Abstract
Malignant melanoma is known by its rapid progression and poor response to currently applied treatments. Despite the well-documented melanoma immunogenicity, the results of immunotherapeutic clinical trials are not satisfactory. This poor antitumor reactivity is due to the development of chronic inflammation in the tumor microenvironment characterized by infiltrating leukocytes and soluble mediators, which lead to an immunosuppression associated with cancer progression. Using the ret transgenic mouse melanoma model that closely resembles human melanoma, we demonstrated increased levels of chronic inflammatory factors in skin tumors and metastatic lymph nodes, which correlated with tumor progression. Furthermore, Gr1+CD11b+ myeloid-derived suppressor cells (MDSC), known to block tumor-reactive T cells, were enriched in melanoma lesions and showed an enhanced immunosuppressive capacity. This MDSC accumulation was associated with a strong TCR ζ-chain downregulation in T cells suggesting that the tumor inflammatory microenvironment supports MDSC recruitment and immunosuppressive activity. Indeed, upon administration of phosphodiesterase-5 inhibitor sildenafil or paclitaxel in non-cytotoxic doses, we observed reduced levels of chronic inflammatory mediators in association with decreased MDSC amounts and immunosuppressive function. This led to a partial restoration of ζ-chain expression in T cells and to a significantly increased survival of tumor-bearing mice. CD8 T-cell depletion resulted in an abrogation of beneficial outcome of both drugs, suggesting the involvement of MDSC and CD8 T cells in the observed therapeutic effects. Our data imply that inhibition of chronic inflammation in the tumor microenvironment should be applied in conjunction with melanoma immunotherapies to increase their efficacy.
Keywords: Melanoma, Tumor microenvironment, Chronic inflammation, Immunosuppression, Myeloid-derived suppressor cells, CITIM 2011
Introduction
Malignant skin melanoma, notorious for its aggressive clinical behavior, proclivity for distant metastasis and poor response to currently applied therapeutics such as chemo- and radio-therapy, is one of the fastest increasing cancers worldwide [1, 2]. Few treatment options are available to patients with metastatic disease, and standard chemotherapeutic agents are generally ineffective. Therefore, development of new alternative strategies for therapy of cutaneous malignant melanoma is extremely important. Immunogenicity of human malignant melanoma is well-documented including an identification of large numbers of melanoma-associated antigens and development of spontaneous tumor regressions in some patients providing thereby direct evidence for the induction of antitumor immunity, in which T cells play a key role [3–5]. However, despite the high potential to stimulate immune responses against melanoma cells, the results of immunotherapeutic clinical studies are not satisfactory. Insufficient antitumor reactivity could be due to different mechanisms dealing with structural and functional changes in both tumor and stroma cells. These mechanisms were reported to include on the side of melanoma cells (1) the absence of co-stimulatory molecules [6], (2) downregulation in the expression of tumor-associated antigens [7], (3) MHC class I molecules [8] and (4) ligands for natural killer (NK) cell receptors [9], as well as (5) an intensive secretion of immunosuppressive factors such as VEGF, TGF-β, IL-10 or nitric oxide [10, 11]. Stroma cell contribution is characterized by strong expansion and accumulation of immunosuppressive cells in the tumor microenvironment such as CD4+CD25+Foxp3+ regulatory T cells [12, 13], myeloid-derived suppressor cells (MDSC) [14, 15], M2 subset of macrophages [10] and regulatory/tolerogenic dendritic cells [16].
This complex immunosuppressive network is thought to be induced by chronic inflammatory conditions developing in the tumor microenvironment (Fig. 1). Indeed, chronic inflammation has been demonstrated to correlate with tumor onset and progression [10, 17–20]. An inflammatory microenvironment ensues during tumor growth due to the secretion of inflammatory mediators (cytokines, chemokines, growth factors, reactive oxygen and nitrogen species and prostaglandins) by the tumor and/or stroma cells [10, 17, 21, 22]. These mediators were found to support tumor development by stimulating protumor mutations, resistance to apoptosis, and angiogenesis [11, 20, 22]. Moreover, many of these chronic inflammatory factors were reported to induce the recruitment and activation of various immunosuppressive leukocytes, in particular MDSC, in tumor lesions [10, 14, 15, 21, 22]. In this review, we discuss the role of chronic inflammation in the recruitment and activation of MDSC in melanoma and therapeutic strategies to neutralize immunosuppressive microenvironment induced by chronic inflammation.
