Abstract
Some anticancer chemotherapeutics, such as anthracyclines and oxaliplatin, elicit immunogenic apoptosis, meaning that dying cancer cells are engulfed by dendritic cells and tumor antigens are efficiently presented to CD8+ T cells, which control residual tumor cells. Immunogenic apoptosis is characterized by pre-apoptotic cell surface exposure of calreticulin (CRT), which usually resides into the endoplasmic reticulum. We investigated the ability of the n3-polyunsaturated fatty acid docosahexaenoic acid (22:6n3, DHA) to induce pre-apoptotic CRT exposure on the surface of the human PaCa-44 pancreatic and EJ bladder cancer cell lines. Cells were treated with 150 μM DHA for different time periods, and, by immunoblot and immunofluorescence, we showed that DHA induced CRT exposure, before the apoptosis-associated phosphatidylserine exposure. As for the known immunogenic compounds, CRT exposure was inhibited by the antioxidant GSH, the pan-caspase zVAD-FMK, and caspase-8 IETD-FMK inhibitor. We provide the first evidence that DHA induces CRT exposure, representing thus a novel potential anticancer immunogenic chemotherapeutic agent.
Keywords: Docosahexaenoic acid, Calreticulin, Immunogenic apoptosis, Immunochemotherapy
Introduction
Despite progress made in recent years in both cancer chemotherapy and immunotherapy, these therapeutic modalities alone have not provided satisfactory long-term clinical results for several types of tumors [1, 2]. Therefore, given the complexity of escape and survival for cancers to develop [3], most oncologists and immunologists have reached the idea that no single chemotherapy or immunotherapy will be effective for treating cancer, at least some cancers, and that chemotherapeutic-mediated tumor cell killing and antitumor immune response can synergize for optimal therapeutic success [1–4]. It has been recently shown that in response to radiotherapy and some chemotherapeutic agents, such as anthracyclines and oxaliplatin, tumor cells undergo immunogenic apoptosis [2]. This means that tumor cells undergo phenotypic changes acting as danger signals (i.e. “eat me” signals) to dendritic cells, which efficiently engulf portions of dying cells and efficiently present tumor antigens to CD8+ T cells, triggering thus a protective antitumor immune response. Such immune response plays a major role in the therapeutic success [4]. Chemotherapeutic agents inducing immunogenic apoptosis are designated as immunogenic chemotherapeutics. In contrast, chemotherapeutic agents incapable of inducing immunogenic apoptosis, such as alkylating agents and cisplatin (CDDP), induce a nonimmunogenic (as opposed to immunogenic) apoptosis and fail to elicit such immune response. The crucial molecular difference between immunogenic and nonimmunogenic apoptosis is that immunogenic cell death is accompanied by the pre-apoptotic cell surface exposure of calreticulin (CRT), a molecule that usually resides in the lumen of the endoplasmic reticulum (ER) [5–7]. Although the exact molecular mechanism mediating the translocation of intracellular CRT to the cell surface is not fully understood, the sequential involvement of ER stress, caspase activation, and exocytosis has been reported [7]. Overall, these data have major implications for tumor immunology, encouraging scientists to discover new anticancer compounds capable of inducing CRT exposure to expand the number of chemotherapeutics endowed with the potential to elicit a CRT-dependent antitumor immune response.
Dietary n3-polyunsaturated fatty acids, such as docosahexaenoic acid (22:6n3, DHA), mostly found in oily cold-water fish, have been shown to exert anticancer activity, representing thus an emerging class of novel potential anticancer agents [8]. DHA inhibits the incidence and development of certain tumors [8] and increases the efficacy of anticancer radio- and chemo-therapies [9–12]. Different biological activities exerted by DHA have been proposed to underlie these effects. DHA is known to promote apoptosis in a variety of cancer cells in vitro and in vivo [8, 13], including pancreatic carcinoma cells [14, 15].
In this study, we investigated whether DHA is capable of inducing immunogenic cell death in the human PaCa-44 pancreatic and EJ bladder carcinoma cell lines via pre-apoptotic CRT exposure.
Materials and methods
Cells and treatments
PaCa-44 pancreatic and EJ bladder carcinoma cell lines, provided by Prof. A. Scarpa (University of Verona, Italy) and purchased from ATCC (Rockville, MD), respectively, were cultured in RPMI-1640 supplemented with 10% FCS, 2 mM l-glutamine, 100 IU/ml penicillin, and 10 mg/ml streptomycin at 37°C under 5% CO2. Cells were treated with 150 μM DHA (Sigma) or 10 μM CDDP (Sigma) for the indicated periods. In some experiments, cells were pre-incubated with the antioxidant GSH (5 mM; Sigma) or caspase inhibitors zVAD-FMK (100 μM; Calbiochem) and IETD-FMK (100 μM; BioVision).
