Abstract
Introduction
Immunization with autologous dendritic cells (DCs) loaded with a heat shock-conditioned allogeneic melanoma cell lysate caused lysate-specific delayed type hypersensitivity (DTH) reactions in a number of patients. These responses correlated with a threefold prolonged long-term survival of DTH+ with respect to DTH− unresponsive patients. Herein, we investigated whether the immunological reactions associated with prolonged survival were related to dissimilar cellular and cytokine responses in blood.
Materials and methods
Healthy donors and melanoma patient’s lymphocytes obtained from blood before and after vaccinations and from DTH biopsies were analyzed for T cell population distribution and cytokine release.
Results/discussion
Peripheral blood lymphocytes from melanoma patients have an increased proportion of Th3 (CD4+ TGF-β+) regulatory T lymphocytes compared with healthy donors. Notably, DTH+ patients showed a threefold reduction of Th3 cells compared with DTH− patients after DCs vaccine treatment. Furthermore, DCs vaccination resulted in a threefold augment of the proportion of IFN-γ releasing Th1 cells and in a twofold increase of the IL-17-producing Th17 population in DTH+ with respect to DTH− patients. Increased Th1 and Th17 cell populations in both blood and DTH-derived tissues suggest that these profiles may be related to a more effective anti-melanoma response.
Conclusions
Our results indicate that increased proinflammatory cytokine profiles are related to detectable immunological responses in vivo (DTH) and to prolonged patient survival. Our study contributes to the understanding of immunological responses produced by DCs vaccines and to the identification of follow-up markers for patient outcome that may allow a closer individual monitoring of patients.
Keywords: Dendritic cells, Melanoma vaccine, Th17 lymphocytes, Regulatory T cells, Cancer immunotherapy
Introduction
Dendritic cell (DCs)-based cancer immunotherapy constitutes a rising alternative for the treatment of a number of solid tumors [1]. The goal of this therapeutic approach is to activate a T cell-mediated immune response against tumor-associated antigens (TAA) in order to eliminate tumor cells. Recently, Sipuleucel-T, a type of active cellular immunotherapy, demonstrated its effectiveness in reducing the risk of death in metastatic castration-resistant prostate cancer patients, raising the interest for developing improved cancer DCs vaccines [1, 2].
However, early clinical trials have shown that DCs immunization, although capable of inducing detectable specific T cell activation, showed poor correlations with objective clinical responses in patients with metastatic lymphoma, melanoma, and prostate cancer [3, 4]. Particularly, synthetic peptides-loaded DCs are not enough in raising significant therapeutic effects [5–8]. For that reason, DCs loaded with allogeneic tumor lysate had constituted an improved way of inducing TAA-specific immune activation and objective clinical responses in treated patients [9–12]. In this regard, one explanation can be related to some properties of tumor cell lysates, which not only provide specific TAA, but also additional danger signals that improve DCs activation and generate a more efficient immunization [13]. In general, the major clinical impact of DCs vaccines has been associated more with prolonged overall patient survival than with objective tumor regressions [12–16]. However, in some studies, it has been observed that cellular immunotherapy has potent effects prolonging patient survival only in a subgroup of patients, but leaving an important group of patients without clinical benefits [12, 13, 17]. The fact that not all treated patients showed the same therapy outcome after identical treatment encourages the search of better molecular and cellular methods that allow the monitoring of clinical and immunological responses [18, 19]. Therefore, the search for predictive biomarkers for identifying patients who benefit from therapies is the current challenge for cellular immunotherapy [20].
One important aspect is related with the type of immunological response generated by different immunotherapies. Currently, there is a consensus that the induction of Th1 profiles or T cell release of cytokines such as IFN-γ and TNF-α is essential to the development of an effective anti-tumor immune response [21–23]. The role of other T helper lymphocyte populations and cytokine profiles in the regulation of the immune response against tumors is currently being explored [24]. Particularly, the role of Th17 subpopulations in cancer has not yet been clearly established. Some evidence in the murine system indicates that IL-17 may promote angiogenesis and tumor growth [25, 26]. However, Th17 lymphocytes are significantly increased in peripheral blood of patients with ovarian carcinoma, prostate cancer, breast, myeloma, lymphoma, colon, gastric, renal, pancreas, lung, and leukemia, and they have been associated with improved tumor regression, suggesting the participation of this subpopulation of T cells in the tumor-associated immune response [27–31].
In line with this, the presence of regulatory T cells has been associated with cancer progression and poor prognosis [32]. These populations have been identified in blood, lymph nodes, ascites, and tumor tissue in different types of cancer [33–35]. Inhibitory cytokines such as TGF-β are elevated in the serum of patients with prostate cancer, pancreatic cancer, breast carcinoma, and melanoma [36, 37]. For example, it has been proposed that TGF-β impairs the effectiveness of immunotherapy with DCs [38] by inhibiting antigen presentation and the migration of these cells into the lymph nodes [38, 39]. Moreover, the blockade of tumor-derived TGF-β enhances antitumor immunity induced by DCs vaccines [40, 41].
We recently demonstrated the clinical utility of heat shock-conditioned tumor cell lysate DCs-based therapy for improving survival in patients with stage IV melanoma [12, 13]. Our results showed that 60 % of patients developed a melanoma antigen-specific DTH reaction associated with a threefold prolonged survival compared with non-responder patients [12, 13]. Additionally, the analysis of regulatory T cell populations in the blood of treated patients showed that DTH responder patients have a fivefold reduced proportion of TGF-β+ regulatory T cells compared with DTH− patients at the end of the treatment [12].
Herein, we studied cellular and soluble factors present in the blood from treated melanoma patients, which may be involved in the differential clinical outcome observed between DTH+ and DTH− vaccinated patients, in order to identify immunological markers relevant for both prognosis and monitoring of the immune and clinical responses of patients treated with DCs-based immunotherapy.
Materials and methods
Patients and healthy donors
Sixty melanoma patients were vaccinated with TAPCells and followed up from April 2002 until March 2009, according to a previously described protocol [12, 13]. The study was performed in agreement with the Helsinki Declaration and approved by the Bioethical Committee for Human Research of the Faculty of Medicine, University of Chile. All patients signed an informed consent. The inclusion criteria for the clinical protocol included (1) histological verified melanoma, (2) Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less, (3) white blood cells and platelets normal counts, seronegative to HIV, Hepatitis B and C. A retrospective analysis was performed using frozen PBMCs from 28 of those patients. PBMCs from healthy donors paired by gender and age were used as a control group. The Bioethical Committee for Human Research of University of Chile approved the study. Patients and healthy donors needed to understand and sign an informed consent statement.
TRIMEL cell lysate
TRIMEL is a cell lysate derived from three allogeneic melanoma cells lines Mel1, Mel2, and Mel3, established from metastatic lymph nodes at the Institute of Biomedical Sciences at University of Chile. Tumor cell lines were heat shocked at 42 °C for 1 h, incubated 2 h at 37 °C, mixed in equal numbers, and then lysed trough repeated freeze–thaw cycles in liquid nitrogen. TRIMEL was then sonicated and irradiated (60 Gy). The protein concentration of the lysate was estimated after dilution in AIM-V medium by the Bradford’s method using a Biophotometer (Eppendorf, Hamburg, Germany).
