Abstract
Prostate cancer cells can produce IL-18 binding protein (IL-18BP) in response to interferon-γ (IFN-γ), which may function to neutralize IL-18, an anti-tumor factor formerly known as IFN-γ inducing factor. The consumption of n-3 polyunsaturated fatty acids (PUFAs) has been associated with a lower risk of certain types of cancer including prostate cancer, although the precise mechanisms of this effect are poorly understood. We hypothesized that n-3 PUFAs could modify IL-18BP production by prostate cancer cells by altering IFN-γ receptor-mediated signal transduction. Here, we demonstrate that n-3 PUFA treatment significantly reduced IFN-γ-induced IL-18BP production by DU-145 and PC-3 prostate cancer cells by inhibiting IL-18BP mRNA expression and was associated with a reduction in IFN-γ receptor expression. Furthermore, IFN-γ-induced phosphorylation of Janus kinase 1 (JAK1), signal transducers and activators of transcription 1 (STAT1), extracellular signal-regulated kinases 1/2 (ERK1/2), and P38 were suppressed by n-3 PUFA treatment. By contrast, n-6 PUFA had no effect on IFN-γ receptor expression, but decreased IFN-γ-induced IL-18BP production and IFN-γ stimulation of JAK1, STAT1, ERK1/2, and JNK phosphorylation. These data indicate that both n-3 and n-6 PUFAs may be beneficial in prostate cancer by altering IFN-γ signaling, thus inhibiting IL-18BP production and thereby rendering prostate cancer cells more sensitive to IL-18-mediated immune responses.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1630-z) contains supplementary material, which is available to authorized users.
Keywords: N-3 PUFAs, IFN-γ signaling, IL-18 binding protein, Prostate cancer cells
Introduction
Prostate cancer is one of the most common solid cancers worldwide. It is estimated that approximately one in 1,600 men in developed countries will develop prostate cancer at some point in their lives with Oceania, Europe, and North America having the highest incidence rates in the world [1]. Furthermore, it is expected that Canada will experience a dramatic increase in new cases of prostate cancer by 2021 [2]. Over the past couple of decades, some immune therapies for prostate cancer have focused on specific cytokines.
Interleukin (IL)-18 is a member of the IL-1 family (originally designated interferon-γ (IFN-γ)-inducing factor) and was first identified as a major immunomodulatory cytokine, activating macrophages to produce IFN-γ and neutrophils to release tumor necrosis factor (TNF) [3, 4]. In addition, IL-18 promotes the proliferation and enhances the cytotoxicity of natural killer (NK) cells [5]. The growth of both subcutaneous and orthotopic prostate cancers is suppressed by IL-18 in a mouse model of RM1 prostate cancer [6], and IL-18 increases the tumor infiltration of neutrophils, macrophages, CD8+ T cells, and CD4+ T cells. This effect is dependent on IFN-γ [6].
IFN-γ is a major T helper (Th)1-type cytokine produced mainly by Th1 cells, cytotoxic T cells, and γδ T cells in the cancer microenvironment [7, 8]. IFN-γ signals through the IFN-γ receptor (IFN-γR) and can inhibit tumor cell proliferation by activating signal transducers and activators of transcription1 (STAT1) homodimers [9], promote apoptosis by increasing the expression of CD95 (Fas) and TNF-related apoptosis-inducing ligand [10], enhance killing of cytotoxic T cells by increasing the surface expression of MHC class I on cancer cells [11], inhibit the generation and activation of regulatory T cells [12], and down-regulate angiogenesis [13].
The anti-tumor effects of IL-18 and IFN-γ can be regulated by IL-18 binding protein (IL-18BP), a soluble inhibitory receptor for IL-18 that is constitutively present in human serum at 4.9 ng [14]. Enhanced production of IL-18BP is associated with resistance to anti-tumor immune responses and correlates with poor prognosis in patients with prostate cancer [15]. In addition, activation of a cell-mediated, tumor-directed immune system may itself activate the production of IL-18BP by cancer cells. Cancer cell-derived IL-18BP may thereby dampen localized cell-mediated immune responses and result in abrogated NK and CD8+ T cell responses typical of prostate cancer [16]. DU-145 and PC-3 human prostate cancer lines produce IL-18BP when they are treated with IFN-γ, which may allow the prostate cancer tissue to evade the immune system [15].
Diets rich in n-3 polyunsaturated fatty acids (PUFAs), such as docosahexaenoic acid (DHA, 22:6, n-3) and eicosapentaenoic acid (EPA, 20:5, n-3), have been shown to reduce the risk of developing prostate cancer [17]. Dietary n-3 PUFA treatment was also reported to potentiate the effect of hormone ablation therapy on androgen-dependent prostate cancer and prevent the development toward androgen-independent prostate cancer [18]. N-3 PUFAs inhibit IFN-γ signaling in macrophages, suggesting that they are able to directly modify IFN-γ-mediated immune responses [19]. The effect of n-3 PUFAs on IFN-γ-induced IL-18BP production by prostate cancer cells has not been studied, and we hypothesized that n-3 PUFAs could modify IL-18BP production by prostate cancer cells, possibly by modifying IFN-γR-mediated signal transduction. In this study, we evaluated the effect of n-3 PUFAs on IFN-γ signaling and IL-18BP production by human prostate cancer cells, DU-145 and PC-3.
Materials and methods
Cell lines
Human prostate DU-145 and PC-3 carcinoma cells were purchased from American type culture collection, maintained in RPMI 1640 medium (Corning cellgro, Manassas, VA, USA), and supplemented with 10 % FBS (Corning cellgro), 100 U/mL penicillin, and 100 μg/mL streptomycin (Corning cellgro). The media were changed every 3–4 days. The percentage of live cells after PUFA-Na treatment was determined by staining the cells with 0.4 % trypan blue (Sigma-Aldrich, Oakville, ON, Canada).
Enzyme-linked immunosorbent assay (ELISA)
DU-145 cells were incubated with 0.4, 2, 10, 50 ng/mL IFN-γ (R&D Systems, Minneapolis, MN, USA) for 24 h, or pre-treated with 10, 50, 100 µM of EPA-Na (Sigma-Aldrich), DHA-Na (Sigma-Aldrich), and arachidonic acid (AA)-Na (20:4, n-6; Sigma-Aldrich) in phosphate-buffered saline (PBS) for 2 and 24 h followed with 24-h treatment of 10 ng/mL IFN-γ. PC-3 cells were pre-treated with 100 µM of EPA-Na, DHA-Na, and AA-Na in PBS for 2 or 24 h followed by a 24-h treatment with 10 ng/mL of IFN-γ. The supernatant was analyzed for IL-18BP release using a commercial ELISA kit (Abcam, Cambridge, MA, USA) according to the manufacturer’s instructions.
