-1. In this regard, previous studies have demonstrated that regulatory T cells (CD24+25+) overexpressed galectin-1 and that blockade of the galectin-1 binding significantly reduced the immunosuppressive effects of these cells [34]. In a second study, galectin-1 was able to negatively regulate Th-1- and Th-17-mediated inflammation through a hypothesised role of Treg [35].
Our study has also shown that lymphocyte infiltrates are a frequent feature of perineural cancers. The presence of lymphocytes, especially CD4 Th1 cells or CD8 cytotoxic T cells, within the primary tumour is often a strong predictor of disease-free and overall survival [36]. T cell infiltrate with a high CD8/Foxp3 ratio was demonstrated to be an independent and favourable predictive factor for disease-free survival in breast cancer patients following neoadjuvant chemotherapy [37]. A dense lymphocytic infiltrate was an independent predictor of absence of sentinel lymph node metastasis in patients with cutaneous melanoma compared with tumours where TILs were absent [38]. Interestingly, the trend in our study was for lymphocyte infiltrates to be associated with poor prognosis. Further subsetting of the lymphocytes, in addition to measuring function, may shed light on this contrasting result. Certainly, immune cells present in the tumour microenvironment can be dysfunctional, demonstrating signalling abnormalities and spontaneous apoptosis. One study reported the relationship between the dysfunctional TILs and their counterparts in the peripheral blood and supported the hypothesis that this immune dysfunction represents immunosuppressive effects of the tumour that can extend beyond the tumour microenvironment and may be linked to tumour aggressiveness [39]. A longitudinal study of risk factors associated with cutaneous head and neck cancer (HNC), in a cohort of 315 patients, showed a dense lymphocytic infiltrate in 26.7 % of the index tumours [40]. Interestingly, in this same study the presence of inflammatory infiltrate correlated with perineural involvement (p < 0.001) and was significantly associated with tumours that recurred (p = 0.005). The association of lymphocyte infiltrate with poor prognosis in our study may partly be explained by the lymphocyte production of immunosuppressive galectin-1. Further studies with larger numbers of patients will be required to firmly establish a link between lymphocytes and survival/recurrence. No individual subset of lymphocytes, amongst T cells, B cells or Fox P3+ cells, was associated with patient outcome. Future studies will focus on other immune subsets known to be immunosuppressive, e.g. NKT. Unlike some other tumours [41–43], the perineural cancers retained expression of HLA class I, suggesting that they can be target cells for a CD8 cytotoxic T cell response if the suppressive tumour microenvironment can be overcome.
In conclusion, our study reinforces the emergence of galectin-1 as an important regulatory molecule mediating tumour–host immune system interaction and fostering an immune-privileged environment allowing the tumour cells to evade host immunity. While galectin-1 has been known to confer an aggressive phenotype to various cancers, this is the first study to draw a link between this molecule and the poorer outcome it portends in the patients with an aggressive subtype of head and neck cutaneous squamous cell carcinoma. To restore immune function, treatments which inhibit the expression or function of galectin-1 should be considered in perineural spread of cancer.
Acknowledgments
We acknowledge the support of the Princess Alexandra Hospital Private Practice Fund.
Abbreviations
- ECM
Extracellular matrix
- HLA
Human leukocyte antigen
- HNC
Head and neck cancer
- MRI
Magnetic resonance imaging
- NKT
Natural killer T cells
- SCC
Squamous cell carcinoma
- TIL
Tumour-infiltrating lymphocyte
- Treg
Regulatory T cells
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Footnotes
Graham R. Leggatt and Benedict J. Panizza have contributed equally to this work.
Graham R. Leggatt and Benedict J. Panizza share last authorship.