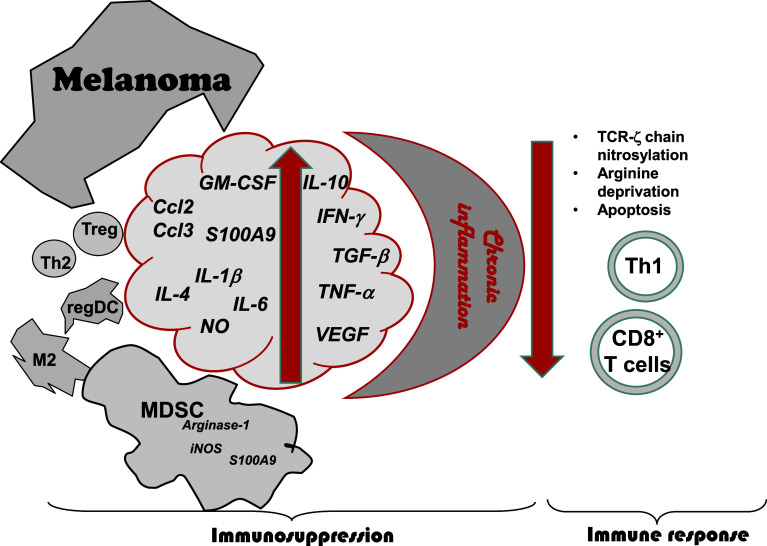
Fig. 1.
Immunosuppression in the melanoma microenvironment. Chronic inflammatory conditions developing in the tumor microenvironment are characterized by inflammatory mediators (cytokines, chemokines, and growth factors), which are secreted by the tumor and stroma cells. These mediators induce the recruitment in tumor lesions and activation of various immunosuppressive leukocytes, including myeloid-derived suppressor cells (MDSC), regulatory T cells (Treg), tumor-associated M2 macrophages regulatory dendritic cells (regDC), and Th2 lymphocytes. In particular, immunosuppressive activity of MDSC reflecting by the activation of inducible nitric oxide synthase (iNOS) and arginase (ARG)-1 can significantly contribute to the inhibition of antitumor responses mediated by effector CD4 (Th1) and CD8 T cells via the induction of TCR ζ-chain downregulation, arginine deprivation and apoptosis
Chronic inflammation and melanoma
Numerous experimental and epidemiological studies carried out in the last two decades indicate a strong link between chronic inflammation and tumor progression [18–20, 23]. The identification of transcription factors (e.g., NF-κB, AP-1 and STAT3) and related proinflammatory molecules such as TNF-α, IL-1, IL-6, IL-8, chemokines, cyclooxygenase-2 (COX-2), matrix metalloproteases and vascular endothelial growth factor (VEGF) have provided the molecular basis for the connection between chronic inflammation and cancer. This connection is indicated by (1) the induction of tumor development under chronic inflammatory conditions and (2) the formation of chronic inflammatory microenvironment in tumor lesions, which strongly stimulates tumor growth and metastasis [17–22]. It has been recently reported that a number of oncogenes are able not only to promote uncontrolled cell proliferation and stimulate their resistance to apoptosis but also to activate a cascade of inflammatory mediators. In particular, the components of the RAS-RAF signaling pathway have been shown to induce the activation of the transcription factor NF-κB and the production of some inflammatory cytokines and chemokines [24, 25]. Since this pathway plays a pivotal role in the initiation and progression of malignant skin melanoma [26, 27], we investigated the production of chronic inflammatory mediators during melanoma development.
This study requires, in particular, the establishment of reliable mouse melanoma models. Conventional animal melanoma models are based on the transplantation of tumor cells (e.g., B16), in which the disease development and tumor–stroma interactions are not comparable with clinical conditions. In contrast to transplantation models, the recently described ret transgenic mouse model closely resemble human melanoma with respect to etiology, tumor genetics, histopathology and clinical development [28]. Mice express the human ret transgene in melanocytes controlled by the mouse metallothionein I promoter/enhancer. Ret kinase belongs to the family of receptor tyrosine kinases [29] and is activated in melanoma developing in ret transgenic mice. Overexpression of Ret kinase is associated with the activation of other kinases (such as mitogen-activated protein kinase and c-Jun) and matrix metalloproteinases located downstream of the Ret kinase [28]. After a short latency (20–70 days; mean, 40 days), transgenic animals developed skin tumors on the head (nose, ears, eyes), neck, back, or tail with metastases in lymph nodes, lungs, liver, brain, kidney, and bone marrow [29, 30]. This metastatic profile resembled that of human malignant melanoma [31]. No new tumor development was ever observed in animals older than 20 weeks. Histologic analysis of primary tumors and metastases revealed the morphology of malignant melanoma.
