Abstract
Carcinoembryonic antigen (CEA) is expressed during embryonic life and in low level during adult life. Consequently, the CEA is recognized by the immune system as a self-antigen and thus CEA-expressing tumors are tolerated. Previously, we constructed a single chain variable fragment using the 6.C4 (scFv6.C4) hybridoma cell line, which gave rise to antibodies able to recognize CEA when C57/Bl6 mice were immunized. Here, the scFv6.C4 ability to prevent the CEA-expressing tumor growth was assessed in CEA-expressing transgenic mice CEA2682. CEA2682 mice immunized with the scFv6.C4 expressing plasmid vector (uP/PS-scFv6.C4) by electroporation gave rise to the CEA-specific AB3 antibody after the third immunization. Sera from immunized mice reacted with CEA-expressing human colorectal cell lines CO112, HCT-8, and LISP-1, as well as with murine melanoma B16F10 cells expressing CEA (B16F10-CEA). Cytotoxic T lymphocytes (CTL) from uP/PS-scFv6.C4 immunized mice lysed B16F10-CEA (56.7%) and B16F10 expressing scFv6.C4 (B16F10-scFv6.C4) (46.7%) cells, against CTL from uP-immunized mice (10%). After the last immunization, 5 × 105 B16F10-CEA cells were injected into the left flank. All mice immunized with the uP empty vector died within 40 days, but uP/PS-scFv6.C4 vaccinated mice (40%) remained free of tumor for more than 100 days. Splenocytes obtained from uP/PS-scFv6.C4 vaccinated mice showed higher T-cell proliferative activity than those from uP vaccinated mice. Collectively, DNA vaccination with the uP-PS/scFv6.C4 plasmid vector was able to give rise to specific humoral and cellular responses, which were sufficient to retard growth and/or eliminate the injected B16F10-CEA cells.
Keywords: DNA vaccine, Single-chain fragment variable, Anti-idiotypic antibody, Immunological tolerance, Carcinoembryonic antigen, Colorectal cancer
Introduction
Human gastrointestinal tract neoplasms as well as non-small-cell lung and breast carcinomas produce elevated levels of the tumor-associated antigen (TAA) carcinoembryonic antigen (CEA), which is a densely glycosylated 180-kDa membrane-bound protein [1, 2]. The CEA is produced at high levels in the fetal colon, but its production in the normal adult colonic epithelium is reduced to trace levels [1]. As CEA is expressed during the fetal development, it is considered a self-antigen. However, significant levels of autoantibodies against CEA were detected in the sera from some patients with CEA-producing tumors [3–7], and this is an important evidence that even the CEA self-antigen can activate the immune system in some conditions giving rise to humoral and cellular responses.
One way to combat CEA-expressing tumors is stimulating the immune system with a CEA epitope mimicking molecules. Such mimicries may be created by immunizing animals with the anti-CEA monoclonal antibodies (mAb) to generate anti-idiotypic (Id) antibodies, which may have similar three-dimensional structures [8–11].
In many animal models, the specific and efficient protective immunity against CEA-producing tumors was observed after vaccination with anti-Id antibodies [12–16]. Chatterjee and co-workers advanced their anti-Id-based vaccines to treat resected colon-cancer patients with an anti-Id monoclonal antibody in the protein form. In all patients (32) followed in this study, a consistent anti-CEA humoral and cellular immune response was observed [17]. Similar protective immunity against other tumor models had also been observed in both preclinical studies and clinical trials [18–21].
We have described an anti-Id monoclonal antibody, designated mAb 6.C4, which was able to mimic CEA functionally. The anti-Id mAb 6.C4 was shown to elicit antibodies that recognized CEA in vitro and in vivo [11, 22]. Using the mAb 6.C4 producer hybridoma, the variable heavy and light chain (scFv) sequences were isolated to construct the scFv6.C4 [13]. C57BL/6 mice immunized with a plasmid vector expressing scFv6.C4 were able to elicit antibodies that recognize CEA.
In this study, we immunized CEA-expressing transgenic mice, CEA2682, [23] with scFv6.C4 to assess its protective activity against CEA-expressing tumor cells.
Materials and methods
Research Ethics Committee approval
All animal work described here was performed in full compliance with the institutional guidelines and was approved by the Research Ethics Committee of the Universidade Federal de São Paulo, Brazil (http://www.unifesp.br/reitoria/ceua; Approval number: CEP 2129/08).
Construction of vectors
To construct the uP/PS-scFv6.C4 plasmid vector, pcDNA3-PS/scFv6.C4 [13] was digested with Hind III and Xba I to release the scFv6.C4, which was inserted into the uP vector [24], previously treated with the same enzymes.
To construct the Sleeping Beauty (SB)-based integrative vector pT2-CAGGS-CEA, the CEA fragment was obtained by digesting pRC/CMV-CEA ([25], which was kindly provided by Dr. Wolfgang Zimmermann, University of Munich, Munich, Germany) with Hind III and Xba I and treated with Klenow polymerase. The CEA fragment was inserted into the pT2-IDUA vector [26], which was previously digested with Ava I (to remove the IDUA fragment) and then treated with Klenow polymerase to make the pT2-CAGGS-CEA vector.
Cell culture, transfection and characterization
Human colorectal carcinoma cell lines CO112 [27], HCT-8 [28] and LISP-1 [29] and murine melanoma cell line B16F10 [30] were maintained (37 °C; 5% CO2 humidified atmosphere) in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen, Grand Island, NY, USA), l-glutamine (2 mM), penicillin (100 units/ml), streptomycin (100 µg/ml) and buffered with sodium bicarbonate (24 mM) plus 2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid (HEPES) buffer (10 mM). The supplemented medium was named RPMIc.