Apoptosis
Apoptosis was assessed by annexin V-FITC (Bender Medsystems) and propidium iodide (PI) [15].
Cell surface protein isolation
Cell surface proteins were biotinylated and isolated using the pinpoint cell surface protein isolation kit (Pierce), according to manufacturer’s instructions.
Immunoblot
Total cell lysates were obtained using a buffer containing 50 mM TRIS–HCl pH 7.6, 150 mM NaCl, 0.5% TRITON X-100, 0.5% Na deoxycolate, 0.1% SDS, and protease inhibitors. Cell surface proteins and total cell lysates were separated by SDS–PAGE, blotted into nitrocellulose (Schleicher and Shyell GmbH), incubated with anti-CRT (MBL International) or anti-β-actin Ac-40 antibody. The reaction was revealed by HRP-secondary antibody (Pierce) and detected by ECL (Amersham).
Flow cytometry
Cells were incubated with anti-CRT or isotype control antibody, then with FITC-secondary antibody, and analyzed by FACSCalibur using CELLQuest.
Statistics
Student’s t test was used for all analyses; P < 0.05 was considered significant.
Results
DHA induces pre-apoptotic CRT exposure in cancer cells
To investigate whether DHA induces CRT exposure on cancer cell surface before the cell manifests apoptosis, we initially determined the kinetics of DHA-induced apoptosis in the PaCa-44 cell line. To this purpose, PaCa-44 cells were incubated with 150 μM DHA dissolved in ethanol solution or with ethanol solution alone for 6, 12, 18, 24, 48, and 72 h, and apoptosis was assessed by immunofluorescence using the phosphatidylserine (PS)-binding annexin V-FITC and the vital dye PI. As shown in Fig. 1a, ~2.2% of cells exhibited apoptosis-associated PS exposure until 24 h of DHA treatment, whereas apoptosis appeared in ~20 and ~54% of PaCa-44 cells at 48 and 72 h, respectively. Therefore, we studied the kinetics of CRT exposure until 24 h of DHA treatment, i.e., before DHA-induced apoptosis. CRT exposure was monitored in plasma membrane proteins and in total cell lysates by immunoblot analysis. As shown in Fig. 1b, a band of 63 kDa corresponding to CRT appeared in cell surface proteins at 24 h of DHA treatment. Moreover, CRT exposure was not accompanied at anytime by a rise of total cell CRT (Fig. 1b). The exposure of CRT to the cell surface was confirmed by immunofluorescence staining and flow cytometry of DHA-treated live PaCa-44 cells (Fig. 1c). We also analyzed DHA-induced CRT exposure in another cancer cell model, such as the EJ bladder cancer cell line. We found that DHA induced CRT exposure in EJ cells and, as for Paca-44 cells, CRT exposure appeared at 24 h of DHA treatment (Fig. 1e), before the apoptosis-associated PS exposure at 48 h (Fig. 1d). As a negative control, Paca-44 and EJ cells were treated with CDDP, which has been reported to exert no CRT-exposing effect (4). According to the literature, CDDP did not induce CRT exposure in both tumor cell lines (Fig. 1a, c–e).
Fig. 1.