References
- 1.Cakir BO, Adamson P, Cingi C. Epidemiology and economic burden of nonmelanoma skin cancer. Facial Plast Surg Clin North Am. 2012;20(4):419–422. doi: 10.1016/j.fsc.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Panizza B, Warren TA, Solares CA, Boyle GM, Lambie D, Brown I. Histopathological features of clinical perineural invasion of cutaneous squamous cell carcinoma of the head and neck and the potential implications for treatment. Head Neck. 2013;36(11):1611–1618. doi: 10.1002/hed.23509. [DOI] [PubMed] [Google Scholar]
- 3.Solares CA, Lee K, Parmar P, O’Rourke P, Panizza B. Epidemiology of clinical perineural invasion in cutaneous squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2012;146(5):746–751. doi: 10.1177/0194599811434897. [DOI] [PubMed] [Google Scholar]
- 4.Lin C, Tripcony L, Keller J, Poulsen M, Martin J, Jackson J, Dickie G. Perineural infiltration of cutaneous squamous cell carcinoma and basal cell carcinoma without clinical features. Int J Radiat Oncol Biol Phys. 2012;82(1):334–340. doi: 10.1016/j.ijrobp.2010.09.040. [DOI] [PubMed] [Google Scholar]
- 5.ten Hove MW, Glaser JS, Schatz NJ. Occult perineural tumor infiltration of the trigeminal nerve. Diagnostic considerations. J Neuroophthalmol. 1997;17(3):170–177. [PubMed] [Google Scholar]
- 6.Panizza B, Solares CA, Redmond M, et al. Surgical resection for clinical perineural invasion from cutaneous squamous cell carcinoma of the head and neck. Head Neck. 2011;34:1622–1627. doi: 10.1002/hed.21986. [DOI] [PubMed] [Google Scholar]
- 7.Mbeunkui F, Johann DJ., Jr Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol. 2009;63(4):571–582. doi: 10.1007/s00280-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaaij-Visser TB, de Wit M, Lam SW, Jimenez CR. The cancer secretome, current status and opportunities in the lung, breast and colorectal cancer context. Biochim Biophys Acta. 2013;1834(11):2242–2258. doi: 10.1016/j.bbapap.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 9.Paltridge JL, Belle L, Khew-Goodall Y. The secretome in cancer progression. Biochim Biophys Acta. 2013;1834(11):2233–2241. doi: 10.1016/j.bbapap.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Astorgues-Xerri L, Riveiro ME, Tijeras-Raballand A, Serova M, Neuzillet C, Albert S, Raymond E, Faivre S. Unraveling galectin-1 as a novel therapeutic target for cancer. Cancer Treat Rev. 2014;40(2):307–319. doi: 10.1016/j.ctrv.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Cedeno-Laurent F, Dimitroff CJ. Galectins and their ligands: negative regulators of anti-tumor immunity. Glycoconj J. 2012;29(8–9):619–625. doi: 10.1007/s10719-012-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viguier M, Advedissian T, Delacour D, Poirier F, Deshayes F. Galectins in epithelial functions. Tissue Barriers. 2014;2:e29103. doi: 10.4161/tisb.29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cedeno-Laurent F, Dimitroff CJ. Galectin-1 research in T cell immunity: past, present and future. Clin Immunol. 2012;142(2):107–116. doi: 10.1016/j.clim.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Schlosser HA, Theurich S, Shimabukuro-Vornhagen A, Holtick U, Stippel DL, Bergwelt-Baildon M. Overcoming tumor-mediated immunosuppression. Immunotherapy. 2014;6(9):973–988. doi: 10.2217/imt.14.58. [DOI] [PubMed] [Google Scholar]
- 17.Bindea G, Mlecnik B, Fridman WH, Pages F, Galon J. Natural immunity to cancer in humans. Curr Opin Immunol. 2010;22(2):215–222. doi: 10.1016/j.coi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Galon J, Fridman WH, Pages F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 2007;67(5):1883–1886. doi: 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- 19.Chiang WF, Liu SY, Fang LY, Lin CN, Wu MH, Chen YC, Chen YL, Jin YT. Overexpression of galectin-1 at the tumor invasion front is associated with poor prognosis in early-stage oral squamous cell carcinoma. Oral Oncol. 2008;44(4):325–334. doi: 10.1016/j.oraloncology.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Ding YM, Dong JH, Chen LL, Zhang HD. Increased expression of galectin-1 is associated with human oral squamous cell carcinoma development. Oncol Rep. 2009;21(4):983–987. doi: 10.3892/or_00000312. [DOI] [PubMed] [Google Scholar]
- 21.Alves PM, Godoy GP, Gomes DQ, Medeiros AM, de Souza LB, da Silveira EJ, Vasconcelos MG, Queiroz LM. Significance of galectins-1, -3, -4 and -7 in the progression of squamous cell carcinoma of the tongue. Pathol Res Pract. 2011;207(4):236–240. doi: 10.1016/j.prp.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Tang CE, Tan T, Li C, Chen ZC, Ruan L, Wang HH, Su T, Zhang PF, Xiao ZQ. Identification of Galectin-1 as a novel biomarker in nasopharyngeal carcinoma by proteomic analysis. Oncol Rep. 2010;24(2):495–500. [PubMed] [Google Scholar]
- 23.Spano D, Russo R, Di Maso V, Rosso N, Terracciano LM, Roncalli M, Tornillo L, Capasso M, Tiribelli C, Iolascon A. Galectin-1 and its involvement in hepatocellular carcinoma aggressiveness. Mol Med. 2010;16(3–4):102–115. doi: 10.2119/molmed.2009.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cimmino F, Schulte JH, Zollo M, Koster J, Versteeg R, Iolascon A, Eggert A, Schramm A. Galectin-1 is a major effector of TrkB-mediated neuroblastoma aggressiveness. Oncogene. 2009;28(19):2015–2023. doi: 10.1038/onc.2009.70. [DOI] [PubMed] [Google Scholar]
