Abstract
Carcinomas (tumors of epithelial origin) are responsible for most of all new cancers in the industrialized countries. Due to the high mortality rate caused by the metastatic spread of aggressive cancer cells, there is an urgent demand in finding new biomarkers, which should detect early formation of metastases and monitor efficacy of systemic adjuvant therapy in a timely manner. It has been considered that the molecular analysis of cells which are shed from tumors into the blood system (circulating tumor cells (CTCs)) might provide new insights for the clinical management of cancer, probably far earlier than using traditional high-resolution imaging technologies. Clinical trials indicated that CTCs can be deployed for diagnostic, monitoring, and prognostic purposes. Furthermore, these cells are discussed to be suitable as predictive markers. In any case, identification of CTCs requires innovative and challenging technologies as detection methods should be specific, sensitive, standardized, and highly reproducible. Although many different approaches have been developed until now, only the CellSearch™ method has been cleared by the American Food and Drug Administration. Although the detection of CTCs has already shown to have a prognostic impact in many tumor entities including breast, prostate, lung and colon cancer, ongoing and future studies are aimed to explore whether CTCs can be used for an individual therapy decision making including novel immunotherapeutic approaches. This review discusses (1) different detection strategies for CTCs, (2) their clinical impact, and (3) the potential use of CTCs guiding the treatment of individual cancer patients.
Keywords: Cancer, EMT, CTCs, DTCs, Metastasis, Biomarker
Introduction
Metastases remain the main cause of cancer-related death. It is thought that only a minority of dispersed cancer cells which left the primary tumor and survived in the circulation are able to seed metastases in distant organs. Furthermore, it is assumed that tumor cells which already moved into remote body parts may reinfiltrate into their tumors of origin enriching the primary tumor with tumor cell populations that have withstood the era of dissemination (“tumor self-seeding”) giving rise to even more aggressive metastatic variants [1, 2]. The bone marrow (BM) appears to be a common organ to which tumor cells from many types of carcinoma (tumors of epithelial origin) home at first [3]. The prognostic relevance of disseminated tumor cells (DTCs) which infiltrated the BM has generally been accepted [4]. These micrometastases may stay in a dormant, non-proliferating state for several years but are also able to recirculate into the bloodstream at which the vascular cell adhesion molecule 1 (VCAM-1) seems to promote the transition from indolent micrometastasis to overt metastasis [5].
In contrast to DTCs, the role of circulating tumor cells (CTCs) detected in blood of cancer patients is not yet entirely understood. Oncologists expect that investigation into CTCs might replace invasive tissue biopsies as detection and molecular characterization of CTCs may give early insights into tumor biology and metastasis formation, probably far earlier than using the current high-resolution imaging technologies [2, 6].
Although CTCs have already been discovered in 1869 [7], molecular examination has just recently gained heightened attention. This phenomenon is explainable by the improved technical progress which took place during the last decades. Current research on CTCs already exhibited that tumor-derived cells which can be found in the bloodstream give valuable information as biomarkers in cancer [8]. For example, different research groups demonstrated a poor prognosis for metastatic breast, colon, and prostate cancer patients who presented basal CTC counts of ≥3 or 5 tumor cells in 7.5 ml of blood [9–11]. At present, CTCs are also discussed as real-time “liquid biopsy” for molecular targeted agents, enabling the identification of patients who will most likely respond to a given therapy (personalized medicine) [2].
However, it remains a technical challenge to identify tumor cell events from the background of normal blood cells. It is estimated that as little as one CTC can be found in the background of up to 108 normal blood cells. To bypass this impediment-sensitive and impediment-specific CTC detection, methods have been developed over the past years [2, 6]. Although various techniques for the detection of CTCs are commercially available or under-development in laboratories all over the world, until now just one method has been cleared by the American Food and Drug Administration (FDA) (CellSearch™). This system facilitates the possibility to capture CTCs in a standardized and highly reproducible manner within the clinical context. The CellSearch system (a Class II product) has received FDA clearance for CTC testing in patients with metastatic breast, prostate, and colon cancer. In order to gain FDA clearance, a system and reagents need to demonstrate analytical validity according to the FDA guidelines. Additionally, a clinical validation study must be conducted that meets all FDA requirements and commitments by the sponsor to the FDA. Finally, FDA-cleared technology is subjected to post-market surveillance, which includes inspections of manufacturing and quality practices, customer complaint handling, and post-approval marketing practices (for more detailed information, please refer to the official FDA site www.fda.gov). Anyway, ongoing and future studies will show which approaches for CTC detection might be used for a CTC-based therapy contributing the development of individualized targeted treatment of cancer patients. This review focuses on (1) the different detection strategies for CTCs. Furthermore, we will (2) describe the clinical impact of tumor cells found in the bloodstream and (3) discuss in particular their potential use as biomarker to guide the treatment of individual cancer patients.