DCs generation and inoculation
Peripheral blood mononuclear cells (PBMC) were obtained from leukapheresis at the Blood Bank Service of University of Chile Clinical Hospital. DCs were generated and characterized as described previously in Lopez et al. [12]. Briefly, adherent monocytes isolated from melanoma patient’s PBMC were cultured in serum-free AIM-V medium (Invitrogen) with rhIL-4 (500 U/mL; US-Biological), and rhGM-CSF (800 U/mL; Schering Plough) for 22 h, and then stimulated for 24 h with TRIMEL (100 μg/mL) alone or plus rhTNF-α (20 U/mL; US-Biological). Patients were inoculated by intradermal injection (id) in the leg or arm closest to intact lymph nodes with 1 mL of TRIMEL/DCs plus Keyhole limpet hemocyanin (KLH, 100 μg; Calbiochem, San Diego, CA). The inoculation protocol consisted of four doses injected on days 0, 10, 30, and 50. At the end of the therapy, we evaluated the immunological response with delayed hypersensitivity test.
Delayed test hypersensitivity
DTH reactions to the TRIMEL were assessed 1 month after the end of the therapy. Skin tests were performed by intradermal injection of 150 μL of TRIMEL lysate (2 mg/mL), 100 μL of control antigens KLH (1 mg/mL), or MULTITEST cell-mediated immunity (CMI; Pasteur-Mérieux, Lyon, France) in saline solutions at different locations of the arm. Saline solution alone (100 μL) was used as a negative control. A positive reaction was defined as skin erythema or induration larger than 5 mm at 48 h after injection.
DTH biopsies
An 8-mm excision was extracted from induration zone of DTH reaction, using a disposable punch (Delasco). Half of the tissue sample was fixed in paraformaldehyde 1 %, and paraffin-embedded specimens were immunostained for immunofluorescence. T cells were isolated from the other half by mechanical disruption and expanded with IL-2 (250 U/mL) for 3 weeks and then analyzed by flow cytometry after re-stimulation with PMA as described below.
Immunofluorescence
The DTH samples in paraffin consisted of slides of 3-μm thickness and incubated with the following combinations of antibodies diluted in blocking solution overnight at 4 °C: (1) mouse anti-CD4 (4B12) (Dako, Denmark dt, Germany) and rabbit anti-IFNγ (ab25101) (Abcam, UK), (2) Rabbit anti-CD4 (SP35) (Bio SB, Santa Barbara, CA. USA) and goat anti-IL-17 (AF-317-NA) (R & D Systems, MN, USA). The samples were washed with PBS and incubated with the following secondary antibodies, Alexa Fluor 546 Donkey anti-mouse, Alexa Fluor 488-Chicken anti rabbit, and Alexa Fluor 546 Donkey anti-goat (Molecular Probes, Invitrogen, Eugene, OR, USA). The samples were mounted with fluorescence mounting medium (Dako, Carpinteria, CA, USA). The observation and photographic documentation was performed in the confocal microscope LSM 5 Pascal software run by Axiovert (Carl Zeiss, Jenna).
Enzyme-linked immunosorbent assay (ELISA)
Serums were obtained from healthy donors, DTH+ patients and DTH− patients, before (untreated) and after a complete immunotherapy cycle (treated). Interferon gamma was measured using the ELISA KIT II (BD OptEIATM), TGF-β1 soluble protein levels was measured using the ELISA Quantikine Kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s specifications, and IL-17 was measured using an Human IL-17A High Sensitivity ELISA (eBioscience). The samples were read using an ELISA reader (TermoLabsystems, Helsinki, Finland) at 405 nm.
Flow cytometry analysis
PBMC were obtained from 8 DTH+ patients and 8 DTH− patients before (untreated) and after a complete immunotherapy cycle (treated) and from 12 healthy controls, as previously described [12, 13]. DTH-derived T cells were obtained from a biopsy and expanded in RMPI medium with 250 U/mL IL-2 (proleukin, Novartis) during 3 weeks. Th1 (CD4+IFN-γ+), Th17 (CD4+IL-17+), and Th3 (CD4+TGF-β+) profiles were evaluated in PBMC and in cells derived from DTH biopsies. Briefly, IFN-γ and IL-17 analysis was performed previous stimulus with PMA (50 ng/mL) and ionomicyne (1 μg/mL) in the presence of Brefeldin A (1 μg/mL, eBioscience, SanDiego, CA) for 5 h. Cells were incubated with anti-CD4-FITC (RPA-T4) antibody (eBioscience, CA, USA); then, the cells were permeabilized with permeabilization buffer (eBioscience, CA, USA) and incubated with anti-IL-17-PE (eBio64DEC17), and anti-IFN-γ-PerCpCy5.5 (Clone: 4S.B3) (eBioscience, CA) for intracellular staining. For the Th3 population analysis, the cells were stimulated with LPS (100 ng/mL, Sigma Aldrich, GA) during 16 h in the presence of Brefeldin A (1 μg/mL, eBioscience, San Diego, CA) for the last 4 h. Then, TGF-β on CD4+ T cells was evaluated using a primary anti-LAP-TGF-β antibody (R&D Systems, Minneapolis, MN) followed by an anti-IgG1-FITC antibody (R&D Systems, Minneapolis, MN) and anti-CD4-PerCPCy5.5 antibody (eBioscience, SanDiego, CA). For unspecific antibody binding, the anti-IgG1-PE (Mouse IgG1, κ) and anti-IgG1-PerCPCy5.5 (Mouse IgG1 κ,) (eBioscience, CA) were used as Isotype controls. Finally, cells were analyzed on a FACSort flow cytometer (Becton–Dickinson, Franklin Lakes, NJ), and data were analyzed with the WinMDI software.
Statistical analysis
Data were expressed as mean ± SEM. After confirming normal distribution with Skewness/Kurtosis statistic test, paired and unpaired Student’s t-test was used to analyze the differences between the proportion of T helper cell populations of responder and non-responder patients. Statistical analysis was performed using Stata 7.0 software. Statistical differences were considered significant for values of p < 0.05. For correlation analysis, correlation test was used and R value was obtained. For further analysis, fold change was obtained by a ratio of: T helper proportion after treatment (untreated)/T helper proportion before treatment (treated).
Results
Th3 population is augmented in melanoma patients PBL before treatment compared with normal donors and decreased in DTH+ but not in DTH− patients after DC vaccination
Previously, we reported that populations of natural Treg (CD4+CD25+FoxP3+), Tr1 (CD4+IL-10+), and Th3 (CD4+TGF-β+) regulatory T cells augmented in non-responder (DTH−) patients after DC-based immunotherapy. In contrast, a significant inhibition of Th3, TGF-β producing CD4+ T cells could be observed in patients developing a tumor lysate-specific DTH reaction after vaccination [12]. Here, we evaluated whether Th3 population in PBMC from a representative group of 28 DCs-vaccinated melanoma patients, obtained from a cohort of sixty patients treated between April 2002 to March 2009 and followed up for a median of 34 months, could result in a prognostic marker for the therapy response (Table 1). The group was constituted by peripheral blood lymphocytes (PBL) from 14 DTH+ and 14 DTH− patients, which showed similar survival differences as reported in previous works [12, 13].
Table 1.