To promote permanent expression of CEA or green fluorescent protein (GFP) in B16F10 cells, 5 × 105 cells in Nucleofector solution (100 µl) containing pT2-CAGGS-CEA (4 µg) and pCMV-SB100 (4 µg), or pT2-HB-AMAXA-GFP (4 µg) and pCMV-SB100 (4 µg) were transferred into a cuvette and submitted to the U008 (Amaxa Nucleofector II Device; Lonza, Basel, Switzerland) program. The transformed cells were named with basis on the transferred genes B16F10-CEA or B16F10-GFP. The pRC/CMV-CEA and pEGFP-N3 (Clontech; Mountain View, CA, USA) vectors were used as transient controls, being nucleofected as described above. The nucleofected cells were maintained in RPMIc and incubated at 37 °C in a humidified incubator with 5% CO2. Gene expression was analyzed at different times after transfection.
Vector integration analysis by polymerase chain reaction (PCR)
CEA cDNA integration into the cell genome was assessed by PCR using the following primers (final concentration: 0.4 mM): 5′-CATTTGCAACAGCTACAGTC-3′ and 5′-AGTGCAGTGGTATCAGAAAC-3′. The thermocycler was programmed to 94 °C (1 min) and 30 cycles of 94 °C (1 min), 58 °C (1 min) and 72 °C (1 min). After the final cycle, the reaction continued (72 °C; 7 min) and the reaction product was then maintained at 4 °C. As an internal control of reaction, the glyceraldehyde-3-phosphate dehydrogenase housekeeping gene was used with the following primers: 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′. The PCR products were analyzed by electrophoresis (1% agarose gel with ethidium bromide).
Immunocytochemistry of HCT-8, CO112, LISP-1 and B16F10-CEA cells
Cells were seeded on the coverslips placed in a 24-well plate and incubated in a humidified chamber with 5% CO2 for 24 h. The media were aspirated, and the cells were fixed in paraformaldehyde (4%; 1 h). After washing with phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4; pH 7.2), NH4Cl (50 mM; pH 8.0) was added and the plate was left for 15 min. Cells were washed again with PBS, before permeabilization (10 min) with PBS containing 0.5% Triton X-100 (PBS-Triton). After new washings, the cells were blocked (30 min) with PBS containing bovine serum albumin (BSA; 10%) and non-fat milk (8%). Cells were incubated (4 °C; humidified chamber; overnight) with diluted (1:50) sera from uP-PS/scFv6.C4 immunized mice. Then, the coverslips were washed with PBS containing Tween-20 (0.25%; PBS-Tween) and incubated for 1 h with diluted biotinylated secondary antibody rabbit anti-mouse IgG (Dako; 1:100).
All coverslips were washed with PBS-Tween. The coverslips coated with HCT-8, CO112, or LISP-1 cells were incubated for 1 h with diluted Alexa 594-streptavidin (Invitrogen; 1:1000). After washings, cell nuclei were labeled during 15 min with diluted 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen; 1:1000). In the case of coverslips coated with B16F10-CEA cells, the coverslips were incubated for 1 h with diluted HRP-streptavidin (Dako; 1:500), and the reaction was developed in a dark chamber at room temperature for 10 min with AEC solution (0.5 mg/ml; Sigma) and H2O2 (0.01%) in acetate buffer (0.05 M sodium acetate; pH 5.5). In both cases, at the end, coverslips were rinsed with distilled water and mounted with Fluoromount aid (Sigma).
Quantification of CEA-specific antibody response
Sera from vaccinated mice were evaluated for anti-CEA antibodies (AB3 = AB1′) by ELISA. For this purpose, one hundred microliters of a solution containing 0.5 µg/ml of CEA (Sigma–Aldrich, Germany) in PBS was added to each well of 96-well plates and maintained at 37 °C for 1 h. Plates were blocked with 1% (w/v) BSA (Sigma–Aldrich) in PBS (PBS-BSA) at room temperature for 1 h. The mouse sera diluted in PBS-BSA 0.1% (1:100) were added and incubated at 37 °C for 2 h. The wells were washed three times with PBS containing 0.05% Tween-20 (PBS-Tween), and anti-IgG murine antibody conjugated with biotin (Sigma–Aldrich) diluted in PBS-BSA 0.1% (1:5000) was added and incubated for 2 h at 37 °C. After new washings, horseradish peroxidase-streptavidin (Dako, Carpinteria, CA, EUA) diluted in PBS-Tween (1:1000) was added and incubated for 30 min at room temperature in a dark chamber. The wells were washed as described before adding the ortho-phenylenediamine (3 mg/ml) in 50 mM citrate–phosphate buffer (51.4 mM Na2HPO4, 24.3 mM acetic acid, pH 5.0) containing 0.03% H2O2. The reactions were stopped with 2 N H2SO4. The optical density at 492 nm was read in an ELISA reader (Spectra Max M2e, Molecular Devices, EUA). Triplicates of each sample were performed.
Mouse tumor model
Human CEA-transfected B16F10 cells (5 × 103–5 × 105 cells per mouse and 3 mice per group) were injected (left flank; subcutaneous route) into CEA-expressing transgenic mice (CEA2682; kindly donated by Wolfgang Zimmermann; University of Munich; Munich, Germany) [23], and the tumor size was periodically measured with a caliper during 40 days. If a tumor reaches 1000 mm3, this mouse is euthanized.