Detection of circulating tumor cells: a technical challenge
Enrichment of circulating tumor cells
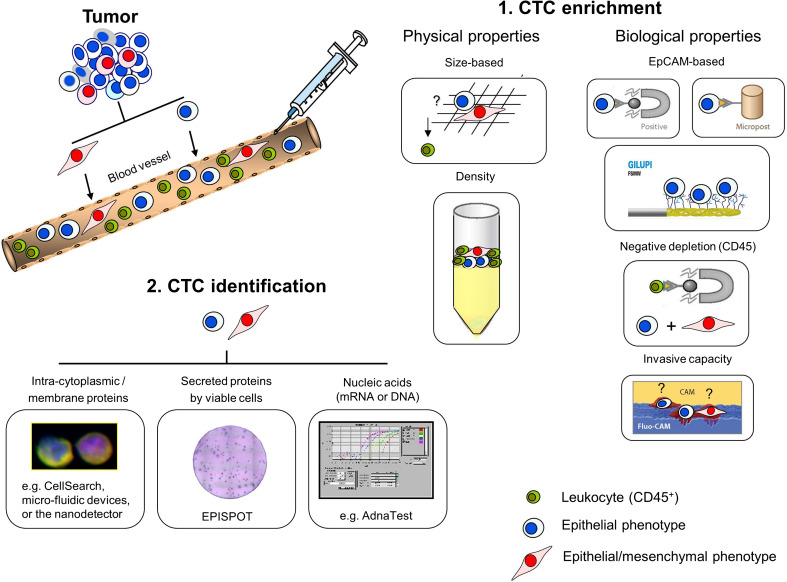
Due to the low concentration of CTCs and the large background of normal hematopoietic cells, most approaches for CTC detection require an enrichment step before application of the identification (Fig. 1, 1. CTC enrichment). Enrichment of tumor cells includes a considerable panel of different procedures which are usually based on physical or biological properties [2]. Tumor cell enrichment by physical properties comprehends approaches like the gradient centrifugation (e.g. FICOLL or OnkoQuick). This principle allows a marker-independent cell selection but leads to a high loss of tumor cells conducting to false-negative results in clinical samples [12]. Alternatively, a size-based enrichment of CTCs can be applied to detect a greater extent of tumor cells out of one sample (e.g., ISET, isolation by size of epithelial tumor cells). The ISET system is supposed to detect a single tumor cell in one ml of blood [13]. Using this approach, CTCs could already be found in 80 % (32/40) of patients with non-small cell lung cancer [14]. Furthermore, Micro-Electro-Mechanical Systems (MEMS) may be used to combine a size-based enrichment with an electrically lysis enabling the identification and molecular characterization of CTCs on a single platform (recovery rate up to 90 %) [15]. Anyway, although the membrane filter devices seem to detect CTCs with a high recovery rate, their clinical impact has to be proven in larger clinical studies. In addition, this enrichment procedure is handicapped by the fact that leukocytes might agglutinate the filter pores or the variable size of the different tumor cell populations. However, CTC enrichment by physical properties allows a label-free tumor cell selection, making these approaches applicable methods for both epithelial tumors (e.g., breast, colon, prostate or lung cancer) and non-epithelial tumors (e.g., malignant melanomas).
Fig. 1.
Enrichment and identification of CTCs from the blood of cancer patients. Tumor cells leave the primary tumor and circulate through the bloodstream. CTC enrichment (1) is either based on physical or biological properties. Physical properties include size-based procedures (e.g., ISET) or density centrifugation steps (e.g., FICOLL or Oncoquick). Biological properties include the targeting of specific cell surface markers like EpCAM for positive selection (e.g., CellSearch™, microfluidic devices or the nanodetector) or CD45 for negative depletion. Further, invasive capacities of the tumor cells can be utilized (e.g., Vita Assay). CTC identification (2) is based on the immunocytochemistry analyses of specific intra-cytoplasmic or membrane proteins (e.g., CellSearch™, mircofluidic devices, and the nanodetector), either by the detection of secreted proteins (EPISPOT) or by PCR targeting tumor cell–specific nucleic acids (e.g., AdnaTest). CTC circulating tumor cells, EpCAM epithelial cell adhesion molecule, EPISPOT EPithelial ImmunoSPOT, Fluo-CAM fluorescent cell adhesion matrix, ISET insulation by size of epithelial tumor cells, PCR polymerase chain reaction
In contrast to the physical properties, biological enrichment features often rely on a positive cell selection targeting tumor-associated antigens of which the epithelial cell adhesion molecule (EpCAM) is a common tumor-specific surface target for carcinoma patients, whereas the melanoma-associated chondroitin sulfate proteoglycan (MCSP) or CD146 might be used for immunomagnetic enrichment of circulating melanoma cells. Clinical relevance of CTCs captured via the EpCAM protein could be validated in several studies [9–11]. The CellSearch™ assay enriches CTCs by using magnetic particles coated with antibodies against the EpCAM protein. Based on the FDA clearance, this system can be used as “standard” for all other detection methods. EpCAM-based microfluidic devices like the CTC or the HerringBone (HB) Chip seem to capture tumor cells with a high purity as well [16–18]. Just recently, the GILUPI GmbH has introduced a novel nanodetector to capture EpCAM-positive tumor cells specifically out of cancer patients. This nanodetector might overcome present limitations of other diagnostic methods (e.g., the blood volume) as the detector will be positioned through a cannula into the arm vein for an intended contact time of 30 min increasing the chances of diagnostic sensitivity [19].
Although epithelial marker-based enrichment targeting the EpCAM molecule has shown clinical relevance in various studies, this preparation disregards the issue that some tumor cell subpopulations may escape from EpCAM-based detection due to a process termed epithelial-to-mesenchymal transition (EMT) [20]. EMT was first reported as a feature of embryogenesis but has recently also been discussed to be activated during cancer invasion and metastasis formation [21]. EMT is characterized by the loss of epithelial cell properties, including the loss of cell–cell adhesion and the basoapical polarity. As EMT-like changes (e.g., downregulation of EpCAM) have already been discovered on DTCs and CTCs [22–25], current EpCAM-based technologies have to be revised for the detection of CTC subpopulations having undergone EMT-associated processes.