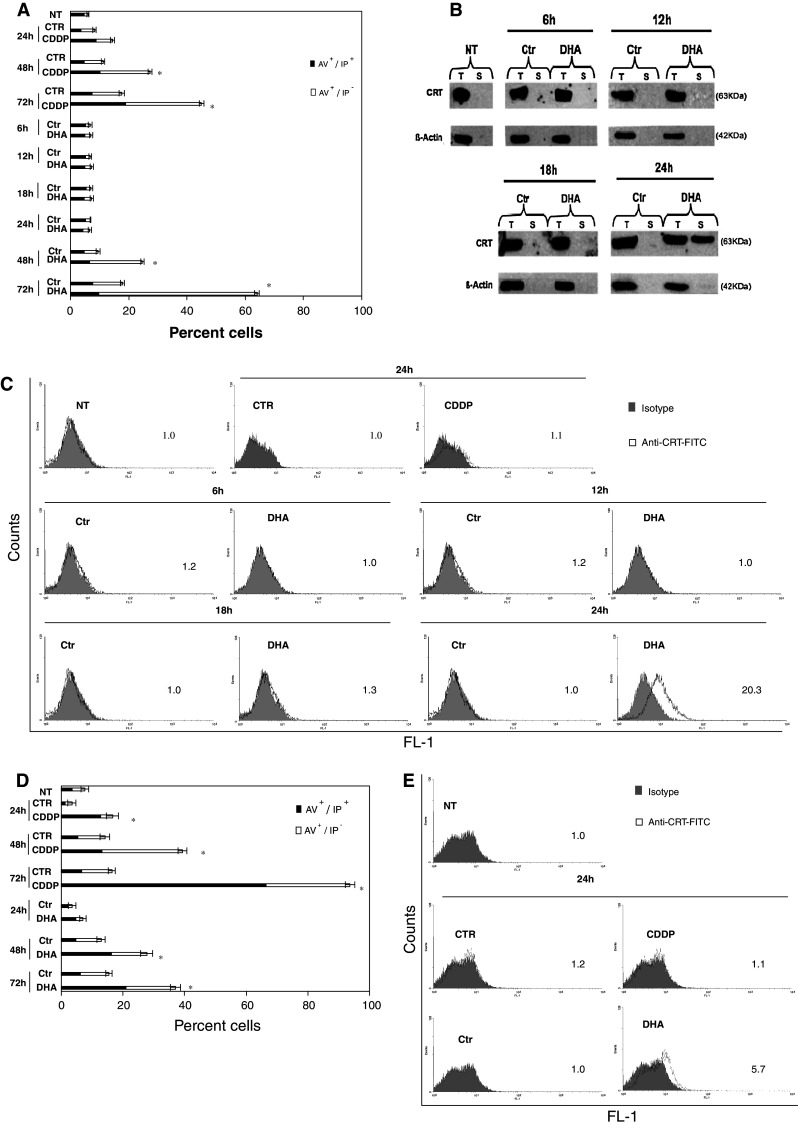
DHA induces pre-apoptotic CRT exposure. PaCa-44 and EJ cells were incubated with 150 μM DHA or, as negative control, with 10 μM CDDP for the indicated period, followed by: a immunofluorescence staining of PaCa-44 cells using annexin V-FITC (AV)/iodure propide (IP) and cytofluorimetry; results are expressed as the mean ± SD of three experiments; b immunoblot using anti-CRT antibody in total cell lysates (T) or surface proteins (S) of PaCa-44 cells; β-actin was used as both loading and intracellular protein control; representative experiment out of three; c immunofluorescence and flow cytometry on viable PaCa-44 cells using anti-CRT or isotype control antibody; numbers indicate mean fluorescence intensity (MFI) ratio, calculated as the ratio between MFI of positive cells and MFI of control; representative experiment out of three; d immunofluorescence staining of EJ cells using AV/IP and cytofluorimetry; results are expressed as the mean ± SD of three experiments; e immunofluorescence and flow cytometry on viable EJ cells using anti-CRT or isotype control antibody; numbers indicate MFI ratio; representative experiment out of three. NT not treated, CTR control for CDDP: PBS alone, CDDP CDDP dissolved in PBS, Ctr control for DHA: ethanol solution alone, DHA DHA dissolved in ethanol solution. *P < 0.05
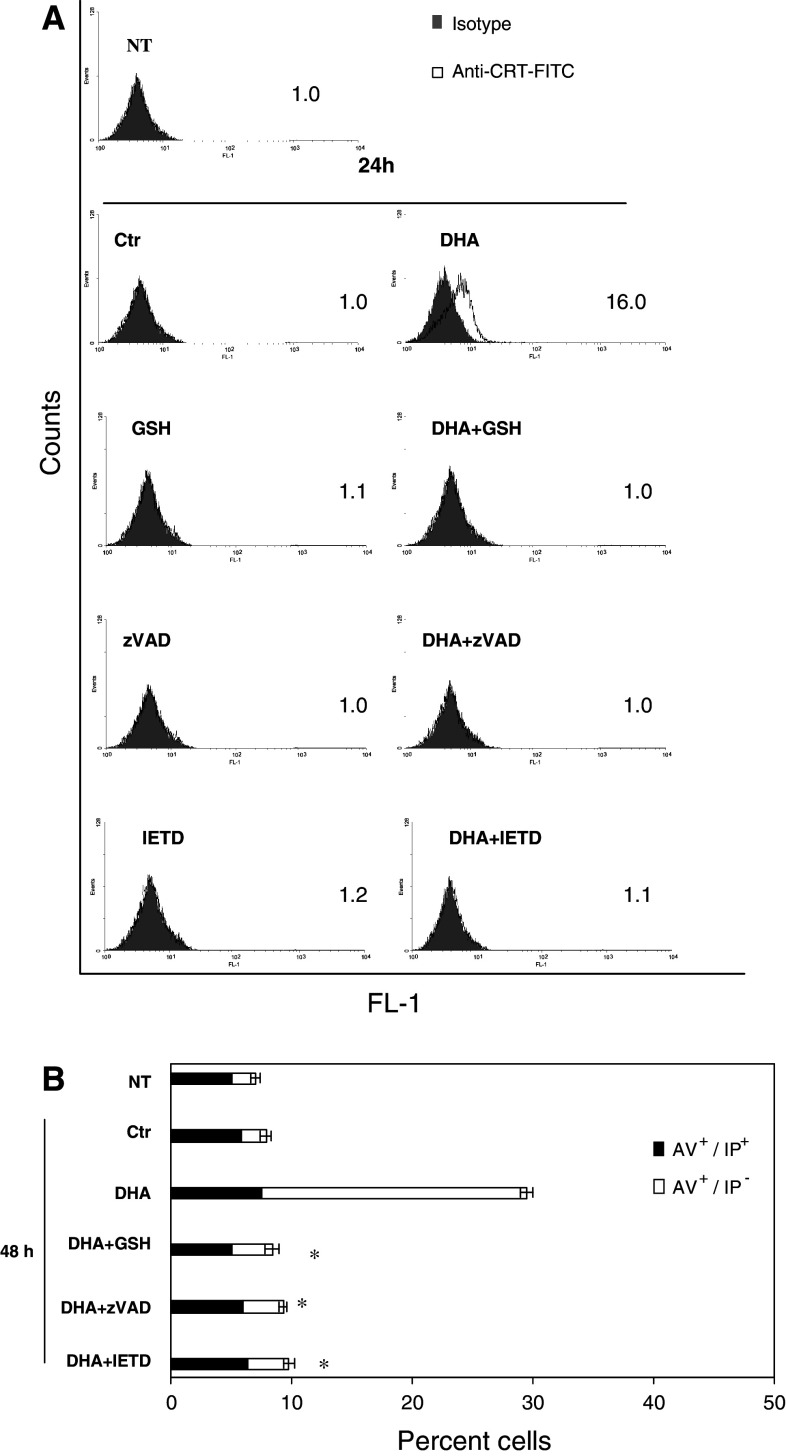
Reactive oxygen species and caspases mediated the exposure of CRT induced by DHA