Preventive DNA vaccination and tumor cell challenge
CEA2682 mice were immunized by injection of 50 µg of uP-PS/scFv6.C4 or uP plasmid DNA in 50 µl PBS at each quadriceps muscle. Six electric pulses (100 V, 40 ms duration per pulse, 1 s interval) were applied through needle electrodes (Electroporator T820—BTX Genetronics, San Diego, CA, EUA) that were placed around DNA injection site. Four subsequent immunizations were performed with 2-week intervals. Blood samples were collected 7 days after each immunization to quantify anti-anti-idiotypic (anti-anti-Id or AB3) antibody production. Fifteen days after the last immunization, some mice were euthanized to obtain spleens for assessment of cellular response by T-cell proliferation and cytotoxicity assays. To assess the protective effect of the vaccination, mice were challenged with s.c. injection of 5 × 105 B16F10-CEA cells in the left flank, 10 days after the last immunization. Immediately after challenge, mice received a new boost with the same immunization dose. All animals were euthanized 15 days after tumor cell injection. Tumor volume was estimated according to the following formula: V = A × B × C (A: length, B: width, C: height) and expressed in mm3.
In vitro T-cell proliferation assay
Fifteen days after the B16F10-CEA injection, splenocytes were collected and treated with the ammonium chloride potassium buffer (0.15 M NH4Cl; 10 mM KHCO3; 0.1 mM disodium, EDTA; pH 7.4) to lyse the red blood cells. For the proliferation assay, 7 × 106 splenocytes suspended in PBS (140 µl) were labeled at 37 °C for 15 min with 2.5 µM carboxyfluorescein diacetate succinimidyl ester (CFSE) (CellTrace™; CFSE Cell Proliferation Kit; Invitrogen; Carslbad, CA, USA). Cells were centrifuged at 1500×g for 5 min and washed three times with RPMI containing 10% FBS. In a 96-well plate (U-bottom shape), 4 × 105 cells in 200 µl RPMIc were plated per well. The cells were stimulated with 2.0 µg/ml CEA (Sigma–Aldrich) or scFv6.C4, obtained from the supernatant of HEK293 cells transfected with uP-PS/scFv6.C4 by the calcium phosphate method, or 2.5 µg/ml concanavalin A (ConA; Sigma–Aldrich). Non-stimulated cells were used as a negative control. All experiments were carried out in triplicate. After 6 days of incubation at 37 °C in a CO2 chamber, the cells were washed with MAC buffer (PBS containing 2 mM EDTA and 0.5% BSA; pH 7.2) and labeled in a dark chamber at 4 °C for 45 min with anti-CD4-PercP (1:200), anti-CD8-APC (1:200) or anti-CD45R/B220-PE (1:200) antibodies (BD Bioscience; San Jose, CA, USA). The labeled cells were washed three times and suspended in 200 µl MAC buffer. The samples were analyzed by flow cytometry (BD FACS Canto; Mississauga, ON, USA), using the BD FACSDiva (BD Bioscience; Sao Paulo, SP, Brazil) and Flow Jo (Three Star; Ashland, OR, USA) softwares.
Cytotoxicity assay
The B16F10-CEA and B16F10-scFv6.C4 cells were used as targets. Initially, 5 × 103 cells of each condition were seeded per well in 96-well plates containing RPMIc. After 24 h, 5 × 105 splenocytes were added per well in the presence of 2.0 µg/ml CEA for effector stimulation. After 5 days, 10 µl 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 5 mg/ml; Invitrogen) were added per well and incubated for 3 h at room temperature. The supernatant was discarded and 100 µl dimethyl sulfoxide (DMSO) were added, and the solution was left under slow agitation in a dark chamber for 15 min. Optical densities (λ = 595 nm) of samples were read using a Spectra Max (model M2e; Orleans Drive Sunnyvale, CA, USA) spectrophotometer, and the lysis percentage was determined using the following formula:
CVE: OD595nm with target cellsRCV: OD595nm without target cells
Statistical analysis
Statistical analyses were performed using the GraphPad (v. 5.0; La Jolla, CA, USA) software. Data were analyzed using analysis of variance (ANOVA) followed by the Bonferroni test. The data were presented as mean ± standard deviation (SD). A value of P < 0.05 was considered to be significant. The survival analysis was performed using the Mantel–Cox method.
Results
In vivo tumor model with B16F10 cells expressing CEA
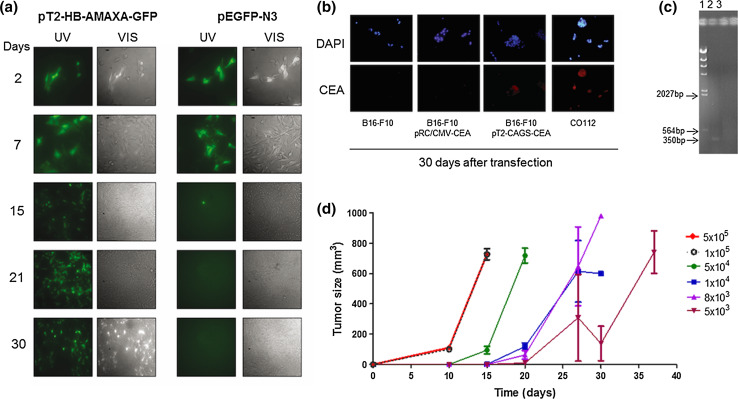
To establish an animal model and evaluate scFv6.C4 vaccination, first we focused on creating a mouse tumor cell line expressing human CEA permanently. The murine melanoma cell line B16F10 [30], which is a well-known cell line in tumor biology due to its high capacity of tumorigenesis in vivo, was nucleofected. Initially, the GFP expressing vectors pEGFP-N3 and pT2-HB-AMAXA-GFP, which are non-integrative and integrative vectors, respectively, were used to evaluate transfection efficiency and transgene expression duration in B16F10 cells. Transfection efficiency, which was assessed 2 days after nucleofection by counting GFP positive cells, was about 85–90% in both vectors (not shown). GFP gene expression in pEGFP-N3 transfected cells could be observed until day 7, but no GFP positive cell was observed at day 15 (Fig. 1a). On the other hand, about 50% were GFP positive cells in the plate of the pT2-HB-AMAXA-GFP transfected cells on day 30, indicating long-term GFP gene expression and high nucleofection efficiency.