In contrast to the selection of tumor cells via epithelial antigens targeting of the leukocyte-specific antigen, CD45 (negative depletion) could be used to identify CTCs that do not display the classical epithelial phenotype anymore. So far, vital tumor cells have been successfully discovered and cultured for longer than 6 months using this preparation [26].
Additionally, viable tumor cells have also been laboratory-confirmed using the Vita Assay. By this approach, invasive tumor cells could be separated from non-tumor and dead cells by the viable tumor cell’s active ingestion of a specific cell adhesion matrix (CAM) [27]. Although the detection of EpCAM-negative or viable CTCs might enable valuable new insights for the understanding of cancer biology, independent studies have to be done to determine the functionality of the different assays. Furthermore, clinical relevance of the different captured CTC subpopulations has to be explored.
Identification of circulating tumor cells
In order to verify enriched CTCs from the undesired background of leukocytes, morphologic identification can be combined with immunocytochemistry analyses (ICC) (Fig. 1, 2 CTC identification). The fluorescence staining of epithelial-specific antigens like cytokeratins (e.g., CK-8, -18, and -19), epithelial-specific adhesion molecules (e.g., EpCAM or E-Cadherin), or tumor-specific surface proteins (e.g., estrogen receptor (ER), progesterone receptor (PR), or the prostate-specific membrane antigen (PSMA)) can be used for the detection and characterization of CTCs from a patient-derived sample after the enrichment. The CellSearch™ system uses a combined EpCAM selection with staining of CK-8, CK-18, and CK-19, the leukocyte antigen CD45, and the nuclear counterstain DAPI (4,6-diamino-2-phenylindole) at which CTCs are defined as (EpCAM+), CK+/DAPI+/CD45− [28]. ICC identification of (EpCAM+) CK+/DAPI+ and CD45− cells is also used by the microfluidic devices (CTC /HB Chip) and the novel nanodetector of the GILUPI GmbH [16–19]. However, just recently, it has been published that cytokeratin expression may change during EMT-associated processes on some cancer cell subpopulations. Joosse and colleagues found that cytokeratins currently targeted for CTC detection are downregulated in particular in breast cancer patients with unfavorable outcome, suggesting that targeting of more cytokeratins would improve the detection of clinically relevant CTCs [29].
Alternative to the identification based on ICC analyses staining cytokeratins, molecular detection by real-time polymerase chain reaction (PCR) amplification might be used to verify CTCs based on a tumor-specific multimarker gene profile. The AdnaGen™ test system combines an epithelial-based preselection with a reverse transcription PCR for the specific characterization of the CTC-associated gene expression. This system identifies CTCs based on (1) tumor markers like EpCAM, MUC1 (mucin-1), and HER2 (human epidermal growth factor receptor 2), (2) EMT-associated markers like PI3 Kα (phosphatidylinositol 3-kinase alpha), Akt-2, and Twist1, and (3) stemness indicators like ALDH1 (aldehyde dehydrogenase 1) [30, 31]. Furthermore, the CELLTRACKS™ CMC Kit or multimarker qRT-PCR assays targeting transcripts like the melanoma antigen recognized by T cells 1 (MART-1), the melanoma antigen gene A3 family (MAGE-A3), and the β-1,4-N-acetylgalactosaminyl transferase (GalNAc-T) could also be used for CTC detection in melanoma patients [32, 33].
The EPISPOT assay (EPithelial Immuno SPOT) might be used to detect and characterize tumor cells based on the detection of marker proteins which are shed or released specifically by viable cancer cells. Using this approach, CTCs or DTCs could be identified in clinical samples of breast, prostate, and colon carcinomas detecting a various panel of secreted proteins like CK-19, MUC1, PSA (prostate-specific antigen), or the stemcell factor FGF-2 (fibroblast growth factor-2) [34].
Taken together, although CTCs can be enriched and identified using a wide variety of different strategies, until now just the CellSearch™ approach has been cleared by the FDA. Future studies will show which other methods will become applicable for a routine clinical practice. Table 1 summarizes all mentioned technologies for CTC detection weighing their potential pros and cons for their use in clinical studies.
Table 1.