Reactive oxygen specie (ROS) and caspases, in particular caspase-8, are implicated in antracyclin- and oxiplatin-induced CRT exposure [7]. To investigate the relevance of these molecules in DHA-induced CRT exposure, PaCa-44 cells, treated with 150 μM DHA for 24 h, were pre-incubated with the antioxidant GSH, the pan-caspase zVAD-FMK or caspase-8 IETD-FMK inhibitor, and CRT exposure was analyzed by immunofluorescence and flow cytometry. As shown in Fig. 2a, both oxidative stress and caspase (including caspase-8) inhibition prevented the CRT-exposing activity of DHA. We also showed that both ROS and caspases, including caspase-8, are also implicated in DHA-induced apoptosis (Fig. 2b).
Fig. 2.
DHA-induced CRT exposure is ROS- and caspase-dependent. PaCa-44 cells, treated with 150 μM DHA for the indicated period, were pre-incubated with antioxidant GSH, pan-caspase zVAD-FMK or caspase-8 IETD-FMK inhibitor, and immunofluorescence and flow cytometry was performed using: a anti-CRT or isotype control antibody; numbers indicate MFI ratio; representative experiment out of three; b annexin V-FITC (AV) and iodure propide (IP); results are expressed as the mean ± SD of three experiments. NT not treated, Ctr control: ethanol solution alone, DHA DHA dissolved in ethanol solution. *P < 0.05
Discussion
In this study, we provide the first evidence that DHA induces pre-apoptotic exposure of CRT on the surface of cancer cells. Since only a few (anthracyclins and oxaliplatin) cytotoxic chemotherapeutics exert CRT-exposing activity [5–7], our study have major implications for tumor immunology, expanding thus the number of immunogenic anticancer agents endowed with the potential to elicit a CRT-dependent antitumor immune response.