Characteristics of vaccinated patients (2002–2009)
| Patient code | Gender | Age (years) | AJCC stage | Primary tumor localization | Metastasis | Additional treatment | DTH | Clinical status | Overall survival (months) |
|---|---|---|---|---|---|---|---|---|---|
| MT015 | M | 30 | IV | Right ventral abdomen | M1a | RT + IL2 | + | Deceased | 40.0 |
| MT018 | F | 63 | IV | Left ankle exterior side | M1b | None | + | Deceased | 36.0 |
| MT019 | M | 19 | IV | Right eye | M1c | Local RT | − | Deceased | 5.0 |
| MT020 | F | 51 | IV | Right uveal tumor | M1c | Local RT | + | Deceased | 41.0 |
| MT021 | F | 61 | IV | Scalp | M1a | None | + | Stable | 80.0 |
| MT023 | M | 40 | IV | Right Malar | M1a | Surgery + RT + IL-2 | − | Deceased | 11.0 |
| MT026 | M | 54 | IV | Ventral left thigh | M1a | Surgery + IL-2 | + | Stable | 78.0 |
| MT027 | M | 47 | IV | Supraumbilical right side | M1b | Surgery | + | Stable | 78.0 |
| MT028 | F | 70 | IV | Vulva | M1b | None | + | Deceased | 7.0 |
| MT029 | M | 42 | IV | Periumbilical | M1b | None | + | Deceased | 16.0 |
| MT030 | F | 81 | IV | Ventral left knee | M1c | Local RT | + | Deceased | 28.0 |
| MT031 | F | 42 | IV | Right eyelid, local extension | M1a | Local RT | − | Deceased | 62.0 |
| MT034 | F | 71 | IV | Subungueal big toe right foot | M1b | None | − | Deceased | 15.0 |
| MT035 | F | 66 | IV | Left dorsal calf | M1a | Surgery + IFN-a | − | Stable | 71.0 |
| MT037 | M | 55 | IV | Right anterior cervical region | M1a | Surgery | − | Deceased | 6.0 |
| MT038 | F | 48 | IV | Right dorsal subscapular hemithorax | M1c | None | + | Deceased | 20.0 |
| MT039 | F | 42 | IV | Ventral right foot | M1a | None | − | Deceased | 13.0 |
| MT040 | M | 59 | IV | Left dorsal thoracic | M1c | Local RT | − | Deceased | 7.0 |
| MT042 | M | 52 | IV | Right inguinal region | M1c | RT + temodal | − | Deceased | 6.0 |
| MT043 | F | 71 | IV | Right heel | M1a | Surgery + DTIC | + | Deceased | 20.3 |
| MT044 | F | 25 | IV | Left scapula | M1a | Local RT | + | Deceased | 8.0 |
| MT045 | M | 19 | IV | ND | M1a | Local RT | + | Progressor | 33.5 |
| MT046 | M | 69 | IV | Back | M1b | None | − | Deceased | 6.0 |
| MT048 | F | 44 | IV | Left forearm | M1c | None | + | Deceased | 9.4 |
| MT049 | M | 54 | IV | Back | M1a | None | + | Stable | 27.0 |
| MT050 | F | 52 | IV | Left foot sole | M1c | RT + temodal | + | Deceased | 3.0 |
| MT053 | F | 72 | IV | Left ankle | M1c | None | − | Deceased | 3.3 |
| MT055 | F | 36 | IV | Left hand | M1c | None | − | Deceased | 9.3 |
| MT058 | F | 52 | IV | Left ankle | M1a | RT | + | Progressor | 27.4 |
| MT060 | M | 42 | IV | Right leg | M1a | IL-2 + RT | + | Progressor | 22.9 |
| MT061 | M | 67 | IV | Urethral meatus | M1c | CTX | − | Deceased | 10.7 |
| MT062 | F | 47 | IV | Dorsal | M1a | CTX | + | Stable | 23.6 |
| MT064 | F | 45 | IV | Pharynx | M1c | CTX | − | Deceased | 15.1 |
| MT065 | F | 40 | IV | Scalp | M1c | CTX | − | Deceased | 5.7 |
| MT066 | M | 54 | IIIC | Abdominal wall | ND | RT + DTIC | + | Stable | 23.1 |
| MT072 | M | 42 | IV | Dorsal | M1c | None | − | Deceased | 10.6 |
| MT076 | F | 29 | IV | Left foot | M1b | RT | + | Stable | 19.2 |
| MT079 | F | 64 | IV | Left flank | M1c | None | − | Deceased | 9.0 |
| MT080 | M | 74 | IV | Palate | M1c | ND | + | Stable | 18.3 |
| MT083 | M | 57 | IIIC | Right hand | N3 | None | − | Progressor | 16.5 |
| MT084 | M | 47 | IIIC | Right hand | N3 | None | + | Stable | 16.3 |
| MT088 | F | 70 | IV | Uveal melanoma | M1c | RT + TAM + DTIC | + | Deceased | 7.4 |
| MT091 | M | 70 | IV | Scalp | M1c | None | + | Stable | 15.5 |
| MT094 | F | 48 | IV | Uveal melanoma | M1c | RT | + | Stable | 14.9 |
| MT096 | M | 46 | IIIA | Dorsal | N2a | None | + | Stable | 14.4 |
| MT101 | M | 41 | IV | Right leg | M1a | None | + | Stable | 13.8 |
| MT102 | F | 46 | IV | Right thigh | M1b | DTIC + Ipimilumab | + | Stable | 14.0 |
| MT103 | F | 47 | IIIB | Left foot | N2b | None | + | Stable | 13.7 |
| MT105 | F | 56 | IIIA | Right left | N2a | None | − | Stable | 13.5 |
| MT112 | M | 67 | IIIA | Dorsal | N2a | None | + | Stable | 11.9 |
| MT114 | M | 47 | IV | Thoracic wall | M1c | RT + IFN-α | − | Deceased | 10.7 |