Fig. 1.
Establishment of a tumor model with B16F10-CEA. a B16F10 cells were nucleofected with pT2-HB-AMAXA-GFP and pCMV-SB100 or pEGFP-N3, and GFP expression was monitored during 30 days by fluorescence microscopy. b B16F10 cells were nucleofected with pT2-CAGGS-CEA and pCMV-SB100 (B16F10-CEA) or pRC/CMV-CEA, and CEA expression was detected by immunocytochemistry after 30 days. The CO112 and B16F10 cells were used as positive and negative controls, respectively. c Integration of CEA in the genome was analyzed by PCR 4 weeks after nucleofection. 1: λ Hind III ladder. 2: B16F10-CEA. 3: B16F10. The expected amplicon is 350 bp (base pair). d Tumor growth rate was determined after s.c. injection of B16F10 cell suspension in the left flank. Each time point corresponds to 3 mice per group. Representative data of 2 biological replicate experiments are shown
Based on these results, B16F10 cells were transfected with the CEA-expressing vectors, pRC/CMV-CEA or pT2-CAGGS-CEA, which are non-integrative and integrative systems, respectively. By immunocytochemistry analyses, CEA expression was observed only in the cells transfected with pT2-CAGGS-CEA and the naturally expressing cell line CO112 (Fig. 1b). The estimated transfection efficiency was about 90% (not shown). Integration of the CEA expression cassette was confirmed by PCR, only in the pT2-CAGGS-CEA-transfected B16F10 cells (Fig. 1c).
To establish the conditions of the in vivo experiments, several concentrations of B16F10-CEA cells were injected into the left flank of CEA2682 transgenic mice. With the highest concentration (5 × 105 cells), a visible tumor was observed around day 10 post-injection, reaching a volume of 800-mm3 15 days after injection (Fig. 1c). Injection of lower numbers of cells postponed the onset of tumor formation, and also decreased the tumor growth rate, as can be noted by the decrease in the curve slope. Based on these observations, we used 5 × 105 B16F10-CEA cells in the following experiments.
Humoral and cellular immune responses induced by uP-PS/scFv6.C4 DNA vaccination
To evaluate the immune response induced after vaccination with the uP/PS-scFv6.C4 vector, the CEA2682 mice were immunized four times with 2-week intervals. The DNA vaccination with plasmid solution was performed by i.m. injection followed by electroporation based on our previous experience [31].
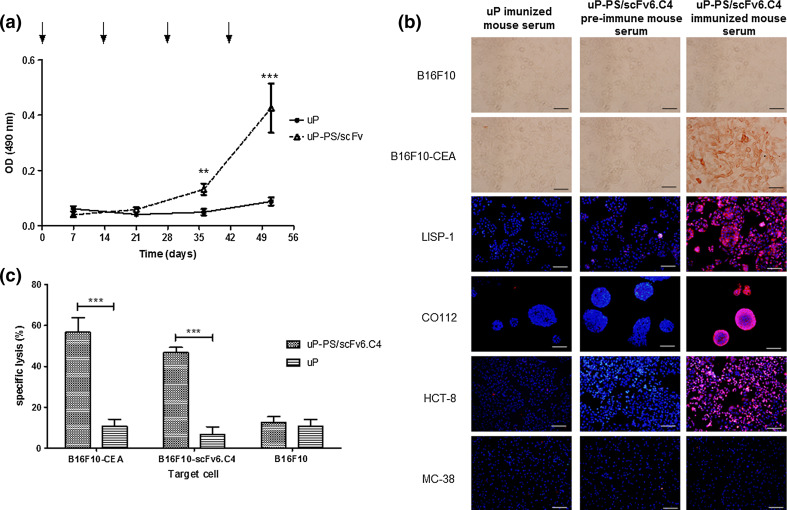
Blood samples were collected 1 week after each immunization to determine the AB3 antibody titers. A significant AB3 titer was detected after the third immunization, and the highest titer was achieved after the fourth immunization (Fig. 2a). However, additional immunization did not increase the antibody titers (not shown).
Fig. 2.
Humoral and cellular responses raised after DNA vaccination with uP-PS/scFv6.C4. a CEA-specific AB3 antibodies from mice immunized with uP-PS/scFv6.C4 were detected by ELISA. Blood samples were collected 7 days after each immunization and diluted 1:100 for assays. Sera from mice immunized with uP vector were used as control. Arrows indicate day of immunization. Values for OD490nm are expressed as mean ± SD of each group (n = 12 per group). Representative data of 2 biological replicate experiments are shown. ***p ≤ 0.001 and **p ≤ 0.01. b Immunocytochemistry of human colorectal adenocarcinoma cell lines, murine melanoma cell lines (B16F10 and B16F10-CEA) and murine colon tumor cell line (MC38) using sera from uP-PS/scFv6.C4-immunized mice. Sera from uP-immunized mice and pre-immune sera from uP-PS/scFv6.C4-immunized mice were used as negative control. Bar 100 µm. c CEA-specific CTL activity was determined using splenocytes isolated 7 days after the last immunization. B16F10-CEA, B16F10-scFv6.C4, and B16F10 cells were used as targets (target:effector ratio: 1:50). Data are expressed as mean ± SD of each group of mice (3 mice per group). Representative data of 2 biological replicate experiments are shown. ***p ≤ 0.001
To assess CEA-specificity of the AB3 antibody, sera obtained after the last immunization were tested by immunocytochemistry against the CEA-expressing human colorectal cell lines CO112, HCT-8, and LISP-1 and murine cell lines B16F10-CEA. Pre-immune sera or sera from mice immunized with the uP vector were used as negative controls (Fig. 2b). All the CEA-expressing cell lines were strongly marked with sera from uP-scFv6.C4-immunized mice, whereas pre-immune sera (Fig. 2b) or sera from mice vaccinated with the uP vector did not show any reaction. These results confirm the effectiveness of the scFv6.C4 recombinant protein to mimic CEA.