Gas chromatography–mass spectrometry (GC–MS)
EPA-Na, DHA-Na, and AA-Na in PBS were added to cell cultures to a final concentration of 100 µM, and the cultures were incubated at 37 °C for 24 h. Lipid was extracted and methylated by a method modified from Kang et al. [20]. Briefly, 5 × 105 cells were mixed with 1 mL hexane and 1 mL 14 % BF3/MeOH after 24 h PUFA treatment. After blanketing with nitrogen, samples were heated at 100 °C for 1 h and then centrifuged on 200×g for 1 min after adding 1 mL H2O. The upper hexane layer containing fatty acid methyl esters was collected for GC–MS analysis.
GC–MS was performed in an Agilent 6890N Network GC System (Agilent Technologies, Mississauga, ON, Canada) with a split/splitless injector (Agilent Technologies) and DB-23 column (Agilent Technologies). Fatty acid components were quantified by a flame ionization detector (Agilent Technologies) and qualified by a MS detector (Agilent Technologies). Supelco 37 component fatty acid methyl ester mix standard (Sigma-Aldrich) was used to check the performance of the GC instrument. Samples were analyzed at 130 °C for 1.0 min, 130–170 °C at 6.5 °C/min, 170–215 °C at 2.75 °C/min, 215 °C for 12 min, 215–230 °C at 40 °C/min, and 230 °C for 3 min with hydrogen as the carrier gas.
RNA isolation, cDNA synthesis, and quantitative polymerase chain reaction (qPCR)
DU-145 cells were pre-treated with 100 μM of PUFA-Na for 24 h and then stimulated for an additional 4 h with 10 ng/mL of IFN-γ. Total RNA was isolated from cells using TRI reagent (Sigma-Aldrich). First strand cDNA was generated from 1 μg RNA with a mixture of 200 U Moloney murine leukemia virus reverse transcriptase (Life Technologies), 500 μg/mL oligo (dT) primer (Promega, Madison, WI, USA), 10 mM dNTP Mix (Promega), 0.1 M DTT (Life Technologies), and 5× first strand buffer (Life Technologies). Duplex qPCR amplification of the IL-18BP and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) genes in each sample were performed using qPCR master mix containing 100 ng cDNA reaction mixture, 1× PCR Buffer (Life Technologies), 50 mM MgCl2 (Life Technologies), 10 mM dNTP mix (Life Technologies), and 5 U/µL Taq DNA polymerase (Life Technologies). The primer sets were designed using Primer-BLAST. All reactions were performed in triplicate for 40 cycles. The expression of IL-18BP was normalized using the Ct of GAPDH and was calculated by the method [21].
Protein isolation and western blot analysis
To evaluate the effect of n-3 PUFAs on IFN-γ receptor expression, DU-145 cells were treated with 100 μM of PUFA-Na for 24 h. To determine the effect of n-3 PUFAs on signal transduction of IFN-γ pathway, DU-145 cells were pre-treated with 100 μM of PUFA-Na for 24 h and then stimulated for an additional 30 min with 10 ng/mL of IFN-γ. Protein was extracted from cells by lysing the cells with lysis buffer containing 1 % triton X-100 (Sigma-Aldrich), 1× complete protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN, USA), 1× protease inhibitor cocktail (Sigma-Aldrich), 50 μg/mL 3,4 dichloroisocoumarin (Roche Molecular Biochemicals), 1 mM benzamidine (Sigma-Aldrich), 1 mM sodium orthovanadate (Sigma-Aldrich), 5.4 mM sodium pyrophosphate (Sigma-Aldrich), and 50 mM sodium fluoride (Sigma-Aldrich) in tris-buffered saline (TBS). After measuring the protein concentration using the Bradford protein assay (Sigma-Aldrich), the protein concentration of each sample was adjusted to the same level using lysis buffer. Finally, the samples were boiled for 5 min in 4× NuPage lithium dodecyl sulfate sample buffer (Life Technologies).
Protein samples were electrophoresed on a NuPAGE Novex 4–12 % Bis–Tris Gel (Life Technologies) at 200 V for 30 min. Separated proteins were transferred to a nitrocellulose membrane (Life Technologies) at 30 V for 1 h. The membrane was removed and washed with TBST (TBS containing 0.05 % tween-20). After blocking with 5 % nonfat milk/TBST at 4 °C over night, the membrane was probed with rabbit polyclonal antihuman IFN-γRα (IFN-γR1, Santa Cruz Biotechnology, Santa Cruz, CA, Canada), rabbit polyclonal antihuman Janus kinase 1 (JAK1) (BD Biosciences, Mississauga, ON, Canada), rabbit polyclonal antihuman pho-JAK1 (Cell Signaling Technology, Danvers, MA, USA), rabbit polyclonal antihuman STAT1 (BD Biosciences), rabbit polyclonal antihuman pho-STAT1 (BD Biosciences), rabbit polyclonal antihuman extracellular signal-regulated kinase1/2 (ERK1/2; Cell Signaling Technology), rabbit polyclonal antihuman pho-ERK1/2 (Sigma-Aldrich), rabbit polyclonal antihuman c-Jun N-terminal kinase (JNK; Cell Signaling Technology), rabbit polyclonal antihuman pho-JNK (Cell Signaling Technology), rabbit polyclonal antihuman P38 (Cell Signaling Technology), rabbit polyclonal antihuman pho-P38 (Sigma-Aldrich), and mouse monoclonal antihuman actin (Sigma-Aldrich) antibody diluted with 5 % nonfat milk/TBST at room temperature for 3 h. The membrane was washed with TBST three times for 15 min each and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology) and horseradish peroxidase-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology) diluted in 5 % nonfat milk/TBST at room temperature for 1 h. After washing with TBST three times for 15 min each, the blot was developed in chemiluminescent peroxidase substrate (Sigma-Aldrich) for 1 min and exposed to UV light in a ChemiDoc XRS system (BD Biosciences) for image acquisition.
Statistical analysis
Data were analyzed using SPSS 11.5 software (IBM Corporation, Armonk, NY, USA). One-way ANOVA followed with the Bonferroni posttest was performed to determine the significance of the observed differences. The statistical significance was set at p < 0.05.