| MT115 | F | 46 | IB | Left shoulder | NA | None | + | Stable | 11.4 |
| MT118 | F | 40 | IV | Left shoulder | M1a | ND | + | Progressor | 11.2 |
| MT120 | M | 36 | IV | Dorsal | M1b | DTIC | + | Progressor | 10.9 |
| MT122 | M | 23 | IIIC | Left leg | ND | None | + | Stable | 10.1 |
| MT123 | M | 43 | IV | Left flank | M1c | RT | − | Deceased | 10.1 |
| MT125 | F | 54 | IIIC | Right leg | N3 | None | + | Progressor | 7.6 |
| MT126 | F | 39 | IB | Left axillary | NA | None | + | Stable | 7.2 |
| MT127 | F | 37 | IV | Left foot | M1b | None | + | Progressor | 7.2 |
| MT128 | F | 34 | IIIB | Right shoulder | N2c | RT | + | Stable | 6.5 |
Immunologic response to therapy and post-vaccine survival
M male, F female, AJCC American Joint Committee on Cancer, ND not determinate, NA not applicable, CTX cyclophosphamide, DTIC dacarbazine, RT radiotherapy, TAM tamoxifen. Bold font = patients included in T cell population analysis
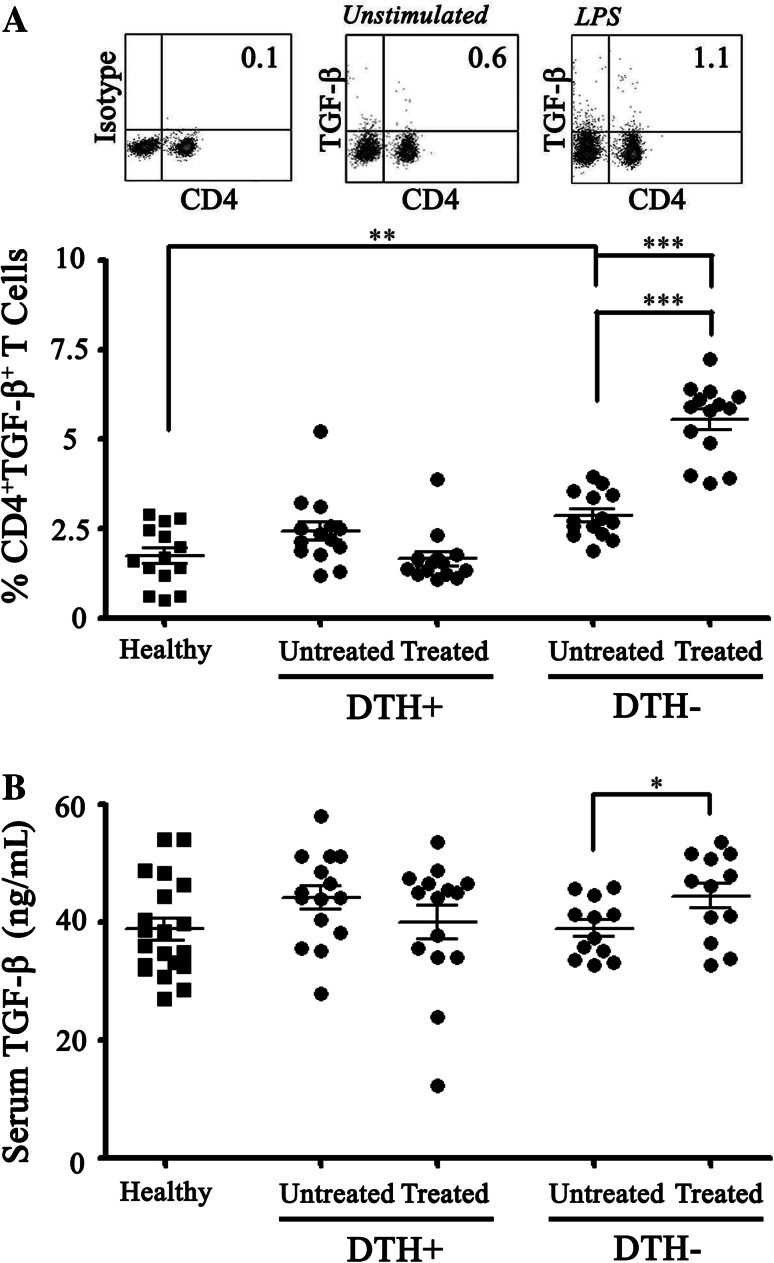
In this respect, the group of 28 melanoma patients showed significantly higher percentages of CD4+TGFβ+ T cells in PBLs than a control group of 28 healthy donors both before and after treatment [2.64 % ± 0.16 vs 1.72 % ± 0.22 and 3.40 % ± 0.41 vs 1.72 % ± 0.22, respectively (p < 0.01)]. Moreover, the separated analysis of DTH+ and DTH− patients showed that although no significant differences could be detected between the two groups before vaccine treatment, a significant increase of Th3 population was observed in the DTH− patients after vaccination compared with DTH+ patients or normal donors (5.54 % ± 0.28; 1.65 % ± 0.19; and 1.72 % ± 0.22, respectively; p < 0.01) (Fig. 1a). Significantly, the Th3 population among DTH+ patients decreased to similar levels than healthy controls at the end of immunotherapy (Fig. 1a). Additionally, we evaluated the levels of TGF-β-free cytokine in each patient’s sera. We found that TGF-β levels in sera were similar in melanoma patients than in healthy donors. DTH− patients showed significant increase in the TGF-β levels post-immunotherapy (39 ng/mL ± 0.28 vs 42 ng/mL ± 0.17; p < 0.05) (Fig. 1b) but DTH+ patients did not show such an increase.
Fig. 1.
Th3 population is augmented in PBL from untreated melanoma patients compared with normal donors and decreased in DTH+ but not in DTH− patients after DCs vaccination. PBMC from 14 healthy donors (Healthy) were stained with anti-CD4 and anti-LAP-TGF-β antibodies and analyzed by flow cytometry. The obtained results were compared with Th3 proportion from 14 DTH+ patients and 8 DTH− patients before (untreated) and after a complete immunotherapy cycle (treated). Th3 percentage on healthy donors compared with melanoma untreated or treated patients. a Comparison of expression of Th3 percentage in PBMC between healthy donors, DTH− and DTH+ patients before (untreated) and after immunotherapy (treated). b Detection of free TGFβ in serum of DTH− and DTH+ patients was performed by ELISA assay. Statistical analyzes were performed using one-way ANOVA and unpaired Student’s t-test (**p < 0.01, ***p < 0.001)
CD4+ IFNγ+ T lymphocyte population is more potently induced after DCs vaccination in DTH+ than in DTH− melanoma patients
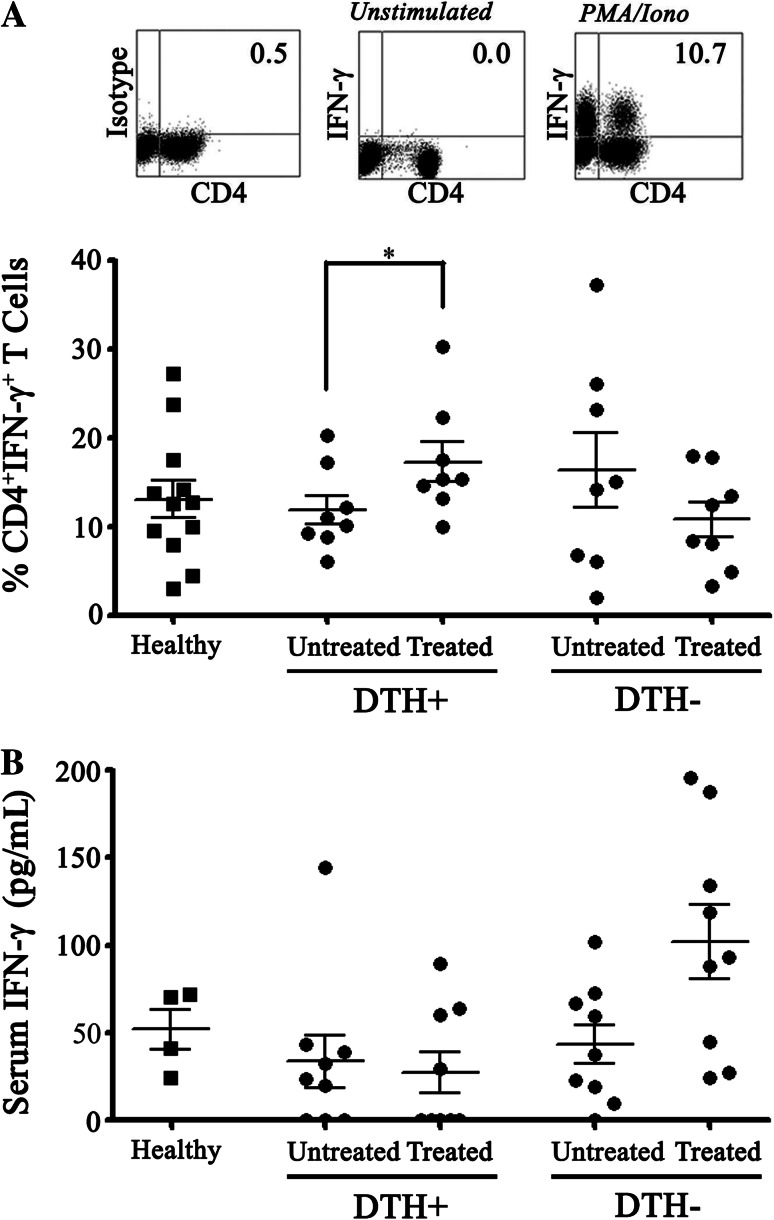
It is known that Th1 profile plays an important role in an effective immune response against tumor cells. Particularly, the central role of IFN-γ has been established in different models [23]. To determine the presence of IFN-γ producing T cell population in our patients, we evaluated the level of CD4+IFNγ+ T cells in PBMC of DTH+ and DTH− patients. First, we did not find significant differences in the proportions of Th1 cells between healthy donors and melanoma patients’ PBMC obtained before treatment (Fig. 2a). However, a significant increase in the percentages of CD4+IFN-γ+ T cells was observed in DTH+ patients after DCs treatment compared with pre-vaccination (11.98 % ± 1.60 before treatment, to 17.83 % ± 2.23 after treatment, p < 0.05) (Fig. 2a). A non-statistical reduction in the percentages of CD4+IFN-γ+ T cells was observed after vaccination in the DTH− group (Fig. 2a).