To address the involvement of chronic inflammatory mediators in melanoma progression, we investigated the production of relevant cytokines and growth factors in primary skin melanomas and metastatic lymph nodes as well as in the cell line, which was established from primary skin melanomas isolated from ret transgenic mice (Ret melanoma cells). This cell line showed an expression of IL-6, VEGF and TGF-β1 at the mRNA level, whereas the IL-10 mRNA expression was not detected [32]. Considerable amounts of VEGF and TGF-β1 proteins were found in supernatants from cultured Ret melanoma cells by ELISA. Analyzing the primary tumors, we demonstrated IL-6, VEGF and TGF-β1 production at the mRNA and protein levels [32]. Notably, the amount of VEGF displayed a significant positive correlation with the tumor weight as an indicator of progression. Moreover, concentrations of IL-6 and VEGF were significantly elevated in the serum from transgenic tumor-bearing mice compared with non-transgenic littermates [32]. Furthermore, we found an association of elevated concentrations of IL-1β and GM-CSF in both skin melanomas and metastatic lymph nodes with accelerated melanoma growth [33]. Importantly, increased concentrations of the chemokine Ccl-2 (MCP-1) have been also detected in melanoma lesions of transgenic mice [34]. All these above-mentioned factors have been reported to be critical for driving MDSC migration into tumor lesions and for keeping their suppressive phenotype in the tumor microenvironment, lymphatic organs and peripheral blood [14, 15, 21–23, 35]. Interestingly, primary melanomas and metastatic lymph nodes accumulated high amounts of IFN-γ [33]. This cytokine (known to be released by activated T cells) was reported to enhance the MDSC recruitment into the chronic inflammatory area and to stimulate nitric oxide (NO) production by these cells [36–38].
Role of MDSC immunosuppressive melanoma microenvironment
MDSCs have been described as an extremely heterogeneous population of immature myeloid cells that are precursors of DC, macrophages and granulocytes [10, 14, 15]. Whereas in mice, they coexpress Gr1 and CD11b markers, the situation with human MDSC is much more complex due to the absence of human analog of Gr1 [39]. In mice, it has been reported that MDSC consist of granulocytic CD11b+Ly6G+Ly6Clow and monocytic CD11b+Ly6G+/−Ly6Chigh subsets that could differ in their immunosuppressive pathways [14, 39]. Main mechanisms of MDSC-mediated immunosuppression in tumor-bearing hosts are dealing with the inhibition of antitumor T-cell responses through the upregulated activity of inducible NO synthase (iNOS) and arginase (ARG)-1. This leads to depletion of l-arginine that is essential for protein synthesis by T cells [40] and increased production of NO and reactive oxygen species [14, 15, 40, 41]. NO was shown to mediate T-cell apoptosis and modulate signaling processes that are crucial for T-cell functions [42, 43]. More recently, it has been demonstrated an ability of NO to induce the nitrosylation of T-cell receptors (TCR) on tumor-infiltrating lymphocytes (TIL) [44] and even that of chemokines in the tumor microenvironment impairing thereby the T-cell migration [45]. Other recently described mechanisms by which MDSC can inhibit T-cell functions involve (1) the sequestration of cystine blocking the delivery of another critical amino acid cysteine to T cells [46] and (2) the reduction in T-cell migration to lymph nodes via the downregulation of l-selectin, which is responsible for T-cell homing [47].
Studying MDSC in skin melanomas and lymphoid organs from transgenic mice revealed a remarkable elevation of their numbers [33]. Similar observations were previously reported in different transplantation tumor models and patients with cancer [10, 14, 15, 39, 48, 49]. These findings indicate that an increased production of numerous inflammatory factors may recruit MDSC into melanoma lesions in ret transgenic mice. In addition, MDSC accumulation has been described in the mouse chronic inflammation model [22]. Importantly, the enhanced MDSC frequency may lead to decreased amounts of mature DC [14, 15]. Indeed, it has been recently reported a significant decrease in numbers of these cells in melanoma lesions and lymphoid organs from ret transgenic mice [32]. All these data suggest a linkage among developing tumors, chronic inflammation and immunosuppression. MDSC from tumor-bearing transgenic mice showed also an increased NO production and ARG-1 expression that was associated with their strong capacity to suppress T-cell proliferation in in vitro assay [33].
One of the principal characteristics of MDSC-mediated inhibition of T-cell activities is associated with a striking decrease in TCR ζ-chain expression [50, 51], which plays a critical role in coupling the TCR-mediated antigen recognition to diverse signal transduction pathways [52]. We demonstrated a strong diminution of TCR ζ-chain levels in T-cells infiltrating melanoma lesions and localized in lymphoid organs of ret transgenic mice [33]. A downregulation of ζ-chain expression has been previously reported not only in cancer patients [53, 54], but also in chronic inflammation models [50, 52], suggesting again a remarkable resemblance of both pathological conditions. Furthermore, a direct MDSC effect on TCR ζ-chain expression has been found in vitro [33]. Upon coculture of MDSC derived from skin tumors or bone marrow of tumor-bearing transgenic mice, normal T cells showed not only reduced proliferative activity but also lower levels of ζ-chain expression. Based on these observations, we suggest that measuring ζ-chain expression levels in T cells from tumor-bearing host directly ex vivo without their withdrawal from the immunosuppressive microenvironment for in vitro cultures could provide a more accurate characteristic of their functional capacity.