Technologies for CTC detection
| Tumor entity | Name | CTC enrichment (target) | CTC identification | Pros | Cons | Reference |
|---|---|---|---|---|---|---|
| Epithelial tumors | AdnaGen™ | Immunomagnetic selection (EpCAM/MUC1) | Multiplex PCR for tumor- (EpCAM, Her2, MUC1), EMT- (TWIST1, Akt2, PI3 Kα) or stem cell–associated transcripts (ALDH) |
High sensitivity and specificity Storage of the sample for 24 h |
EpCAM might be downregulated during EMT Larger clinical studies needed (AdnaGen, CTC-Chip and GILUPI device) |
[30, 31] |
| CellSearch™ | Coupled ferrofluids (EpCAM) |
ICC for CK, the leukocyte antigen CD45, and DAPI (CellSearch: other markers like Her2 and EGFR or FISH analyses possible) |
FDA-cleared High sensitivity and specificity Storage of the sample for 96 h |
[9–11] | ||
| CTC and HB Chip | Antibody-coated microposts (EpCAM) | High sensitivity and specificity | [16–18] | |||
| GILUPI device | Immunomagnetic selection using a nanodetector placed into the arm vein (EpCAM) |
Large blood volume (~1,5 l) High sensitivity and specificity |
[19] | |||
| Epithelial and non-epithelial tumors | EPISPOT | Density gradient centrifugation or negative depletion | Detection of secreted proteins by viable tumor cells (e.g., CK19, MUC1, PSA, FGF-2) |
Antibody-independent approach Detection of viable CTCs |
Larger clinical studies needed | [33] |
| ISET™/MEMS |
Size based (>8 μm) |
PCR, ICC, or cell culturing possible |
Antibody-independent approach High sensitivity and specificity |
Sample must be processed within 4 h Larger clinical studies needed |
[13–15] | |
| Vita Assay™ |
Ingestion of fluorescent CAM fragments |
Antibody-independent approach |
Larger clinical studies needed Low sensitivity |
[27] | ||
| E.g., RosetteSep™ | Depletion of CD45+ leukocytes | PCR, ICC, cell culturing or EPISPOT possible | [26] | |||
| OncoQuick™ | Density gradient centrifugation | [12] | ||||
| Melanoma | n.d. | Immunomagnetic selection (e.g. MCSP) | qRT-PCR targeting MART-1, MAGE-A3 or GalNAc-T |
Melanoma-specific approach High sensitivity |
Larger clinical studies needed | [33] |
| CELLTRACKS™ CMC kit | Immunomagnetic selection (CD146) | ICC for DAPI, HMW-MAA, CD45, CD34 and Ki67 (CMCs CD146+, HMW-MAA+, CD45-, CD34-, Ki67∓) | [32] |
ALDH1 aldehyde dehydrogenase 1, CAM cell adhesion matrix, CK cytokeratin, CMC circulating melanoma cell, CTC circulating tumor cell, DAPI 4`,6-diamidino-2-phenylindole, EpCAM epithelial cell adhesion molecule, EPISPOT EPithelial ImmunoSPOT, FGF-2 fibroblast growth factor-2, FISH fluorescent in situ hybridisation, GalNAc-T β-1,4-N-acetylgalactosaminyl transferase, h hour, HER2 human epidermal growth factor receptor 2, HMW-MAA molecular weight melanoma-associated antigen, ICC immunocytochemistry analyses, ISET isolation by size of epithelial tumor cells, MART-1 melanoma antigen recognized by T cells 1, MAGE-A3 melanoma antigen gene A3 family, MCSP melanoma-associated chondroitin sulfate proteoglycan, MUC1 mucin-1, n.d. not defined, PCR polymerase chain reaction, PI3 Kα phosphatidylinositol 3-kinase alpha, PSA prostate-specific antigen, qRT-PCR quantitative real-time PCR
Clinical relevance of CTCs
Various clinical trials pursue to uncover the impact of CTCs as new biomarkers in cancer therapy. Although a surprisingly high percentage of CTC-negative blood samples have been reported even in patients with overt metastases, encouraging studies using the CellSearch™ system have indicated that tumor cells found in the bloodstream predict progression-free and overall survival in patients with metastatic breast, prostate, and colorectal cancer [9–11]. The appearance of CTCs has also been associated with a shorter survival rate in metastatic cancer patients when tumor cells were detected using other CTC assays [2, 6]. Furthermore, it could be shown that the molecular characterization of CTCs might help to identify patients at higher risk of metastasis [35, 36]. Anyway, besides prognostication, there is an unmet need in finding biomarkers which should monitor the efficacy of systemic adjuvant therapy in real time. At present, the examination of the success or the failure of an anticancer therapy requires sophisticated laboratory and imaging technologies. Hence, reliable enumeration of CTCs could provide another scope for the use of therapeutic monitoring as changes in CTC appearance might reflect the success or the failure more rapidly than using the current approaches.
In breast cancer, clinical data already presented that the assessment of CTCs is an earlier and more reproducible indication of the disease status compared to radiologic approaches [37]. Complementary, using the CellSearch™ system, it could be demonstrated that CTC counts at any time point during therapy are a precise indication for disease progression in metastatic breast cancer patients [38]. The CellSearch™ system was also used to enumerate CTCs in blood from breast cancer patients, showing the potential role of this assay in assessing response to a preventive vaccine-based immunotherapeutic approach [39]. Interestingly, CTC counts further provided an earlier assessment of therapy response compared to the commonly used PSA test in prostate cancer [12, 40, 41].
As tumor cell detection is hindered by the fact that CTCs might be missed due to (1) the low sensitivity of some current available detection methods, (2) the EMT-associated loss of specific marker proteins necessary for CTC enrichment, or (3) by the low (unknown) frequency of the release rate of CTCs into the bloodstream, a real challenge is the detection of minimal residual disease in cancer patients with no clinical or radiographic evidence of distant metastasis (stage M0). Several studies have focused on CTCs in blood of early breast cancer with detection rates varying from 8 to 41 % [6, 42].
Xenidis et al. [43] showed that the detection of CK-19 mRNA post-chemotherapy was significantly associated with a reduced overall and disease-free survival in blood samples from non-metastatic breast cancer patients. Analyzing 115 non-metastatic breast cancer patients, Bidard and colleagues discovered that the detection of CTCs before chemotherapy is an independent prognostic factor for distant metastasis-free and overall survival [44]. Furthermore, recent data from Georgoulias et al. [45] indicate that targeting of chemotherapy-resistant CK19 mRNA-positive CTCs with “secondary adjuvant” trastuzumab treatment resulted in a significantly reduced probability of disease relapse and increased disease-free interval compared to early breast cancer patients receiving only standard treatment. However, question remains whether CTC detection in the (neo)adjuvant setting will provide valuable information for treatment decisions in cancer patients.
Summing up, besides the prognostic value of the baseline CTC counts (i.e., before initiation of therapy), measurements of the dynamic changes in CTC counts during (neo)adjuvant therapy might provide real-time information on the efficacy of therapy. Ongoing clinical trials like the German adjuvant SUCCESS study or the neoadjuvant GEPARQuattro and GEPARQuinto study will help clarifying whether observed decreases in CTC detection rates are associated with measurable benefit for the individual cancer patients [46, 47] and will give new insights into the use of CTCs as predictive markers.