Results
N-3 PUFA treatment increased n-3 PUFA level in DU-145 cells
The effect of fatty acid treatment on DU145 and PC-3 cell viability was determined using trypan blue assay. None of the fatty acids (EPA-NA, DHA-Na, or AA-Na) that were tested had any significant effect on DU145 or PC-3 cell viability after 2-h and 24-h treatments (supplementary Figure 1). To determine whether the DU-145 cells were absorbing the PUFAs in culture, we ascertained the fatty acid profile of the DU-145 whole cell lysates after treatment. Compared to the untreated control, treatment with 100 µM of EPA-Na significantly increased the amount of EPA, docosapentaenoic acid (DPA), and DHA in the cell lysates. Treatment with DHA-Na (100 μM) increased the amount of EPA and DHA in the cell lysates. Treatment with AA-Na (100 µM) increased the amount of AA in the cell lysates. Notably, EPA-Na and DHA-Na treatment decreased AA content in the cell lysates. EPA-Na, DHA-Na, and AA-Na treatments (100 µM) all significantly decreased oleic acid (18:1, n-9) and linoleic acid (18:2, n-6) levels. EPA-Na and DHA-Na treatments resulted in a significantly decreased n-6/n-3 ratio. AA-Na treatment significantly increased the n-6/n-3 ratio (Table 1).
Table 1.
Fatty acid composition of PUFA-treated DU-145 cells
| Fatty acids | Composition (% of total long chain fatty acids identified) | |||
|---|---|---|---|---|
| Control | EPA | DHA | AA | |
| 16:0 | 24.99 ± 0.04 | 26.78 ± 0.15 | 24.82 ± 0.91 | 30.90 ± 0.15 |
| 18:0 | 17.12 ± 0.08 | 14.77 ± 0.06 | 14.38 ± 0.64 | 17.12 ± 0.03 |
| 18:1, n-9 | 41.35 ± 0.22 | 16.75 ± 0.14*** | 16.61 ± 0.50*** | 19.66 ± 0.34*** |
| 18:2, n-6 | 2.89 ± 0.08 | 1.01 ± 0.01*** | 0.95 ± 0.02*** | 0.31 ± 0.06*** |
| 20:4, n-6 (AA) | 5.46 ± 0.22 | 3.52 ± 0.13*** | 2.70 ± 0.05*** | 25.52 ± 0.34*** |
| 20:5, n-3 (EPA) | 0.42 ± 0.03 | 7.07 ± 0.15*** | 1.05 ± 0.06*** | 0.60 ± 0.01 |
| 22:5, n-3 (DPA) | 4.98 ± 0.55 | 25.99 ± 0.09*** | 5.27 ± 2.36 | 3.97 ± 0.91 |
| 22:6, n-3 (DHA) | 2.80 ± 0.13 | 4.12 ± 0.11* | 34.22 ± 0.88*** | 1.91 ± 0.06 |
| n-6/n-3 ratio | 1.02 ± 0.09 | 0.122 ± 0.01** | 0.09 ± 0.01** | 4.04 ± 0.64*** |
* p < 0.05, compared with control, one-way ANOVA
** p < 0.01, compared with control, one-way ANOVA
*** p < 0.001, compared with control, one-way ANOVA
N-3 PUFAs reduced IL-18BP production by DU-145 cells and PC-3 cells
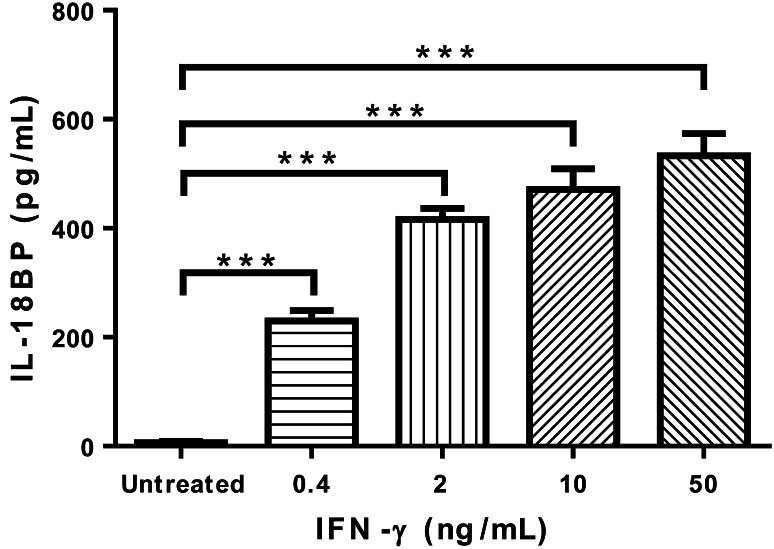
DU-145 cells produce IL-18BP after IFN-γ stimulation in a dose-dependent manner, and a 24-h treatment with 0.4, 2, 10, 50 ng/mL IFN-γ all significantly induced IL-18BP production by DU-145 cells (Fig. 1).
Fig. 1.
IFN-γ induces IL-18BP production by DU-145 cells. ELISA measurement of IL-18BP production by DU-145 cells induced by IFN-γ treatment at indicated concentrations for 24 h. ***p < 0.001, compared with untreated cells, one-way ANOVA followed with Bonferroni posttest. Error bars represent SEM (n = 3)
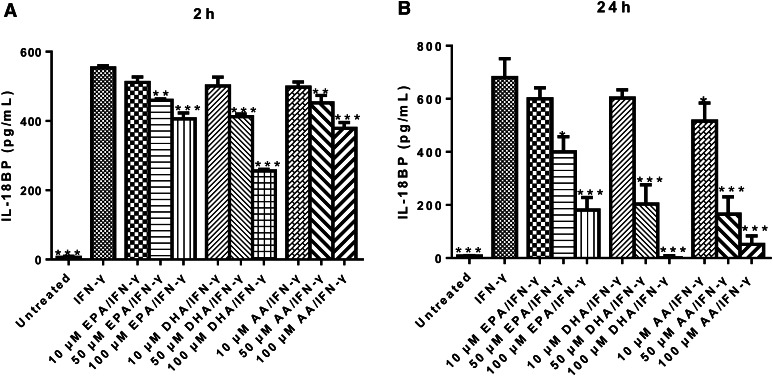
To evaluate the effect of n-3 PUFAs on IFN-γ-induced IL-18BP production, DU-145 cells were pre-treated with PUFA-Na for 2 or 24 h, then stimulated with IFN-γ for 24 h, and IL-18BP production was measured by ELISA. Treatment with all three PUFAs (EPA-Na, DHA-Na, and AA-Na) for 2 h and 24 h showed a dose-dependent inhibition of IFN-γ-induced IL-18BP production (Fig. 2a, b).
Fig. 2.