Neutralization of immunosuppression in melanoma
Numerous therapeutic strategies were applied during the last years to decrease MDSC-mediated immunosuppression under different pathological conditions including cancer and chronic inflammation [55]. These strategies include the reduction in MDSC frequencies by (1) stimulating their differentiation into DC and macrophages [56], (2) impairing their generation from earlier precursors [57], and (3) reducing their accumulation [58, 59]. In addition, their immunosuppressive function has been reported to be attenuated by (1) inhibiting the activities of iNOS and ARG-1 in MDSC [60, 61] and (2) interfering with the IL-13/IL-4Rα/STAT6 signaling pathway [60, 62]. It has recently been reported that phosphodiesterase (PDE)-5 inhibitors [sildenafil (Viagra) and tadalafil (Cialis)], which are widely used for the treatment of erectile dysfunction, pulmonary hypertension and cardiac hypertrophy [63], could exert antitumor effects in various transplantation mouse models by blocking MDSC immunosuppressive functions (e.g., downregulation of iNOS and ARG-1 activities) that resulted in the TIL enrichment and activation [60, 64, 65]. To overcome the chronic inflammatory conditions associated with MDSC activation in the tumor microenvironment of ret transgenic mice, the PDE-5 inhibitor sildenafil was applied in animals with established skin tumors. Chronic drug administration resulted in a significant increase in the survival of tumor-bearing mice [33]. Importantly, a significant reduction in key inflammatory mediators such as IL-1β, VEGF, GM-CSF, IL-6, Ccl2, and Ccl3 in primary tumors was found upon the treatment, demonstrating the earlier unknown anti-inflammatory effect of sildenafil [33]. Moreover, this drug was able to decrease in metastatic lymph nodes the number of MDSC, expressing the proinflammatory protein S100A9 that is known to be critically involved in the accumulation and retention of these cells [66, 67]. Although moderate, these changes in the concentration of numerous chronic inflammatory factors in the melanoma microenvironment could be responsible for observed diminution in MDSC frequencies [33]. In accordance with previous publication on transplantable tumor mouse models [60], the biochemical mechanism of sildenafil effects in transgenic mice involved the accumulation of cyclic GMP as well as the decrease in NO production and ARG-1 expression in MDSC, leading to the inhibition of their immunosuppressive activity [33].
Since MDSC have been recently described to promote angiogenesis in tumor-bearing hosts [68], one cannot exclude that the inhibition of MDSC functions could lead to the suppression of tumor angiogenesis. However, we found that the observed effects of sildenafil were strongly associated not only with an increase in TIL amounts but also with an enhancement of TCR ζ-chain expression in these cells [33]. Moreover, a systemic depletion of CD8+ T cells in treated mice with depleting anti-CD8 monoclonal antibodies resulted in a complete abrogation of antitumor effects induced by sildenafil, highlighting the mechanism of the therapy dealing with the restoration of antitumor T-cell reactivity [33]. Interestingly, applying tetramer staining failed to observe an accumulation of melanoma antigen-specific CD8+ T lymphocytes or elevated ζ-chain expression in these cells as compared with total CD8+ TIL, indicating that the T-cell recovery mediated by the treatment with sildenafil was independent of their antigen specificity. As levels of TCR ζ-chain expression in TIL have been recently reported as a prognostic and survival biomarker in cancer patients [53, 54], it could be useful for the evaluation of the host immune status and of the efficiency of tumor immunotherapies.
Importantly, the PDE-5 expression was detected in tumor-derived MDSC but not in the Ret melanoma cells, suggesting thereby that sildenafil cannot stimulate the proliferation of tumor cells due to the accumulation of cyclic GMP. In addition, no impairment of PDE-6 expression was detected in the mouse retina after chronic drug administration, indicating the absence of possible side effects related to PDE-6 inhibition [69]. These data suggest that PDE-5 inhibitors could be safely applied neutralizing immunosuppression in melanoma microenvironment.