CTCs: a real-time liquid biopsy?
Molecular characterization of CTCs might provide valuable insights for the understanding and individual treatment of cancer patients. One of the major questions remains whether the modification in treatment decisions based on the CTC status will result in a measurable benefit for the individual cancer patient. Preliminary results indicated that the molecular profiling of CTCs can be used to detect specific gene mutations or proteins which are relevant for the efficacy of cancer-related therapies adverting for the use of CTCs as real-time liquid biopsy. For example, therapeutic targets like the EGF receptor could be quantified by immunofluorescence on the surface of CTCs from blood samples of metastatic lung cancer patients, identifying patients who might respond to specific EGFR inhibitors [48]. Furthermore, by non-invasive genotyping in patients with non-small lung cancer, Maheswaran and co-workers could identify a specific T790M mutation in the EGFR gene of CTCs, which confers to the drug resistance of EGFR inhibitors like erlotinib or gefitinib [18]. Besides, Hannemann and colleagues were able to develop a robust protocol for the quantitative genomic analysis of single tumor cells. In their study, a heterogeneous intra- and inter-patient status of the EGFR gene was found on DTCs and CTCs [49]. However, the authors claimed that the molecular analysis of only one or two cells per blood sample can lead to results not reflecting the complex situation in the cancer patient. Recent data from Gasch et al. demonstrate a considerable intra- and inter-patient heterogeneity of genetic alterations for therapeutic targets like EGFR, KRAS, or PIK3CA, which might help to explain the variable response rates to EGFR inhibition in colon cancer patients [50]. Furthermore, Attard and colleagues performed multicolor fluorescence in situ hybridization (FISH) investigating the ERG, AR, and PTEN gene loci on CTCs and observed a genetic heterogeneity for PTEN and AR in contrast to the ERG loci in patients with castration-resistant prostate cancer (CRPC) [51].
The HER2 oncogene-encoded protein p185HER2 seems to be another promising target for a CTC-based biomarker validation as it is a well-described marker where the metrics for HER2 expression/amplification on primary breast carcinomas have been correlated with response to HER2-targeting drugs like trastuzumab or lapatinib [52]. However, several studies have shown an interesting discordance between the HER2 status of the primary tumor and CTCs in the same patients, making this protein a feasible marker for a therapy determination based on the individual CTC profile [47]. The German multicenter, randomized, phase III study (DETECT III) has started just recently to compare a standard therapy alone versus standard therapy plus lapatinib, randomizing metastatic breast cancer patients with initially HER2-negative primary breast carcinomas and HER2-positive CTCs. The results of this important intervention study will provide outstanding information for the use of CTCs as predictive markers in breast cancer.
During the last years, considerable research effort has also been concentrated on the detection of mesenchymal markers on CTCs as tumor cells disseminate using the phenotypic switching mechanism of EMT-like processes. EMT-like processes include the loss of cell–cell adhesion, downregulation of epithelial markers, and upregulation of mesenchymal markers, leading to an increased cell motility [22]. Just recently, Azab and colleagues could demonstrate that hypoxic conditions in solid tumors promote metastasis through the activation of proteins involved in the EMT process [53]. Furthermore, EMT-like changes are also thought to induce stem cell properties, making EMT-associated markers up-and-coming targets for the treatment decisions based on a CTC profile. So far, EMT- and stemness-associated markers like ALDH1, CXCR4, EGFR, FOXC2, N-Cadherin, Snail1, Twist1, ZEB2, or Vimentin have already been described to be upregulated on DTCs or CTCs in animal models as well as in patient-derived samples [24, 36, 54–56]. Therefore, EMT-associated markers could be used (1) as promising new targets in cancer therapy in future studies and (2) further might help for the identification of CTC subpopulations having undergone EMT-like processes.
Anyhow, from the current knowledge, it is not possible to deduce how many CTCs may be a reasonable number to analyze to capture inter- and intra-lesional tumor heterogeneity. New devices such as the GILUPI nanodetector [19] or leukapheresis [57] that allow the in vivo capture of CTCs from more large blood volumes (>1 l) will provide information on how much blood would be needed to capture a sufficient number of CTCs. It is conceivable that this amount might differ from patient to patient or between different solid tumor entities depending on the actual burden of CTCs, for example, patients with colon cancer appear to have lower amounts of CTCs in their peripheral blood than patients with other tumor entities, which might be due to the function of the liver as filter organ for tumor cells released from the colon.
Conclusion
Tumor cells are now detectable in the peripheral blood and they are thought to provide beneficial information for the clinical management of cancer patients as (1) stratification markers to estimate the risk for metastatic relapse and (2) as monitoring markers to estimate a change in therapy years before, the appearance of disease progression is visible using current clinical approaches. In addition, CTCs are also discussed to be (3) suitable as predictive biomarkers providing valuable new insights for an individual cancer treatment strategy. However, due to the rareness of CTCs found in a blood sample, extremely sensitive and specific analytical methods are required for their detection.
Clinical studies demonstrated that tumor cells found in the bloodstream can be deployed for diagnostic, monitoring, and prognostic purposes. Furthermore, preliminary data indicated that molecular analysis of CTCs might be used to detect specific markers which are relevant for the efficacy of cancer-related therapies, adverting for the use CTCs as predictive markers.