N-3 PUFA treatment inhibits IFN-γ-induced IL-18BP production by DU-145 cells. a ELISA determination of IL-18BP production by DU-145 cells pre-treated with indicated concentrations of PUFA-Na for 2 h, followed with 10 ng/mL IFN-γ treatment for 24 h. b ELISA measurement of IL-18BP production by DU-145 cells pre-treated with indicated concentrations of PUFA-Na for 24 h, followed with 10 ng/mL IFN-γ treatment for 24 h. *p < 0.05; **p < 0.01; ***p < 0.001, compared with cells treated with IFN-γ alone, one-way ANOVA followed with Bonferroni posttest. Error bars represent SEM (n = 3)
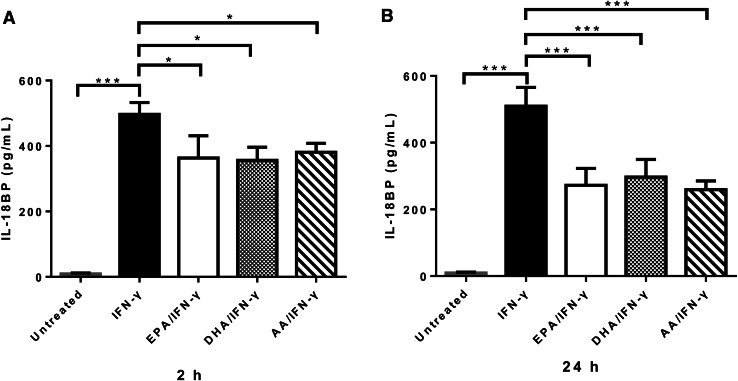
Similarly, IFN-γ-induced IL-18BP production by PC-3 cells was significantly decreased by 100 µM of EPA-Na, DHA-Na, and AA-Na for 2 h (Fig. 3a) and 24 h (Fig. 3b).
Fig. 3.
N-3 PUFA treatment reduces IFN-γ-induced IL-18BP production by PC-3 cells. a ELISA determination of IL-18BP production by PC-3 cells pre-treated with 100 µM PUFA-Na for 2 h, followed with 10 ng/mL IFN-γ treatment for 24 h. b ELISA measurement of IL-18BP production by PC-3 cells pre-treated with 100 µM PUFA-Na for 24 h, followed with 10 ng/mL IFN-γ treatment for 24 h. *p < 0.05; ***p < 0.001, compared with cells treated with IFN-γ alone, one-way ANOVA followed with Bonferroni posttest. Error bars represent SEM (n = 3)
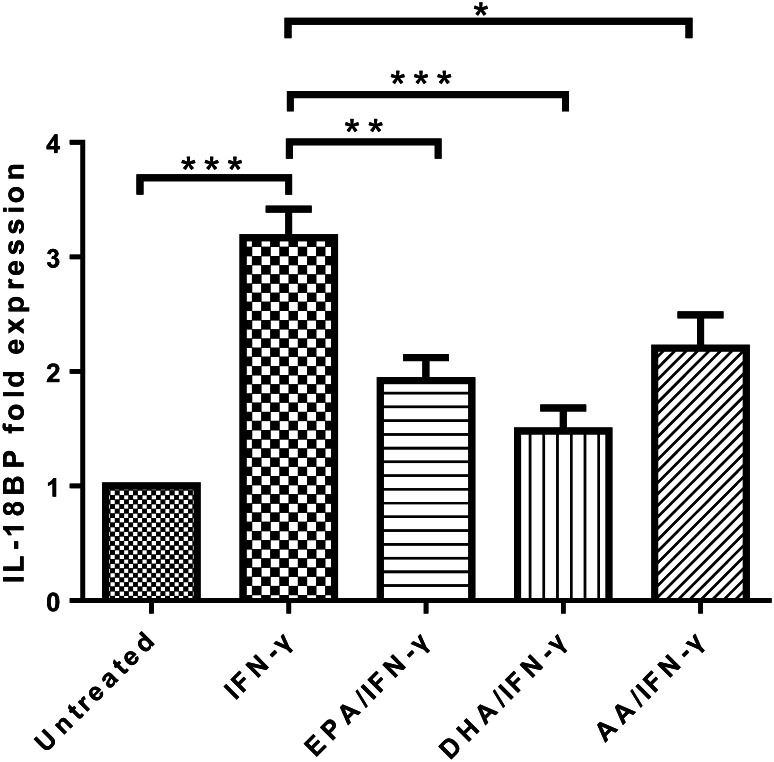
N-3 PUFAs inhibited IFN-γ-induced IL-18BP mRNA expression
We performed qPCR to see if the suppressing effect of n-3 PUFAs on IL-18BP production by IFN-γ-treated DU-145 cells was transcription-dependent. IFN-γ treatment for 4 h increased IL-18BP mRNA expression by DU-145 cells. However, 24 h pre-treatments with 100 μM of EPA-Na, DHA-Na, and AA-Na all significantly suppressed the increased IL-18BP mRNA expression in DU-145 cells induced by IFN-γ treatment (Fig. 4).
Fig. 4.
N-3 PUFA treatment suppresses IFN-γ-induced IL-18BP mRNA expression in DU-145 cells. qPCR measurement of IL-18BP mRNA expression in DU-145 cells pre-treated with 100 μM PUFA-Na for 24 h, followed with 10 ng/mL IFN-γ treatment for 4 h. *p < 0.05; **p < 0.01; ***p < 0.001, compared with cells treated with IFN-γ alone, one-way ANOVA followed with Bonferroni posttest. Error bars represent SEM (n = 4)
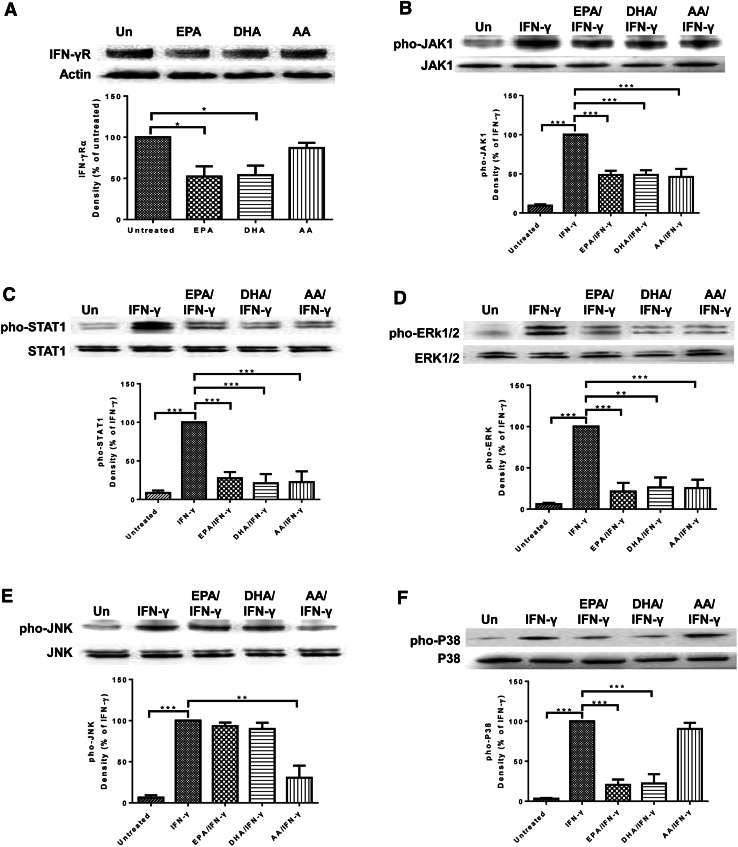
N-3 PUFAs inhibited the expression of IFN-γR and supressed IFN-γR-mediated signal transduction
Next, we measured the effect of n-3 PUFA treatment on IFN-γR expression and IFN-γR-mediated signal transduction in DU-145 cells. Western blot analysis of IFN-γR expression showed that EPA and DHA, but not AA, treatment significantly decreased IFN-γR expression by DU-145 cells (Fig. 5a). To evaluate the effect of n-3 PUFAs on IFN-γR-mediated signal transduction, we treated the cells with PUFAs for 24 h and then stimulated them with IFN-γ for 30 min. EPA treatment significantly decreased IFN-γ-induced phosphorylation of JAK1 (Fig. 5b), STAT1 (Fig. 5c), ERK1/2 (Fig. 5d), and P38 (Fig. 5f). Similarly, DHA treatment significantly decreased phosphorylation of JAK1 (Fig. 5b), STAT1 (Fig. 5c), ERK1/2 (Fig. 5d), and P38 (Fig. 5f) induced by IFN-γ. AA treatment reduced phosphorylation of JAK1 (Fig. 5b), STAT1 (Fig. 5c), ERK1/2 (Fig. 5d), and JNK (Fig. 5e).