Another strategy to overcome immunosuppressive tumor microenvironment could involve the application of chemotherapeutic drugs in particular doses. Conventional chemotherapy based on maximum tolerated doses (MTD) is currently used to eliminate quickly proliferating tumor cells. However, this kind of chemotherapy usually induces severe side effects including general toxicity and profound immunosuppression in tumor-bearing hosts supporting rapid proliferation of chemoresistant tumor cells. Reduction in the doses of cytotoxic drugs has been proposed to limit some undesirable effects of conventional chemotherapy in cancer patients [70]. Indeed, it has been recently reported that the application of chemotherapeutics in moderately low doses (20–33% of MTD) can induce so-called immunogenic death of cancer cells involving the cell surface alterations and the release of soluble immunogenic factors from dying tumor cells [71]. In addition, the treatment for cancer with cytotoxic drugs in further reduced, non-cytotoxic and non-cytostatic doses (3–5% of MTD) doses has been recently described and designated as chemoimmunomodulation [72, 73]. In contrast to conventional therapies, this approach induces no suppression of proliferation of tumor cells, hematopoietic cells or immune cells in vitro. However, it mediates the development of antitumor immune responses by stimulating immune cell functions and changing the immunogenicity of tumor cells. Thus, the treatment for human DC with different chemotherapeutic agents including vincristine, paclitaxel and methotrexate in ultra-low, non-cytotoxic concentrations resulted in the stimulation of DC maturation and their ability to induce T-cell proliferation and activation [72, 74]. In vivo, chemotherapeutic drugs, including paclitaxel administered in ultra-low non-cytotoxic doses, displayed a direct stimulatory effect on DC reflected by an upregulation of components of the MHC class I antigen processing machinery that correlated with an increased expression of MHC class II and co-stimulatory molecules such as CD80, CD86, and CD40 [74, 75]. Moreover, chemoimmunomodulation with paclitaxel prior to intratumoral DC vaccination in the Lewis lung carcinoma model has been demonstrated to inhibit tumor growth and to increase tumor infiltration with CD4+ and CD8+ T cells [76].
The administration of paclitaxel in non-cytotoxic doses into ret transgenic mice with established skin melanomas led to a significant prolongation of mouse survival and retardation of melanoma a progression. Similar to the effects of sildenafil in the same mouse melanoma model, we observed a striking inhibition of the production of chronic inflammatory mediators in melanoma lesions such as IL-β, TNF-α, IFN-γ, and IL-10. These changes were found to be associated with a reduction in MDSC frequencies in primary skin tumors and their ability to produce an immunosuppressive molecule NO compared to the untreated group. Moreover, the ability of CD11b+Gr1+ MDSC isolated from skin melanoma upon the chemoimmunomodulation with paclitaxel to suppress T-cell proliferation has been shown to be markedly lower than that of MDSC from untreated tumor-bearing animals. As a result, the number of CD8 T cells infiltrating melanoma lesions was elevated, and the expression level of TCR ζ-chain in these cells was upregulated as compared to the untreated mice. Finally, the depletion of CD8 T cells in paclitaxel-treated animals resulted in a complete abolishment of the beneficial effect of this drug indicating thereby a critical role of CD8 T cells in the mechanism of antitumor effects of paclitaxel in ultra-low, non-cytotoxic doses.
Conclusion
Taken together, malignant melanoma represents a complex association of chronic inflammatory factors and proinflammatory cells that play a critical role in generating immunosuppressive microenvironment typical for tumor-bearing mice and melanoma patients. This immunosuppressive network is able to destroy melanoma-specific T cells or severely attenuate their antitumor functions despite the strong immunogenic capacity of melanoma cells. By using a transgenic mouse melanoma model, which resembles human cutaneous melanoma, we demonstrated a remarkable increase in levels of inflammatory mediators and in amounts of highly immunosuppressive MDSCs in melanoma lesions that correlated with diminished TCR ζ-chain expression in tumor infiltrating T cells. The treatment with sildenafil or chemoimmunomodulation with paclitaxel enabled neutralization of chronic inflammatory milieu associated with decreased MDSC numbers and suppressive activity. Importantly, this resulted in a partial restoration of antitumor T-cell activities leading to a significant retardation of spontaneous melanoma progression (Fig. 2). We thus suggest that a key prerequisite for an effective melanoma immunotherapy should involve monitoring the initial immune status of patients including the measurement of chronic inflammatory and immunosuppressive cytokines (e.g., IL-1β, IL-6, IL-10, TNF-α, and IFN-γ), chemokines (like Ccl-2 and Ccl3) and growth factors (such as GM-CSF, VEGF, and TGF-β). Based on this information, the neutralization of immunosuppressive melanoma microenvironment should be performed before applying any immunologic treatments.
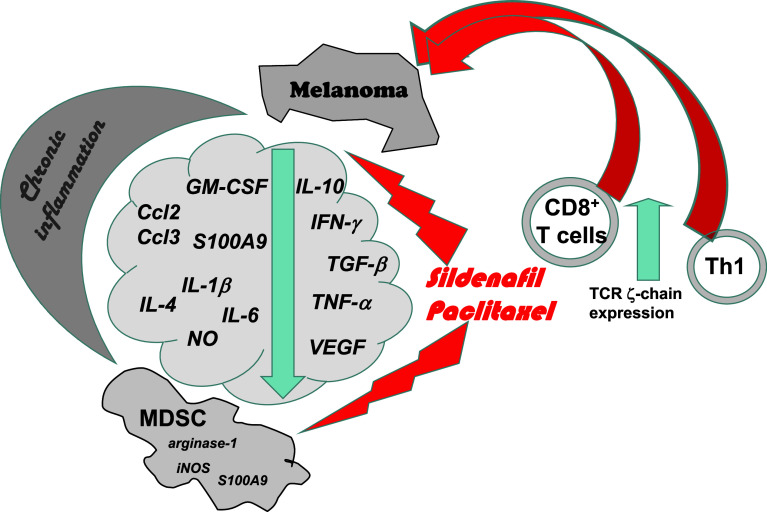
Fig. 2.