Monitoring of CTCs during and after therapy might provide beneficial information for the clinical management of a personalized cancer treatment, and CTCs are currently implemented into more than 400 clinical trials at which the integration of CTCs into immunotherapeutic approaches should be promising. Anyhow, the shrinkage or complete eradication of overt metastases might be an unrealistic goal that may block the further clinical development of many interesting approaches. In contrast, targeting of small micrometastases or minimal residual disease, as indicated by the presence of CTCs after surgical resection of the primary tumor, might be achievable. Thus, changes in CTC counts might serve as surrogate marker for the assessment of therapeutic efficacy and can be used as liquid biopsy for target identification. Ongoing and future clinical trials have to prove the impact of a CTC-based stratification and monitoring of therapy. These results will answer the key question whether the modification in treatment decisions based on the CTC profile will lead to a measurable benefit in clinical outcome for cancer patients.
Acknowledgments
Prof. Pantel would like to thank the European Research Council Advanced Investigator Grant No. ERC-2010-AdG_20100317 DISSECT (K.P.).
Conflict of interest
Prof. Pantel receives honoria and grant support by Veridex. Tobias M. Gorges declares that he has no conflict of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Tenth Annual Meeting of the Association for Cancer Immunotherapy (CIMT), held in Mainz, Germany, May 23–25, 2012. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massagué J. Tumor self-seeding by circulating cancer cells. Cell. 2009;139(7):1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alix-Panabières C, Schwarzenbach H, Pantel K. Circulating tumor cells and circulating tumor DNA. Annu Rev Med. 2012;63:199–215. doi: 10.1146/annurev-med-062310-094219. [DOI] [PubMed] [Google Scholar]
- 3.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4(6):448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 4.Riethdorf S, Wikman H, Pantel K. Review: biological relevance of disseminated tumor cells in cancer patients. Int J Cancer. 2008;123:1991–2006. doi: 10.1002/ijc.23825. [DOI] [PubMed] [Google Scholar]
- 5.Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, Yan J, Hua Y, Tiede BJ, Lu X, Haffty BG, Pantel K, Massagué J, Kang Y. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell. 2011;20(6):701–714. doi: 10.1016/j.ccr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8(5):329–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 7.Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J. 1869;14:146–147. [Google Scholar]
- 8.Alix-Panabières C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res. 2008;14(16):5013–5021. doi: 10.1158/1078-0432.CCR-07-5125. [DOI] [PubMed] [Google Scholar]
- 9.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben M, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumour cells, disease progression and survival in metastatic breast cancer. New Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 10.De Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumour cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 11.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse MA, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ. Prognostic significance of circulating tumour cells in patients with metastatic colorectal cancer. Ann Oncol. 2009;20:1223–1229. doi: 10.1093/annonc/mdn786. [DOI] [PubMed] [Google Scholar]
- 12.Balic M, Dandachi N, Hofmann G, Samonigg H, Loibner H, Obwaller A, van der Kooi A, Tibbe AG, Doyle GV, Terstappen LW, Bauernhofer T. Comparison of two methods for enumerating circulating tumor cells in carcinoma patients. Cytometry B Clin Cytom. 2005;68(1):25–30. doi: 10.1002/cyto.b.20065. [DOI] [PubMed] [Google Scholar]
- 13.Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schütze K, Capron F, Franco D, Pazzagli M, Vekemans M, Lacour B, Bréchot C, Paterlini-Bréchot P. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol. 2000;156(1):57–63. doi: 10.1016/S0002-9440(10)64706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebs MG, Hou JM, Sloane R, Lancashire L, Priest L, Nonaka D, Ward TH, Backen A, Clack G, Hughes A, Ranson M, Blackhall FH, Dive C. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol. 2012;7(2):306–315. doi: 10.1097/JTO.0b013e31823c5c16. [DOI] [PubMed] [Google Scholar]
- 15.Zheng S, Lin H, Liu JQ, Balic M, Datar R, Cote RJ, Tai YC. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J Chromatogr A. 2007;1162(2):154–161. doi: 10.1016/j.chroma.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 16.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, Ryan P, Balis UJ, Tompkins RG, Haber DA, Toner M. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450(7173):1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ, Bell DW, Digumarthy S, Muzikansky A, Irimia D, Settleman J, Tompkins RG, Lynch TJ, Toner M, Haber DA. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359(4):366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK, Floyd FP, Jr, Gilman AJ, Lord JB, Winokur D, Springer S, Irimia D, Nagrath S, Sequist LV, Lee RJ, Isselbacher KJ, Maheswaran S, Haber DA, Toner M. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci USA. 2010;107(43):18392–18397. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parkinson DR, Dracopoli N, Gumbs Petty B, Compton C, Cristofanilli M, Deisseroth A, Hayes DF, Kapke G, Kumar P, Lee JS, Liu MC, McCormack R, Mikulski S, Nagahara L, Pantel K, Pearson-White S, Punnoose EA, Roadcap LT, Schade AE, Scher HI, Sigman CC, Kelloff GJ. Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med. 2012;10(1):138. doi: 10.1186/1479-5876-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinberg RA. Twisted epithelial–mesenchymal transition blocks senescence. Nat Cell Biol. 2008;10:1021–1023. doi: 10.1038/ncb0908-1021. [DOI] [PubMed] [Google Scholar]