Fig. 5.
N-3 PUFA treatment inhibits IFN-γR expression and IFN-γ signaling in DU-145 cells. a Western blot analysis of IFN-γRα expression in DU-145 cells pre-treated with 100 μM PUFA for 24 h. b–f Western blot measurement of pho-JAK1 and JAK1 (b), pho-STAT1 and STAT1 (c), pho-ERK1/2 and ERK1/2 (d), pho-JNK and JNK (e), and pho-P38 and P38 (f) in DU-145 cells pre-treated with 100 μM PUFA for 24 h, followed with 10 ng/mL IFN-γ treatment for 30 min. Data are representative of three independent experiments with similar result. *p < 0.05; **p < 0.01; ***p < 0.001, compared with untreated cells (a) or cells treated with IFN-γ alone (b–f), one-way ANOVA followed with Bonferroni posttest. Error bars represent SEM (n = 3). Un untreated
Discussion
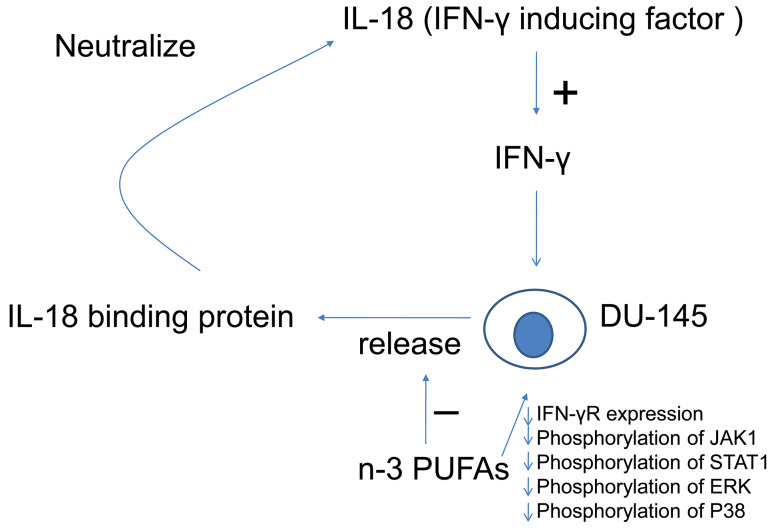
Our data indicate that n-3 PUFAs modified the IFN-γ/IL-18BP/IL-18/IFN-γ loop (Fig. 6). IFN-γ dose-dependently stimulated DU-145 prostate cancer cells to produce IL-18BP in a transcription-dependent manner, confirming an earlier study [15]. We further showed that DU-145 cells incorporated higher amounts of n-3 PUFAs when they were cultured with soluble forms of the fatty acids, and IFN-γ-induced IL-18BP production by DU-145 prostate cancer cells was diminished by n-3 PUFA treatment in a transcription-dependent manner. A similar effect was observed with the PC-3 prostate cancer line. Our data also showed that n-3 PUFAs decreased the expression of IFN-γR and inhibited the signal transduction initiated by functional IFN-γR in DU-145 prostate cancer cells. IFN-γ-induced phosphorylation of JAK1, STAT1, ERK1/2, and P38 was inhibited by n-3 PUFA treatments. Supplementation of AA, an n-6 PUFA, had a similar suppressing effect on IFN-γ-induced IL-18BP production and mRNA expression. Although n-6 PUFA treatment did not change IFN-γR expression, n-6 PUFA inhibited JAK1, STAT1, ERK1/2, and JNK phosphorylation under IFN-γ stimulation suggesting that n-6 PUFA may influence membrane-proximal events such as IFN-γR dimerization or directly inhibit JAK1/2 autophosphorylation. Therefore, n-3 and n-6 PUFAs have fundamentally different effects on IFN-γ signaling, and this warrants further investigation.
Fig. 6.
Summary of the balancing effect of n-3 PUFAs on the IFN-γ/IL-18BP/IL-18/IFN-γ loop. N-3 PUFAs decrease IFN-γR expression and inhibit IFN-γ-mediated signal transduction in DU-145 cells, so that suppress IFN-γ-induced IL-18BP production by DU-145 cells. With less IL-18BP, there is more functional IL-18 to maintain IFN-γ level, which has anti-tumor effects
Certainly, IFN-γ is a complex cytokine with pleiotropic effects on prostate cancer cells. IFN-γ inhibits the DU-145 cell cycle by inducing the expression of cyclin-dependent kinase inhibitor p21WAF1 [22] and dose-dependently inhibits DU-145 and PC-3 cell proliferation by down-regulating neu/HER-2 expression, but has no effect on LNCaP prostate cancer cell proliferation [23]. Some cancer cells escape the cell-mediated immune response by decreasing the expression of Fas and thus avoiding Fas-mediated cell death [24]. However, IFN-γ up-regulates Fas and activates Fas-mediated killing of LNCaP and PC-3 cells [25]. Furthermore, an in vitro study has shown that IFN-γ has the ability to reduce the metastatic ability of DU-145 cells, possibly due to changes in the expression of adhesion molecules [22]. The clinical use of IFN-γ in treating cancer such as melanoma has been tested in some studies, and IFN-γ has shown promising effects [26]. However, IFN-γ has also been shown to have dual effects on some forms of transformed cells in vivo. He et al. has reported that injecting mice with plasmids that produce low levels of IFN-γ (10 µg) can promote the growth of H22 hepatoma, MA782/5S mammary adenocarcinoma, and B16 melanoma in these mice. When the mice were injected with a tenfold higher amount of IFN-γ-producing plasmid constructs (100 µg), the growth of the H22 hepatoma cells was inhibited [27].