Overcoming melanoma-induced immunosuppression. Therapy with PDE-5 inhibitor sildenafil or chemoimmunomodulation with paclitaxel in non-toxic doses enables neutralization of chronic inflammatory milieu associated with decreased MDSC numbers and suppressive activity. This leads to a restoration of antitumor activities of T-cell effector CD4 (Th1) and CD8 T cells (indicated by an enhancement of TCR ζ-chain levels), inducing a significant retardation of spontaneous melanoma progression. Thus, the neutralization of immunosuppressive melanoma microenvironment should be performed before applying any immunologic treatments
Acknowledgments
This project has been funded by the DKFZ-MOST Cooperation in Cancer Research (grant CA128, to Viktor Umansky), Dr. Mildred Scheel Foundation for Cancer Research (grant 108992, to Viktor Umansky), the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Immunotherapy of Cancer (to Viktor Umansky).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Second International Conference on Cancer Immunotherapy and Immunomonitoring (CITIM 2011), held in Budapest, Hungary, 2nd–5th May 2011. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.MacKie RM, Hauschild A, Eggermont AM. Epidemiology of invasive cutaneous melanoma. Ann Oncol. 2009;20(Suppl 6):vi1–vi7. doi: 10.1093/annonc/mdp252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garbe C, Peris K, Hauschild A, Saiag P, Middleton M, Spatz A, Grob JJ, Malvehy J, Newton-Bishop J, Stratigos A, Pehamberger H, Eggermont A. Diagnosis and treatment of melanoma: European consensus-based interdisciplinary guideline. Eur J Cancer. 2010;46:270–283. doi: 10.1016/j.ejca.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 3.Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol. 2010;37:473–484. doi: 10.1053/j.seminoncol.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandolfi F, Cianci R, Lolli S, Dunn IS, Newton EE, Haggerty TJ, Boyle LA, Kurnick JT. Strategies to overcome obstacles to successful immunotherapy of melanoma. Int J Immunopathol Pharmacol. 2008;21:493–500. doi: 10.1177/039463200802100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parmiani G, Castelli C, Santinami M, Rivoltini L. Melanoma immunology: past, present and future. Curr Opin Oncol. 2007;19:121–127. doi: 10.1097/CCO.0b013e32801497d7. [DOI] [PubMed] [Google Scholar]
- 6.Fujii S, Shimizu K, Hemmi H, Steinman RM. Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol Rev. 2007;220:183–198. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 7.Dissemond J, Kothen T, Mörs J, Weimann TK, Lindeke A, Goos M, Wagner SN. Downregulation of tapasin expression in progressive human malignant melanoma. Arch Dermatol Res. 2003;295:43–49. doi: 10.1007/s00403-003-0393-8. [DOI] [PubMed] [Google Scholar]
- 8.Ferrone S, Marincola FM. Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol Today. 1995;16:487–494. doi: 10.1016/0167-5699(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 9.Burke S, Lakshmikanth T, Colucci F, Carbone E. New views on natural killer cell-based immunotherapy for melanoma treatment. Trends Immunol. 2010;31:339–345. doi: 10.1016/j.it.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18:11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lázár-Molnár E, Hegyesi H, Tóth S, Falus A. Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine. 2000;12:547–554. doi: 10.1006/cyto.1999.0614. [DOI] [PubMed] [Google Scholar]
- 12.Antony PA, Restifo NP. CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J Immunother. 2005;28:120–128. doi: 10.1097/01.cji.0000155049.26787.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 14.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shurin MR, Naiditch H, Zhong H, Shurin GV. Regulatory dendritic cells: new targets for cancer immunotherapy. Cancer Biol Ther. 2011;11:988–992. doi: 10.4161/cbt.11.11.15543. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 18.Rook GA, Dalgleish A. Infection, immunoregulation, and cancer. Immunol Rev. 2011;240:141–159. doi: 10.1111/j.1600-065X.2010.00987.x. [DOI] [PubMed] [Google Scholar]