Fig. 2.
CD4+ IFNγ+ T lymphocyte population is more potently induced after DCs vaccination in DTH+ than in DTH− melanoma patients. PBMC from DTH+ and DTH− patients before (untreated) and after (treated) a complete immunotherapy cycle were activated with PMA/Ionomicyne, incubated with antibodies against CD4 and IFNγ and analyzed by flow cytometry. a Panel displaying density plots from one representative analysis of Th1 profile (upper). Th1 percentage based on CD4+ IFNγ+ T cells in DTH+ (untreated and treated, n = 8) and DTH− (untreated and treated, n = 8) patients. b An ELISA assay showing the IFNγ secretion in DTH− and DTH+ patients. Statistical analyzes were performed using paired Student’s t-test (*p < 0.05)
In addition, to determine whether the Th1 cell population induction observed in the responder patients correlated with systemic levels of IFN-γ, soluble levels of this cytokine in serum before and after the treatment were also evaluated. Our results showed that significant changes of IFN-γ in serum could not be detected between the different groups, before and after vaccination. The obtained values were 33.53 ng/mL ± 14.93 before treatment, 26.95 ng/mL ± 11.78 after treatment for DTH+ patients (p = 0.5625) and 47.56 ng/mL ± 11.88 before treatment, 89.87 ng/mL ± 20.07 after treatment for DTH− patients; (p = 0.0547) (Fig. 2b).
CD4+IL-17+ T lymphocyte population is more potently induced after DCs vaccination in DTH+ than in DTH− melanoma patients
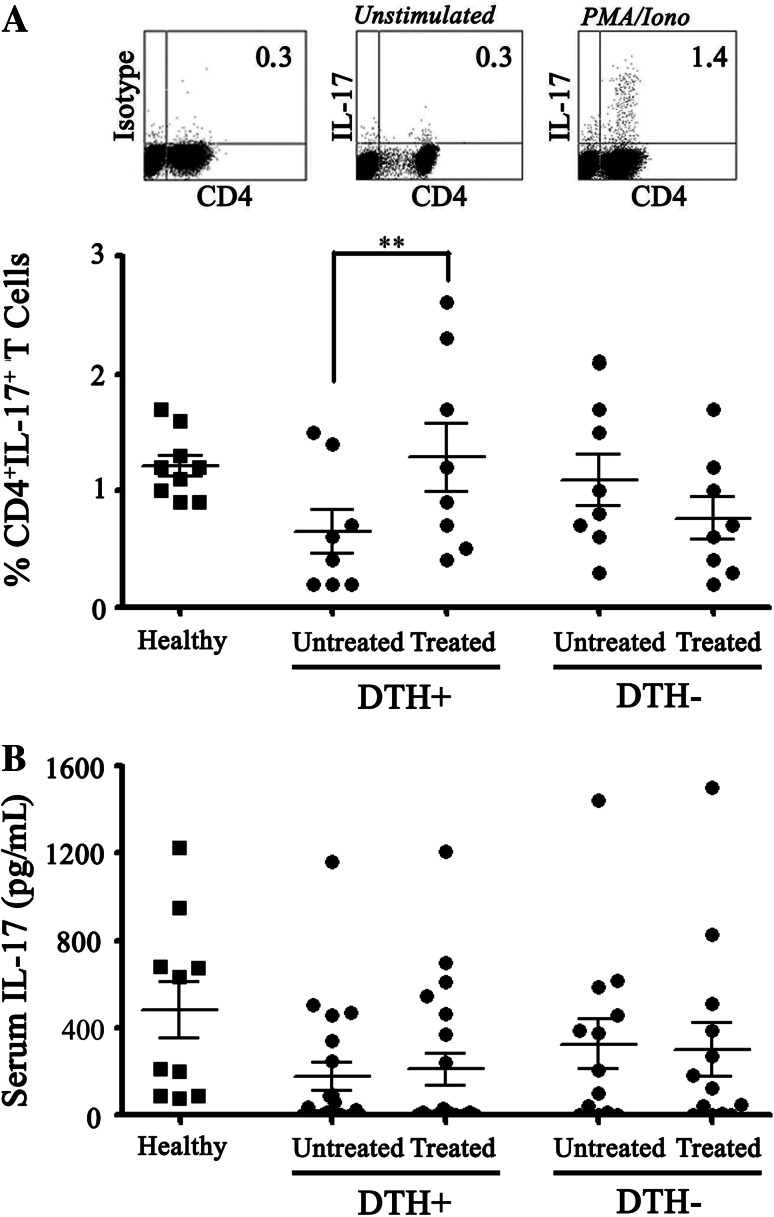
The involvement of the Th17 profile in the antitumor immune response induced by DCs vaccines is an important question that remains open. In this respect, several murine and human models have evaluated the IL-17 presence in tumors and PBLs to determine the role of Th17 population in the antitumor immune responses. Although still controversial, Th17 cells may apparently contribute to the elimination of tumor cells [27–31], a process that could be related to the induction of a pro-inflammatory tumor environment [42]. Based on that fact, we assessed the proportion of Th17 population in PBLs of vaccinated melanoma patients. In this regard, DTH+ patients, but not DTH− ones, showed an increase in CD4+IL-17+ T cells proportion after treatment (0.65 % ± 0.19 to 1.29 % ± 0.29, p < 0.01) (Fig. 3a). Additionally, IL-17 levels were evaluated on patient sera pre- and post-treatment, but no differences were observed between the different groups nor when compared with healthy controls (Fig. 3b).
Fig. 3.