To evaluate the CTL immune response, two target cells, B16F10-CEA or B16F10-scFv6.C4, were initially constructed to express CEA and scFv6.C4, respectively.
About 10% of B16F10 cells transfected with the empty vector uP (which was used as a control) were lysed by splenocytes from mice immunized with uP/scFv6.C4 or uP. Such percentage was considered as nonspecific basal activity. Furthermore, splenocytes obtained from CEA2682 immunized with uP vector presented basal CTL activity upon B16F10-CEA and B16F10-scFv6.C4 cells. On the other hand, uP-PS/scFv6.C4-immunized CEA2682 mice showed a strong CTL activity, lysing B16F10-CEA (56.7%) and B16F10-scFv6.C4 (46.7%) cells (Fig. 2c). The percentage of lysis in the CEA-transfected cells was higher than in the scFv6.C4-transfected ones. However, the difference was not statistically significant.
Protection by uP-PS/scFv6.C4 DNA vaccination against tumor challenge
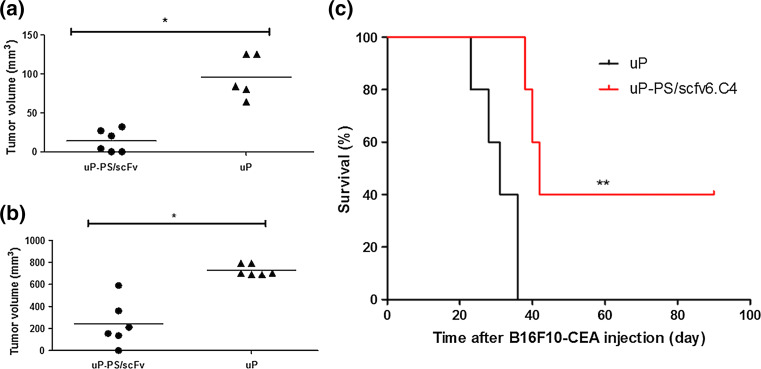
To assess the effectiveness of the preventive DNA uP-PS/scFv6.C4 vaccine, 5 × 105 B16F10-CEA cells were injected into the left flank of mice 10 days after the last immunization, and the tumor growth was measured periodically. In mice vaccinated with the uP vector, tumor volumes were about 180 mm3 on the 10th day, reaching about 800 mm3 on the 15th day (Fig. 3). Soon after the last tumor measurement, these mice were euthanized due to tumor overgrowth. On the other hand, tumor volume in mice vaccinated with uP-PS/scFv6.C4 was only about 20 mm3 on the 10th day, reaching 210 mm3 on the 15th day. This difference indicates that this vaccination strongly prevented tumor growth in comparison to the control group. The difference between these two groups was statistically significant (p < 0.05) (Fig. 3).
Fig. 3.
Protection by uP-PS/scFv6.C4 DNA vaccination against B16F10-CEA tumor challenge. CEA-expressing CEA2682 mice were immunized four times with uP-PS/scFv6.C4 or uP, and ten days after the last immunization 5 × 105 B16F10-CEA cells were injected subcutaneously. Representative data of 2 biological replicate experiments are shown below. Tumor growth was measured with a caliper 10 days (a) and 15 days (b) later (n = 6 per group). In other immunized mice, 8 × 103 B16F10-CEA cells were injected subcutaneously and tumor growth was followed for 100 days (n = 5 per group). c To estimate survival rate by the Mantel–Cox method, mice with tumors smaller than 500 mm3 were considered as survivors. *p ≤ 0.01, **p ≤ 0.01. n = 5 per group
The preventive DNA vaccine study was also carried out challenging mice with a lower number of B16F10-CEA cells (8 × 103 cells) following the same immunization protocol as used above. In mice immunized with the uP vector, visible tumors appeared between the 16th and 30th days after injection, and the tumor volume reached 800 mm3 in less than 10 days later. However, visible tumors were observed only on the 34th day after tumor injection in 3 out of 5 uP-PS/scFv6.C4 vaccinated mice, and no visible tumors were seen over 100 days of observation in the rest of the vaccinated mice (Fig. 3c). These results showed that the vaccine regimen with the uP-PS/scFv6.C4 vector is able to delay tumor growth either increasing animal survival or even preventing tumor growth completely.
To assess specific T- and B-cell proliferative activities in vitro, splenocytes from vaccinated and B16F10-CEA-challenged mice were harvested and stimulated with CEA or scFv6.C4. ConA (2.5 µg/ml) and no stimulator were used as positive and negative controls, respectively.
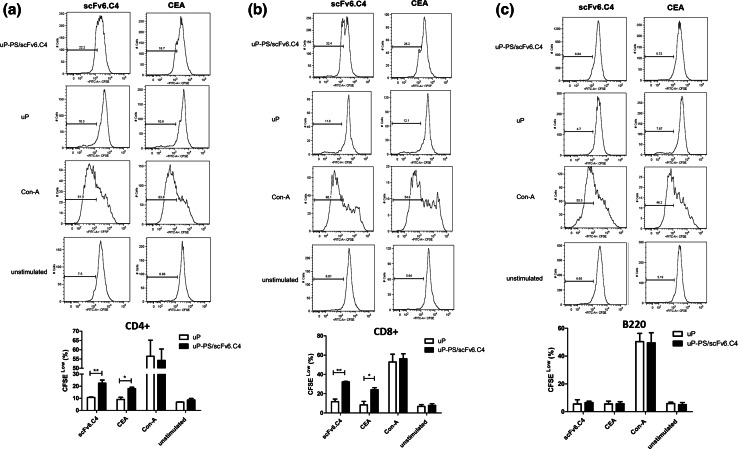
CD4+ and CD8+ T-cells from uP-immunized mice proliferated about 10% after stimulation with CEA or scFv6.C4; meanwhile, in the cells from the uP/PS-scFv6.C4-immunized mice, CEA stimulated 22.4 ± 2.6% CD4+ and 24.2 ± 2.0% CD8+ cells, and scFv6.C4 stimulated 22.5 ± 1.0% CD4+ and 32.2 ± 0.6% CD8+ cells (Fig. 4). As the unstimulated CD4+ and CD8+ cells had about 10% proliferation and ConA-stimulated cells about 50%, the main T-cells’ proliferative activity from uP/PS-scFv6.C4-immunized mice should be specific to CEA and scFv6.C4.