- 19.Cramer DW, Finn OJ. Epidemiologic perspective on immune-surveillance in cancer. Curr Opin Immunol. 2011;23:265–271. doi: 10.1016/j.coi.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med. 2010;10:369–373. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 21.Allavena P, Germano G, Marchesi F, Mantovani A. Chemokines in cancer related inflammation. Exp Cell Res. 2011;317:664–673. doi: 10.1016/j.yexcr.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Baniyash M. Chronic inflammation, immunosuppression and cancer: new insights and outlook. Semin Cancer Biol. 2006;16:80–88. doi: 10.1016/j.semcancer.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19:209–216. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 25.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haluska F, Pemberton T, Ibrahim N, Kalinsky K. The RTK/RAS/BRAF/PI3K pathways in melanoma: biology, small molecule inhibitors, and potential applications. Semin Oncol. 2007;34:546–554. doi: 10.1053/j.seminoncol.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Lomas J, Martin-Duque P, Pons M, Quintanilla M. The genetics of malignant melanoma. Front Biosci. 2008;13:5071–5093. doi: 10.2741/3065. [DOI] [PubMed] [Google Scholar]
- 28.Kato M, Takahashi M, Akhand AA, Liu W, Dai Y, Shimizu S, Iwamoto T, Suzuki H, Nakashima I. Transgenic mouse model for skin malignant melanoma. Oncogene. 1999;17:1885–1888. doi: 10.1038/sj.onc.1202077. [DOI] [PubMed] [Google Scholar]
- 29.Eng C. RET proto-oncogene in the development of human cancer. J Clin Oncol. 1999;17:380–383. doi: 10.1200/JCO.1999.17.1.380. [DOI] [PubMed] [Google Scholar]
- 30.Umansky V, Abschuetz O, Osen W, Ramacher M, Zhao F, Kato M, Schadendorf D. Melanoma-specific memory T cells are functionally active in ret transgenic mice without macroscopic tumors. Cancer Res. 2008;68:9451–9458. doi: 10.1158/0008-5472.CAN-08-1464. [DOI] [PubMed] [Google Scholar]
- 31.Houghton A, Polsky D. Focus on melanoma. Cancer Cell. 2002;2:275–278. doi: 10.1016/S1535-6108(02)00161-7. [DOI] [PubMed] [Google Scholar]
- 32.Zhao F, Falk C, Osen W, Kato M, Schadendorf D, Umansky V. Activation of p38 mitogen-activated protein kinase drives dendritic cells to become tolerogenic in ret transgenic mice spontaneously developing melanoma. Clin Cancer Res. 2009;15:4382–4390. doi: 10.1158/1078-0432.CCR-09-0399. [DOI] [PubMed] [Google Scholar]
- 33.Meyer C, Sevko A, Ramacher M, Bazhin AV, Falk CS, Osen W, Borrello I, Kato M, Schadendorf D, Baniyash M, Umansky V. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci USA. 2011;108:17111–17116. doi: 10.1073/pnas.1108121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimpfler S, Sevko A, Ring S, Falk C, Osen W, Frank K, Kato M, Mahnke K, Schadendorf D, Umansky V. Skin melanoma development in ret transgenic mice despite the depletion of CD25+Foxp3+ regulatory T cells in lymphoid organs. J Immunol. 2009;183:6330–6337. doi: 10.4049/jimmunol.0900609. [DOI] [PubMed] [Google Scholar]
- 35.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V. Tumor-induced tolerance and immune suppression depend on the C/EBPb transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Bronstein-Sitton N, Vaknin I, Ezernitchi AV, Leshem B, Halabi A, Houri-Hadad Y, Greenbaum E, Zakay-Rones Z, Shapira L, Baniyash M. Sustained exposure to bacterial antigen induces interferon gamma-dependent T cell receptor zeta down-regulation and impaired T cell function. Nat Immunol. 2003;4:957–964. doi: 10.1038/ni975. [DOI] [PubMed] [Google Scholar]
- 37.Rössner S, Voigtländer C, Wiethe C, Hänig J, Seifarth C, Lutz MB. Myeloid dendritic cell precursors generated from bone marrow suppress T cell responses via cell contact and nitric oxide production in vitro. Eur J Immunol. 2005;35:3533–3544. doi: 10.1002/eji.200526172. [DOI] [PubMed] [Google Scholar]
- 38.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 40.Bronte V, Zanovello P. Regulation of immune responses by l-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 41.Rodríguez PC, Ochoa AC. T cell dysfunction in cancer: role of myeloid cells and tumor cells regulating amino acid availability and oxidative stress. Semin Cancer Biol. 2006;16:66–72. doi: 10.1016/j.semcancer.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Umansky V, Schirrmacher V. Nitric oxide-induced apoptosis in tumor cells. Adv Cancer Res. 2001;82:107–131. doi: 10.1016/S0065-230X(01)82004-2. [DOI] [PubMed] [Google Scholar]
- 43.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 44.Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol. 2010;184:3106–3116. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, De Palma A, Mauri P, Monegal A, Rescigno M, Savino B, Colombo P, Jonjic N, Pecanic S, Lazzarato L, Fruttero R, Gasco A, Bronte V, Viola A. Chemokine nitrosylation prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate l-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183:937–944. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 49.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 50.Ezernitchi AV, Vaknin I, Cohen-Daniel L, Levy O, Manaster E, Halabi A, Pikarsky E, Shapira L, Baniyash M. TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. J Immunol. 2006;177:4763–4772. doi: 10.4049/jimmunol.177.7.4763. [DOI] [PubMed] [Google Scholar]