- 21.Bednarz-Knoll N, Alix-Panabières C, Pantel K. (2012) Plasticity of disseminating cancer cells in patients with epithelial malignancies. Cancer Metastasis Rev. [Epub ahead of print] doi:10.1007/s10555-012-9370-z [DOI] [PubMed]
- 22.Bartkowiak K, Effenberger KE, Harder S, Andreas A, Buck F, Peter-Katalinic J, Pantel K, Brandt BH. Discovery of a novel unfolded protein response phenotype of cancer stem/progenitor cells from the bone marrow of breast cancer patients. J Proteome Res. 2010;9(6):3158–3168. doi: 10.1021/pr100039d. [DOI] [PubMed] [Google Scholar]
- 23.Gorges TM, Tinhofer I, Drosch M, Roese L, Zollner TM, Krahn T, Ahsen O. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer. 2012;12(1):178. doi: 10.1186/1471-2407-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, Herold CI, Marcom PK, George DJ, Garcia-Blanco MA. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res. 2011;9(8):997–1007. doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kallergi G, Papadaki MA, Politaki E, Mavroudis D, Georgoulias V, Agelaki S. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 2011;13(3):R59. doi: 10.1186/bcr2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Fusi A, Klopocki E, Schmittel A, Tinhofer I, Nonnenmacher A, Keilholz U. Negative enrichment by immunomagnetic nanobeads for unbiased characterization of circulating tumor cells from peripheral blood of cancer patients. J Transl Med. 2011;9:70. doi: 10.1186/1479-5876-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan T, Zhao Q, Chen JJ, Chen WT, Pearl ML. Clinical significance of circulating tumor cells detected by an invasion assay in peripheral blood of patients with ovarian cancer. Gynecol Oncol. 2009;112(1):185–191. doi: 10.1016/j.ygyno.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riethdorf S, Fritsche H, Müller V, Rau T, Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Jänicke F, Jackson S, Gornet T, Cristofanilli M, Pantel K. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the cell search system. Clin Cancer Res. 2007;13(3):920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 29.Joosse SA, Hannemann J, Spötter J, Bauche A, Andreas A, Müller V, Pantel K. Changes in keratin expression during metastatic progression of breast cancer: impact on the detection of circulating tumor cells. Clin Cancer Res. 2012;18(4):993–1003. doi: 10.1158/1078-0432.CCR-11-2100. [DOI] [PubMed] [Google Scholar]
- 30.Barrière G, Riouallon A, Renaudie J, Tartary M, Rigaud M. Mesenchymal and stemness circulating tumor cells in early breast cancer diagnosis. BMC Cancer. 2012;12:114. doi: 10.1186/1471-2407-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasimir-Bauer S, Hoffmann O, Wallwiener D, Kimmig R, Fehm T. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res. 2012;14(1):R15. doi: 10.1186/bcr3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao C, Bui T, Connelly M, Doyle G, Karydis I, Middleton MR, Clack G, Malone M, Coumans FA, Terstappen LW. Circulating melanoma cells and survival in metastatic melanoma. Int J Oncol. 2011;38(3):755–760. doi: 10.3892/ijo.2011.896. [DOI] [PubMed] [Google Scholar]
- 33.Hoshimoto S, Shingai T, Morton DL, Kuo C, Faries MB, Chong K, Elashoff D, Wang HJ, Elashoff RM, Hoon DS. Association between circulating tumor cells and prognosis in patients with stage III melanoma with sentinel lymph node metastasis in a phase III international multicenter trial. J Clin Oncol. 2012;30(31):3819–3826. doi: 10.1200/JCO.2011.40.0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alix-Panabières C. EPISPOT assay: detection of viable DTCs/CTCs in solid tumor patients. Recent Results Cancer Res. 2012;195:69–76. doi: 10.1007/978-3-642-28160-0_6. [DOI] [PubMed] [Google Scholar]
- 35.Wülfing P, Borchard J, Buerger H, Heidl S, Zänker KS, Kiesel L, Brandt B. HER2-positive circulating tumor cells indicate poor clinical outcome in stage I–III breast cancer patients. Clin Cancer Res. 2006;12(6):1715–1720. doi: 10.1158/1078-0432.CCR-05-2087. [DOI] [PubMed] [Google Scholar]
- 36.Bednarz N, Eltze E, Semjonow A, Rink M, Andreas A, Mulder L, Hannemann J, Fisch M, Pantel K, Weier HU, Bielawski KP, Brandt B. BRCA1 loss preexisting in small subpopulations of prostate cancer is associated with advanced disease and metastatic spread to lymph nodes and peripheral blood. Clin Cancer Res. 2010;16(13):3340–3348. doi: 10.1158/1078-0432.CCR-10-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV, Terstappen LW, Hayes DF. Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006;12(21):6403–6409. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- 38.Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LW. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12(14 Pt 1):4218–4224. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- 39.Stojadinovic A, Mittendorf EA, Holmes JP, Amin A, Hueman MT, Ponniah S, Peoples GE Quantification and phenotypic characterization of circulating tumor cells for monitoring response to a preventive HER2/neu vaccine-based immunotherapy for breast cancer: a pilot study. Ann Surg Oncol. 2007;14(12):3359–3368. doi: 10.1245/s10434-007-9538-x. [DOI] [PubMed] [Google Scholar]