CD4+IL-17+ T lymphocyte population is more potently induced after DCs vaccination in DTH+ than in DTH− melanoma patients. PBMC from DTH+ and DTH− patients before (untreated) and after (treated) a complete immunotherapy cycle were activated with PMA/Ionomicyne and analyzed by flow cytometry using antibodies against CD4 and IL-17. a Upper panel density plots from one representative analysis of Th17 profile. Lower panel Th17 percentage based on CD4+IL-17+ T cells in DTH+ (untreated and treated, n = 8), and DTH− (untreated and treated, n = 8) patients. b IL-17 ELISA assay in DTH− and DTH+ patients’ serum. Statistical analyzes were performed using paired Student’s t-test (*p < 0.05)
IFN-γ and IL-17 expressing CD4+ T lymphocytes are highly present in DTH biopsies from DCs-vaccinated patients
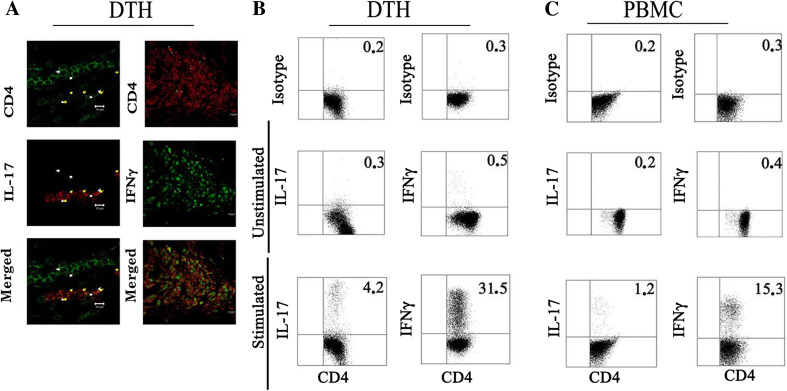
DTH reactions against tumor cell lysates post-vaccination have been associated to memory CD4+ and CD8+ T cell responses [13]. In fact, DTH biopsies from vaccinated patients included a proportion of CD4+CD45RO+ and CD8+CD45RO+ memory T cells [13]. To determine whether the melanoma lysate-specific T cell infiltration at DTH site detected in some patients may correlate with observations in peripheral blood, responder patients were challenged with TRIMEL in an arm proximal to axillary lymph nodes. We then evaluated the presence of pro-inflammatory cytokines associated with T cells in DTH tissues. Immunofluorescence analysis of paraffin-embedded DTH biopsies determined the effective co-expression of IL-17 and IFN-γ cytokines within the infiltrating CD4+ T cells (Fig. 4a).
Fig. 4.
IFN-γ and IL-17 expressing CD4+ T lymphocytes are highly present in DTH biopsies from DCs-vaccinated patients. Biopsy punches were obtained from the induration zone of immunotherapy-treated melanoma patients with positive DTH against TRIMEL. a A portion of the biopsy sample was used to analyze the CD4+ T cells and IFN-γ and IL-17 expression by immunofluorescence. The other half was mechanically disrupted and the obtained cells were expanded in RPMI medium supplemented with IL-2 for three weeks. In parallel, autologous PBMC were expanded at the same manner. IL-17 and IFNγ expression were induced by PMA and ionomycin in the presence of Brefeldin A for 5 h in b DTH-derived T cells and c PBMC-derived T cells, stained and analyzed by flow cytometry as indicated in “Materials and methods”. Percentages of double-positive cells were expressed on the upper right corner. Dot plot is representative of one of three patients
In order to identify the cytokine profile of CD4+ T cells derived from DTH biopsies expanded in vitro with a low-dose IL-2, CD4+IFNγ+, CD4+IL17+, and regulatory T cells were evaluated by flow cytometry after stimulation with PMA in the presence of Brefeldin A and compared with those present in IL-2 expanded autologous PBL. We were able to detect a fourfold augmented IL17-release of CD4+ T cell population in DTH-derived cells compared with autologous PBL (4.2 vs 1.2 %, respectively) and a doubled amount of IFN-γ in DTH-derived respect to PBL-derived T cells (31.5 vs 15.3 %) (Fig. 4b). In contrast, regulatory CD4+ T cell subpopulations, Tregs CD4+CD25+FOXP3+, Tr1 CD4+IL-10+, or TH3 CD4+TGFβ+ could not be observed in the analyzed DTH biopsies (data not shown).
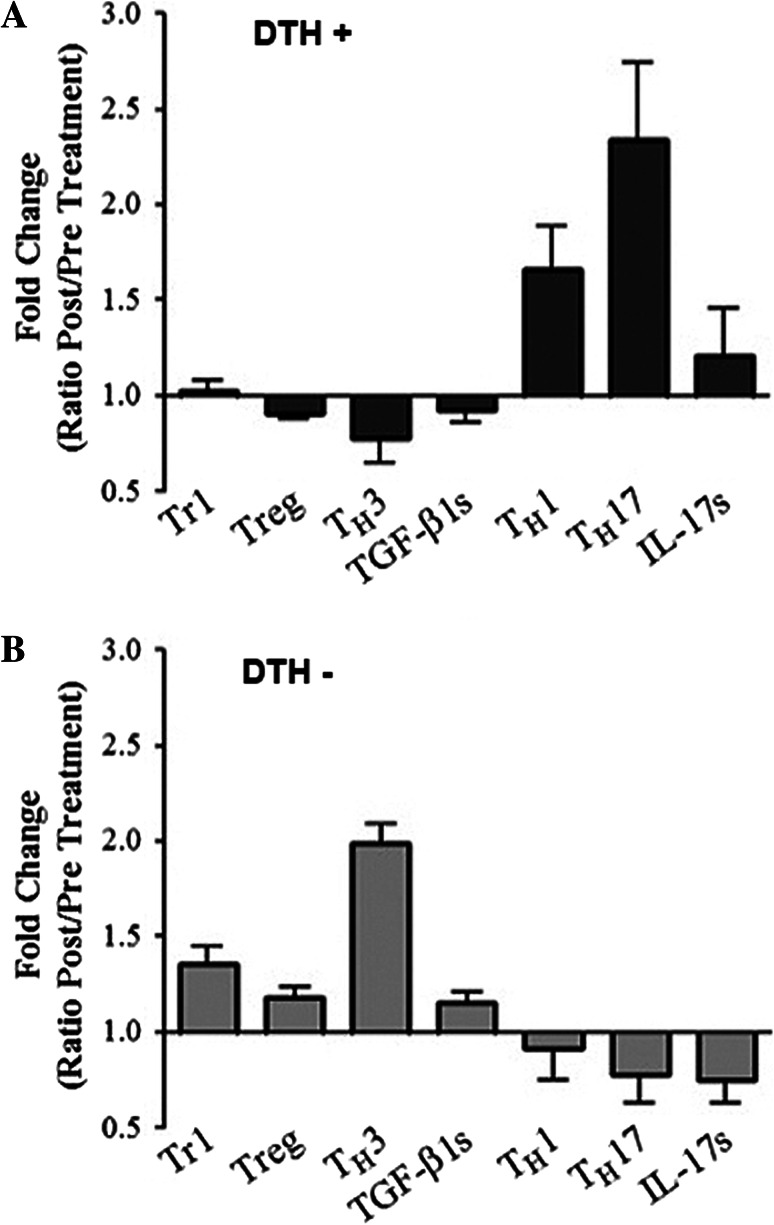
Th1 Th17 cytokine profiles are associated with DTH+ reactions and prolonged patient survival, while TH3 profiles are associated with immunological unresponsiveness
Finally, we investigated the T lymphocytes mean fold change in cytokine release as a ratio between pre- and post-vaccine values in the PBLs of DTH+ and DTH− patients. The analysis showed that DTH− patients have a significantly higher proportion of Tr1, Treg (foxp3+CD4+T cells), and Th3 (TGF-β-producing T cells) than DHT+ patients (Fig. 5a, b). Moreover, a minor but significant increased amount of soluble TGF-β could be detected in the PBLs of DTH− patients with respect to DTH+ ones (Fig. 5a, b). In contrast, a significantly increased proportion of Th1 (IFN-γ releasing CD4+ T cells) and Th17 (IL-17 releasing CD4+ T cells) could be detected in DTH+ patients compared with DTH− patients (Fig. 5a, b). These results allowed us to establish a correlation between DTH response and a defined pattern of specific cytokine responses in both patient subgroups (Fig. 5a, b).