Fig. 4.
Proliferation assay of splenocytes. CEA-expressing CEA2682 mice were immunized with uP-PS/scFv6.C4 or uP, as described in the legend of Fig. 3 and Materials and Methods section. Fifteen days later, these mice were euthanized to obtain splenocytes, which were labeled with 2.5 µM CSFE and stimulated with CEA or scFv6.C4 for 6 days. Frequency of CD4+ (a), CD8+ (b) and CD45R/B220 (c) CFSE-low cells were determined by flow cytometry. Data are expressed as mean ± SD of each group of mice (4 per group). Representative data of 2 biological replicate experiments are shown. *p ≤ 0.05, **p ≤ 0.01
The B-cell proliferation after stimulation with CEA or scFv6.C4 was less than 10%, irrespective of vaccinated groups. A similar B-cell proliferation rate was seen without stimulation (Fig. 4c), showing no specificity of B cells from uP/PS-scFv6.C4-immunized mice to CEA and scFv6.C4. These results indicate that the immune protection seen after vaccination is mostly due to T-cell activities.
Discussion
Active immunotherapy using a TAA epitope surrogate to stimulate immune system is an attractive strategy because, in principle, TAA-expressing tumors may be recognized by the patients’ immune system as nonself, consequently, be pursed and destroyed. Based on this principle, some groups constructed CEA epitope surrogates and showed elicitation of humoral and cellular responses [8, 10, 13].
In our previous study, we described the construction of scFv6.C4, a CEA epitope surrogate, using RNA isolated from the hybridoma cell line 6.C4 and the expression vector pcDNA3-PS/scFv6.C4 [11, 13]. C57BL/6 wild-type mice immunized with pcDNA3-PS/scFv6.C4 raised a specific humoral immune response against CEA, and this response was shown by both immunocytochemistry using the human colon adenocarcinoma cell line CO112 [27] and immunohistochemistry using patient tumor biopsies [13]. However, scFv6.C4 ability to elicit immune response and provide protection against tumor challenge in CEA-expressing transgenic mice has not yet been demonstrated.
The vector delivery mode and transgene expression level are important variables that significantly affect the immune response of vaccinated animals [32, 33]. In this study, we used uP-PS/scFv6.C4 instead of pcDNA3-PS/scFv6.C4 vector because the uP vector was constructed with the strong CMV promoter and enhancer, without eukaryotic reporter or selection gene, which drives the transgene expression level much higher than pcDNA3 vector does [24]. In addition, electroporation instead of simple i.m. vector injection was adopted to transfer the uP-PS/scFv6.C4 vector into the skeletal muscle because in our previous study we observed a high level of gene transfer and a consequent high level of transgene expression [31]. Although electroporation appears to be very invasive, it has already been used in clinical studies for DNA vaccines and immunotherapies without significant side-effects or discomfort [34].
To test our DNA vaccine in vivo, we used the genetically modified tumor cell line B16F10 to permanently express human CEA gene with the SB system. This cell line was chosen for our study because it has the same genetic background of the CEA2682 mouse cells [30] and high capacity to both grow in vivo when injected subcutaneously and form a well-localized solid tumor that can be easily measured with a caliper. In addition, this cell line can be used to create a metastatic model by simple injection in the tail vein [35], which is our next challenge to show the functioning of our vaccine in the final stage of cancer.
Figure 3 shows a clear evidence of the immune protection after vaccination with uP-PS/scFv6.C4 vector. However, this protection was not enough to eliminate the 5 × 105 B16F10-CEA injected cells, and all vaccinated mice had to be euthanized later due to tumor overgrowth. Classically, as efficiency of any therapeutics is dose dependent, we have hypothesized that the number of injected B16F10 cells was higher than the immunity level provided by vaccination. To test this hypothesis, we challenged the vaccinated mice injecting 8 × 103 B16F10-CEA cells, which delayed about 10 more days in comparison to the 5 × 105 cell injection to form a similar tumor size (Fig. 1c). After challenge with less B16F10-CEA cells, 40% of vaccinated mice remained free of tumor during all period of experimentation. These results clearly show that this vaccination raised a specific immunity against CEA. However, the level of immune response, which depends on the immunization protocol, and the challenge with tumor cells, which depends on the type and number of injected cells, must be quantitatively evaluated by varying these parameters to estimate vaccination efficiency. Such parameters become more relevant when planning clinical trials, because individual physiological and pathological variabilities are higher than those of the experimental conditions used here by us and by other investigators who use isogenic animals and a tumor cell line for tumorigenesis. For example, in a phase-III study (343 colorectal patients) with the 3H1 anti-Id antibody, a CEA epitope surrogate [8], they showed strong (only about 12%), weak (31%), and no (30%) immune responses, with median survivals of 28.2, 15.8 and 8.3 months, respectively [36]. These data show that vaccination with the CEA epitope surrogate raised the immune response enough to combat the last-stage of tumor progression in these patients. However, the best outcomes depend on raising a strong immune response, which depends on the relationship between immunization protocol and tumor progression state, beyond other clinical factors such as comorbidity and aging.