- 40.Saad F, Pantel K. The current role of circulating tumor cells in the diagnosis and management of bone metastases in advanced prostate cancer. Future Oncol. 2012;8(3):321–331. doi: 10.2217/fon.12.3. [DOI] [PubMed] [Google Scholar]
- 41.Scher HI, Morris MJ, Basch E, Heller G. End points and outcomes in castration-resistant prostate cancer: from clinical trials to clinical practice. J Clin Oncol. 2011;29(27):3695–3704. doi: 10.1200/JCO.2011.35.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lianidou ES, Markou A. Circulating tumor cells as emerging tumor biomarkers in breast cancer. Clin Chem Lab Med. 2011;49(10):1579–1590. doi: 10.1515/CCLM.2011.628. [DOI] [PubMed] [Google Scholar]
- 43.Xenidis N, Markos V, Apostolaki S, Perraki M, Pallis A, Sfakiotaki G, Papadatos-Pastos D, Kalmanti L, Kafousi M, Stathopoulos E, Kakolyris S, Mavroudis D, Georgoulias V. Clinical relevance of circulating CK-19 mRNA-positive cells detected during the adjuvant tamoxifen treatment in patients with early breast cancer. Ann Oncol. 2007;18(10):1623–1631. doi: 10.1093/annonc/mdm208. [DOI] [PubMed] [Google Scholar]
- 44.Bidard FC, Mathiot C, Delaloge S, Brain E, Giachetti S, de Cremoux P, Marty M, Pierga JY. Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol. 2010;21(4):729–733. doi: 10.1093/annonc/mdp391. [DOI] [PubMed] [Google Scholar]
- 45.Georgoulias V, Bozionelou V, Agelaki S, Perraki M, Apostolaki S, Kallergi G, Kalbakis K, Xyrafas A, Mavroudis D. Trastuzumab decreases the incidence of clinical relapses in patients with early breast cancer presenting chemotherapy-resistant CK-19mRNA-positive circulating tumor cells: results of a randomized phase II study. Ann Oncol. 2012;23(7):1744–1750. doi: 10.1093/annonc/mds020. [DOI] [PubMed] [Google Scholar]
- 46.Rack B, Schindlbeck C, Andergassen U, Lorenz R, Zwingers T, Schneeweiss A, Lichtenegger W, Beckmann MW, Sommer H, Pantel K, Friese K, Janni W. Use of circulating tumor cells (CTCs) in peripheral blood of breast cancer patients before and after adjuvant chemotherapy to predict risk for relapse: the SUCCESS Trial 7. Clin Oncol. 2020;28:7s. [Google Scholar]
- 47.Riethdorf S, Müller V, Zhang L, Rau T, Loibl S, Komor M, Roller M, Huober J, Fehm T, Schrader I, Hilfrich J, Holms F, Tesch H, Eidtmann H, Untch M, von Minckwitz G, Pantel K. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res. 2010;16(9):2634–2645. doi: 10.1158/1078-0432.CCR-09-2042. [DOI] [PubMed] [Google Scholar]
- 48.Punnoose EA, Atwal SK, Spoerke JM, Savage H, Pandita A, Yeh RF, Pirzkall A, Fine BM, Amler LC, Chen DS, Lackner MR. Molecular biomarker analyses using circulating tumor cells. PLoS ONE. 2010;5(9):e12517. doi: 10.1371/journal.pone.0012517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hannemann J, Meyer-Staeckling S, Kemming D, Alpers I, Joosse SA, Pospisil H, Kurtz S, Görndt J, Püschel K, Riethdorf S, Pantel K, Brandt B. Quantitative high-resolution genomic analysis of single cancer cells. PLoS ONE. 2011;6(11):e26362. doi: 10.1371/journal.pone.0026362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gasch C, Bauernhofer T, Pichler M, Langer-Freitag S, Reeh M, Seifert AM, Mauermann O, Izbicki JR, Pantel K, Riethdorf S. Heterogeneity of epidermal growth factor receptor status and mutations of KRAS/PIK3CA in circulating tumor cells of patients with colorectal cancer. Clin Chem. 2013;59(1):252–260. doi: 10.1373/clinchem.2012.188557. [DOI] [PubMed] [Google Scholar]
- 51.Attard G, Swennenhuis JF, Olmos D, Reid AH, Vickers E, A’Hern R, Levink R, Coumans F, Moreira J, Riisnaes R, Oommen NB, Hawche G, Jameson C, Thompson E, Sipkema R, Carden CP, Parker C, Dearnaley D, Kaye SB, Cooper CS, Molina A, Cox ME, Terstappen LW, de Bono JS. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69(7):2912–2918. doi: 10.1158/0008-5472.CAN-08-3667. [DOI] [PubMed] [Google Scholar]
- 52.Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2011;9(1):16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 53.Azab AK, Hu J, Quang P, Azab F, Pitsillides C, Awwad R, Thompson B, Maiso P, Sun JD, Hart CP, Roccaro AM, Sacco A, Ngo HT, Lin CP, Kung AL, Carrasco RD, Vanderkerken K, Ghobrial IM. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood. 2012;119(24):5782–5794. doi: 10.1182/blood-2011-09-380410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mego M, Mani SA, Lee BN, Li C, Evans KW, Cohen EN, Gao H, Jackson SA, Giordano A, Hortobagyi GN, Cristofanilli M, Lucci A, Reuben JM. Expression of epithelial-mesenchymal transition-inducing transcription factors in primary breast cancer: the effect of neoadjuvant therapy. Int J Cancer. 2012;130(4):808–816. doi: 10.1002/ijc.26037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Powell AA, Talasaz AH, Zhang H, Coram MA, Reddy A, Deng G, Telli ML, Advani RH, Carlson RW, Mollick JA, Sheth S, Kurian AW, Ford JM, Stockdale FE, Quake SR, Pease RF, Mindrinos MN, Bhanot G, Dairkee SH, Davis RW, Jeffrey SS. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS ONE. 2012;7(5):e33788. doi: 10.1371/journal.pone.0033788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eifler RL, Lind J, Falkenhagen D, Weber V, Fischer MB, Zeillinger R. Enrichment of circulating tumor cells from a large blood volume using leukapheresis and elutriation: proof of concept. Cytometry B Clin Cytom. 2011;80(2):100–111. doi: 10.1002/cyto.b.20560. [DOI] [PubMed] [Google Scholar]