Here, we showed that DHA induced CRT exposure at 24 h, preceding hence DHA-induced apoptosis at 48 h. Indeed, as for the other immunogenic chemotherapeutics [5–7], we observed a dissociation between CRT and PS exposure, confirming that CRT exposure occurs upstream of apoptosis as part of a specific danger-signaling system. Moreover, according to the mechanisms reported for the known CRT-exposing compounds [5–7], CRT translocation by DHA occurred in the absence of a general increase in the abundance of intracellular CRT and was dependent on both ROS production and caspase activation, including caspase-8. As previously reported by others and us [14–16], both ROS and caspases, in particular caspase-8, are also implicated in DHA-induced apoptosis, meaning that, although CRT exposure occurs at a pre-apoptotic stage, the immunogenic and the lethal response could be coupled at these levels.
Several studies have documented that dietary DHA increases the efficacy of anticancer radio- and chemo-therapies [9–12]. In particular, DHA improves the effects of proven immunogenic as well as nonimmunogenic therapies. Although different properties of DHA have been proposed to underlie this activity, the mechanisms remain still unclear. Based on our present data, we might suppose that DHA potentiates or induces a CRT-dependent antitumor immune response in immunogenic or nonimmunogenic therapeutic regimens, respectively.
In conclusion, DHA induces CRT exposure in tumor cells, representing thus another immunogenic pro-apoptotic compound endowed with the potential to elicit a CRT-dependent antitumor response. Future functional studies are needed to test this hypothesis and to explore the potential usefulness of DHA as immunogenic adjuvant or therapeutic agent in cancer therapy.
Acknowledgments
This study was supported by a grant from “Ministero delle Politiche Agricole Alimentari e Forestali,” the “Agricultural and food-biodiversity” project. We thank Dr. Lara Costantini for skillful assistance.
Conflict of interest
We do not have any financial/commercial conflict of interest.
References
- 1.Lake RA, Robinson WS. Immunotherapy and chemotherapy—a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 2.Haynes NM, van der Most RG, Lake RA, Smyth MJ. Immunogenic anti-cancer chemotherapy as an emerging concept. Curr Opin Immunol. 2008;20:545–571. doi: 10.1016/j.coi.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 4.Zitvogel L, Kepp O, Senovilla L, et al. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010;16:3100–3104. doi: 10.1158/1078-0432.CCR-09-2891. [DOI] [PubMed] [Google Scholar]
- 5.Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 7.Panaretakis T, Kepp O, Brockmeier U, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28:578–590. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 9.Colas S, Paon L, Denis F, et al. Enhanced radiosensitivity of rat autochthonous mammary tumors by dietary docosahexaenoic acid. Int J Cancer. 2004;109:449–454. doi: 10.1002/ijc.11725. [DOI] [PubMed] [Google Scholar]
- 10.Bougnoux P, Hajjaji N, Ferrasson MN, et al. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: a phase II trial. Br J Cancer. 2009;101:1978–1985. doi: 10.1038/sj.bjc.6605441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley MO, Webb NL, Anthony FH, et al. Tumor targeting by covalent conjugation of a natural fatty acid to paclitaxel. Clin Cancer Res. 2001;7:3229–3238. [PubMed] [Google Scholar]
- 12.El-Mesery M, Al-Gayyar M, Salem H, et al. Chemopreventive and renal protective effects for docosahexaenoic acid (DHA): implications of CRP and lipid peroxides. Cell Div. 2009;4:6–23. doi: 10.1186/1747-1028-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serini S, Piccioni E, Merendino N, Calviello G. Dietary polyunsaturated fatty acids as inducers of apoptosis: implications for cancer. Apoptosis. 2009;14:135–152. doi: 10.1007/s10495-008-0298-2. [DOI] [PubMed] [Google Scholar]
- 14.Merendino N, Molinari R, Loppi B, Pessina G, D’ Aquino M, Tomassi G, Velotti F. Induction of apoptosis in human pancreatic cancer cells by docosahexaenoic acid. Ann NY Acad Sci. 2003;1010:361–364. doi: 10.1196/annals.1299.143. [DOI] [PubMed] [Google Scholar]
- 15.Merendino N, Loppi B, D’Aquino M, Molinari R, Pessina G, Romano C, Velotti F. Docosahexaenoic acid induces apoptosis in the human PaCa-44 pancreatic cancer cell line by active reduced glutathione extrusion and lipid peroxidation. Nutr Cancer. 2005;52:225–233. doi: 10.1207/s15327914nc5202_12. [DOI] [PubMed] [Google Scholar]
- 16.Kang KS, Wang P, Yamabe N, et al. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase-8 activation. PLoS One. 2010;5:e10296. doi: 10.1371/journal.pone.0010296. [DOI] [PMC free article] [PubMed] [Google Scholar]