Cancer progression is a complex relationship between the host’s immune response and the immune-evasion strategies of the transformed cells. This push–pull relationship requires the constant and coordinated production of several factors, one of which is the IL-18/IL-18BP protein axis. In this study, we have shown that IFN-γ stimulation resulted in IL-18BP production by DU-145 and PC-3 cells. IL-18BP functions to neutralize IL-18 [28], which is known as a stimulator of IFN-γ production [29]. IL-18 may contribute to the elimination of cancer by inducing the production of IFN-γ. Furthermore, it has been reported that IL-18 directly activates NK cells and cytotoxic T cells in the tumor environment [30]. Therefore, producing IL-18BP might be an attempt of cancer cells to counteract the harmful stimuli delivered by IL-18 through IFN-γ.
To our knowledge, our study is the first to report the inhibitory effect of n-3 and n-6 PUFAs on IFN-γR-mediated signal transduction in the context of prostate cancer cells. Some previous studies have shown that treatment with n-3 PUFAs inhibits proliferation of some types of cancer cells, including breast, lung, colon, and prostate cancer cells [31–33]. In our study, fatty acid treatment (up to 100 μM) did not significantly alter cell viability (supplementary Figure 1). We also measured the fatty acid profile of cells cultured with n-3 and n-6 PUFAs to determine whether PUFA treatment changed the fatty acid content of prostate cancer cells in vitro. The results showed that EPA treatment increased EPA, DPA, and DHA levels in the whole cell lysates. DHA treatment increased EPA and DHA levels, and AA treatment increased AA level in the cells. EPA and DHA treatments all decreased the n-6/n-3 ratio in the DU-145 cells, and AA treatment increased the n-6/n-3 ratio, suggesting the successful incorporation of PUFAs into the cells.
IL-18BP production by IFN-γ-treated DU-145 and PC-3 cells was inhibited by EPA, DHA, and AA treatments, indicating that PUFAs inhibited IFN-γ signaling. Our data indicate that n-3 PUFAs may modulate IFN-γ-induced IL-18BP production by down-regulating surface IFN-γR, a process that can occur in vivo. Feng et al. [34] has shown that a high n-3 PUFA diet in mice reduces IFN-γR surface expression by peritoneal macrophages and splenocytes. However, Bonilla et al. [35] reported that although n-3 PUFA treatment in vitro inhibits IFN-γ-induced STAT1 phosphorylation and pro-inflammatory cytokine production, it is not dependent upon a change in the surface expression of IFN-γR.
In contrast to the effects of n-3 PUFAs, AA did not change IFN-γR expression but modified the downstream signaling of the JAK/STAT pathway. A functional IFN-γR contains two IFN-γR1 chains for ligand-binding and two IFN-γR2 chains for signal transduction. JAK1 and JAK2 are attached to the intracellular carboxyl termini of IFN-γR1 and IFN-γR2, respectively. The pathway is initiated by the binding of antiparallel dimerized IFN-γ to the extracellular region of IFN-γR1, which causes a conformational change in IFN-γR. Inactive JAK2 kinase (attached to IFN-γR2) is activated by autophosphorylation, which in turn phosphorylates JAK1 (attached to IFN-γR1). Activated JAK1 phosphorylates the IFN-γR1 chain and forms two docking sites for the Src homology (SH)2 domains of STAT1. The homodimer of phosphorylated STAT1 functions as a transcription factor [36]. In addition to the classical JAK/STAT cascade described above, the mitogen-activated protein kinase pathway is also involved in IFN-γR-mediated signal transduction [37, 38].
It is possible that n-6 PUFAs modify signaling in any number of areas along these pathways. Certainly, AA treatment inhibited IFN-γ-induced phosphorylation of JAK1, STAT1, ERK1/2, and JNK. It has been postulated that AA promotes tumorigenesis by serving as the precursor of pro-inflammatory mediators such as 2-series prostaglandins and 4-series leukotrienes [39, 40]. However, the association between AA and the risk of cancer, such as breast and prostate cancers, is still not clear [41], and a derivative of AA, 6 iodo-δ-lactone, inhibits proliferation of colon cancer cells [42]. Therefore, AA may promote the growth of some neoplastic cells while inhibiting the proliferation of others, processes that are likely dependent upon the tissue microenvironment. Nevertheless, PUFAs such as EPA, DHA, and AA may regulate receptor expression and signal transduction by increasing the fluidity of the plasma membrane or activating fatty acid receptors such as G protein-coupled receptor 40 and 120 and peroxisome proliferator-activated receptors (PPARs) [43–47].
In this study, we showed that n-3 PUFAs modified the IFN-γ/IL-18BP/IL-18/IFN-γ loop, an important immune response to cancer. N-3 PUFAs inhibited IL-18BP production and mRNA expression in DU-145 prostate cancer cells when these cells were stimulated with IFN-γ. IFN-γR expression, and phosphorylation of JAK1, STAT1, ERK1/2, and P38 were inhibited by n-3 PUFAs. N-6 PUFA treatment has similar inhibiting effect on IFN-γ-induced IL-18BP production and mRNA expression in DU-145 cells. However, IFN-γR expression was not changed by AA, and JAK1, STAT1, ERK1/2, and JNK activation were altered. Our results indicate that n-3/n-6 PUFAs are an important modulator of prostate cancer cell production of IL-18BP.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr. Adriana Catalli for technical support. This work was supported by The Canadian Institute for Health Research, the National Research Council Canada, and the Canadian Breast Cancer Foundation-Atlantic.
Conflict of interest
The authors declare that they have no conflicts of interest.