Cellular response assessed by a specific cell lysis assay showed a slightly higher activity on cells expressing CEA than scFv6.C4 (Fig. 2c), which was not statistically significant. On the other hand, the T-cell proliferation assay showed higher stimulation with scFv6.C4 than CEA, thus indicating an apparent higher affinity of target cells to scFv6.C4 than to CEA. Nevertheless, as we have used scFv6.C4 from the supernatant of HEK293 cells transfected with uP-PS/scFv6.C4 the actual scFv6.C4 concentration is unknown; therefore, these results allow us to only conclude that a cellular response occurred without, however, power for quantitative comparison. Production, purification and quantification of the recombinant protein scFv6.C4 are technical limitations we faced in this study.
Collectively, we showed that preventive DNA vaccination performed by electroporation of the uP-PS/scFv6.C4 vector raised humoral and cellular responses in CEA-expressing transgenic mice, which were sufficient to retard and/or eliminate injected B16F10-CEA cells.
Acknowledgements
Priscila M. A. Denapoli and Bianca F. Zanetti were recipients of the Brazilian National Council for Scientific and Technological Development (CNPq) and São Paulo Research Foundation (FAPESP) scholarships, respectively.
Funding
This study was funded by FAPESP (Grant Number: 2012/21861-1).
Abbreviations
- B16F10-CEA
Murine melanoma cell line B16F10 expressing CEA
- B16F10-scFv6.C4
Murine melanoma cell line B16F10 expressing scFv6.C4
- CEA
Carcinoembryonic antigen
- CEA2682
CEA-expressing transgenic mice
- CFSE
Carboxyfluorescein diacetate succinimidyl ester
- FAPESP
Sao Paulo research foundation
- RPMIc
RPMI 1640 medium with supplements
- SB
Sleeping beauty
- scFv
Single-chain variable fragment
- scFv6.C4
Single-chain variable fragment 6.C4
- SD
Standard deviation
- uP/PS-scFv6.C4
scFv6.C4 expressing plasmid vector
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Thompson JA, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991;5:344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- 2.Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 3.Ura Y, Ochi Y, Hamazu M, et al. Studies on circulating antibody against carcinoembryonic antigen (CEA) and CEA-like antigen in cancer patients. Cancer Lett. 1985;25:283–295. doi: 10.1016/S0304-3835(15)30008-2. [DOI] [PubMed] [Google Scholar]
- 4.Konstadoulakis MM, Syrigos KN, Albanopoulos C, et al. The presence of anti-carcinoembryonic antigen (CEA) antibodies in the sera of patients with gastrointestinal malignancies. J Clin Immunol. 1994;14:310–313. doi: 10.1007/BF01540984. [DOI] [PubMed] [Google Scholar]
- 5.Albanopoulos K, Armakolas A, Konstadoulakis MM, et al. Prognostic significance of circulating antibodies against carcinoembryonic antigen (anti-CEA) in patients with colon cancer. Am J Gastroenterol. 2000;95:1056–1061. doi: 10.1111/j.1572-0241.2000.01982.x. [DOI] [PubMed] [Google Scholar]
- 6.Haidopoulos D, Konstadoulakis MM, Antonakis PT, et al. Circulating anti-CEA antibodies in the sera of patients with breast cancer. Eur J Surg Oncol. 2000;26:742–746. doi: 10.1053/ejso.2000.0996. [DOI] [PubMed] [Google Scholar]
- 7.Ladd J, Lu H, Taylor AD, et al. Direct detection of carcinoembryonic antigen autoantibodies in clinical human serum samples using a surface plasmon resonance sensor. Colloids Surf B Biointerfaces. 2009;70:1–6. doi: 10.1016/j.colsurfb.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee SK, Tripathi PK, Chakraborty M, et al. Molecular mimicry of carcinoembryonic antigen by peptides derived from the structure of an anti-idiotype antibody. Cancer Res. 1998;58:1217–1224. [PubMed] [Google Scholar]
- 9.Tsujisaki M, Hinoda Y, Tokuchi S, et al. The analysis of internal image-bearing anti-idiotypic monoclonal antibody in relation to carcinoembryonic antigen. J Immunol. 1993;150:508–516. [PubMed] [Google Scholar]
- 10.Gaida FJ, Pieper D, Roder UW, et al. Molecular characterization of a cloned idiotypic cascade containing a network antigenic determinant specific for the human carcinoembryonic antigen. J Biol Chem. 1993;268:14138–14145. [PubMed] [Google Scholar]
- 11.de Moraes JZ, Carneiro CR, Buchegger F, et al. Induction of an immune response through the idiotypic network with monoclonal anti-idiotype antibodies in the carcinoembryonic antigen system. J Cell Biochem. 1992;50:324–335. doi: 10.1002/jcb.240500313. [DOI] [PubMed] [Google Scholar]
- 12.Tripathi PK, Qin H, Deng S, et al. Antigen mimicry by an anti-idiotypic antibody single chain variable fragment. Mol Immunol. 1998;35:853–863. doi: 10.1016/S0161-5890(98)00072-8. [DOI] [PubMed] [Google Scholar]
- 13.Pignatari GC, Takeshita D, Parise CB, et al. Carcinoembryonic antigen (CEA) mimicry by an anti-idiotypic scFv isolated from anti-Id 6.C4 hybridoma. J Biotechnol. 2007;127:615–625. doi: 10.1016/j.jbiotec.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Schwegler C, Dorn-Beineke A, Nittka S, et al. Monoclonal anti-idiotype antibody 6G6.C4 fused to GM-CSF is capable of breaking tolerance to carcinoembryonic antigen (CEA) in CEA—transgenic mice. Cancer Res. 2005;65:1925–1933. doi: 10.1158/0008-5472.CAN-04-3591. [DOI] [PubMed] [Google Scholar]