Fig. 5.
Th1 and Th17 cytokine profiles are associated with DTH+ reactions and prolonged patient survival, while TH3 profile is associated with immunological unresponsiveness. Fold change ratio obtained calculating the difference between pre- and post-vaccine values for each patient for Tr1 (CD4+IL-10+), Treg (CD4+CD25+Foxp3+), TH3 (CD4+TGF-β+), TGF-βs (soluble TGF-β), Th1 (CD4+ IFN-γ+), Th17 (CD4+ IL-17+) are showed for a DTH+ and b DTH− patients. Statistical analyzes were performed using non-paired student’s t-test (p < 0.05, **p < 0.01, ***p < 0.001)
Discussion
DCs vaccines constitute a promising approach for the treatment of melanoma. Besides the clinical impact observed in some patients, primarily related to prolonged survival, immunizations are almost innocuous with very limited adverse effects. However, a major problem is related to the variability in clinical and immunological patient response to the treatments. In fact, several studies show that a proportion but not all patients are capable of developing immune or clinical responses that impact disease stability and survival [19, 20]. In this respect, we have previously shown that autologous DCs loaded with a conditioned allogeneic melanoma cell lysate induced tumor-specific immune responses associated with prolonged survival in a subgroup of stage IV melanoma patients [12, 13]. In fact, DTH immunological reactions correlated with a threefold prolonged long-term survival compared with DTH− patients [12, 13]. These differences can be due to several factors such as, the type of tumor, the patient genetic background and/or the immunological status of the patient. Herein, we investigated whether our DCs vaccine is associated with dissimilar cellular and cytokine responses that impact the clinical outcome. The analysis of T cell populations in PBLs obtained from melanoma patients before and after immunizations showed a significant increased proportion of IL-10 and TGF-β-producing T cells in non-responder patients compared with responder ones [12]. These results indicate that patients suffering metastatic disease may have imbalanced reactions between the tumor and the immune system generating distinct responses following vaccination. It is known that the immune system, while killing some tumor cells, modulates the transformation of the cancer cells evading the immune response and develop, at this turn, properties that affect the immune system interaction with tumors, in a process named immunoediting [43]. To investigate the capacity of PBLs from patients to generate effective immune responses, we measured CD4+ T cell capacity to produce IFN-γ and IL-17 upon in vitro stimulation. Our results showed that cells from DTH+ patients have an increased capacity to produce both cytokines compared with DTH− patients. Although differences in cytokine production were undetectable in serum, probably due to dilution, it was clear that DTH+ patients showed an increased proportion of CD4+ T cells producing IFN-γ, defined as a Th1 response. The Th1 response has been largely associated with an effective anti-tumor reaction relating to macrophage activation and particularly to CTL-mediated tumor lysis [44]. Interestingly, no difference was observed in the proportion of Th1 reactive cells before vaccination, indicating that the vaccine is capable of inducing a more potent Th1 response in the group of responder patients compared with DTH− ones. Importantly, similar behavior was observed when IL-17 producing T cells were studied. In fact, again, no difference was observed at serum levels, but an important increase in Th17 population was observed in DTH+ patients but not in DTH− patients. These results may indicate that IL-17-producing T cells are important in the vaccine-induced anti-tumor response and probably are also associated with the anti-melanoma DTH response. Although much evidence associates Th17 response with angiogenesis and pro-tumor activity, these observations may be related to the early stages in the tumor development [25, 26]. Accumulating evidence shows that Th17 may be part of the arsenal of anti-tumor responses maintaining its role in autoimmune reactions [27–31, 42]. Further analysis showed that a proportion of IFN-y/IL-17 producing CD4+ T cells can be detected in the blood of DTH+ patients. Our results suggest that Th1 and Th17 responses act concomitantly in vaccinated patients and they are not antagonic.
Regarding TGF-β producing T cells, we wanted to study whether the increased proportion of Th3 cells in non-responder patients correlated with the melanoma condition or whether they were only a product of vaccination. We could not detect differences at serum level between patients and normal donors, discarding the measuring of serum-associated cytokines as molecular markers of progression. However, our analysis showed that untreated stage IV melanoma patients have a significant augmented proportion of TGF-β producing CD4+ T cells (Th3 cells) compared with an equivalent control group of healthy donors. It has been demonstrated that TGF-β is over-expressed in melanoma patients compared with healthy donors and that increased levels can be induced by immunization with MAA-associated gp9639 [39]. Elevated concentration of TGF-β has been correlated with the specific inhibition of CTL and NK cell-mediated cytotoxicity [45].
Moreover, although no differences were observed between DTH+ and DTH− patients regarding Th3 populations before treatment, a significant decrease of Th3 proportion in blood was detected in DTH+ responder patients, while a potent augment of same cells was observed in DTH− patients. The correlation between melanoma-specific DTH reaction and the reduction of TGF-β-expressing T cells indicates that these cells are able to limit the immunological anti-melanoma response. Moreover, the level of Th3 cells in melanoma patients potentially constitutes an easy and relevant prediction factor for the outcome of DCs-based therapy.
DTH reaction against TRIMEL has been clearly associated with patient immune response and clinical survival [12, 13]. Control tests showed that the majority of vaccinated patients are immunocompetent because they can show DTH reactions against control antigens [12]. Moreover, we have previously demonstrated that DTH tissues were infiltrated with memory CD45+/CD4+ and CD45+/CD8+ T cells [13]. Here, we investigated the presence of Th17 and Th1 CD4+ T cells in DTH biopsies. Interestingly, immunofluorescense analysis revealed an amplified presence of IFN-γ and IL-17 producing CD4+ cells in the DTH infiltration compared with PBLs from the same patient. This result may further support that Th1/Th17 responses are clearly related to DTH reaction and are indirectly associated with prolonged patient survival.
Taken together, our results demonstrated that DTH+ and DTH− patients have a different reaction capacity and develop different immunological status that can be related to clinical outcome. In fact, DTH+ patients showed an increased capacity of generating a Th1/Th17 response compared with non-responder patient and also a significant reduction of TGF-β production and Th3 response. Although, as we recently demonstrated, genetic polymorphisms in genes related to the immune system like TLR4 can influence clinical outcome [46], the immune system/tumor interaction may also state the immune response generated by vaccines. However, the present work will contribute to an overall better understanding of different cellular and molecular factors associated with a more effective immune response and will help to develop new follow-ups and prognostic molecular marker tools based on reported immunological observations, which can be more appropriate for the concept of personalized medicine.
Acknowledgments
Grants from the National Fund for Scientific and Technological Development (FONDECYT 1090243, 1090238, 3090044), the Fund for the Promotion of Scientific and Technological Development (FONDEF DO5I10366), and the Millennium Science Initiative from the Ministry supported this work for the Economy, Development and Tourism (P09/016-F).