Abbreviations
- IFN-γR
Interferon-γ receptor
- IL
Interleukin
- IL-18BP
IL-18 binding protein
- DHA
Docosahexaenoic acid
- DPA
Docosapentaenoic acid
- ELISA
Enzyme-linked immunosorbent assay
- EPA
Eicosapentaenoic acid
- ERK
Extracellular signal-regulated kinase
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- GC–MS
Gas chromatography–mass spectrometry
- JAK
Janus kinase
- JNK
c-Jun N-terminal kinase
- MHC
Major histocompatibility complex
- NK
Natural killer
- PBS
Phosphate-buffered saline
- PPARs
Peroxisome proliferator-activated receptors
- PUFAs
Polyunsaturated fatty acids
- qPCR
Quantitative polymerase chain reaction
- SH
Src homology
- STAT
Signal transducers and activators of transcription
- TBS
Tris-buffered saline
- TBST
TBS containing 0.05 % tween-20
- Th
T helper
- TNF
Tumor necrosis factor
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Quon H, Loblaw A, Nam R. Dramatic increase in prostate cancer cases by 2021. BJU Int. 2011;108(11):1734–1738. doi: 10.1111/j.1464-410X.2011.10197.x. [DOI] [PubMed] [Google Scholar]
- 3.Leung BP, Culshaw S, Gracie JA, Hunter D, Canetti CA, Campbell C, Cunha F, Liew FY, McInnes IB. A role for IL-18 in neutrophil activation. J Immunol. 2001;167(5):2879–2886. doi: 10.4049/jimmunol.167.5.2879. [DOI] [PubMed] [Google Scholar]
- 4.Darwich L, Coma G, Pena R, Bellido R, Blanco EJ, Este JA, Borras FE, Clotet B, Ruiz L, Rosell A, Andreo F, Parkhouse RM, Bofill M. Secretion of interferon-gamma by human macrophages demonstrated at the single-cell level after costimulation with interleukin (IL)-12 plus IL-18. Immunology. 2009;126(3):386–393. doi: 10.1111/j.1365-2567.2008.02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srivastava S, Pelloso D, Feng H, Voiles L, Lewis D, Haskova Z, Whitacre M, Trulli S, Chen YJ, Toso J, Jonak ZL, Chang HC, Robertson MJ. Effects of interleukin-18 on natural killer cells: costimulation of activation through Fc receptors for immunoglobulin. Cancer Immunol Immunother. 2013;62(6):1073–1082. doi: 10.1007/s00262-013-1403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tse BW, Russell PJ, Lochner M, Forster I, Power CA. IL-18 inhibits growth of murine orthotopic prostate carcinomas via both adaptive and innate immune mechanisms. PLoS ONE. 2011;6(9):e24241. doi: 10.1371/journal.pone.0024241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198(3):433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt MJ, Philippidou D, Reinsbach SE, Margue C, Wienecke-Baldacchino A, Nashan D, Behrmann I, Kreis S. Interferon-gamma-induced activation of Signal Transducer and Activator of Transcription 1 (STAT1) up-regulates the tumor suppressing microRNA-29 family in melanoma cells. Cell Commun Signal. 2012;10(1):41. doi: 10.1186/1478-811X-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin EC, Shin WC, Choi Y, Kim H, Park JH, Kim SJ. Effect of interferon-gamma on the susceptibility to Fas (CD95/APO-1)-mediated cell death in human hepatoma cells. Cancer Immunol Immunother. 2001;50(1):23–30. doi: 10.1007/s002620000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Street D, Kaufmann AM, Vaughan A, Fisher SG, Hunter M, Schreckenberger C, Potkul RK, Gissmann L, Qiao L. Interferon-gamma enhances susceptibility of cervical cancer cells to lysis by tumor-specific cytotoxic T cells. Gynecol Oncol. 1997;65(2):265–272. doi: 10.1006/gyno.1997.4667. [DOI] [PubMed] [Google Scholar]
- 12.Caretto D, Katzman SD, Villarino AV, Gallo E, Abbas AK. Cutting edge: the Th1 response inhibits the generation of peripheral regulatory T cells. J Immunol. 2010;184(1):30–34. doi: 10.4049/jimmunol.0903412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayakawa Y, Takeda K, Yagita H, Smyth MJ, Van Kaer L, Okumura K, Saiki I. IFN-gamma-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, alpha-galactosylceramide. Blood. 2002;100(5):1728–1733. [PubMed] [Google Scholar]
- 14.Palladino I, Salani F, Ciaramella A, Rubino IA, Caltagirone C, Fagioli S, Spalletta G, Bossu P. Elevated levels of circulating IL-18BP and perturbed regulation of IL-18 in schizophrenia. J Neuroinflammation. 2012;9:206. doi: 10.1186/1742-2094-9-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita K, Ewing CM, Isaacs WB, Pavlovich CP. Immunomodulatory IL-18 binding protein is produced by prostate cancer cells and its levels in urine and serum correlate with tumor status. Int J Cancer. 2011;129(2):424–432. doi: 10.1002/ijc.25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ray M, Hostetter DR, Loeb CR, Simko J, Craik CS. Inhibition of Granzyme B by PI-9 protects prostate cancer cells from apoptosis. Prostate. 2012;72(8):846–855. doi: 10.1002/pros.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC, Giovannucci EL. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80(1):204–216. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- 18.McEntee MF, Ziegler C, Reel D, Tomer K, Shoieb A, Ray M, Li X, Neilsen N, Lih FB, O’Rourke D, Whelan J. Dietary n-3 polyunsaturated fatty acids enhance hormone ablation therapy in androgen-dependent prostate cancer. Am J Pathol. 2008;173(1):229–241. doi: 10.2353/ajpath.2008.070989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irons R, Fritsche KL. Omega-3 polyunsaturated fatty acids impair in vivo interferon- gamma responsiveness via diminished receptor signaling. J Infect Dis. 2005;191(3):481–486. doi: 10.1086/427264. [DOI] [PubMed] [Google Scholar]
- 20.Kang JX, Wang J. A simplified method for analysis of polyunsaturated fatty acids. BMC Biochem. 2005;6:5. doi: 10.1186/1471-2091-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Hobeika AC, Etienne W, Cruz PE, Subramaniam PS, Johnson HM. IFNgamma induction of p21WAF1 in prostate cancer cells: role in cell cycle, alteration of phenotype and invasive potential. Int J Cancer. 1998;77(1):138–145. doi: 10.1002/(SICI)1097-0215(19980703)77:1<138::AID-IJC21>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Kominsky SL, Hobeika AC, Lake FA, Torres BA, Johnson HM. Down-regulation of neu/HER-2 by interferon-gamma in prostate cancer cells. Cancer Res. 2000;60(14):3904–3908. [PubMed] [Google Scholar]