- 15.Tsujisaki M, Imai K, Tokuchi S, et al. Induction of antigen-specific immune response with use of anti-idiotypic monoclonal antibodies to anti-carcinoembryonic antigen antibodies. Cancer Res. 1991;51:2599–2604. [PubMed] [Google Scholar]
- 16.Losman MJ, Novick KE, Goldenberg DM, Monestier M. Mimicry of a carcinoembryonic antigen epitope by a rat monoclonal anti-idiotype antibody. Int J Cancer. 1994;56:580–584. doi: 10.1002/ijc.2910560419. [DOI] [PubMed] [Google Scholar]
- 17.Foon KA, John WJ, Chakraborty M, et al. Clinical and immune responses in resected colon cancer patients treated with anti-idiotype monoclonal antibody vaccine that mimics the carcinoembryonic antigen. J Clin Oncol. 1999;17:2889–2895. doi: 10.1200/JCO.1999.17.9.2889. [DOI] [PubMed] [Google Scholar]
- 18.Foon KA, Chakraborty M, John WJ, et al. Immune response to the carcinoembryonic antigen in patients treated with an anti-idiotype antibody vaccine. J Clin Invest. 1995;96:334–342. doi: 10.1172/JCI118039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner U, Kohler S, Reinartz S, et al. Immunological consolidation of ovarian carcinoma recurrences with monoclonal anti-idiotype antibody ACA125: immune responses and survival in palliative treatment. See the biology behind: K. A. Foon and M. Bhattacharya-Chatterjee, are solid tumor anti-idio. Clin Cancer Res. 2001;7:1154–1162. [PubMed] [Google Scholar]
- 20.Alfonso M, Diaz A, Hernandez AM, et al. An anti-idiotype vaccine elicits a specific response to N-glycolyl sialic acid residues of glycoconjugates in melanoma patients. J Immunol. 2002;168:2523–2529. doi: 10.4049/jimmunol.168.5.2523. [DOI] [PubMed] [Google Scholar]
- 21.Reinartz S, Wagner U. Current approaches in ovarian cancer vaccines. Minerva Ginecol. 2004;56:515–527. [PubMed] [Google Scholar]
- 22.de Moraes JZ, Gesztesi JL, Westermann P, et al. Anti-idiotypic monoclonal antibody AB3, reacting with the primary antigen (CEA), can localize in human colon-carcinoma xenografts as efficiently as AB1. Int J Cancer. 1994;57:586–591. doi: 10.1002/ijc.2910570424. [DOI] [PubMed] [Google Scholar]
- 23.Eades-Perner A-M, Van der Putten H, Hirth A, et al. Mice transgenic for the human carcinoembryonic antigen gene maintain its spatiotemporal expresion pattern. Cancer Res. 1994;54:4169–4176. [PubMed] [Google Scholar]
- 24.Sacramento CB, Cantagalli VD, Grings M, et al. Granulocyte-macrophage colony-stimulating factor gene based therapy for acute limb ischemia in a mouse model. J Gene Med. 2009;11:345–353. doi: 10.1002/jgm.1298. [DOI] [PubMed] [Google Scholar]
- 25.Pelegrin A, Terskikh A, Hayoz D, et al. Human carcinoembryonic antigen cDNA expressed in rat carcinoma cells can function as target antigen for tumor localization of antibodies in nude rats and as rejection antigen in syngeneic rats. Int J Cancer. 1992;52:110–119. doi: 10.1002/ijc.2910520120. [DOI] [PubMed] [Google Scholar]
- 26.Aronovich EL, Bell JB, Belur LR, et al. Prolonged expression of a lysosomal enzyme in mouse liver after Sleeping Beauty transposon-mediated gene delivery: implications for non-viral gene therapy of mucopolysaccharidoses. J Gene Med. 2007;9:403–415. doi: 10.1002/jgm.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mach JP, Carrel S, Merenda C, et al. In vivo localisation of radiolabelled antibodies to carcinoembryonic antigen in human colon carcinoma grafted into nude mice. Nature. 1974;248:704–706. doi: 10.1038/248704a0. [DOI] [PubMed] [Google Scholar]
- 28.Tompkins WA, Watrach AM, Schmale JD, et al. Cultural and antigenic properties of newly established cell strains derived from adenocarcinomas of the human colon and rectum. J Natl Cancer Inst. 1974;52:1101–1110. doi: 10.1093/jnci/52.4.1101. [DOI] [PubMed] [Google Scholar]
- 29.Solimene AC, Carneiro CR, Melati I, Lopes JD. Functional differences between two morphologically distinct cell subpopulations within a human colorectal carcinoma cell line. Braz J Med Biol Res. 2001;34:653–661. doi: 10.1590/S0100-879X2001000500014. [DOI] [PubMed] [Google Scholar]
- 30.Fidler IJ. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975;35:218–224. [PubMed] [Google Scholar]
- 31.Parise CB, Lisboa B, Takeshita D, et al. Humoral immune response after genetic immunization is consistently improved by electroporation. Vaccine. 2008;26:3812–3817. doi: 10.1016/j.vaccine.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 32.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer. 2008;8:108–120. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 33.Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 34.Bodles-Brakhop AM, Heller R, Draghia-Akli R. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol Ther. 2009;17:585–592. doi: 10.1038/mt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Overwijk WW, Restifo NP (2001) B16 as a mouse model for human melanoma. Curr Protoc Immunol. doi:10.1002/0471142735.im2001s39 [DOI] [PMC free article] [PubMed]
- 36.Chong G, Bhatnagar A, Cunningham D, et al. Phase III trial of 5-fluorouracil and leucovorin plus either 3H1 anti-idiotype monoclonal antibody or placebo in patients with advanced colorectal cancer. Ann Oncol. 2006;17:437–442. doi: 10.1093/annonc/mdj090. [DOI] [PubMed] [Google Scholar]