- 25.Bacigalupo ML, Manzi M, Rabinovich GA, Troncoso MF. Hierarchical and selective roles of galectins in hepatocarcinogenesis, liver fibrosis and inflammation of hepatocellular carcinoma. World J Gastroenterol. 2013;19(47):8831–8849. doi: 10.3748/wjg.v19.i47.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang D, Yuan Z, Xue X, Lu Z, Zhang Y, Wang H, Chen M, An Y, Wei J, Zhu Y, Miao Y, Jiang K. High expression of Galectin-1 in pancreatic stellate cells plays a role in the development and maintenance of an immunosuppressive microenvironment in pancreatic cancer. Int J Cancer. 2012;130(10):2337–2348. doi: 10.1002/ijc.26290. [DOI] [PubMed] [Google Scholar]
- 27.Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A, Mordoh J, Fainboim L, Podhajcer OL, Rabinovich GA. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5(3):241–251. doi: 10.1016/S1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- 28.Rabinovich GA, Croci DO. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity. 2012;36(3):322–335. doi: 10.1016/j.immuni.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Kuo PL, Hung JY, Huang SK, Chou SH, Cheng DE, Jong YJ, Hung CH, Yang CJ, Tsai YM, Hsu YL, Huang MS. Lung cancer-derived galectin-1 mediates dendritic cell anergy through inhibitor of DNA binding 3/IL-10 signaling pathway. J Immunol. 2011;186(3):1521–1530. doi: 10.4049/jimmunol.1002940. [DOI] [PubMed] [Google Scholar]
- 30.Juszczynski P, Ouyang J, Monti S, Rodig SJ, Takeyama K, Abramson J, Chen W, Kutok JL, Rabinovich GA, Shipp MA. The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc Natl Acad Sci USA. 2007;104(32):13134–13139. doi: 10.1073/pnas.0706017104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banh A, Zhang J, Cao H, Bouley DM, Kwok S, Kong C, Giaccia AJ, Koong AC, Le QT. Tumor galectin-1 mediates tumor growth and metastasis through regulation of T-cell apoptosis. Cancer Res. 2011;71(13):4423–4431. doi: 10.1158/0008-5472.CAN-10-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le QT, Shi G, Cao H, Nelson DW, Wang Y, Chen EY, Zhao S, Kong C, Richardson D, O’Byrne KJ, Giaccia AJ, Koong AC. Galectin-1: a link between tumor hypoxia and tumor immune privilege. J Clin Oncol. 2005;23(35):8932–8941. doi: 10.1200/JCO.2005.02.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Brule FA, Waltregny D, Castronovo V. Increased expression of galectin-1 in carcinoma-associated stroma predicts poor outcome in prostate carcinoma patients. J Pathol. 2001;193(1):80–87. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH730>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 34.Garin MI, Chu CC, Golshayan D, Cernuda-Morollon E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4 + CD25 + T cells. Blood. 2007;109(5):2058–2065. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 35.Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum LG, Rabinovich GA. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8(8):825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 36.Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29(8):1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 37.Ladoire S, Arnould L, Mignot G, Coudert B, Rebe C, Chalmin F, Vincent J, Bruchard M, Chauffert B, Martin F, Fumoleau P, Ghiringhelli F. Presence of Foxp3 expression in tumor cells predicts better survival in HER2-overexpressing breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2011;125(1):65–72. doi: 10.1007/s10549-010-0831-1. [DOI] [PubMed] [Google Scholar]
- 38.Taylor RC, Patel A, Panageas KS, Busam KJ, Brady MS. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. 2007;25(7):869–875. doi: 10.1200/JCO.2006.08.9755. [DOI] [PubMed] [Google Scholar]
- 39.Reichert TE, Strauss L, Wagner EM, Gooding W, Whiteside TL. Signaling abnormalities, apoptosis, and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clin Cancer Res. 2002;8(10):3137–3145. [PubMed] [Google Scholar]
- 40.Kyrgidis A, Tzellos TG, Kechagias N, Patrikidou A, Xirou P, Kitikidou K, Bourlidou E, Vahtsevanos K, Antoniades K. Cutaneous squamous cell carcinoma (SCC) of the head and neck: risk factors of overall and recurrence-free survival. Eur J Cancer. 2010;46(9):1563–1572. doi: 10.1016/j.ejca.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 41.Csiba A, Whitwell HL, Moore M. Distribution of histocompatibility and leucocyte differentiation antigens in normal human colon and in benign and malignant colonic neoplasms. Br J Cancer. 1984;50(5):699–709. doi: 10.1038/bjc.1984.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seliger B, Hohne A, Knuth A, Bernhard H, Ehring B, Tampe R, Huber C. Reduced membrane major histocompatibility complex class I density and stability in a subset of human renal cell carcinomas with low TAP and LMP expression. Clin Cancer Res. 1996;2(8):1427–1433. [PubMed] [Google Scholar]
- 43.Seliger B, Hohne A, Knuth A, Bernhard H, Meyer T, Tampe R, Momburg F, Huber C. Analysis of the major histocompatibility complex class I antigen presentation machinery in normal and malignant renal cells: evidence for deficiencies associated with transformation and progression. Cancer Res. 1996;56(8):1756–1760. [PubMed] [Google Scholar]