- 51.Rodríguez PC, Zea AH, Culotta KS, Zabaleta J, Ochoa JB, Ochoa AC. Regulation of T cell receptor CD3zeta chain expression by l-arginine. J Biol Chem. 2002;277:21123–21129. doi: 10.1074/jbc.M110675200. [DOI] [PubMed] [Google Scholar]
- 52.Baniyash M. TCR zeta-chain downregulation: curtailing an excessive inflammatory immune response. Nat Rev Immunol. 2004;4:675–687. doi: 10.1038/nri1434. [DOI] [PubMed] [Google Scholar]
- 53.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Higashi H, Iwashige H, Aridome K, Hokita S, Aikou T. CD3-zeta chain expression of intratumoral lymphocytes is closely related to survival in gastric carcinoma patients. Cancer. 2002;94:1437–1442. doi: 10.1002/cncr.10346. [DOI] [PubMed] [Google Scholar]
- 54.Whiteside TL. Down-regulation of zeta-chain expression in T cells: a biomarker of prognosis in cancer? Cancer Immunol Immunother. 2004;53:865–878. doi: 10.1007/s00262-004-0521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ugel S, Delpozzo F, Desantis G, Papalini F, Simonato F, Sonda N, Zilio S, Bronte V. Therapeutic targeting of myeloid-derived suppressor cells. Curr Opin Pharmacol. 2009;9:470–481. doi: 10.1016/j.coph.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 56.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, Chen SH. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–228. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 60.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Santo C, Serafini P, Marigo I, Dolcetti L, Bolla M, Del Soldato P, Melani C, Guiducci C, Colombo MP, Iezzi M, Musiani P, Zanovello P, Bronte V. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc Natl Acad Sci USA. 2005;102:4185–4190. doi: 10.1073/pnas.0409783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 63.Ghofrani HA, Osterloh IH, Grimminger F. Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov. 2006;5:689–702. doi: 10.1038/nrd2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Capuano G, Rigamonti N, Grioni M, Freschi M, Bellone M. Modulators of arginine metabolism support cancer immunosurveillance. BMC Immunol. 2009;10:1. doi: 10.1186/1471-2172-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tartour E, Pere H, Maillere B, Terme M, Merillon N, Taieb J, Sandoval F, Quintin-Colonna F, Lacerda K, Karadimou A, Badoual C, Tedgui A, Fridman WH, Oudard S. Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 2011;30:83–95. doi: 10.1007/s10555-011-9281-4. [DOI] [PubMed] [Google Scholar]
- 69.Laties A, Zrenner E. Viagra (sildenafil citrate) and ophthalmology. Prog Retin Eye Res. 2002;21:485–506. doi: 10.1016/S1350-9462(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 70.Nowak AK, Lake RA, Robinson BW. Combined chemoimmunotherapy of solid tumours: improving vaccines? Adv Drug Deliv Rev. 2006;58:975–990. doi: 10.1016/j.addr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–160. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 72.Kaneno R, Shurin GV, Kaneno FM, Naiditch H, Luo J, Shurin MR. Chemotherapeutic agents in low non-cytotoxic concentrations increase immunogenicity of human colon cancer cells. Cell Oncol (Dordr) 2011;34:97–106. doi: 10.1007/s13402-010-0005-5. [DOI] [PubMed] [Google Scholar]
- 73.Kaneno R, Shurin GV, Tourkova IL, Shurin MR. Chemomodulation of human dendritic cell function by anti-neoplastic agents in low non-cytotoxic concentrations. J Transl Med. 2009;7:58. doi: 10.1186/1479-5876-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shurin GV, Tourkova IL, Kaneno R, Shurin MR. Chemotherapeutic agents in non-cytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol. 2009;183:137–144. doi: 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shurin GV, Tourkova IL, Shurin MR. Low-dose chemotherapeutic agents regulate small rho GTPase activity in dendritic cells. J Immunother. 2008;31:491–499. doi: 10.1097/CJI.0b013e318176fae4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhong H, Han B, Tourkova IL, Lokshin A, Rosenbloom A, Shurin MR, Shurin GV. Low-dose paclitaxel prior to intratumoral dendritic cell vaccine modulates intratumoral cytokine network and lung cancer growth. Clin Cancer Res. 2007;13:5455–5462. doi: 10.1158/1078-0432.CCR-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]