References
- 1.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17(11):3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 2.Di Lorenzo G, Buonerba C, Kantoff PW. Immunotherapy for the treatment of prostate cancer. Nat Rev Clin Oncol. 2011;8(9):551–561. doi: 10.1038/nrclinonc.2011.72. [DOI] [PubMed] [Google Scholar]
- 3.Ridgway D. The first 1000 dendritic cell vaccines. Cancer Invest. 2003;21:873–886. doi: 10.1081/CNV-120025091. [DOI] [PubMed] [Google Scholar]
- 4.Ardavin C, Amigorena S, Reis e Sousa C. Dendritic cells: immunobiology and cancer immunotherapy. Immunity. 2004;20:17–23. doi: 10.1016/S1074-7613(03)00352-2. [DOI] [PubMed] [Google Scholar]
- 5.Schadendorf D, Ugurel S, Schuler-Thurner B, Nestle FO, Enk A, Bröker EB, et al. DC study group of the DeCOG. Dacarbazine (DTIC) versus vaccination with autologous peptide- pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol. 2006;17(4):563–570. doi: 10.1093/annonc/mdj138. [DOI] [PubMed] [Google Scholar]
- 6.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuessel S, Meye A, Schmitz M, Zastrow S, Linné C, Richter K, et al. Vaccination of hormone-refractory prostate cancer patients with peptide cocktail-loaded dendritic cells: results of a phase I clinical trial. Prostate. 2006;66:811–821. doi: 10.1002/pros.20404. [DOI] [PubMed] [Google Scholar]
- 9.Escobar A, López M, Serrano A, Ramirez M, Perez C, Aguirre A, et al. Dendritic cell immunizations alone or combined with low doses of interleukin-2 induce specific immune responses in melanoma patients. Clin Exp Immunol. 2005;142(3):555–568. doi: 10.1111/j.1365-2249.2005.02948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palucka A, Ueno H, Connolly J, Kerneis-Norvell F, Blanck JP, Johnston DA, et al. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8 T-cell immunity. J Immunother. 2006;9:545–557. doi: 10.1097/01.cji.0000211309.90621.8b. [DOI] [PubMed] [Google Scholar]
- 11.Salcedo M, Bercovici N, Taylor R, Vereecken P, Massicard S, Duriau D, et al. Vaccination of melanoma patients using dendritic cells loaded with an allogeneic tumor cell lysate. Cancer Immunol Immunother. 2006;55(7):819–829. doi: 10.1007/s00262-005-0078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez MN, Pereda C, Segal G, Muñoz L, Aguilera R, González FE, et al. Prolonged survival of dendritic cell-vaccinated melanoma patients correlates with tumor-specific delayed type IV hypersensitivity response and reduction of tumor growth factor β-expressing T cells. J Clin Oncol. 2009;27(6):945–952. doi: 10.1200/JCO.2008.18.0794. [DOI] [PubMed] [Google Scholar]
- 13.Aguilera R, Saffie C, Tittarelli A, González FE, Ramírez M, Reyes D, et al. Heat shock induction of tumor-derived danger signals mediate rapid monocyte differentiation to clinically effective dendritic cells. Clin Cancer Res. 2011;17(8):2474–2483. doi: 10.1158/1078-0432.CCR-10-2384. [DOI] [PubMed] [Google Scholar]
- 14.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 15.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364(22):2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA- targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridolfi L, Petrini M, Fiammenghi L, Granato AM, Ancarani V, Pancisi E, et al. Dendritic cell-based vaccine in advanced melanoma: update of clinical outcome. Melanoma Res. 2011;21(6):524–529. doi: 10.1097/CMR.0b013e32834b58fa. [DOI] [PubMed] [Google Scholar]
- 18.Hales RK, Banchereau J, Ribas A, Tarhini AA, Weber JS, Fox BA, et al. Assessing oncologic benefit in clinical trials of immunotherapy agents. Ann Oncol. 2010;21(10):1944–1951. doi: 10.1093/annonc/mdq048. [DOI] [PubMed] [Google Scholar]
- 19.Bilusic M, Gulley JL. Endpoints, patient selection, and biomarkers in the design of clinical trials for cancer vaccines. Cancer Immunol Immunother. 2012;61(1):109–117. doi: 10.1007/s00262-011-1141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogino S, Galon J, Fuchs CS, Dranoff G. Cancer immunology-analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8(12):711–719. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4 (+) T cells in the antitumor immune response. J Exp Med. 1998;188(12):2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ossendorp F, Toes RE, Offringa R, van der Burg SH, Melief CJ. Importance of CD4(+) T helper cell responses in tumor immunity. Immunol Lett. 2000;74:75–77. doi: 10.1016/S0165-2478(00)00252-2. [DOI] [PubMed] [Google Scholar]
- 23.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54(8):721–728. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derhovanessian E, Adams V, Hähnel K, Groeger A, Pandha H, Ward S, et al. Pretreatment frequency of circulating IL-17+ CD4+ T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cáncer patients. Int J Cancer. 2009;125:1372–1379. doi: 10.1002/ijc.24497. [DOI] [PubMed] [Google Scholar]
- 25.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101(7):2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 26.Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175(9):6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 27.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112(2):362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci USA. 2008;105:15505–15510. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhodapkar KM, Barbuto S, Matthews P, Kukreja A, Mazumder A, Vesole D, et al. Dendritic cells mediate the induction of polyfunctional human IL17-producing cells (Th17–1 cells) enriched in the bone marrow of patients with myeloma. Blood. 2008;112:2878–2885. doi: 10.1182/blood-2008-03-143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inozume T, Hanada K, Wang QJ, Yang JC. IL-17 secreted by tumor reactive T cells induces IL-8 release by human renal cancer cells. J Immunother. 2009;32:109–117. doi: 10.1097/CJI.0b013e31819302da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127(4):759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 33.Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69(5):2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 34.Shen LS, Wang J, Shen DF, Yuan XL, Dong P, Li MX, et al. CD4(+)CD25(+)CD127(low/-) regulatory T cells express Foxp3 and suppress effector T cell proliferation and contribute to gastric cancers progression. Clin Immunol. 2009;131(1):109–118. doi: 10.1016/j.clim.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother. 2009;58(3):449–459. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wan YY, Flavell RA. Yin-Yang functions of transforming growth factor-β and T regulatory cells in immune regulation. Immunol Rev. 2007;220:199–213. doi: 10.1111/j.1600-065X.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGF beta. Nat Rev Immunol. 2010;10(8):554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobie JJ, Wu RS, Kurt RA, Lou S, Adelman MK, Whitesell LJ. Transforming growth factor beta inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res. 2003;63:1860–1864. [PubMed] [Google Scholar]
- 39.Kao JY, Gong Y, Chen CM, Zheng QD, Chen JJ. Tumor-derived TGF-β reduces the efficacy of dendritic cell/tumor fusion vaccine. J Immunol. 2003;170:3806–3811. doi: 10.4049/jimmunol.170.7.3806. [DOI] [PubMed] [Google Scholar]
- 40.Nam JS, Terabe M, Mamura M, Kang MJ, Chae H, Stuelten C, et al. An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res. 2008;68(10):3835–3843. doi: 10.1158/0008-5472.CAN-08-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, et al. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2007;117(5):1305–1313. doi: 10.1172/JCI30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilke CM, Bishop K, Fox D, Zou W. Deciphering the role of Th17 cells in human disease. Trends Immunol. 2011;32(12):603–611. doi: 10.1016/j.it.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quezada SA, Peggs KS, Simpson TR, Allison JP. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev. 2011;241(1):104–118. doi: 10.1111/j.1600-065X.2011.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13(2):95–109. doi: 10.1016/S1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 45.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, Berzofsky JA. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198(11):1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tittarelli A, González FE, Pereda C, Mora G, Muñoz L, Saffie C, García T, Díaz D, Falcón C, Hermoso M, López MN, Salazar-Onfray F (2012) Toll-like receptor 4 gene polymorphism influences dendritic cell in vitro function and clinical outcomes in vaccinated melanoma patients. Cancer Immunol Immunother. doi:10.1007/s00262-012-1268-7 (in press) [DOI] [PMC free article] [PubMed]