- 24.Igney FH, Krammer PH. Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukoc Biol. 2002;71(6):907–920. [PubMed] [Google Scholar]
- 25.Selleck WA, Canfield SE, Hassen WA, Meseck M, Kuzmin AI, Eisensmith RC, Chen SH, Hall SJ. IFN-gamma sensitization of prostate cancer cells to Fas-mediated death: a gene therapy approach. Mol Ther. 2003;7(2):185–192. doi: 10.1016/S1525-0016(02)00040-0. [DOI] [PubMed] [Google Scholar]
- 26.Hastie C. Interferon gamma, a possible therapeutic approach for late-stage prostate cancer? Anticancer Res. 2008;28(5B):2843–2849. [PubMed] [Google Scholar]
- 27.He YF, Wang XH, Zhang GM, Chen HT, Zhang H, Feng ZH. Sustained low-level expression of interferon-gamma promotes tumor development: potential insights in tumor prevention and tumor immunotherapy. Cancer Immunol Immunother. 2005;54(9):891–897. doi: 10.1007/s00262-004-0654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinarello CA. Targeting interleukin 18 with interleukin 18 binding protein. Ann Rheum Dis. 2000;59(Suppl 1):17–20. doi: 10.1136/ard.59.suppl_1.i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golab J. Interleukin 18–interferon gamma inducing factor–a novel player in tumour immunotherapy? Cytokine. 2000;12(4):332–338. doi: 10.1006/cyto.1999.0563. [DOI] [PubMed] [Google Scholar]
- 30.Micallef MJ, Tanimoto T, Kohno K, Ikeda M, Kurimoto M. Interleukin 18 induces the sequential activation of natural killer cells and cytotoxic T lymphocytes to protect syngeneic mice from transplantation with Meth A sarcoma. Cancer Res. 1997;57(20):4557–4563. [PubMed] [Google Scholar]
- 31.Begin ME, Ells G, Das UN, Horrobin DF. Differential killing of human carcinoma cells supplemented with n-3 and n-6 polyunsaturated fatty acids. J Natl Cancer Inst. 1986;77(5):1053–1062. [PubMed] [Google Scholar]
- 32.Corsetto PA, Montorfano G, Zava S, Jovenitti IE, Cremona A, Berra B, Rizzo AM. Effects of n-3 PUFAs on breast cancer cells through their incorporation in plasma membrane. Lipids Health Dis. 2011;10:73. doi: 10.1186/1476-511X-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang T, Fang S, Zhang HX, Xu LX, Zhang ZQ, Yuan KT, Xue CL, Yu HL, Zhang S, Li YF, Shi HP, Zhang Y. N-3 PUFAs have antiproliferative and apoptotic effects on human colorectal cancer stem-like cells in vitro. J Nutr Biochem. 2013;24(5):744–753. doi: 10.1016/j.jnutbio.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 34.Feng C, Keisler DH, Fritsche KL. Dietary omega-3 polyunsaturated fatty acids reduce IFN-gamma receptor expression in mice. J Interferon Cytokine Res. 1999;19(1):41–48. doi: 10.1089/107999099314405. [DOI] [PubMed] [Google Scholar]
- 35.Bonilla DL, Ly LH, Fan YY, Chapkin RS, McMurray DN. Incorporation of a dietary omega 3 fatty acid impairs murine macrophage responses to Mycobacterium tuberculosis. PLoS ONE. 2010;5(5):e10878. doi: 10.1371/journal.pone.0010878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramana CV, Gil MP, Schreiber RD, Stark GR. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol. 2002;23(2):96–101. doi: 10.1016/S1471-4906(01)02118-4. [DOI] [PubMed] [Google Scholar]
- 37.Valledor AF, Sanchez-Tillo E, Arpa L, Park JM, Caelles C, Lloberas J, Celada A. Selective roles of MAPKs during the macrophage response to IFN-gamma. J Immunol. 2008;180(7):4523–4529. doi: 10.4049/jimmunol.180.7.4523. [DOI] [PubMed] [Google Scholar]
- 38.Matsuzawa T, Kim BH, Shenoy AR, Kamitani S, Miyake M, Macmicking JD. IFN-gamma elicits macrophage autophagy via the p38 MAPK signaling pathway. J Immunol. 2012;189(2):813–818. doi: 10.4049/jimmunol.1102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakanishi M, Rosenberg DW. Roles of cPLA2alpha and arachidonic acid in cancer. Biochim Biophys Acta. 2006;1761(11):1335–1343. doi: 10.1016/j.bbalip.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang P, Cartwright CA, Li J, Wen S, Prokhorova IN, Shureiqi I, Troncoso P, Navone NM, Newman RA, Kim J. Arachidonic acid metabolism in human prostate cancer. Int J Oncol. 2012;41(4):1495–1503. doi: 10.3892/ijo.2012.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakai M, Kakutani S, Horikawa C, Tokuda H, Kawashima H, Shibata H, Okubo H, Sasaki S. Arachidonic acid and cancer risk: a systematic review of observational studies. BMC Cancer. 2012;12:606. doi: 10.1186/1471-2407-12-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomasz L, Oglio R, Rossich L, Villamar S, Perona M, Salvarredi L, Dagrosa A, Pisarev MA, Juvenal GJ. 6 Iodo-delta-lactone: a derivative of arachidonic acid with antitumor effects in HT-29 colon cancer cells. Prostaglandins Leukot Essent Fatty Acids. 2013;88(4):273–280. doi: 10.1016/j.plefa.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Rietjens IM, van Tilburg CA, Coenen TM, Alink GM, Konings AW. Influence of polyunsaturated fatty acid supplementation and membrane fluidity on ozone and nitrogen dioxide sensitivity of rat alveolar macrophages. J Toxicol Environ Health. 1987;21(1–2):45–56. doi: 10.1080/15287398709531001. [DOI] [PubMed] [Google Scholar]
- 44.Brown M, Anderson KM, Patel H, Hopfinger AJ, Harris JE. Eicosatetraynoic and arachidonic acid-induced changes in cell membrane fluidity consonant with differences in computer-aided design-structures. Biochim Biophys Acta. 1992;1105(2):285–290. doi: 10.1016/0005-2736(92)90206-2. [DOI] [PubMed] [Google Scholar]
- 45.Mancini AD, Poitout V. The fatty acid receptor FFA1/GPR40 a decade later: how much do we know? Trends Endocrinol Metab. 2013;24(8):398–407. doi: 10.1016/j.tem.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Mobraten K, Haug TM, Kleiveland CR, Lea T. Omega-3 and omega-6 PUFAs induce the same GPR120-mediated signalling events, but with different kinetics and intensity in Caco-2 cells. Lipids Health Dis. 2013;12:101. doi: 10.1186/1476-511X-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, Moorhead JF, Varghese Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005;67(3):867–874. doi: 10.1111/j.1523-1755.2005.00151.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.